Abstract
The conidiomatal differences among six root-inhabiting Cryptosporiopsis species were studied at an ultrastructral level and a dichotomous key was then provided. Cryptosporiopsis brunnea is delimited by its conidiomata having a distinct sterile excipular covering tissue without adhesive amorphous material and smooth to pitted macroconidia. Cryptosporiopsis radicicola produces only excipular covering conidioma-like tissue with adhesive amorphous material and setae. Synnematous conidiomata with abundant macroconidia dominate the colony of C. ericae. Cryptosporiopsis rhizophila is different in its globose to subglobose conidiomata, consisting of loosely aggregated vegetative hyphae developing macroconidial conidiophores. Cryptosporiopsis grisea, being the only teleomorph-connected species, differs from the others in its distinct columnar surface structures composed of entangling hyphae and rising well above the aerial mycelium, and unique platform-like hymenium consisting of tightly packed hyphal stroma interspersed with macroconidial conidiophores and localized microconidia conidiophores. Cryptosporiopsis melanigena is distinguished by the production of abundant chlamydospores that secede schizolytically.
Introduction
Species of Cryptosporiopsis Bubák and Kabát, with teleomorphs in Pezicula Tul. and C. Tul., have been collected predominantly from bark, wood and/or roots of trees and shrubs in temperate and boreal forests of the northern hemisphere (Verkley Citation1999). Some of these species are recognized as plant pathogens (Butin Citation1983; Kowalski Citation1983; Taylor Citation1983; Kehr Citation1991) and six are known as endophytes in the roots of woody plants (Kowalski and Bartnik Citation1995; Kowalski et al. Citation1998; Verkley Citation1999; Verkley et al. Citation2003; Sigler et al. Citation2005): viz., Cryptosporiopsis grisea (Pers.) Petr., the anamorph of the wood and bark endophyte Pezicula cinnamomea (DC.) Sacc., capable of spreading into roots of dying trees (Kowalski Citation1983); Cryptosporiopsis melanigena Kowalski and Halmschlager and Cryptosporiopsis radicicola Kowalski and Bartnik, isolated originally from the roots of Quercus (Kowalski and Bartnik Citation1995; Kowalski et al. Citation1998); Cryptosporiopsis rhizophila Verkley and Zijlstra, Cryptosporiopsis ericae Sigler, and Cryptosporiopsis brunnea Sigler, all from the roots of ericaceous plants (Verkley et al. Citation2003; Sigler et al. 2005). Only C. grisea has been linked to a teleomorph. Recently, C. radicicola and C. ericae were reported as common endophytes in the roots of Populus tremuloides Michx. (tembling aspen, hereafter aspen) (Wang et al. Citation2007; Tsuneda et al. Citation2009).
With the addition of C. brunnea and C. ericae, the dichotomous key presented by Verkley et al. (Citation2003) to the four previously described root endophytic species is insufficient for the purpose of routine identification. Furthermore, a recent study using light (LM) and scanning electron microscopy (SEM) showed that some key characters were variable and others associated with morphogenesis and structure of conidiomata had been overlooked (Wang et al. Citation2007). Consequently, I have made a detailed re-examination of the morphological attributes of all six root-inhabiting species and searched for new diagnostic characters associated with conidiomatal morphogenesis. Here, I illustrate additional characters that distinguish the six root-inhabiting species of Cryptosporiopsis, and provide a dichotomous key that incorporates these new data along with some revisions to previously reported distinctions based on other morphological features.
Materials and methods
Ex-type strains of C. brunnea, C. ericae, C. melanigena, C. radicicola, C. rhizophila and one representative of C. grisea (ex-type not found) were obtained from the University of Alberta Microfungus Collection and Herbarium (UAMH). Two additional strains from aspen roots (C. ericae, UAMH 10920, and C. radicicola, UAMH 10864), whose phylogenetic dispositions had been confirmed previously (Wang et al. Citation2007), were also examined (). Strains were grown on corn meal agar (CMA), malt extract agar (MEA) and oatmeal agar (OA), at 20 or 5 °C in the dark (Wang et al. Citation2007). Replicate plates of all three media overlaid with a sterile cellophane membrane (Innovia Films, Atlanta, GA, USA) were inoculated with each of the eight strains to examine cellulolytic abilities and whether the membrane would promote conidiomatal production.
Table 1. Eight strains of root-inhabiting Cryptosporiopsis species examined in this study
Conidial size of each Cryptosporiopsis isolate was measured under a light microscope with 100 replicates and compared by using Student's t-test ().
Table 2. Growth rate and colonial characteristics of six root-associated species of Cryptosporiopsis
Table 3. Statistical analyses of conidial sizes among eight strains of root-inhabiting Cryptosporiopsis species (Student's t-test, n = 100)
Within 1–3 months of inoculation, sporulating cultures were prepared using methods described in Tsuneda et al. (Citation2004), and then examined and photographed at 10 or 15 KV using a Hitachi S-510 scanning electron microscope (Hitachi, Tokyo, Japan).
Results
There were few distinctive cultural characteristics across the six species. Cryptosporiopsis rhizophila had a significantly slower growth rate (12 mm in diameter after 14 days on MEA) than the other five isolates (30–55 mm in diameter after 14 days on MEA). Colonies of C. grisea differed in being yellow at first and olivaceous after 14 day, and with yellowish brown exudates. No exudate was formed in cultures of the other five species ().
Cryptosporiopsis brunnea, C. radicicola, and C. ericae readily formed conidiomata on MEA after 1 month at 22 °C in the dark. Neither C. grisea nor C. rhizophila produced conidiomata on MEA, although C. grisea did so on CMA after 3–6 months at 22 °C in the dark, and C. rhizophila on CMA overlaid with cellophane membrane after 1 month at 5 °C in the dark. Under a dissecting microscope, conidiomata were recognizable as tiny, cream to brown dots on the agar surface. Cryptosporiopsis melanigena produced macroconidia on MEA but conidiomata were never observed.
Conidiomata of both C. brunnea () and C. radicicola () were distinct in having a saucer-shaped excipulum-like structure that opened to expose a layer of active conidiogenous cells (). In C. brunnea, excipular tissue was composed of abundant, incurved sterile hyphae that were branched, tapered and determinate in growth, 3–3.4 μm at the base, 0.8–2.8 μm at the tip, and 37–60 μm long (). As the excipular tissue opened (), it ruptured and became discontinuous by the time the conidioma had fully expanded (). The layer of sporulating tissue was composed of tightly packed conidiogenous cells (phialides) bearing macroconidia. Some conidiophores elongated beyond the upper level of macroconidia, producing phialides in a more or less sympodial fashion (, arrow). Macroconidia were smooth to pitted, ellipsoidal, straight or slightly curved, rounded at the apex, narrowly truncate and apiculate at the base, nonseptate, hyaline becoming yellowish with age, 25–35 × 7–9 μm (, arrowheads; and ). Typical microconidia, chlamydospores and setae were not observed and cellulolytic activity was not evident. In C. radicicola, the excipular tissue consisted of two elements, i.e. branched vegetative hyphae, mostly 3–6 μm wide, and much finer setose hyphae, mostly 1–2 μm wide, cemented together with adhesive amorphous material (). As in C. brunnea, conidiomata were initially closed but gradually opened as the conidiogenous layer matured ( and ). In the conidiogenous layer, both macro- and micro-conidia were abundant and sparse setose hyphae also occurred. The macro- and micro-conidiogenous cells (both phialidic) were tightly packed and embedded in adhesive amorphous material (). Macroconidia were straight to curved, aseptate, and 15–32 × 5–8 μm (). Microconidia were mostly ellipsoidal, straight, and 2–6.5 × 1–2 μm (). Some conidia were intermediate between macro- and micro-conidia in size and shape. Setose hyphae occurring in the conidiogenous layer were smooth, septate, hyaline, blunt, up to 94 μm long and ca. 2.5 μm wide at the base. Chlamydospores were not found. No notable differences in morphology, including the conidiomatal structure, were found between the two strains of C. radicicola and both were capable of degrading cellophane membrane.
Figures 1–6. Scanning electron micrographs of Cryptosporiopsis brunnea (ex-type, UAMH 10106) on MEA after 1 month at 22 °C. (1) Immature conidioma containing a few macroconidia (arrowheads). (2) Near mature conidioma with an opening, excipular covering tissue (arrow). (3) Fully expanded conidioma exposing the conidiogenous layer with numerous macroconidia. The arrow indicates the excipular hyphae. (4) Enlarged view of sterile excipular hyphae. (5) More or less alternately proliferated macroconidiogenous cells (arrow). (6) Smooth and pitted macroconidia. Bars = 40 μm (); 20 μm ( and ); 30 μm (); 6 μm ( and ).
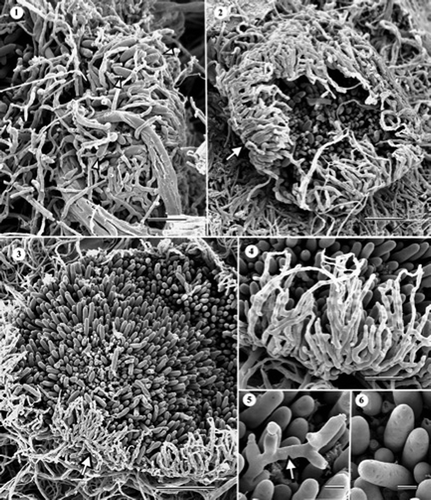
Figures 7–15. Scanning electron micrographs (SEM) of Cryptosporiopsis radicicola (ex-type, UAMH 10729, CBS 640.94) on MEA after 1 month at 22 °C. (7) Conidioma with an opening excipular covering tissue (arrow). (8) Mature conidioma. (9) Enlarged view of excipular tissue consisting of thicker hyphal and finer setose elements, partially embedded in adhesive amorphous material. (10) Part of conidiogenous layer showing abundant macro- and micro-conidia, and sparse setose hyphae. Conidiogenous cells are embedded in amorphous material. –. SEM of C. ericae (BWC-43-127a, UAMH 10920) on MEA after 1 month at 22 °C. (11) Developing synnema. (12) Synnema forming numerous macro- and micro-conidia. (13) Sporodochium-like structure resulting from the merger of two synnemata. The arrow indicates the thickened stipe. (14) Enlarged view of a portion of a conidial head showing macro- and micro-conidia, and sparse setose hyphae. (15) Chains of chlamydospores. Septum schizolysis was infrequent (arrowheads). Bars = 20 μm (); 40 μm (); 10 μm (, and ); 5 μm (); 15 μm ( and 13); 33 μm ().
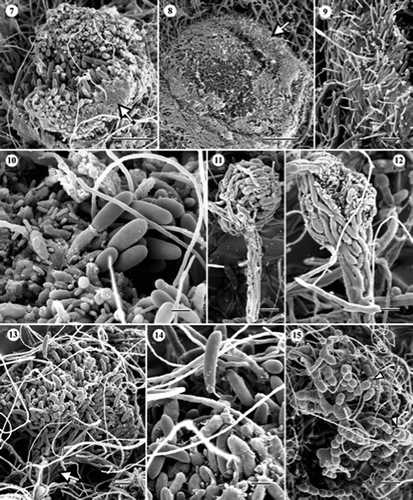
Figures 16–21. Scanning electron micrographs of Cryptosporiopsis rhizophila (ex-type, UAMH 10730, CBS 109839) on MEA after 1 month at 22°C. (16) Conidiomata of various developmental stages. The arrow indicates an immature one. (17) Two adjacent conidiomata, each with a stipe (arrows). Arrowheads indicate bundles of setose hyphae, one arising from the conidiogenous head and the other from the stipe. (18) Two merged conidiomata appearing like a sporodochium. (19) Macroconidia (arrows) and microconidia (arrowheads). (20) Microconidiogenous cell (arrow). (21) Macroconidiogenous cells (arrows) after secession of conidia surrounded by tufts of setose hyphae. Bars = 70 μm (); 55 μm (); 50 μm (); 3 μm (); 6 μm (); 22 μm ().
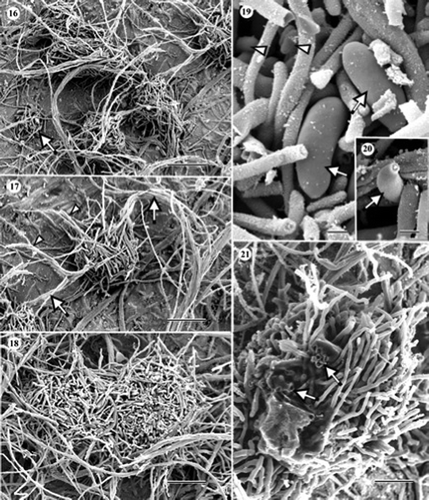
Figures 22–29. Scanning electron micrographs of Cryptosporiopsis grisea (UAMH 10860, CBS 482.97) on CMA after 3 months at 22 °C. (22) Conidiomatal initial. (23) Immature conidioma with a developing conidiogenous layer embedded in adhesive amorphous material (arrow). (24) Obliquely fractured immature conidiogenous layer showing sterile stromatic tissue (arrow). (25)–(27) Obliquely fractured mature conidiogenous layer showing macroconidia arising from the stromatic tissue. Arrows in indicate macroconidia-bearing remnants of amorphous material. The arrow in indicates macroconidiogenous cell. (28) Top view of a mature conidioma. (29) Macro- and micro-conidia (arrows). Bars = 25 μm (); 17 μm (); 20 μm (); 12 μm ( and ); 6 μm ( and ); 30 μm ().
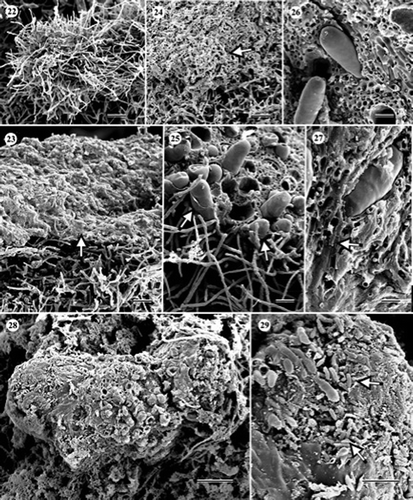
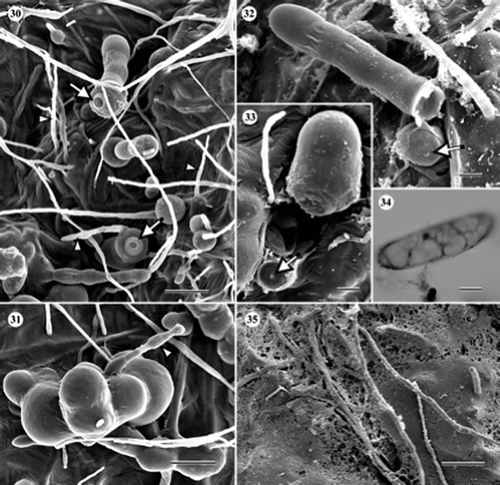
Figures 30–35. Scanning electron and light micrographs of Cryptosporiopsis melanigena (ex-type, UAMH 10731, CBS 898.97) on CMA overlaid with cellophane membrane after 3 months at 22 °C. (30) Variously shaped chlamydospores and setose hyphae (arrowheads). Arrows indicate secession scars. (31) Chlamydospore giving rise to a germ tube (arrowhead). (32, 33). Chlamydospores seceded by septum schizolysis. Arrows indicate septal pores at the exposed apexes of conidiogenous cells. (34) Macroconidium. (35) Cellophane being degraded by hyphae. Bars = 17 μm (); 12 μm (); 6 μm (); 4 μm ( and ); 30 μm ().
Conidiomata in C. ericae (–) were mostly synnematous, although stalks were obscured when two or more conidiomata formed in close proximity (see in Wang et al. (Citation2007)) to form a structure resembling a sporodochium. A few single conidiomata appeared sporodochial (see in Wang et al. (Citation2007)) but their occurrence was rare. Synnemata consisted of few to many erect conidiophores, up to 185 μm long, and a conidiogenous head, initially enclosed, and 64–125 μm in diameter (–). Both macro- and micro-conidiogenous cells were phialidic, producing abundant conidia in basipetal succession (see –9 in Wang et al. (Citation2007)), although relative abundances of macro- and micro-conidia varied among synnemata. Macroconidia were straight to slightly curved, up to three septate with age, and 16–35 × 4–8 μm (). Microconidia were mostly oblong, aseptate, and 2–6 × 1–1.5 μm ( and ). Chlamydospores were initially hyaline, becoming darkly pigmented with age, globose (ca. 22 μm in diameter) to ellipsoidal, 19 × 17 μm, usually in chains or in clusters, and occasionally seceded schizolytically (). Setose hyphae were hyaline, darkly pigmented with age, 5.2–7.8 μm wide, and up to 100 μm long. No significant morphological differences were noted between the two strains of C. ericae and both strains degraded cellophane membrane. In C. rhizophila, conidiomata of various developmental stages were found at the base of radiating coaxial hyphal strands after incubating cultures for about 6 months ( and ). Strands were composed of three to more hyphae (, arrows) and conidiogenous heads were subglobose and mostly 80–90 μm in diameter ( and ). Single or bundled setose hyphae, which were determinate in growth, often arose from both stalks and heads of the conidiomata (, arrowheads). When two or more conidiomata grew together, the resulting structure resembled a hemispherical sporodochium (). Both macro- and micro-conidia occurred in conidiomata of C. rhizophila but were much fewer than in C. ericae. Macroconidia were ellipsoidal, slightly curved, rounded at the apex, truncate at the base, smooth, aseptate, and 19–22 × 5–6 μm (). Microconidia were ellipsoidal, smooth, aseptate, and 1–3 × 1–1.5 μm (). Conidiogenous cells of both forms of conidia arose singly or in bundles ( and ). They appeared phialidic but basipetal, successive conidiation could not be confirmed. Tips of macro- and micro-conidiogenous cells were 1.4–1.7 μm () and less than 1 μm () in diameter, respectively. In mature conidiomata, conidiogenous cells were often surrounded by tufts of setose hyphae (). Chlamydospores were not found and cellulolytic activity was not evident.
During conidioma morphogenesis in C. grisea (–), a spherical mass of entangled vegetative hyphae formed first and subsequently gave rise to numerous, sterile aerial hyphae (). They became embedded in abundant amorphous material to form a crustose upper layer () that was highly stromatic (). Mature conidiomata were subspherical to irregular in shape (), having abundant conidiogenous cells that were interspersed among the sterile elements (–; obliquely fractured views). Macroconidia predominated in the conidiogenous layer and microconidia occurred in localized areas ( and ). Macroconidia were ellipsoidal to oblong, straight to slightly curved, rounded at the ends, apiculate at the base, mostly aseptate but sometimes with one to three septae (), thin-walled, hyaline when liberated, and 24–38 × 5–12 μm. Macroconidiogenous cells were phialidic, borne on single, narrow conidiophores, ca. 2 μm wide. Microconidia were filiform, straight or slightly curved, truncate at the base and rounded at the apex, and 4.2–8.5 × 0.8–1 μm (). I could not find microconidiogenous cells and could not confirm the nature of conidiogenesis. Chlamydospores were absent and cellulolytic activity was not evident.
None of the cultures of C. melanigena (–) produced conidiomata in this study but the species was distinguishable from the others by the production of abundant chlamydospores. Chlamydospores were globose (10–12 μm in diameter) to oblong (16–35×8–12 μm), and formed singly or in clusters (–). In older cultures, chlamydospores germinated to form germ tubes ( and ), and often seceded schizolytically ( and ). Macroconidia were sparse, slightly curved, aseptate, and 16–29×4.8–7.2 μm (). Microconidia were not found. The mode of conidiogenesis could not be confirmed. Setose hyphae were smooth, straight, and up to 144 μm long. Degradation of cellophane membrane was evident ().
Conidial sizes of all six species were analyzed statistically (). Macroconidia were significantly shorter in C. rhizophila, and significantly broader in C. grisea than in other species. Of particular interest were the significant differences in size in both macro- and micro-conidia between strains within C. radicicola and C. ericae. There were also some discrepancies between my measurements and those reported in the original species descriptions (). For example, there was no overlap between my initial measurements and those given in Sigler et al. (Citation2005) for macroconidia of C. brunnea. I later found that the size of macroconidia in young cultures (55-day-old) matched that given in Sigler et al. (Citation2005) and that conidia taken from much older colonies (1-year-old) were much larger. The following dichotomous key is based on the foregoing morphological characteristics.
Key to the six root-inhabiting Cryptosporiopsis species in culture
| 1. | Colonies on MEA initially white, becoming pale to reddish to dark brown, or black with age; conidiomata acervular, or synnematous, or sporodochial. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .2 | ||||
| 2. | Colonies on MEA initially yellowish green, becoming brownish, cinnamon or olivaceous, and producing yellowish brown exudates, conidiomata distinctly sclerotized . . . . . . . . . . . . . . . . . . C. grisea | ||||
| 3. | Conidiomata acervular** . . . . . . . . . . . . . . . . . . . . . 3 | ||||
| 4. | Conidiomata synnematous or forming at the base of radiating coaxial hyphal strands . . . . . . . . . . . . . 5 | ||||
| 5. | Chlamydospores abundant, often seceding schizolytically, macroconidia often produced from conidiogenous cells arising from vegetative hyphae; microconidia absent . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . C. melanigena | ||||
| 6. | Chlamydospores uncommon . . . . . . . . . . . . . . . . . . 4 | ||||
| 7. | Excipulum composed of incurved, branched, tapered vegetative hyphae and without adhesive amorphous material; macroconidia smooth to pitted; microconidia and setose hyphae absent . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . C. brunnea | ||||
| 8. | Excipulum composed of both branched vegetative hyphae and finer setose hyphae cemented together with adhesive amorphous material; macroconidia smooth without pitting; microconidia abundant; setose hyphae present but sparse . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . C. radicicola | ||||
| 9. | Conidiomata synnematous with a stalk of aggregated vegetative hyphae and a head consisting mostly of branched macro- and micro-conidiophores and conidiogenous cells with sparse setose hyphae; both macro- and micro-conidia abundant; chlamydospores catenate, and occasionally seceding schizolytically, colonies on MEA dark brown and 45 mm after 14 days . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . C. ericae | ||||
| 10. | Conidiomata globose to subglobose consisting of relatively sparse, often integrated macro- and micro-conidiophores, and forming at the base of radiating coaxial hyphal strands; both macro- and micro-conidia sparse, colonies on MEA pale brown and 12 mm after 14 days . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . C. rhizophila * | ||||
*Two or more synnemata often aggregate to form a structure resembling a sporodochium.
**According to Kowalski et al. (Citation1998).
Discussion
Most root-associated species of Cryptosporiopsis showed a strong tendency to develop dark brown to black colonies on agar media, making them difficult to distinguish, but C. grisea was unique in producing mycelium that was yellow at first and later olivaceous. Rate of growth was only distinctive in C. rhizophila, which grew markedly slower compared to the other five strains on MEA. A similar slow growth rate in C. rhizophila was also noted on MEA by Verkley et al. (Citation2003). Cultures of C. rhizophila and C. melanigena were white on CMA. More deeply pigmented colonies developed only after serial transfers to fresh MEA and after prolonged incubation at room temperature in the dark.
Sutton (Citation1980) mentioned that there is little difference between Cryptosporiopsis species and the characters used to distinguish taxa are few and unreliable, and conidial size is only occasionally useful. The plasticity of conidia during the development stages of Cryptosporiopsis species does not necessarily indicate different species. SEM observations of various developmental stages of six species of Cryptosporiopsis provided useful information regarding conidiomatal characteristics, some of which could not be detected from squash mounts and LM examination. For example, mature conidiomata of C. brunnea were originally described merely as hemispherical (Sigler et al. Citation2005) and those of C. radicicola as synnema-like or sporodochial (Kowalski and Bartnik Citation1995). However, I was able to observe that conidiomata of both species differed from those of other species in having excipular tissue and, furthermore, that they were distinguishable from each other by differences in the structural attributes of this tissue, such as the presence of setose elements and amorphous matrix material. Also, microconidia were abundant in C. radicicola but absent in C. brunnea. Immature conidiomata of C. radicicola were sometimes synnema-like (see – in Wang et al. (Citation2007)), as Kowalski and Bartnik (Citation1995) described, or pycnidium-like (see – in Wang et al. (Citation2007)) but fully developed ones were not (see and in Wang et al. (Citation2007)). Sigler et al. (Citation2005) reported that conidiomata of C. ericae were sporodochial or consisted of raised synnema-like tufts, while Verkley et al. (Citation2003) described those of C. rhizophila as superficial, hemispherical sporodochia, which may become surrounded by tufts of setose hyphae, or erect synnema-like columns. My SEM observations, however, showed that the conidiomata of only C. ericae were synnematous. Exceptions occurred in C. ericae with some unusual forms (see in Wang et al. (2007)), and when two or more conidiomata coalesced to form a structure resembling sporodochium. Synnemata in C. ericae were similar in being of the “determinate-parallel type” (Seifert Citation1985), consisting of a more or less compacted group of erect and sometimes fused conidiophores bearing conidia at the apex. The tufts of coaxial strands in C. rhizophila were referred to by Verkley et al. (Citation2003) as “synnema-like”, but this term is inappropriate in the absence of a single stipe of fused hyphae and/or a capitulum of conidiogenous cells and conidia. In some instances, conidiomata of C. rhizophila grew together to form a structure resembling the “hemispherical sporodochium” referred to by Verkley et al. (Citation2003). Therefore, C. ericae and C. rhizophila are similar in producing conidiomatal structures resembling sporodochia. However, the two species differed in that (i) the conidial head of C. ericae consisted primarily of macro- and micro-conidiogenous cells while the conidiogenous basal structure of C. rhizophila contained sterile hyphae amongst the conidiogenous cells and resulting in fewer macro- and micro-conidia, and (ii) the setose, determinate hyphae arising both from the conidiogenous head and the stalk of C. rhizophila were absent in C. ericae. Due to the setose hyphae, which complicated the conidioma structure, SEM observations of different developmental stages were particularly helpful in revealing the conidiomatal nature of C. rhizophila.
The structure of the conidioma of Cryptosporiopsis grisea was unique. According to Groves (Citation1938), conidiomata formed in culture were stromatic, at first rounded, then becoming more or less cylindrical to cylindric–conic, then spreading out widely, sometimes becoming dish-shaped. I confirmed these features and was able to clarify further structural details by observing developmental stages and mature conidiomata fractured along different planes. This approach showed that the sporogenous layer was crustose, consisting of a tightly packed, hyphal stroma interspersed with macro- and micro-conidial conidiogenous cells, and formed beneath a blanket of amorphous material.
Distinctive differences in the shape (straight or curved) and surface morphology of conidia were generally not visible, although the mature macroconidia of C. brunnea were often pitted, albeit somewhat faintly, rather than smooth, which was typical for the other species. There were some significant differences in conidial sizes between some species (), but these data must be interpreted with caution. Significant differences in length of both macro- and micro-conidia were evident between different strains in both C. ericae and C. radicicola (p< 0.05), and there were substantial differences between my measurements and those in original descriptions. Finally macroconidia obtained from old cultures were much larger than those from young colonies in C. brunnea. Kowalski et al. (Citation1998) also noted that macroconidia of C. melanigena were different in shape, size, color and structure of cytoplasm (granular vs. eguttulate) depending on the age of cultures. Therefore, the shape and size of conidia are characters that should be used cautiously for making distinctions among species.
Verkley et al. (Citation2003) considered the presence of chlamydospores a key characteristic to distinguish the four root-inhabiting species of Cryptosporiopsis and treated C. radicicola as a chlamydospore-producing species. Sigler et al. (Citation2005) claimed that the absence of chlamydospores in C. ericae distinguished it from C. radicicola. Previous observations (Wang et al. Citation2007) and those reported here do not agree with this distinction because, in contrast, chlamydospores were absent in C. radicicola and present in C. ericae in my material. This indicates that the presence or absence of chlamydospores is not a reliable feature to use in recognizing these species. An exception occurs perhaps with C. melanigena. Although this species did not produce conidiomata, it was readily distinguishable from others by the abundant production of one- to multi-celled, thick-walled spores that often seceded by septum schizolysis. The use of the term chlamydospores require some clarifications because it has been variously defined depending on authors (Riggs and Mims Citation2000; Tsuneda and Currah Citation2006). Carmichael (Citation1971), for example, restricted the use of the term for resistant cells that are non-deciduous, lacking any mechanism for liberation. If this definition were to be adopted here, the thick-walled spores of C. melanigena would not be considered chlamydospores because of their release from the parent hypha by septum schizolysis. Broader definitions refer to chlamydospores as darkly pigmented, thick-walled and developing in a terminal or intercalary position on a hypha, and mode of dehiscence is not considered (e.g. Riggs and Mims Citation2000). The broader definition is applied here.
Sigler et al. (Citation2005) reported that both C. ericae and C. brunnea were able to decompose cellophane membrane overlaid onto CMA. Using the same technique, I examined cellophane-decomposing abilities of the eight strains by LM and SEM and confirmed cellulolytic ability in C. ericae but not in C. brunnea. Cryptosporiopsis melanigena was also capable of degrading cellophane membrane, a characteristic not mentioned in the original description. These results suggest that cellulolytic ability assayed by this method is not reliable.
Based on the above results, I conclude that conidioma structure appears to be the most reliable morphological character for distinguishing the root-inhabiting species of Cryptosporiopsis. However, my observations and measurements were based on very limited material (one isolate of each species in most cases). The lack of sporulation in the isolate of C. melanigena and somewhat irregular colonial characteristics of C. rhizophila and C. melanigena (e.g. pale colonies) indicate that culture degeneracy in these strains might have altered their typical characteristics. Also, due to the very limited sampling, I was not able to consider character variation within species. A broader approach to sampling, along with a parallel analysis of DNA sequence variation, would no doubt clarify the reliability of the characters I used to construct the key. However, Wang et al. (Citation2007) showed, at least, that conidiomatal characteristics were reliable in dividing 44 isolates of Cryptosporiopsis from aspen roots between C. ericae and C. radicicola, and this was corroborated by observations on two additional and distinctive isolates of each of these species in this study.
Acknowledgements
This research was supported by Natural Sciences and Engineering Research Council of Canada (NSERC) Discovery Grant and by Alberta's Conservation Association's grant. I am grateful to Dr Akihiko Tsuneda for SEM technical support.
References
- Butin , H. 1983 . Krankheiten der Wald- und Parkbaume , Stuttgart : Georg Thieme .
- Carmichael , JW. 1971 . “ Blastospores, aleuriospores, chlamydospores ” . In Taxonomy of fungi imperfecti , Edited by: Kendrick , WB . 309 Toronto : University of Toronto Press .
- Groves , JW. 1938 . The perfect stage of Catinula turgida . Mycologia , 30 : 46 – 53 .
- Kehr , RD. 1991 . Pezicula canker of Quercus rubra L., caused by Pezicula cinnamomea (DC.) Sacc. I. Symptoms and pathogenesis . Eur J Forest Pathol. , 21 : 218 – 233 .
- Kowalski , T. 1983 . Vorkommen von Pilzen in durch Luftverunreinigung geschädigten Wäldern im Oberschlesischen und Krakauer Industriegebiet IX. Mykoflora von Quercus robur L. und Q. rubra L. an einem Standort mit mittlerer Immisionsbelastung . Eur J Forest Pathol. , 13 : 46 – 59 .
- Kowalski , T and Bartnik , C. 1995 . Cryptosporiopsis radicicola sp. nov. from roots of Quercus robur . Mycol Res. , 99 : 663 – 666 .
- Kowalski , T , Halmschlager , E and Schrader , K. 1998 . Cryptosporiopsis melanigena sp. nov., a root-inhabiting fungus of Quercus robur and Q. petraea . Mycol Res. , 102 : 347 – 354 .
- Riggs , W and Mims , CW. 2000 . Ultrastructure of chlamydospore development in the plant pathogenic fungus Thielaviopsis basicola . Mycologia , 92 : 123 – 129 .
- Seifert , KA. 1985 . A monograph of Stilbella and some allied Hyphomycetes . Stud Mycol. , 27 : 1 – 236 .
- Sigler , L , Allan , T , Lim , SR , Berch , S and Berbee , M. 2005 . Two new Cryptosporiopsis species from roots of ericaceous hosts in western North America . Stud Mycol. , 53 : 53 – 62 .
- Sutton , BC. 1980 . The Coelomycetes , Kew (Surrey, UK) : Commonwealth Mycological Institute .
- Taylor , GS. 1983 . Cryptosporiopsis canker of Acer rubrum: some relationships among host, pathogen and vector . Plant Dis. , 67 : 984 – 986 .
- Tsuneda , A and Currah , RS. 2006 . Toward a deeper understanding of the nature of pleomorphism in conidial fungi . Rep Tottori Mycol Inst. , 44 : 1 – 52 .
- Tsuneda , A , Hambleton , S and Currah , RS. 2004 . Morphology and phylogenetic placement of Endoconidioma, a new endoconidial genus from trembling aspen . Mycologia , 96 : 1128 – 1135 .
- Tsuneda , A , Wang , W , Tsuneda , I and Currah , RS. 2009 . Endomembrane system of aspen root cells plays a key role in defense against a common fungal root endophyte, Cryptosporiopsis radicicola . Mycologia , 101 : 182 – 189 .
- Verkley , GJM. 1999 . A monograph of the genus Pezicula and its anamorphs . Stud Mycol. , 44 : 1 – 180 .
- Verkley , GJM , Zijlstra , JD , Summerbell , RC and Berendse , F. 2003 . Phylogeny and taxonomy of root-inhabiting Cryptosporiopsis species, and C. rhizophila sp. nov., a fungus inhabiting roots of several Ericaceae . Mycol Res. , 107 : 689 – 698 .
- Wang , W , Tsuneda , A , Gibas , CF and Currah , RS. 2007 . Cryptosporiopsis species isolated from the roots of aspen in central Alberta: identification, morphology, and interactions with the host, in vitro . Can J Bot. , 85 : 1214 – 1226 .