Abstract
Soil characteristics may be an important factor in structuring fungal communities. We relate the above- and below-ground distribution of the ectomycorrhizal fungi (EMF) Pisolithus spp. to changes in soil chemistry in a Quercus suber plantation located in the Maâmora forest (Morocco). Intrageneric variability of 115 fruit bodies was studied, using morphological characterization, restriction fragment length polymorphism (RFLP) analyses and internal transcribed spacer (ITS) sequencing. Two Pisolithus spp. genotypes were identified: 97% of the fruit bodies represent Pisolithus arhizus; the remaining 3% correspond to Pisolithus species 4, as previously described by Martin et al. [New Phytologist 153 (2002) 345-357]. Multivariate analysis (PCA) showed that species structure was strongly correlated with soil characteristics. P. arhizus fruit bodies were frequently found in the Eastern part of the plot at low molasic clay, N and P contents, while Pisolithus species 4 were collected only in the Western part at the high molasic clay, N and P contents. To determine whether this change in fruit body structure was expressed at the belowground level, we used morphological and molecular techniques to characterize the Pisolithus ectomycorrhizas. Only ectomycorrhizas of P. arhizus were identified at the low P–N–molasic clay site and disappeared completely at the high P–N–red clay site, where no Pisolithus species 4 mycorrhizas were found. However, autocorrelation among soil parameters makes it difficult to isolate the effects of individual parameters. These results show the local-scale impact of natural spatial heterogeneity on an ectomycorrhizal fungal genus.
Introduction
Species belonging to the genus Pisolithus are widespread throughout the world and common on acidic (siliceous or slate-derived) or basic (calcareous and marsh-gypsum) soils. In association with a variety of angiosperms and gymnosperms, Pisolithus forms ectomycorrhizal symbiosis (Marx Citation1977), which plays a major role in the biology and ecology of tree-hosts (Smith and Read Citation1997). However, the effects of tree inoculation may differ greatly according to the strain of Pisolithus used (Burgess et al. Citation1994). As a matter of fact, controlled mycorrhization, using selected isolates of Pisolithus, has been performed worldwide with eucalypts, pines and acacias seedlings to improve plantation quality (e.g. Garbaye et al. Citation1988; Duponnois and Ba Citation1999).
Taxa within the genus Pisolithus have been widely regarded as conspecific and grouped as Pisolithus tinctorius (Pers.) Coker and Couch (Chambers and Cairney Citation1999). Large variations in ecology, morphology of basidioma and basidiospore, mycorrhizal ability, physiology and geographical distribution of Pisolithus collections have, however, been reported, suggesting the existence of several species (Calonge and Demoulin Citation1975; Lamhamedi et al. Citation1990; Burgess et al. Citation1994, Citation1995). Advances in molecular biology, using various genomic markers (mostly ITS), have considerably improved knowledge of the worldwide phylogeography of Pisolithus (Anderson et al. Citation1998; Martin et al. Citation2002). In the Mediterranean region, there are at least five ecological species of Pisolithus (Diez et al. Citation2001). In Morocco, the distribution of Pisolithus has proven to be dependent on the presence of cork oak, holm oak, pine, eucalypt and Cistus shrubs (Abourouh Citation1992; Bakkali et al. Citation2009).
In the Maâmora forest (Morocco), the cork oak woodlands have suffered a worrying regression despite intensive plantation programs. The success of cork oak establishment and growth after outplanting is especially related to nursery cultural techniques, including controlled mycorrhization with selected ectomycorrhizal fungi (e.g. Marx Citation1977; Le Tacon et al. Citation1992). Due to ecological specialization among ectomycorrhizal fungi (Goodman and Trofymow Citation1998), the establishment of selection programs to find mycorrhizal isolates locally adapted to nursery conditions and to planting sites is necessary (Garbaye et al. Citation1988; Duponnois and Ba Citation1999). In this field, selection of indigenous Mediterranean strains of Pisolithus adapted to local soil features of planting sites is recommended (Diez et al. Citation2001). Various authors have also analyzed the influence of nitrogen, carbon, phosphorus, temperature, pH and water stress on the growth of ectomycorrhizal fungi (Smith and Read Citation1997 and references therein). Soil physical and chemical characteristics, especially carbon and nitrogen sources, greatly influence the survival and growth of ectomycorrhizal fungi in the field as well as under controlled conditions (Lilleskov et al. Citation2002).
The objectives of this study were to: (1) assess the effects of soil abiotic characteristics on the genetic diversity of the Pisolithus fruit bodies and ectomycorrhizas associated with cork oak in the Maâmora forest, and (2) select indigenous Pisolithus strains adapted to local soil.
Materials and methods
Site description
The studied site (EVI 11) is a 1992 cork oak plantation using acorn seedlings. It is located at Bir Chleuh, Eastern Maâmora (Morocco) between coordinates 34°02′07″N and 34°02′23″N and 6°08′00″W and 6°08′29″W, and an elevation range 245–266 m. The plantation has an area of 40 ha with a density of ∼2500 trees per ha (4×1 m). The site was previously colonized by the invasive weed shrub Acacia mollissima Willd. Mean annual temperature and precipitation are, respectively, 22°C and 375 mm, with a marked dry season from May to September. The soil is predominantly sandy and the herbaceous stratum vegetation is dominated by Cistus salviifolius L., Helianthemum guttatum Mill. and Chamaerops humilis L.
Fruit bodies and ectomycorrhizas sampling
A total of 115 Pisolithus spp. fruit bodies were collected over 3 years and their exact positions in the plot were noted. To collect ectomycorrhizas, soil samples were carefully removed starting from the base of 50 random fruit bodies in three different directions. Lateral roots were followed until the Pisolithus ectomycorrhizal roots were reached. Both fine roots and ectomycorrhizas were found principally in the upper 40 cm layer of the soil. In the laboratory, fine roots were gently washed to remove adhering soil particles. Pisolithus-like ectomycorrhizas were visually identified as golden yellow clusters of ectomycorrhizas with smooth surface, and often emanating mycelial strands. Mean abundance, expressed as a percentage, of Pisolithus-like ectomycorrhizas was estimated for each selected fruit body position and a subsample Pisolithus ectomycorrhiza was collected and stored at –20°C.
Soil analyses
Three soil samples were collected at each ectomycorrhiza sampling locations. A total of 50 bulked soils were obtained. These soils were air-dried and sieved (2 mm) before analyses. Soil pH was measured in 1:2.5 (w/v) deionized water. Total nitrogen (N) was measured by the Kjeldhal method. Available P was determined by the P-Olsen method (Olsen Citation1963). Clay percent was determined by densimetry. Soil humidity was measured every month for 1 year, with soil samples being collected at four different places in the plot chosen for the presence of Pisolithus ectomycorrhizas and/or fruit bodies in the neighbourhood. Samples were weighed, air-dried overnight at 105°C and reweighed. Humidity (%) was then calculated as follows: (wet soil weight – dry soil weight) × 100/wet soil weight.
Cultivation of Pisolithus spp. isolates
In vitro cultures were obtained by aseptically transferring internal pieces of Pisolithus spp. basidioma onto modified Melin Norkrans (MMN) agar medium (Marx and Bryan Citation1975). They were maintained on the culture medium in the dark at 25°C by sub-culture every 6 months. All isolates are conserved within the fungal collections of the INRA (Montpellier, France) and the Forest Research Center (Rabat, Morocco).
Total DNA extraction and PCR amplification of ITS rDNA
Total DNA was extracted from mycelia, fruit bodies and single ectomycorrhizas using the CTAB protocol (Gardes and Bruns Citation1993; Henrion et al. Citation1994) or the DNeasy plant mini kit according to the manufacturer's recommendations (QIAgen SA). The internal transcribed spacer (ITS) of the nuclear ribosomal DNA (rDNA) was amplified by using ITS1 and ITS4 primers (White et al. Citation1990). PCR amplification was carried out on a PCR-100 thermocycler (MJ Research, Inc. Watertown, MA, USA) using 50 μl reaction volumes each containing 1 μl DNA template, 3 μl MgCl2 (25 mM), 5 μl dNTP (2 mM), 5 μl of each primer (5 μM), 5 μl of 10× Taq buffer and 0.6 units of Taq DNA polymerase (Eurogentec, Belgium). Purified water (25.4 μl) was added to the PCR mix (49 μl) to improve the success of amplifications. Cycling parameters were 1 cycle of 95°C for 5 min, 35 cycles including 94°C for 30 s, 55°C for 30 s, 72°C for 2 min with a final extension at 72°C for 10 min. Negative controls (no DNA template) were included in all PCR experiments to check for DNA contamination.
RFLP analysis of ITS diversity
An 8-μl aliquot of amplified ITS were mixed with 2 μl of the react mix, containing 5 units of HinfI, MspI or TaqI restriction endonucleases (Gibco BRL, Life Technologies), and adjusted to 10 μl with deionized water according to the manufacturer's recommendations. ITS and digested ITS products were, respectively, migrated by electrophoresis on 2% and 3% regular (Sigma) and Nusieve (FMC) agarose gels, stained with ethidium bromide and photographed using the Oncor-Appligene Imager 2.02. A digested 100-bp molecular weight marker (Boehringer Mannhein) was used as ladder.
ITS rDNA sequencing
The double-stranded ITS products were purified using the QIAquick PCR purification kit (QIAgen) in accordance with the manufacturer's instructions. Both strands were sequenced separately using the BigDye Terminator cycle sequencing kit, the AmpliTaq DNA Polymerase FS (Perkin Elmer Applied Biosystems, Foster City, CA, USA) and the ITS1 or ITS4 primers. Sequencing products were analyzed using the automated ABI PRISM 310 DNA Genetic Analyzer (Perkin Elmer Applied Biosystems) at the DNA Sequencing Facilities of INRA-Nancy (France). The sequencing data were edited using Sequencher (Genes Codes Corporation, Ann Arbor, MI, USA) for Macintosh computers. Sequences were compared using the BlastN program at the National Center for Biotechnology Information (NCBI; www.ncbi.nlm.nih.gov/BLAST/index) (Altschul et al. Citation1997). Phylogenetic analysis used the maximum likelihood method implemented in the PhyML program at www.phylogeny.fr(Dereeper et al., 2008). The percentage of homology and the relation with species named by Martin et al. (Citation2002) were noted. ITS sequences of Paxillus involutus (AF167700) and Suillus luteus (L54110) were used as outgroup taxa.
Statistical analysis
To study the correlation between Pisolithus spp. structure and measured soil parameters, a multivariate analysis (PCA) was carried out on soil characteristics of the 50 random fruit body positions. Soil characteristics used in analyses were pH, clay type (Cl), nitrogen (N) and phosphorus (P) contents and percent abundance ectomycorrhization (M). Statistical analysis was carried out on SPSS 11.5 for Windows.
Results
Anatomical and morphological data from fruit bodies were in accordance with the presence of one single species harboring all the characters of Pisolithus arhizus (Scop.) Rauschert (syn: P. tinctorius), as deposited in RBG in Kew and PC in Paris. Notwithstanding, examination of the morphological traits of in vitro cultures, such as color, growth and mycelium density, suggests at least two types of isolates.
The sequences of rDNA ITS regions, obtained for ITS-RFLP type 1 and ITS-RFLP type 2 (), were compared with rDNA ITS of Pisolithus reference strains, as described by Martin et al. (Citation2002). The phyML tree separated the isolates into two groups (). Group I contained ITS-RFLP type 1 and the sequences (AF374709, AF374630, AF374712) of types strains (H237, MH177, H525) of P. arhizus named species 6, while group II contained ITS-RFLP type 2 and types strains Gr 13 (AF228650), Pt 03 (AF228648) and Pt 04 (AF228649) of Pisolithus sp., named species 4 (Martin et al. Citation2002). Pisolithus arhizus was the dominant ectomycorrhizal fungus representing 87% of collected fruit bodies. The remaining 13% of fruit bodies corresponded to Pisolithus sp. species 4.
Figure 1. Maxkimum likelihood phylogenetic tree of Pisolithus species based on ITS sequences. Paxillus involutus and Suillus luteus were used as an outgroup. Bootstrap values calculated (using phyML) are shown at tree nodes, only for values over 70%.
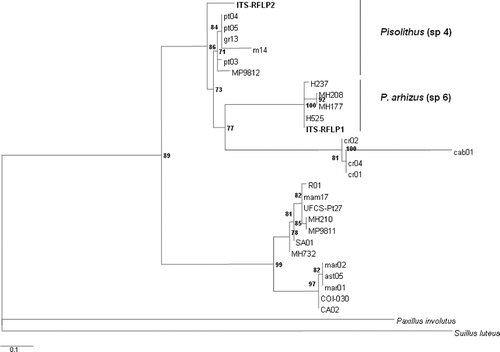
Table 1. ITS and ITS-RFLP fragment sizes of Pisolithus arhizus and Pisolithus sp. from the Bir Chleuh plantation
Spatial analyses of the 50 Pisolithus fruit bodies revealed a specific distribution of the two species. P. arhizus was frequent in the Eastern part of the plot and disappeared completely at the Western part. According to soil characteristics, there were significance differences between the two Pisolithus niches (). The P. arhizus fruit bodies niche was characterized by an average pH of 6.1 ± 0.01, a low percentage of molasic clay (11.5 ± 0.06%) and low N and P contents (48.8 ± 1.24 and 75.8 ± 1.14 mg/kg, respectively). In contrast, Pisolithus sp. 4 was collected only in the Western part of the plantation where no fruit bodies of Pisolithus arhizus were found. The soil in this area had an average of pH of 5.8 ± 0.03 and was composed of red clay (18.5 ± 0.53%) with relatively high N and P contents (134 ± 2.13 and 194.8 ± 6.59 mg/kg, respectively). The higher red clay content under Pisolithus sp. 4 fruit bodies is linked with a higher humidity level in the soil during winter ().
Figure 2. Soil humidity variations in Pisolithus arhizus and Pisolithus sp. niches in the Bir Chleuh plantation over 1 year (2004–2005). Bars with the same letter are not significantly different at p<0.05.
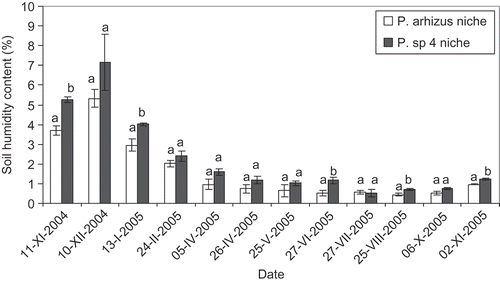
Table 2. Soil analysis data in Pisolithus arhizus and Pisolithus sp. niches
To examine the data in more details, the randomly chosen 50 fruit body positions were compared according to soil parameters (pH, Clay, N, P and M), using the PCA, a data analysis that plots the Pisolithus fruit bodies in multidimensional space. The PC axis 1 of the principal component analysis explained 82% of the variation. On this axis, as shown in , there was a tendency to separate the Pisolithus fruit body groups according to soil characteristics. In fact, P. arhizus samples appeared to be associated with a nutrient-poor niche, whereas Pisolithus sp 4 samples were associated to a nutrient-rich niche.
Figure 3. Plot of factor scores for the 50 Pisolithus spp. fruit bodies along principal components axes 1 and 2. Vectors indicate quantitative soil parameters (pH, clay, N, P, M).
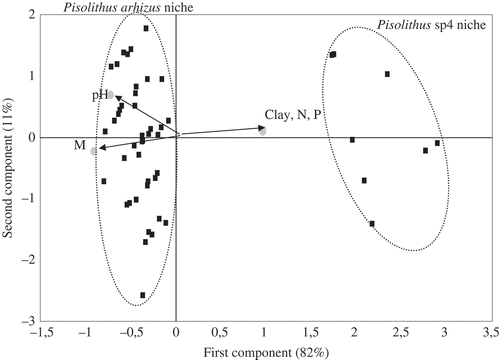
Furthermore, there was a parallel between the above- and below-ground distribution of P. arhizus. Ectomycorrhizas disappeared completely in the rich niche where no ectomycorrhizas of Pisolithus sp. 4 were found.
In the present study, each of the parameters had a strong positive or negative correlation with axis 1. Correlation analyses of the relationships between soil factors were performed, and it was apparent that pH, clay percent, N and P content were significantly correlated with each other. The strong autocorrelation among the soil parameters () makes it difficult to determine the influence of the individual parameters in this combined analysis.
Table 3. Correlation analysis between soil characteristics giving r and p values
Discussion
Based on RFLP analysis of nuclear ITS, Farmer and Sylvia (Citation1998), Anderson et al. (Citation1998b) and Martin et al. (Citation1998) suggested that the genus Pisolithus is a complex of species presenting similar anatomical and morphological features. Indeed, ITS-RFLP polymorphisms as well as morphological variations were observed between in vitro cultures of Pisolithus spp. (Lamhamedi et al. Citation1990). ITS-RFLP, sequencing and morphological analyses of Pisolithus spp. from the Bir Chleuh plantation enabled us to distinguish two species: P. arhizus and Pisolithus sp., respectively named “species 6” and “species 4” by Martin et al. (Citation2002). In the present study, multivariate analysis PCA suggested a strong influence of soil characteristics on the distribution of the two Pisolithus species. In natural environments, the ecology and geographical distribution of ectomycorrhizal species are similar to these of the host species with which they are associated (Martin et al. Citation2002; Moyersoen et al. Citation2003). There is no evidence in this study of an influence of host specificity upon the observed relationship between the Pisolithus species and soil parameters.
The restricted distribution of P. arhizus to the low-nutrient niche agrees with other studies which reported that this fungus is well adapted to disturbed sites (McAfee and Fortin 1988). However, Pisolithus sp. 4 is likely to be adapted to an acidified and high-nutrient niche.
In the Bir Chleuh plantation, the host range of P. arhizus includes Quercus suber, whereas the ectomycorrhizal status of Pisolithus sp. 4 remains doubtful since no ectomycorrhizas of this species were observed on the roots of Q. suber over a detailed 3-year survey. The same result were obtained by “cross inoculations” of cork oak and holm oak from various phylogeographical origins with Pisolithus spp. (including strains of species 6 and 4, and of a species associated with Eucalyptus sp.): the last two species did not generate mycorrhizas with the Mediterranean oaks, as opposed to the strains of species 6 (Mousain et al. unpublished data). On another hand, “species 4” is known to form ectomycorrhizas with pines and oaks in basic soils (Martin et al. Citation2002). Even if the genus Pisolithus, as with several other ectomycorrhizal species, is able to grow saprotrophically (Maijala et al. Citation1991; Bending and Read Citation1995), it is probable that Pisolithus sp. (species 4) is an inconsistent ectomycorrhizal partner of Quercus suber in acidic soils, permitting formation of erratic fruit bodies but not typical Pisolithus-like ectomycorrhizas. Such a situation has already been recognized for Paxillus involutus, which can grow saprotrophically but needs connections with a plant host to produce fruit bodies (Laiho Citation1970). A saprotrophic growth mode was also evident for individual genets of Suillus pungens, enabling its survival for short periods in the absence of a plant host, for example, after wildfires, by persisting on the dead host-root systems or other dead organic matter (Pierluigi et al. Citation1998). Finally, as various levels of saprotrophic abilities have been demonstrated for several ectomycorrhizal fungi (Laiho Citation1970; Pierluigi et al. Citation1998; Maijala et al. Citation1991; Durall et al. Citation1994; Bending and Read Citation1995), species 4 of Pisolithus could be of this type in the present case.
Contrary to Moyersoen and Beever (Citation2004), who reported the co-existence of three species of Pisolithus associated with one single host (Kunzea ericoides), under similar ecological conditions without mutual competitive exclusion in the Bir Chleuh plantation, fruit bodies of Pisolithus spp., “species 4” and “species 6”, co-existed in different soil conditions. Furthermore, Pisolithus species 4, considered “basophilous” by Diez et al. (Citation2001), was found in acid soils (pH 5.5–5.8) in Maâmora and in a cork oak plantation at Montesquieu-des-Albères (Eastern Pyrenees, France). Bowen (Citation1994) reported that different ecological and physiological factors affect the growth of mycorrhizal fungi, in addition to mycorrhizal formation and functioning, in nature. In experimentally fertilized conifer forests, increased N led to a decrease in total production and diversity of ectomycorrhizal fruit bodies above-ground and to shifts in the composition of ectomycorrhizal communities present on roots (Jonsson et al. Citation2000; Peter et al. Citation2001). Indeed, N supply level is a critical factor in fruit body production and the colonization of roots by ectomycorrhizal fungi in a range of terrestrial ecosystems (Peter et al. Citation2001; Lilleskov et al. Citation2001, 2002). Increasing nutrient status (phosphorus, in particular) could reduce infection levels by Pisolithus spp. and induce growth responses in Eucalyptus diversicolor (Marx et al. Citation1982, Beckjord et al. Citation1985; Bougher and Malajczuk Citation1990), as in some N and P richer parts of the Bir Chleuh plantation.
In total, two species of Pisolithus, P. arhizus and species 4 (Diez et al. Citation2001) have been identified in the Bir Chleuh cork oak plantation. Species 4, from which no ectomycorrhiza has been observed, was masked among a community mostly represented by the ectomycorrhizal P. arhizus. In this case, the two Pisolithus species can be structured not only according to the tree-host species but also to their ecology (soil abiotic characteristics) and probably their biology (ectomycorrhizal versus saprotrophic). Clearly, the habitats of the two species are distinct, as a function of clay type, soil humidity and contents in N and P. This diversity could be exploited to enhance the performances rehabilitation programs by integrating the Pisolithus isolates adapted to local soil in the mycorrhizal inoculation programs in forest nurseries.
Acknowledgements
This work was supported by the PRAD (Projet de Recherche Agronomique pour le Développement) 04-13 “Diversité, écologie et utilisation des Pisolithus spp. pour la gestion durable des subéraies marocaines (Pisum)”. The authors thank Benaïssa Kerdouh, El Mnouar El Ayyachi (CRF, Agdal-Rabat), Elvire Legname, Serge Conventi and Catherine Pernot (INRA, Montpellier) for their technical support and Franck Richard (CEFE/CNRS, Montpellier) for helpful discussions about the manuscript.
References
- Abourouh , M. 1992 . Essai de mycorhization en pépinière par les spores de Pisolithus tinctorius . Ann Rech For Maroc , 26 : 126 – 137 .
- Altschul , SF , Madden , TL , Schaffer , AA , Zhang , J , Miller , W and Lipman , DJ . 1997 . Gapped BLAST and PSI–BLAST: a new generation of protein database search programs . Nucleic Acids Res. , 25 : 3389 – 3402 .
- Anderson , IC , Chambers , SM and Cairney , JWG . 1998a . Molecular determination of genetic variation in Pisolithus isolates from a defined region in New South Wales . Australia. New Phytol. , 138 : 151 – 162 .
- Anderson , IC , Chambers , SM and Cairney , JWG . 1998b . Use of molecular methods to estimate the size and distribution of mycelial individuals of the ectomycorrhizal basidiomycete . Pisolithus tinctorius. Mycol Res. , 102 : 295 – 300 .
- Anderson , IC , Chambers , SM and Cairney , JWG . 2001 . ITS–RFLP and ITS sequence diversity in Pisolithus from central and eastern Australia sclerophyll forests . Mycol Res. , 105 : 1304 – 1312 .
- Bakkali Yakhlef , S , Mousain , D , Duponnois , R , Ducousso , M , Belkouri , A , Kerdouh , B , Perrineau , M and Abourouh , M . 2009 . Molecular phylogeny of Pisolithus species from Moroccan forests woodlands . Symbiosis , 49 ( 3 ) : 157 – 162 .
- Beckjord , PR , Melhuish , JH and McIntosh , MS . 1985 . Effects of nitrogen and phosphorus fertilization on growth and formation of ectomycorrhizae of Quercus alba and Q. rubra seedlings by Pisolithus tinctorius and Scleroderma aurantium . Can J Bot. , 63 : 677 – 1680 .
- Bending , GD and Read , J . 1995 . The structure and function of the vegetative mycelium of ectomycorrhizal plants. V. Foraging behaviour and translocation of nutrients from exploited litter . New Phytologist , 130 : 401 – 409 .
- Bougher , NL and Malajczuk , N . 1990 . Effects of high soil moisture on formation of ectomycorrhizas and growth of karri (Eucalyptus diversicolor) seedlings inoculated with Descolea maculate, Pisolithus tinctorius and Laccaria laccata . New Phytologist , 114 : 87 – 91 .
- Bowen , GD . 1994 . “ The ecology of ectomycorrhiza formation and functioning ” . In Management of mycorrhizas in agriculture, horticulture and forestry , Edited by: Robson , AD , Abbott , LK and Malajczuk , N . 61 – 68 . Dordrecht : Kluwer .
- Burgess , T , Dell , B and Malajczuk , N . 1994 . Variation in mycorrhizal development and growth stimulation by 20 Pisolithus isolates inoculated onto Eucalyptus grandis W. Hill ex Maiden . New Phytol. , 127 : 731 – 739 .
- Burgess , T , Malajczuk , N and Dell , B . 1995 . Variation in Pisolithus based on basidiome and basidiospore morphology, culture characteristics and analysis of polypeptides using ID–SDS–PAGE . Mycol Res. , 99 : 1 – 13 .
- Calonge , FD and Demoulin , V . 1975 . Les Gastéromycètes d'Espagne . Bull Trimest Soc Mycol France , 91 : 247 – 292 .
- Chambers , SM and Cairney , JWG . 1999 . “ Pisolithus ” . In Ectomycorrhizal fungi. Key genera in profile , Edited by: Cairney , JWG and Chambers , SM . 1 – 31 . Springer : Berlin .
- Diez , J , Anta , B , Manjon , JL and Honrubia , M . 2001 . Genetic variability of Pisolithus isolates associated with native hosts and exotic eucalyptus in the western Mediterranean region . New Phytol , 149 : 577 – 587 .
- Duponnois , R and Ba , AM . 1999 . Growth stimulation of Acacia mangium Willd. by Pisolithus sp. in some Senegalese soils . For Ecol Manag. , 119 : 209 – 215 .
- Durall , DM , Todd , AW and Trappe , JM . 1994 . Decomposition of 14C–labelled substrates by ectomycorrhizal fungi in association with Douglas fir . New Phytol , 127 : 725 – 729 .
- Farmer , DJ and Sylvia , DM . 1998 . Variation in the ribosomal DNA internal transcribed spacer of a diverse collection of ectomycorrhizal fungi . Mycol Res. , 102 : 859 – 865 .
- Garbaye , J , Delwaulle , JC and Diangana , D . 1988 . Growth response of eucalyptus in the Congo to ectomycorrhizal inoculation . For Ecol Manag. , 24 : 151 – 157 .
- Gardes , M and Bruns , T . 1993 . ITS primers with enhanced specificity for basidiomycetes: application to the identification of mycorrhizae and rusts . Mol Ecol. , 2 : 113 – 118 .
- Goodman , DM and Trofymow , JA . 1998 . Distribution of ectomycorrhizas in microhabitats in mature and old–growth stands of Douglas–fir on South–eastern Vancouver island . Soil Biol Biochem. , 30 : 2127 – 2138 .
- Henrion , B , Di Battista , C , Bouchard , D , Vairelles , D , Thompson , BD , Le Tacon , F and Martin , F . 1994 . Monitoring the persistence of Laccaria bicolor as an ectomycorrhizal symbiont of nursery–grown Douglas–fir by PCR of the rDNA intergenic spacer . Mol Ecol. , 3 : 571 – 580 .
- Horton , TR and Bruns , TD . 2001 . The molecular revolution in ectomycorrhizal ecology: peeking into the black–box . Mol Ecol. , 10 : 1855 – 1871 .
- Jonsson , L , Dahlberg , A and Brandrud , TE . 2000 . Spatio–temporal distribution of an ectomycorrhizal community in an oligotrophic Swedish Picea abies forest subjected to experimental nitrogen addition: Above– and below–ground views . For Ecol Manag. , 132 : 143 – 156 .
- Laiho , O . 1970 . Paxillus involutus as a mycorrhizal symbiont of forest trees . Acta For Fennica , 106 : 1 – 73 .
- Lamhamedi , MS , Fortin , JA , Kope , HH and Kropp , BR . 1990 . Genetic variation in ectomycorrhiza formation by Pisolithus arhizus on Pinus pinaster and Pinus banksiana . New Phytol , 115 : 689 – 697 .
- Le Tacon , F , Alvarez , IF , Bouchard , D , Henrion , B , Jackson , RM , Luff , S , Parlade , JI , Pera , J , Stenström , E , Villeneuve , N and Walker , C . 1992 . “ Variations in field response of forest trees to nursery ectomycorrhizal inoculation in Europe ” . In Mycorrhizas in ecosystems , Edited by: Read , DJ , Lewis , D , Fitter , A and Alexander , I . 119 – 134 . Wallington, UK : CAB International .
- Lilleskov , EA , Fahey , TJ and Lovett , GM . 2001 . Ectomycorrhizal fungal aboveground community change over an atmospheric nitrogen deposition gradient . Ecol Applic. , 11 : 397 – 410 .
- Lilleskov , EA , Hobbie , EA and Fahey , TJ . 2002 . Ectomycorrhizal fungal taxa differing in response to nitrogen deposition also differ in pure culture organic nitrogen use and natural abundance of nitrogen isotopes . New Phytol. , 159 : 219 – 231 .
- Maijala , P , Fagerstedt , KV and Raudaskoski , M . 1991 . Detection of extracellular cellulolytic and proteolytic activity in ectomycorrhizal fungi and Heterobasidion annosum (Fr.) Bref . New Phytol , 117 : 643 – 648 .
- Martin , F , Delaruelle , C and Ivory , M . 1998 . Genetic variability in intergenic spacers of ribosomal DNA in Pisolithus isolates associated with pine, eucalyptus and Afzelia in lowland Kenyan forests . New Phytol , 139 : 341 – 352 .
- Martin , F , Diez , J , Dell , B and Delaruelle , C . 2002 . Phylogeography of the ectomycorrhizal Pisolithus species as inferred from nuclear ribosomal DNA ITS sequences . New Phytol , 153 : 345 – 357 .
- Marx , DH . 1977 . Tree host range and world distribution of the ectomycorrhizal fungus Pisolithus tinctorius . Can J Microbiol. , 23 : 217 – 233 .
- Marx , DH and Bryan , WC . 1975 . Growth and ectomycorrhizal development of loblolly pine seedlings in fumigated soil infested with the fungal symbiont Pisolithus tinctorius . For Sci. , 21 : 245 – 254 .
- Marx , DH , Ruehle , JL , Kenney , DS , Cordell , CE , Riffle , JW , Molina , RJ , Pawuk , WH , Navratil , S , Tinus , RW and Goodwin , OC . 1982 . Commercial vegetative inoculum of Pisolithus tinctorius and inoculation techniques for development of ectomycorrhizae on container–grown tree seedlings . For Sci. , 28 : 373 – 400 .
- Moyersoen , B and Beever , RE . 2004 . Abundance and characteristics of Pisolithus ectomycorrhizas in New Zealand geothermal areas . Mycologia , 96 : 1225 – 1232 .
- Moyersoen , B , Beever , RE and Martin , F . 2003 . Genetic diversity of Pisolithus in New Zealand indicates multiple long–distance dispersal from Australia . New Phytol , 160 : 569 – 579 .
- Olsen , JS . 1963 . Energy storage and the balance of producers and decomposers in ecological ecosystems . Ecology , 14 : 323 – 331 .
- Peter , M , Ayer , F and Egli , S . 2001 . Fire and vegetation effects on productivity and nitrogen cycling across a forest–grassland continuum . Ecology , 82 : 1703 – 1719 .
- Pierluigi , B , Bruns , TD and Gardes , M . 1998 . Genetic structure of a natural population of the ectomycorrhizal fungus Suillus pungens . New Phytol , 138 : 533 – 542 .
- Smith , SE and Read , DJ . 1997 . Mycorrhizal symbiosis , 2nd , London : Academic Press .
- Taylor , AFS , Martin , F and Read , DJ . 2000 . “ Fungal diversity in ectomycorrhizal communities of Norway spruce [Picea abies (L.) Karst.] and beech (Fagus sylvatica L.) along North–South transects in Europe ” . In Carbon and nitrogen cycling in European forest ecosystems , Edited by: Schulze , ED . 343 – 365 . Ecological Studies, Heidelberg : Springer .
- White , TJ , Bruns , TD , Lee , SS and Taylor , JW . 1990 . “ Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics ” . In PCR protocols: a guide to methods and application , Edited by: Innis , MA , Gelf , DH , Sninsky , JJ and White , TJ . 315 – 322 . San Diego : Academic Press .