Abstract
Filamentous fungi and oomycetes are major pathogens of plants and the principal cause of destructive damage to ecosystems and agriculture. During colonization of their hosts, these phytopathogens produce a large array of effectors to break down host cellular components, evade host immune responses and modulate host cell survival. The identification of diverse effectors from different ascomycete, basitiomycete and oomycetes pathogens offers an in-depth view of effector function and evolution. Effectors produced by plant pathogens operate at a few interaction interfaces and are trafficked with several logistic strategies. This review summarizes the various aspects of effector genomics, such as different trafficking routes, modular molecular construction, dynamic genome environment, various modes of host-modulation and intricate co-evolution with hosts.
Introduction
Microbes have coevolved with land plants for approximately 480 million years (Heckman et al. Citation2001) and they have shaped the evolution of host plants with myriads of mutualistic and pathogenic interactions (Chisholm et al. Citation2006). Plants are constantly challenged by a large array of microbes. Filamentous fungi and oomycetes are the major pathogens of plants and they are the principal threat to ecosystems and agriculture (Latijnhouwers et al. Citation2003). A successful pathogen performs a series of complex tasks, such as locating its host, breaking down the host cell wall barriers, the acquisition of nutrients, and suppression of host immune responses. Because these filamentous pathogens grow outside the confinement of the plasma membrane of host cells, they must interact with the host across several molecular membrane barriers. Effectors are molecules secreted by pathogens to modulate the host's physiology at these interaction interfaces. Some effectors act at the interface whereas others are delivered into host cytoplasm.
Interaction interfaces for effectors
During the colonization process, a few molecular communication interfaces are established between the pathogen and host cells. Depending on where the molecular interaction takes place, two different types of interfaces can be defined – one extracellular and the other intracellular. These interfaces are crucial in determining the logistics and action of the microbial effectors.
Extracellular space refers to the space between host cells or the column within the xylem in a host. Many different types of microbes colonize this space in plants (). The apoplastic space refers to the free diffusion space between plant cell walls. This is the interface where the interaction between Cladosporidium fulvum and a tomato plant takes space. All characterized effectors from this fungus, including all identified avirulence proteins, are located at the apoplastic space (Rivas and Thomas Citation2005). Another example of intercellular interface is the xylem of host plants. The pathogen Fusarium oxysporum colonizes the xylem and secretes effectors into the transport system of the host (Gordon and Martyn Citation1997).
Figure 1. Effectors and interfaces of plant–pathogen interactions. (A) Extracellular interfaces. The Hartig net is typical of ectomycorrhizal fungi. (B) Intracellular interfaces. Invasive hyphae are developed by the rice blast fungus. The plant cells are represented by squares or columns. Fungal or oomycete tissues are represented by threads and ovals. Effectors are indicated with black dots.
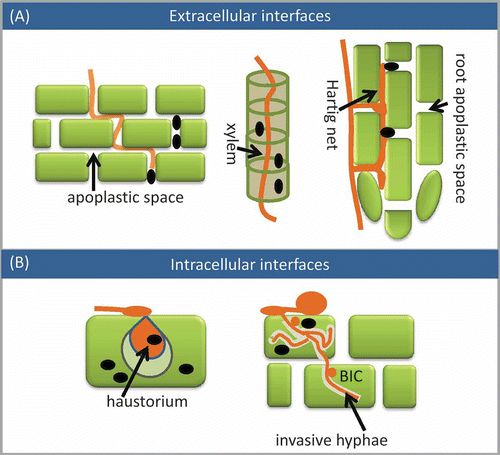
Within the apoplastic space of host roots, the ectomycorrhizal fungi develop an elaborate hyphal net called the Hartig net (). The fungus exchanges nutrients with its host within the Hartig net. To form this mutalistic relationship, the fungus also secretes effectors to modulate host metabolisms and immune responses. These so-called mycorrhizal-induced small secreted proteins (MiSSP) operate on this tissue net interface to maintain the symbiosis (Martin et al. Citation2008).
The other interaction interface is located inside the host cell wall. These are specialized structures, such as haustoria and infection hyphae, formed by biotrophs or semi-biotrophs (). Biotrophs are microbes that feed on living host tissue and replicate within a living host. A haustorium is developed when the pathogen penetrates the host cell wall and invaginates the host plasma membrane. A group of phylogenetically distinct pathogens, i.e. the oomycete downy mildews and Phytophthora, the fungi powdery mildews and rusts, are all able to develop haustoria during infection. Haustoria serve as the essential interface for nutrient acquisition and modulation of the host metabolism (Catanzariti et al. Citation2007). Many effectors are located within or around harstoria; for example, members of the large oomycete RXLR families are induced in these structures (Armstrong et al. Citation2005). Another intracellular structure is invasive hyphae (IH), which is developed by the rice blast pathogen Magnaporthe oryzae within rice cells. Similar to haustoria, the invading hyphae are enclosed in host-derived membranes and they can multiply in the cell and, subsequently, invade new living host cells (Mosquera et al. Citation2009). Delivery of the effector from the IH is carried out in two steps: first from an initial IH and then from a specialized secretory structure called the biotrophic interfacial complex (BIC) (Khang et al. Citation2010). The effectors, PWL2 (which prevents pathogenicity toward weeping lovegrass) and BAS1 (biotrophy-associated secreted protein 1), were identified from these structures (Khang et al. Citation2010).
Logistics of crossing barriers
To be deployed at any interaction interfaces, effector proteins have to, at least, cross the pathogen plasma membrane barrier. Effector proteins from fungal and oomycete pathogens are trafficked by the eukaryotic (type II) secretory pathway. This pathway operates as a universal mechanism across all major life forms, which involves exocytosis of secretory vesicles from the Golgi apparatus. Most known effectors from fungi and oomycetes have a canonical N-terminal type II secretion signal (Kamoun Citation2007). The bioinformatic identification of the signal has enabled the development of computational tools to estimate the sum of secreted proteins, the so-called secretome (Kamoun Citation2006). Using this method, a large number of effectors functioning in the extracellular environment have been identified from various pathogens . For example, the SIX (secreted in xylem) genes, which function in the xylem (Lievens et al. Citation2009), AVR and ECP (extracellular proteins), functioning in the apoplastic space of the leaf and RXLR effectors, functioning within the host cytoplasm, all carry such a signal (Wubben et al. Citation1994; Rivas and Thomas Citation2005). However, this type-II secretory prediction method may not uncover effectors that are trafficked by alternative routes. The most notable example is effectors from the barley powdery mildew fungus, Blumeria graminis, which lack canonical eukaryotic secretion peptide yet can be experimentally demonstrated to induce cell death in the host cell (Ridout et al. Citation2006).
The intracellular-type of interaction interface actually delivers many effectors a further step into the host cytoplasm. Direct evidence of host targeting have been obtained by immunocytological detection or fluorescent tagging of effectors during the infection process of rust fungi and oomycetes (Whisson et al. Citation2007; Rafiqi et al. Citation2010). These intracellular deliveries require a second crossing of the membrane barrier by the effectors. In several pathosystems, a common trafficking motif has been discovered in heterogeneous effectors for host membrane barrier crossing. The malaria parasite, Plasmodium falciparum, translocates hundreds of heterogeneous effectors into human erythrocytes via a host-targeting signal, PEXEL, at the N-termini of the effector proteins (Hiller et al. Citation2004; Marti et al. Citation2004). Many oomycete effectors harbor a host-targeting RXLR domain, located 30–60 aa downstream of the signal peptide (Whisson et al. Citation2007; Jiang et al. 2008). Both the Plasmodium PEXEL domain and the oomycete RXLR domain are indispensible for the entry of effectors with diverse C-terminal domains into host cells.
The sharing of a trafficking motif by heterogeneous effectors indicates a common trafficking pathway cross the host membrane. In the malaria parasite, for targeting red blood cells, the PEXEL domain is used for enzymatic cleavage and subsequent recognition by protein machinery called translocons (Russo et al. Citation2010). In oomycete, the RXLR motif enables binding of a type of phospholipid, phosphatidylinositol-3-phosphate (PI3P), on the outer surface of plant cell plasma membranes (Kale et al. Citation2010). Unlike malaria parasites, oomycete effector entry into the host cell does not need pathogen-derived machinery. Instead, the uptake process only exploits the plant endogenous endocytosis process. The underlying biochemical significance of these conserved effector delivery motifs has enabled a genome-wide search for host-targeted effectors. Hundreds of effectors have been discovered in both oomycetes and malaria parasites (Jiang et al. 2008; van Ooij et al. Citation2008). In pathogenic fungi, a few secreted avirulence (Avr) proteins are also believed to be delivered into host cells, because they are recognized within the host cell by plant resistance proteins. For the flax rust pathogen, Melampsora lini, the fungal effector delivery into the host has been experimentally shown with the effector AvrL567 and AvrM proteins by fluorescent reporter proteins inside plant cells (Rafiqi et al. Citation2010). The rice blast pathogen, Magnaporthe oryzae, delivers its effector proteins, PWL2 and BAS1, from a specialized BIC structure into the host cytoplasm (Khang et al. Citation2010). The host-targeting signals for these fungal effector proteins show very little primary sequence conservation. Various de novo motif searching methods have so far failed to indentify a shared host targeting motif in fungal pathogens (B. Tyler and R.H.Y. Jiang, Citation2010, unpublished data). It is plausible that a different trafficking pathway is employed by fungal effectors; alternatively, the conservation of targeting signal may reside in a secondary or higher-order protein structure.
After entering the host cytoplasm, some effectors have to cross another membrane barrier to reach the final destination of the trafficking–host organelles. Various bacterial, fungal and oomycete effectors are targeted at host sub-cellular compartments, including the mitochondrion, nucleus and chloroplasts (Torto-Alalibo et al. Citation2010). For example, in the fungus Uromyces fabae, rust transferred protein 1 (Uf-RTP1p) has been shown to enter the host cytoplasm and localize in the nucleus (Kemen et al. Citation2005). In oomycetes, a large, ubiquitous family is known as crinkler (CRN), with several distinct CRN C-termini being shown to localize to the plant nuclei. For the crinkler effector, CRN8, accumulation of the protein in nucleus alone can induce plant cell death (Schornack et al. Citation2010). The sub-cellular localization within the host cell is crucial for the function of the effectors.
Modularity of effector's molecular architecture
Effectors are defined by the route of protein trafficking and their effect on host cells; these different facets of effector function are reflected by different modules in their molecular structure. Many effectors consist of two parts – a trafficking signal and a functional portion. To achieve the different layers of membrane crossing, the signal module can be comprised of multiple parts, such as signal peptide, host-targeting signal and host–organelle targeting signal. For extracellular effectors, only the presence of an N-terminal signal peptide is necessary for secretion. For cytoplasmic effectors, more targeting signals are needed to deliver the effectors. For example, the P. infestans CRN8 effector has three trafficking signals – a signal peptide to cross the pathogen plasma membrane, a LXFLAK signal to target the host cytoplasm and, finally, a nuclear localization signal to localize in host nucleus (Schornack et al. Citation2010).
The functional parts of the effectors are heterogeneous in sequence, reflecting their diverse roles in host modulation. In many cases, the C-termini sequences themselves are comprised of distinct modules. For example, a majority of RXLR effectors carry one or more conserved sequences, termed W, Y and L motifs, in the C-termini (Dou et al., Citation2008; Jiang et al. 2008; Van Poppel et al. Citation2009). For the avirulence effector Avr1b in Phytophthora sojae, mutations in the conserved residues of the W and Y motifs abolish the avirulence interaction of Avr1b with the Rps1b resistance gene in soybean. Another example of C-terminal motifs is the diverse domain structures in the large family of Phytophthora CRN proteins. Over 30 domains can be defined and they occur in a variety of combinations. Expression of a CRN C-terminal domain, designated DXZ, is sufficient to induce cell death, which indicates that this particular domain induces necrosis in the host (Haas et al. Citation2009).
Timing of effector deployment
Due to their inherent role in host modulation, many effectors are expressed or induced during the infection process. This induction pattern serves as a useful criterion in the search for novel effectors without prior knowledge of sequence homology. Effectors operating both extracellularlly and intracellularlly show an induction pattern during the invasion process of P. infestans. The NimbleGen microarray experiment in P. infestans has shown that a few hundred genes were induced at least two-fold during the initial stage of infection (Haas et al. Citation2009). A set of 79 host-targeting RXLR genes were heavily induced, including the known avirulence genes, Avr3a, Avr4 and Avr-blb1. Effector genes functioning outside of host cell, such as protease inhibitors and NPP1-family members, are among the most highly upregulated genes during infection of plants (Haas et al. Citation2009).
Profile of in planta gene expression has been instrumental in discovering the effectors in Ustilago maydis, a ubiquitous basidiomycete pathogen of maize that causes smut disease. U. maydis lacks enzymes or toxins associated with aggressive invasion. For example, it lacks a battery of cell-wall-degrading enzymes that are commonly found in plant pathogenic fungi and oomycetes. Instead, the fungus uses novel secreted protein effectors as virulence determinants to establish infection. These are clusters of genes encoding small secreted proteins with unknown function and many of them form small gene families. Most of the genes contained in these clusters are expressed together and unregulated in infected tissue (Kamper et al. Citation2006). Deletion of these secreted protein encoding gene clusters resulted in significantly altered virulence.
Genome architecture for effector genes
Genome plasticity plays an important role in effector evolution. Because some genomic regions are more prone to evolutionary changes than the other, natural selection can drive effector genes to be located in the regions with more flexibility.
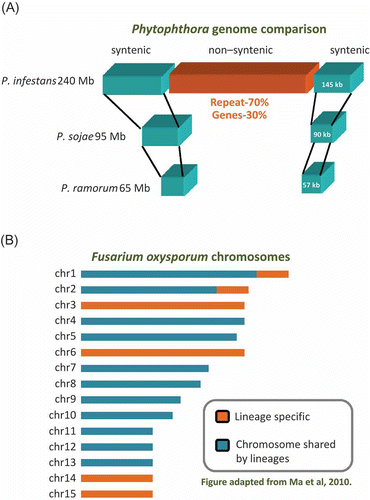
The P. infestans genome has a bi-model mode of evolution, as it can be partitioned in repeat-rich and repeat-poor regions (). For a filamentous micro-organism, its 240-Mb genome is large and complex, with repetitive DNA accounting for 74% of the genome content (Haas et al. Citation2009). There is a discontinuous distribution of gene density; some regions are enriched in genes whereas others are primarily comprised of repeats. Effector genes, such as members of the RXLR and CRN families, are located in the genome milieu with a high repeat content; whereas genes with a clear 1:1 ortholog in other species are located in repeat-poor, gene-dense regions. Genome partitioning facilitates the rapid adaptability of the pathogen effector reservoir and is associated with evolution of new pathogens with different host specificity (Raffaele et al. Citation2010a). This distinct feature of the discontinuous genome can be utilized to identify candidate virulence factors. A number of novel effector genes were identified by a combination of expression profiling and a genome environment approach (Raffaele et al. Citation2010b).
Figure 2. Genome plasticity associated with effector location. (A) The Phytophthora infestans genome is partitioned into repeat-rich non-syntenic regions and repeat-poor syntenic regions. The genome size of each species is listed on the left of the genomic region. Effector genes are frequently associated with the repeat-rich region. (B) The Fusarium oxysporum genome habours lineage-specific chromosomes. The repeat-rich region of the genome coincides with the lineage-specific chromosomes. Pathogenicity-related genes are enriched in the lineage-specific regions.
The genome of the ascomycete Leptosphaeria maculans shows the unusual characteristics of being organized in isochors. Abrupt GC content changes occur in alternating genomic regions. The GC-equilibrated isochores (around 50% GC) are gene-rich, whereas AT-rich isochores (around 40% GC) have few genes but are rich in degenerated repeated elements. The AT-rich isochores seem to be favored by effector genes in L. maculans (Parlange et al. Citation2009). It is within these repeat-rich parts of the genome that three avirulence (AvrLm) genes have been identified, known as “lost in middle of nowhere” genes (Gout et al. Citation2006). These characteristics of residing in an AT-rich isochors and expressing during infection have been used for the bioinformatic discovery of new effectors. This approach has uncovered more than 100 candidate effector genes in this pathogen alone.
Horizontal gene transfer can facilitate the emergence of new pathogens. In Fusarium species, transfer can occur with an entire chromosome, called the lineage-specific (LS) chromome (Fourie et al. Citation2009; Ma et al. Citation2010). These chromosomes have been identified from a comparison of the genomes of three species within the genus Fusarium. In the pathogen F. oxysporum, four entire chromosomes and two large regions of the genome are unique to this species (). These LS regions are rich in transposons but lack genes with house-keeping functions. Interestingly, genes associated with virulence and pathogenicity are also located in these chromosomes (Ma et al. Citation2010). Experimentally, two LS chromosomes can be transferred between two F. oxysporum strains; and the transfer has rendered pathogenicity to the non-pathogenic strain (Ma et al. Citation2010). A single transferring event could transfer a complete package of pathogenicity-related genes to a distinct genetic lineage. Therefore, the LS chromosome mobility between Fusarium genomes underpins the mechanism of host specialization and the rapid emergence of new pathogenic lineages.
One target, many effectors
To survive intracellularlly, it is crucial for pathogenic bacteria to inhibit host phagocytosis by preventing rearrangements of the actin cytoskeleton. The host Rho GTPase mediating cytoskeletal dynamics is targeted frequently by different effectors. Several major bacterial pathogens use the type III secretion system (TTSS) to deliver virulence factors to achieve this task. The bacterial effector proteins in Shigella and Escherichia coli activate the Rho GTPases, whereas YopE and YopT of Yersinia inhibit these Rho family GTPases (Aepfelbacher et al. Citation2005; Schlumberger and Hardt, Citation2005).
Similar to the multiple actions to modify the Rho GTPase in bacterial pathogens, current models of plant–pathogen interactions reveal that pathogens secrete effector proteins which have the same plant defense components. In tomato plant, one common target is the host defense protease Rcr3pim; it is targeted by two unrelated pathogens, the fungus C. fulvum and the oomycete P. infestans. C. fulvum secretes a protease inhibitor Avr2 that targets the host protease Rcr3pim (Shabab et al. Citation2008). The resistance protein Cf2 serves as a ‘guard’ for Rcr3pim; and can initiate a resistance response upon binding of Avr2 to Rcr3pim (Rooney et al. Citation2005). Similar to Avr2, P. infestans secretes several protease inhibitors, including EPIC1 and EPIC2B, that inhibit plant cysteine proteases (Tian et al. Citation2007). However, there are subtle differences between these inhibitors from different pathogens. In contrast to C. fulvum inhibitor, P. infestans inhibitors have separated the inhibitory activity from the elicitor activity because they block enzyme activity but do not trigger a plant host defense response. As increasingly larger amount of effectors are identified in different pathogens, more common host targets, such as Rcr3pim, may be discovered in other plant–pathogen interaction systems.
Manipulating host cell survival
Manipulation of programmed cell death (PCD) is a central task for many microbes in their interactions with hosts. Because biotrophic pathogens need to survive on living host tissue, host cells can launch a PCD response to kill the infected cell and halt the infection process. Both plant and animal host cells use PCD as a powerful defense mechanism against viruses, bacteria, fungi and oomycetes (Tian et al. Citation2007)). To solve this problem, diverse biotrophic pathogens have evolved many mechanisms to suppress host PCD. Similar PCD-modulating tactics are most likely deployed by mutualistic and commensal microbes to negotiate a non-pathogenic relationship with hosts.
For the large oomycete RXLR family, their role in modulating host cell death has been demonstrated in a few members. The P. sojae effector protein Avr1b can contribute positively to virulence. Its virulence role is most likely achieved by suppressing host cell death, as it can prevent PCD in yeast, Glycine max and Nicotiana benthamiana cells from PCD response triggered by a mouse BAX protein (Dou et al. Citation2008). This suppression action can be delineated in the RXLR effector C-terminal W and Y motifs, which are present in more than half of the identified RXLR family members; three other RXLR effectors also suppress PCD in soybean.
Another RXLR effector AVR3a from potato blight pathogen P. infestans was demonstrated to be able to suppress host cell death. Avr3a is translocated into host cells and occurs in two forms: the avirulent form AVR3aKI and the virulent form AVR3aEM (Chisholm et al. Citation2006). Both forms are able to suppress elicitin triggered cell death. The plant E3 ligase CMPG1 is required for the host cell death response. The action of Avr3a is carried out by interacting and stabilizing host CMPG1 and, thus, suppress host PCD during the biotrophic phase of infection (Bos et al. Citation2010).
The host PCD response is sometimes induced rather than suppressed by necrotrophic pathogens, because they kill the host cell and derive their nutrition from dead tissue (Faris et al. Citation2010). Necrotrophic pathogens such as Pyrenophora tritici-repentis produce host-selective toxins (HSTs) specifically to trigger programmed cell death in their hosts. These toxins act as effectors in the fungal population, as the pathogen has a complex race structure with multiple HSTs. A pathogen with a particular toxin is capable of causing disease on specific host genotype with a dominant susceptibility gene (Ciuffetti et al. Citation2010).
Inhibition and counter-inhibition
Much of the plant–pathogen interaction is biochemical in nature. Some of the most prominent antagonistic interactions are played out by extracellular effectors such as hydrolytic enzymes and counteracting inhibitors. Both host and pathogen secrete a range of hydrolases to degrade the other partner's cellular components plus various inhibitors to block the activity to the attacking enzymes.
To colonize a host, pathogens use mechanical and biochemical means to breach the cell walls. Plant cell walls are comprised of different polysaccharides of cellulose, hemicelluloses and pectins. During infection, pathogens secrete a variety of cell-wall-degrading enzymes, such as polygalacturonases (PG), pectin methyl esterases and pectin lyases, endoxylanases and xyloglucan endoglucanases, to loosen and break down the host polymers. In response, plant host secretes enzyme inhibitors, such as xylanase inhibitor protein (XIP) and polygalacturonase-inhibiting proteins (PGIPs), to block the hydrolytic activity of the pathogen (Juge Citation2006). On the other hand, plant hosts also secrete defense-related proteins, such as glucanases and proteases, to eliminate invading pathogens. These host defense enzymes are in turn inhibited by pathogen proteins such as glucanase inhibitor protein-1 (GIP1), Kazal-like inhibitors EPI1 and EPI10 (extracellular protease inhibitor), and AVR2 (Tian et al. Citation2007).
The intense competition between extracellular enzymes and inhibitors has resulted in positive Darwinian selection for variant residues at the contact surface. In the evolution of enzyme-inhibitor pairs, such as PG and PGIP (Raiola et al. Citation2008), very few residues on the protein contact surface are capable of determining the specificity and potency of the interaction. The PG gene in Fusarium verticillioides and Gibberella fujikuroi are highly similar in the sequence. Host PGIPs from monocots have no effect on these fungal PGs whereas, in dicots, PGIPs can inhibit the pathogen PG with varied potency. In addition, the degree of inhibition can be traced to a variation in a single residue on the pathogen enzymes (Raiola et al. Citation2008).
Arms race between effectors and host receptors
In the complex molecular struggle between plants and pathogenic microbes, the ability to detect a pathogen and initiate a defense response has been important in the evolution of plants. Furthermore, the ability to evade host surveillance, while maintaining virulence, has been a central theme in the evolution of pathogens.
For microbes, the invasion process is accompanied by the release of a set of conserved, secreted molecules, called pathogen-associated molecular patterns (PAMPs). Plants can initiate a first layer of defense response upon recognition of these molecules, termed PAMP-triggered immunity (PTI) (Jones and Dangl Citation2006; Boller and He Citation2009). Thus, the PTI has evolved to recognize common features of microbial pathogens. A variety of extracellular surface receptors embedded in the host plasma-membrane perform the surveillance when the pathogens breach the cell wall and expose their PAMPs. To continue the infection process, microbial pathogens must suppress this first layer of defense by delivering effector proteins to suppress PTI, allowing propagation of the pathogen. In response to the delivery of pathogen effector proteins, the plant host launches a second layer of defense via surveillance proteins (R proteins). The host R-proteins act by either directly or indirectly monitoring the presence of certain pathogen effector proteins. This second layer of defense, therefore, is more specialized and the defense mechanism is referred to as effector-triggered immunity (ETI) (Boller and He Citation2009). The layered plant immunity and high specificity of effectors has resulted in co-evolving molecular interactions, which determine the success or failure of an infection process.
The close co-evolution predicts that secreted effectors interacting with plant proteins should rapidly evolve. This rapid evolving feature of effector genes has been observed in several pathogen systems. U. maydis and Sporisorium reilianum are closely related fungal pathogens that parasitize maize plants, and both of them have established an intimate biotrophic relationship with their host by secreting protein effectors. Due to the fast evolving nature of effector genes, the virulence determinants can be uncovered by identifying variable genomic regions between these two similar genomes. The two smut fungi genomes have a remarkable degree of synteny, with an average amino acid identity of 76% for non-secreted proteins. However, the secreted protein encoding genes have a reduced sequence identity of 62% (Schirawski et al. Citation2010). Secreted effectors, including both novel and previously identified virulence clusters, are encoded in genomic regions of low sequence conservation. Moreover, deletion of these clusters in U. maydis showed altered virulence for this pathogen.
Another fast evolving group is the oomycete RXLR effectors, hundreds of these gene family members show very high sequence divergence. They are mostly located in rapid changing parts of the genome and show frequent genomic rearrangements (Jiang et al. 2008). Positive selection seems to drive the rapid divergence of C-termini of closely related paralogs of Phytophthora RXLR genes (Win and Kamoun Citation2008).
The direct evidence of rapid co-evolution of pathogen avirulence genes and host resistance genes has been described in a few interaction systems. The effector ATR13 from the oomycete downy mildew pathogen Hyaloperonospora parasitica shows a high degree of diversity in its alleles. It is recognized by one of the most variable Arabidopsis proteins encoded by the resistance gene RPP13. The different ATR13 alleles can be differentially recognized by RPP1 genes in Arabidopsis. Variation at four amino acid positions in the resistance protein is likely to be involved in this recognition, and it bears the signature of an intricate co-evolution process (Rehmany et al. Citation2005; Allen et al. Citation2008). Another classical example is the interaction between the flax rust fungus effectors and flax resistance L proteins. Both the pathogen AvrL567 genes and the host resistance L5, L6 and L7 genes are highly diverse. There is a high degree of recognition specificity and evidence of diversifying selection acting on these genes (Dodds et al. Citation2006).
Effector cooperation
There is a rich diversity of effector paralogs and alleles in a given pathogen population. Intriguingly, different effectors from the same pathogen may act in synergy. Due to intense selection pressure from the host, the very same effector that suppresses PTI may be recognized by R-proteins and trigger ETI. Therefore, multiple effectors acting together can offer a protective effect to each other. In other words, some effectors may be recognized by a host and elicit an immune response, while other effectors can dampen the defense responses to allow successful infection.
P. infestans effector IPI-O variant analysis supports this synergy concept. There is a high degree of complexity in IPI-O variants naturally occurring in different geographic regions, such as Guatemala, Thailand and the United States. Some alleles, such as IPI-O1, are able to trigger RB-mediated resistance in transgenic potato plants, while another allele, the IPI-O4 variant, is associated with aggressiveness of an isolate (Champouret et al. Citation2009). The ‘aggressiveness allele’ IPI-O4 is able to block the recognition of IPI-O1 by suppressing RB-mediated programmed cell death in Nicotiana benthamiana. Thus, one effector is able to inactive host immunity triggered by another effector (Halterman et al. Citation2010).
The synergy observed in the IPI-O family may, in part, explain the paralogous gene family expansion and rich diversity of alleles in pathogens. During the time-course of an infection, individuals from a large battery of effectors can have different expression peak time (Haas et al. Citation2009; Chen et al. 2010). This ‘shift’ in an individual effector expression profile may also account for their synergetic action.
Concluding remarks
Filamentous fungi and oomycetes are successful pathogens on plants, and the deployment of effectors is a crucial part of the infection process. The identified plant pathogen effectors are heterogeneous in sequence and some of them form rapidly expanding gene families. Effector genes show modularity in their molecular construction and are frequently located in the genome environment with high degrees of plasticity. There are still many questions about effectors remaining to be answered, such as the mechanism of fungal effector trafficking, the intrinsic function of effectors in the host and how the molecular struggle between effectors and host proteins has been played out in evolution and insight into these questions will undoubtedly benefit plant pathology and enable the design of new control strategies.
References
- Aepfelbacher , M , Zumbihl , R and Heesemann , J. 2005 . Modulation of Rho GTPases and the actin cytoskeleton by YopT of Yersinia . Curr Top Microbiol Immunol , 291 : 167 – 175 .
- Allen , RL , Meitz , JC , Baumber , RE , Hall , SA , Lee , SC , Rose , LE and Beynon , JL . 2008 . Natural variation reveals key amino acids in a downy mildew effector that alters recognition specificity by an Arabidopsis resistance gene . Mol Plant Pathol , 9 ( 4 ) : 511 – 523 .
- Armstrong , MR , Whisson , SC , Pritchard , L , Bos , JI , Venter , E , Avrova , AO , Rehmany , AP , Bohme , U , Brooks , K Cherevach , I . 2005 . An ancestral oomycete locus contains late blight avirulence gene Avr3a, encoding a protein that is recognized in the host cytoplasm . Proc Natl Acad Sci USA , 102 ( 21 ) : 7766 – 7771 .
- Boller , T and He , SY . 2009 . Innate immunity in plants: an arms race between pattern recognition receptors in plants and effectors in microbial pathogens . Science , 324 ( 5928 ) : 742 – 744 .
- Bos , JI , Armstrong , MR , Gilroy , EM , Boevink , PC , Hein , I , Taylor , RM , Zhendong , T , Engelhardt , S , Vetukuri , RR Harrower , B . 2010 . Phytophthora infestans effector AVR3a is essential for virulence and manipulates plant immunity by stabilizing host E3 ligase CMPG1 . Proc Natl Acad Sci USA , 107 ( 21 ) : 9909 – 9914 .
- Catanzariti , AM , Dodds , PN and Ellis , JG . 2007 . Avirulence proteins from haustoria-forming pathogens . FEMS Microbiol Lett. , 269 ( 2 ) : 181 – 188 .
- Champouret , N , Bouwmeester , K , Rietman , H , van der Lee , T , Maliepaard , C , Heupink , A , van de Vondervoort , PJ , Jacobsen , E , Visser , RG van der Vossen , EA . 2009 . Phytophthora infestans isolates lacking class I ipiO variants are virulent on Rpi-blb1 potato . Mol Plant Microbe Interact , 22 ( 12 ) : 1535 – 1545 .
- Chen , X , Klemsdal , SS and Brurberg , MB . 2011 . “ Phytophthora cactorum genes up-regulated during cyst germination and strawberry infection ” . In Identification and analysis of , Curr Genet 2011 . in press
- Chisholm , ST , Coaker , G , Day , B and Staskawicz , BJ . 2006 . Host--microbe interactions: shaping the evolution of the plant immune response . Cell , 124 ( 4 ) : 803 – 814 .
- Ciuffetti , LM , Manning , VA , Pandelova , I , Betts , MF and Martinez , JP. 2010 . Host-selective toxins, Ptr ToxA and Ptr ToxB, as necrotrophic effectors in the Pyrenophora tritici-repentis-wheat interaction . New Phytol , 187 ( 4 ) : 911 – 919 .
- Dodds , PN , Lawrence , GJ , Catanzariti , AM , Teh , T , Wang , CI , Ayliffe , MA , Kobe , B and Ellis , JG . 2006 . Direct protein interaction underlies gene-for-gene specificity and coevolution of the flax resistance genes and flax rust avirulence genes . Proc Natl Acad Sci USA , 103 ( 23 ) : 8888 – 8893 .
- Dou , D , Kale , SD , Wang , X , Chen , Y , Wang , Q , Jiang , RH , Arredondo , FD , Anderson , RG , Thakur , PB McDowell , JM . 2008 . Conserved C-terminal motifs required for avirulence and suppression of cell death by Phytophthora sojae effector Avr1b . Plant Cell , 20 ( 4 ) : 1118 – 1133 .
- Faris , JD , Zhang , Z , Lu , H , Lu , S , Reddy , L , Cloutier , S , Fellers , JP , Meinhardt , SW , Rasmussen , JB Xu , SS . 2010 . A unique wheat disease resistance-like gene governs effector-triggered susceptibility to necrotrophic pathogens . Proc Natl Acad Sci USA , 107 ( 30 ) : 13544 – 13549 .
- Fourie , G , Steenkamp , ET , Gordon , TR and Viljoen , A. 2009 . Evolutionary relationships among the Fusarium oxysporum f. sp. cubense vegetative compatibility groups . Appl Environ Microbiol , 75 ( 14 ) : 4770 – 4781 .
- Gordon , TR and Martyn , RD . 1997 . The evolutionary biology of Fusarium oxysporum . Annu Rev Phytopathol. , 35 : 111 – 128 .
- Gout , L , Fudal , I , Kuhn , ML , Blaise , F , Eckert , M , Cattolico , L , Balesdent , MH and Rouxel , T. 2006 . Lost in the middle of nowhere: the AvrLm1 avirulence gene of the Dothideomycete Leptosphaeria maculans . Mol Microbiol , 60 ( 1 ) : 67 – 80 .
- Haas , BJ , Kamoun , S , Zody , MC , Jiang , RH , Handsaker , RE , Cano , LM , Grabherr , M , Kodira , CD , Raffaele , S Torto-Alalibo , T . 2009 . Genome sequence and analysis of the Irish potato famine pathogen Phytophthora infestans . Nature , 461 ( 7262 ) : 393 – 398 .
- Halterman , DA , Chen , Y , Sopee , J , Berduo-Sandoval , J and Sanchez-Perez , A . 2010 . Competition between Phytophthora infestans effectors leads to increased aggressiveness on plants containing broad-spectrum late blight resistance . PLoS One , 5 ( 5 ) : e10536
- Heckman , DS , Geiser , DM , Eidell , BR , Stauffer , RL , Kardos , NL and Hedges , SB . 2001 . Molecular evidence for the early colonization of land by fungi and plants . Science , 293 ( 5532 ) : 1129 – 1133 .
- Hiller , NL , Bhattacharjee , S , van Ooij , C , Liolios , K , Harrison , T , Lopez-Estrano , C and Haldar , K . 2004 . A Host-Targeting Signal in Virulence Proteins Reveals a Secretome in Malarial Infection . Science , 306 ( 5703 ) : 1934 – 1937 .
- Jiang , RH , Tripathy , S , Govers , F and Tyler , BM . 2010 . RXLR effector reservoir in two Phytophthora species is dominated by a single rapidly evolving superfamily with more than 700 members . Proc Natl Acad Sci USA , 105 ( 12 ) : 4874 – 4879 .
- Jones , JD and Dangl , JL . 2006 . The plant immune system . Nature , 444 ( 7117 ) : 323 – 329 .
- Juge , N . 2006 . Plant protein inhibitors of cell wall degrading enzymes . Trends Plant Sci , 11 ( 7 ) : 359 – 367 .
- Kale , SD , Gu , B , Capelluto , DG , Dou , D , Feldman , E , Rumore , A , Arredondo , FD , Hanlon , R , Fudal , I Rouxel , T . 2010 . External lipid PI3P mediates entry of eukaryotic pathogen effectors into plant and animal host cells . Cell , 142 ( 2 ) : 284 – 295 .
- Kamoun , S . 2006 . A catalogue of the effector secretome of plant pathogenic oomycetes . Annu Rev Phytopathol. , 44 : 41 – 60 .
- Kamoun , S . 2007 . Groovy times: filamentous pathogen effectors revealed . Curr Opin Plant Biol. , 10 ( 4 ) : 358 – 365 .
- Kamper , J , Kahmann , R , Bolker , M , Ma , LJ , Brefort , T , Saville , BJ , Banuett , F , Kronstad , JW , Gold , SE Muller , O . 2006 . Insights from the genome of the biotrophic fungal plant pathogen Ustilago maydis . Nature , 444 ( 7115 ) : 97 – 101 .
- Kemen , E , Kemen , AC , Rafiqi , M , Hempel , U , Mendgen , K , Hahn , M and Voegele , RT . 2005 . Identification of a protein from rust fungi transferred from haustoria into infected plant cells . Mol Plant Microbe Interact , 18 ( 11 ) : 1130 – 1139 .
- Khang , CH , Berruyer , R , Giraldo , MC , Kankanala , P , Park , SY , Czymmek , K , Kang , S and Valent , B . 2010 . Translocation of Magnaporthe oryzae effectors into rice cells and their subsequent cell-to-cell movement . Plant Cell , 22 ( 4 ) : 1388 – 1403 .
- Latijnhouwers , M , de Wit , PJ and Govers , F . 2003 . Oomycetes and fungi: similar weaponry to attack plants . Trends Microbiol. , 11 ( 10 ) : 462 – 469 .
- Lievens , B , Houterman , PM and Rep , M . 2009 . Effector gene screening allows unambiguous identification of Fusarium oxysporum f. sp. lycopersici races and discrimination from other formae speciales . FEMS Microbiol Lett. , 300 ( 2 ) : 201 – 215 .
- Ma , LJ , van der Does , HC , Borkovich , KA , Coleman , JJ , Daboussi , MJ , Di Pietro , A , Dufresne , M , Freitag , M , Grabherr , M Henrissat , B . 2010 . Comparative genomics reveals mobile pathogenicity chromosomes in Fusarium . Nature , 464 ( 7287 ) : 367 – 373 .
- Marti , M , Good , RT , Rug , M , Knuepfer , E and Cowman , AF . 2004 . Targeting Malaria Virulence and Remodeling Proteins to the Host Erythrocyte . Science , 306 ( 5703 ) : 1930 – 1933 .
- Martin , F , Aerts , A , Ahren , D , Brun , A , Danchin , EG , Duchaussoy , F , Gibon , J , Kohler , A , Lindquist , E Pereda , V . 2008 . The genome of Laccaria bicolor provides insights into mycorrhizal symbiosis . Nature , 452 ( 7183 ) : 88 – 92 .
- Mosquera , G , Giraldo , MC , Khang , CH , Coughlan , S and Valent , B . 2009 . Interaction transcriptome analysis identifies Magnaporthe oryzae BAS1-4 as biotrophy-associated secreted proteins in rice blast disease . Plant Cell , 21 ( 4 ) : 1273 – 1290 .
- Parlange , F , Daverdin , G , Fudal , I , Kuhn , ML , Balesdent , MH , Blaise , F , Grezes-Besset , B and Rouxel , T. 2009 . Leptosphaeria maculans avirulence gene AvrLm4-7 confers a dual recognition specificity by the Rlm4 and Rlm7 resistance genes of oilseed rape, and circumvents Rlm4-mediated recognition through a single amino acid change . Mol Microbiol , 71 ( 4 ) : 851 – 863 .
- Raffaele , S , Farrer , RA , Cano , LM , Studholme , DJ , MacLean , D , Thines , M , Jiang , RH , Zody , MC , Kunjeti , SG Donofrio , NM . 2010a . Genome evolution following host jumps in the Irish potato famine pathogen lineage . Science , 330 ( 6010 ) : 1540 – 1543 .
- Raffaele , S , Win , J , Cano , LM and Kamoun , S. 2010b . Analyses of genome architecture and gene expression reveal novel candidate virulence factors in the secretome of Phytophthora infestans . BMC Genomics , 11 : 637
- Rafiqi , M , Gan , PH , Ravensdale , M , Lawrence , GJ , Ellis , JG , Jones , DA , Hardham , AR and Dodds , PN . 2010 . Internalization of flax rust avirulence proteins into flax and tobacco cells can occur in the absence of the pathogen . Plant Cell , 22 ( 6 ) : 2017 – 2032 .
- Raiola , A , Sella , L , Castiglioni , C , Balmas , V and Favaron , F . 2008 . A single amino acid substitution in highly similar endo-PGs from Fusarium verticillioides and related Fusarium species affects PGIP inhibition . Fungal Genet Biol , 45 ( 5 ) : 776 – 789 .
- Rehmany , AP , Gordon , A , Rose , LE , Allen , RL , Armstrong , MR , Whisson , SC , Kamoun , S , Tyler , BM , Birch , PR and Beynon , JL . 2005 . Differential recognition of highly divergent downy mildew avirulence gene alleles by RPP1 resistance genes from two Arabidopsis lines . Plant Cell , 17 ( 6 ) : 1839 – 1850 .
- Ridout , CJ , Skamnioti , P , Porritt , O , Sacristan , S , Jones , JD and Brown , JK . 2006 . Multiple avirulence paralogues in cereal powdery mildew fungi may contribute to parasite fitness and defeat of plant resistance . Plant Cell , 18 ( 9 ) : 2402 – 2414 .
- Rivas , S and Thomas , CM . 2005 . Molecular interactions between tomato and the leaf mold pathogen Cladosporium fulvum . Annu Rev Phytopathol. , 43 : 395 – 436 .
- Rooney , HC , Van't Klooster , JW , van der Hoorn , RA , Joosten , MH , Jones , JD and de Wit , PJ. 2005 . Cladosporium Avr2 inhibits tomato Rcr3 protease required for Cf-2-dependent disease resistance . Science , 308 ( 5729 ) : 1783 – 1786 .
- Russo , I , Babbitt , S , Muralidharan , V , Butler , T , Oksman , A and Goldberg , DE . 2010 . Plasmepsin V licenses Plasmodium proteins for export into the host erythrocyte . Nature , 463 ( 7281 ) : 632 – 636 .
- Schirawski , J , Mannhaupt , G , Munch , K , Brefort , T , Schipper , K , Doehlemann , G , Di Stasio , M , Rossel , N , Mendoza-Mendoza , A Pester , D . 2010 . Pathogenicity determinants in smut fungi revealed by genome comparison . Science , 330 ( 6010 ) : 1546 – 1548 .
- Schlumberger , MC and Hardt , WD. 2005 . Triggered phagocytosis by Salmonella: bacterial molecular mimicry of RhoGTPase activation/deactivation . Curr Top Microbiol Immunol , 291 : 29 – 42 .
- Schornack , S , van Damme , M , Bozkurt , TO , Cano , LM , Smoker , M , Thines , M , Gaulin , E , Kamoun , S and Huitema , E. 2010 . Ancient class of translocated oomycete effectors targets the host nucleus . Proc Natl Acad Sci USA , 107 ( 40 ) : 17421 – 17426 .
- Shabab , M , Shindo , T , Gu , C , Kaschani , F , Pansuriya , T , Chintha , R , Harzen , A , Colby , T , Kamoun , S and van der Hoorn , RA. 2008 . Fungal effector protein AVR2 targets diversifying defense-related cys proteases of tomato . Plant Cell , 20 ( 4 ) : 1169 – 1183 .
- Tian , M , Win , J , Song , J , van der Hoorn , R , van der Knaap , E and Kamoun , S. 2007 . A Phytophthora infestans cystatin-like protein targets a novel tomato papain-like apoplastic protease . Plant Physiol , 143 ( 1 ) : 364 – 377 .
- Torto-Alalibo , T , Collmer , CW , Gwinn-Giglio , M , Lindeberg , M , Meng , S , Chibucos , MC , Tseng , TT , Lomax , J , Biehl , B Ireland , A . 2010 . Unifying themes in microbial associations with animal and plant hosts described using the gene ontology . Microbiol Mol Biol Rev , 74 ( 4 ) : 479 – 503 .
- van Ooij , C , Tamez , P , Bhattacharjee , S , Hiller , NL , Harrison , T , Liolios , K , Kooij , T , Ramesar , J , Balu , B Adams , J . 2008 . The malaria secretome: from algorithms to essential function in blood stage infection . PLoS Pathog , 4 ( 6 ) : e1000084
- Van Poppel , PMJA , Jiang , RH , Sliwka , J and Govers , F. 2009 . Recognition of Phytophthora infestans Avr4 by potato R4 is triggered by C-terminal domains comprising W motifs . Mol Plant Pathol , 10 ( 5 ) : 611 – 620 .
- Whisson , SC , Boevink , PC , Moleleki , L , Avrova , AO , Morales , JG , Gilroy , EM , Armstrong , MR , Grouffaud , S , van West , P Chapman , S . 2007 . A translocation signal for delivery of oomycete effector proteins into host plant cells . Nature , 450 ( 7166 ) : 115 – 118 .
- Win , J and Kamoun , S . 2008 . Adaptive evolution has targeted the C-terminal domain of the RXLR effectors of plant pathogenic oomycetes . Plant Signal Behav , 3 ( 4 ) : 251 – 253 .
- Wubben , JP , Joosten , MH and De Wit , PJ . 1994 . Expression and localization of two in planta induced extracellular proteins of the fungal tomato pathogen Cladosporium fulvum . Mol Plant Microbe Interact , 7 ( 4 ) : 516 – 524 .