Abstract
In this review, we described how the plant and fungal cell structures are modified to interface between the host root and ectomycorrhizal (ECM) hyphae and what these processes imply in the forest ecosystem. Cytoplasmic polyphenols induce the colonization of ECM hyphae. The root hairs bend and collapse in contact with hyphae, which adhere closely to mucilage and are embedded in the epidermis. Accordingly, root hairs respond by developing thickened cell walls as the interaction progresses. After first contact with hyphae, their morphology changes with considerable branching and an increase in hyphal diameter. Hyphal morphology of the inner mantle is modified as tissue-like, with compact, flat and repeatedly branched hyphae. Inner mantle hyphae of ECM penetrate the inter-epidermal cells. The Hartig net is linked with inter-epidermal cells (angiosperms) or inter-epidermal and inter-cortical cells (conifers) by the highly branched labyrinthine hyphae. The epidermal cells of angiosperms are radially elongated. Polyphosphate granules are precipitated as an artefact of chemical specimen preparation in the vacuoles of the ECM mantle and Hartig net hyphae. Higher proportions of absorbed phosphate are translocated into the polyphosphate pool in the ECM roots. The wall ingrowth phenomenon, which is a response of root cells to mycorrhizal fungi for development as transfer cells, occurs on the epidermal cells adjacent to the mantle hyphae and the Hartig net hyphae interfacing with the epidermal cell wall, exchanging nutrients between plant and fungus in ECM. Eventually, the plant and fungal cell structures are modified to interface mutually and the outstanding ecological role of symbiont ECM hyphae is to increase the exploitation of phosphorus (P) to host tree from soil.
Introduction
More than 80% of vascular plants worldwide form symbiotic relationships with fungi as mycorrhiza. Symbiotic relationships of mycorrhiza include those associations in which one organism lives outside the root cells (ectomycorrhizae, ECMs), or where one partner lives inside root cell (endomycorrhizae: arbuscular mycorrhizae, ericoid mycorrhiza, arbutoid mycorrhizae, monotropoid mycorrhiza, ectendomycorrhizae and orchid mycorrhizae). ECMs are characterised by the formation of a mantle and a Hartig net of intercellular hyphae to interface with roots of predominantly tree species (Peterson et al. Citation2004).
Roots of all vascular plants are in intimate contact with several substrates (such as soil, water, rock, organic debris or tree litter) (Peterson et al. Citation2004). The litter layer in the fermentation horizon located below the surface consists of predominantly organic residues of the trees and their fungal symbionts and other components of the soil microflora. ECM symbiosis occurs on this layer of forest soil. Nutrient mobilization from natural organic substrates in the fermentation horizon of forest soils may be a core ecological role of the vegetative hyphae of ECM (Perez-Moreno and Read Citation2000).
Plants depend on the ability of roots to interact with microbes for the detection and attraction of partners, prior to direct contact, in many plant–microbe symbioses (Bais et al. Citation2004). During the pre-symbiotic phase, the host plant releases biochemical metabolites to induce fungal growth and root colonization (Buee et al. Citation2000; Jung and Tamai Citation2011). In one of the first stages of host recognition, the mycorrhizal hyphae show extensive branching in the vicinity of host roots before formation of the appressorium (AM fungi) and inner-mantle hyphae (ECM fungi), which are the structures used to penetrate the plant root (Giovannetii et al. Citation1993, Citation1994; Mosse and Hepper Citation1975; Massicotte et al. Citation1989). The branching factor (BF) of hyphae is hypothesised to be a plant signal molecule, such as flavonoids, needed to trigger hyphal morphogenesis that precedes successful root colonization (Buee et al. Citation2000; Giovannetii et al. Citation1996). The critical developmental step in their life cycle is hyphal branching, which helps to ensure contact with the host root and the establishment of symbiosis (Akiyama et al. Citation2005). In this review, we explore current knowledge relating to how the plant and fungal cell structures are modified to interface between the host root and ECM hyphae, and what these processes imply in the forest ecosystem.
Polyphenol and symbiosis
Polyphenols are among the most numerous and widely distributed groups of substances in the plant kingdom. Natural polyphenols can range from simple molecules, such as phenolic acids, phenolic derivatives and flavonoids, to highly polymerised compounds, such as tannins (Bravo Citation1998). Simple phenols (C6), such as thymol, resorcinol and orcinol, are widespread among plant species. They include hydroquinone and derivatives (arbutine and sesamol). In higher plants and ferns, phenolic acids (gallic, vanillic, syringic, and p - hydroxybenzoic) and aldehydes (vanillin, syringaldehyde, and p-hydroxy benzaldehyde) are also common. Phenylpropanoids, such as p-coumaric, caffeic, ferulic, and sinapic acids, and more simple phenols, such as benzoic acid and benzaldehyde derivatives, are usually covalently linked to cell wall polysaccharides or to lignin (Jung Citation1989; Wallace et al. Citation1991). Flavonoids constitute the most common and widely distributed group of plant polyphenols (Harbone and Mabry Citation1982). Proanthocyanidins (condensed tannins) can form insoluble complexes with carbohydrates and protein. They are high-molecular-weight polymers condensed by monomeric flavan-3-oles (e.g. catechin and epicatechin) (Würsch et al. Citation1984).
ECM hyphal growth of Pisolithus tinctorius is stimulated by a polyphenolic compound (Rutin) isolated from root exudates of Eucalyptus globulus ssp. bicostata (Lagrange et al. Citation2001). Flavonoids induce basidiospore germination in the ECM fungus Suillus bovinus (Kikuchi et al. Citation2007). Flavonoids also stimulate hyphal growth during early interactions between roots and arbuscular mycorrhizal (AM) fungi (Nair et al. Citation1991; Siqueira et al. Citation1991; Tsai and Phillips Citation1991) and hyphal development of AM fungi in the presence of optimal CO2 enrichment (Bécard et al. Citation1992; Poulin et al. Citation1993). Flavonoid accumulation in Medicago sativa roots is induced before root colonization and indicates the presence of a fungal-derived signal (Larose et al. Citation2002). Flavonoids induce transcription of nodulation genes in Rhizobium (Peters et al. Citation1986; Peters and Long Citation1988; Redmond et al. Citation1986). In addition, they enhance spore germination and hyphal growth of AM fungi (Gianinazzi-Pearson et al. Citation1989).
Stimulating hyphal growth and spore germination, polyphenols are secreted from lateral root surfaces. These plant root exudates are believed to include low molecular compounds, such as amino acids, organic acids, sugars and polyphenols, and various other secondary metabolites and high molecular compounds, such as mucilage and proteins (Walker et al. Citation2003). Extracts and exudates (abietic acid) derived from Scots pine stimulate basidiospore germination in ECM species (e.g. Suillus) (Fries et al. Citation1987; Fries Citation1988). Changes in the concentrations of individual flavonoids and polyamines (PAs) in cotyledonary seedlings of Scots pine (Pinus sylvestris L.) have been studied during the establishment of an ECM symbiosis with two Suillus variegatus strains in vitro (Niemi et al. Citation2007).
Some polyphenolic compounds are less concentrated in mycorrhizae than in nonmycorrhizal fine roots (Münzenberger et al. Citation1990, Citation1995). Catechin and epicatechin accumulate in apical tissue from proximal tissue of fine roots and some polyphenolic compounds increase in the early mycorrhizal stage and then decrease in the mature mycorrhizal stage of Larix decidua–Suillus tridentinus (Weiss et al. Citation1997). Levels of flavanols and cell-wall-bound ferulate within the cortex are high in the apical portion and low on the proximal side of conifer mycorrhizas (Weiss et al. Citation1999).
Polyphenols in plant cells of semithin sections are stained greenish-blue or green by Toluidine Blue O (TBO) (Feder and O'Brien Citation1968; O'Brien et al. Citation1964; Sakai Citation1973). The elongated epidermal cells of many mycorrhizas also contain phenolic substances in vacuoles that stain greenish-blue with TBO (Ling-lee et al. Citation1977). Cytoplasmic phenolic substances stained with TBO have been observed in epidermal, endodermal and root cap cells of both inoculated and uninoculated short roots of Pinus sp. (Piché et al. Citation1981). Cytoplasmic accumulates of polyphenols are stained a diffuse dark blue (Debeaujon et al. Citation2000; Debeaujon et al. Citation2003; Ranocha et al. Citation2002). In somatic embryogenesis, their vacuoles are filled with polyphenols that stain blue with TBO (Cangahuala-Inocente et al. Citation2004) and stain dark blue around deposits (Gutmann Citation1995; Gutmann et al. Citation1996). Lignified cell walls tend to stain blue-green, whereas unlignified cell walls stain reddish purple with TBO (Ros Barceló et al. Citation1989).
Studies of the role of polyphenolic compounds in the detection and attraction of partners prior to direct contact in many plant–microbe symbioses have revealed that polyphenols stimulate the growth of ECM hyphae (Lagrange et al. Citation2001) and induce transcription of nodulation genes in Rhizobium (Peters et al. Citation1986; Peters and Long Citation1988; Redmond et al. Citation1986). In addition, it has shown that plant sesquiterpenes induce hyphal branching in arbuscular mycorrhizal fungi, helping to ensure contact with the host root and establishment of symbiosis (Akiyama et al. Citation2005).
Cytoplasmic polyphenols inducing ECM hyphae
Suillus variegatus induces significant changes in polyphenol contents in Scots pine seedlings during the formation of mycorrhizae. The roots contain far more catechin and condensed tannins at the beginning of colonization than the needles or stems. A reduction in catechin and condensed tannins toward the end of mycorrhizal formation has been observed (Niemi et al. Citation2007). Phenolics are known to be low in Larix decidua–Laccaria amethystea mycorrhizae in comparison to nonmycorrhizal fine roots. In particular, cell wall-bound ferulic acid is known to be much lower in mycorrhizae than in nonmycorrhizal fine roots (Münzenberger et al. Citation1995). Additionally, the contents of some phenolics are lower in the mycorrhizae of Picea abies–Lactarius deterrimus and P. abies–Laccaria amethystea than in the nonmycorrhizal fine roots. The quantities of p-hydroxybenzoic acid glucoside, picein, catechin and cell wall-bound ferulic acid are substantially reduced in the mycorrhizae (Münzenberger et al. Citation1990). The tissue-specific and development-dependent accumulation of secondary metabolites in the roots and mycorrhizae of Larix deciduas with Suillus tridentinus have been studied by high-performance liquid chromatography and histochemical methods. The major phenolic compounds, catechin and epicatechin, accumulate at high levels during the first 2 weeks of root mycorrhization and are reduced at the mature mycorrhizal stage (Weiss et al. Citation1997).
A recent study (Jung and Tamai Citation2011) is particularly noteworthy and considered here in some detail. In this study, polyphenolic compounds in the epidermal cells varied during the Quercus acutissima–Scleroderma verrucosum ECM development. Epidermal cells of non-inoculated second-order lateral roots were diffusely stained blue with 0.05% (w/v) TBO in 0.1 M phosphate buffer (pH 7.2). Additionally, cytoplasmic polyphenolic compounds accumulated in root hairs, root cap cells, sloughs of root cap cells and apical meristems. Root cap zones formed pointed root apices with slough layers of root cap cells from the continued growth of apical meristems ().
Figure 1. Polyphenolic compound changes to epidermal cells in longitudinal section. (a) Non-inoculated lateral root with pointed root apex. Epidermal cells (EP) are diffusely stained dark by TBO. Cytoplasmic polyphenolic compounds (PC2) accumulate in the root hair (RH), root cap cell (RC), sloughs of root cap cell (SRC), and apical meristem (AM). Scale: 50 μm. (b) Mycorrhizal root at the early mantle stage. Epidermal cells (EP) are stained in the entire cell (PC2), stained partially (PC1), or not stained (PC0) with TBO. Hyphae (HY) adhere to the epidermal (EP) surface, and root hairs (RH) collapse and bend from the hyphae. Scale: 50 μm. (c) Mycorrhizal root at the mature mantle (M) stage. Epidermal cells (EP) are not stained with TBO, whereas the apical meristem (AM), root cap cell (RC), and sloughs of root cap cells (SRC) are stained. Scale: 50 μm. C, cortex; ED, endodermis; VC, vascular cambium; EXH, extraradical hyphae; OMH, outer mantle hyphae; IMH, inner mantle hyphae; RHZ, root hair zone; EZ, elongation zone; MZ, meristematic zone; RCZ, root cap zone; and ECMZ, ectomycorrhizal mantle zone (Jung and Tamai Citation2011).
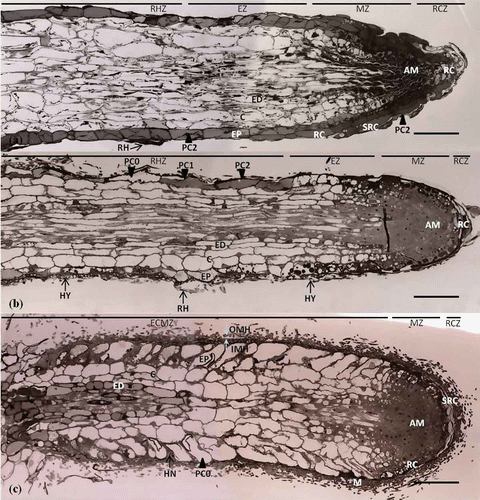
Epidermal cells of mycorrhizal roots at the early-mantled stage were stained entirely (PC2), stained partially (PC1), or not stained (PC0) by TBO. Hyphae adhered to the epidermal surface and to the root hairs, which were bent and collapsed as the result of direct contact with hyphae (). Initially, hyphae proliferated among root hairs in the root hair zone; subsequent development occurred in the elongation zone, meristematic zone and root cap zone (). In the transverse section of the root hair zone, the root hairs were stained entirely, stained partially, or not stained () with TBO. The hyphae penetrated between the root hairs and adhered to the mucilage layer, which was stained dark blue with TBO owing to its polyphenolic compound content. A number of hyphae surrounded the root hairs and the polyphenolic compounds in root hairs were stained partially or entirely. Epidermal cells () were similar to those in the longitudinal section (). In the root cap zone, root cap cells and the sloughs of root cap cells harbored polyphenolic compounds. Hyphae adhered to the mucilage above and within the sloughs of root cap cells ().
Figure 2. Polyphenolic compound changes induced by interactions between hyphae and epidermal cells and root hairs at the early mantle stage. (a) Transverse section of the root hair zone (RHZ). Polyphenolic compounds in root hairs (RH) and epidermal cells (EP) are stained entirely (PC), stained partially (PC1), or not stained (PC0). Hyphae penetrate between root hairs and epidermal cells and adhere to mucilage (MU). Scale: 20 μm. (b) Scanning electron microscopy image. Root hair (asterisk) is bent and collapsed by direct penetration (arrow) of fungal hyphae (arrowhead). Scale: 5 μm. (c) Longitudinal sections of the root hair zone (RHZ). Owing to the interplay between root hairs (RH) and hyphae (HY), polyphenolic compounds are stained partially (PC1). Hyphae are embedded in a mucilage layer stained dark by TBO. Scale: 25 μm. (d) Longitudinal section of the root cap zone (RZ). Polyphenolic compounds in root cap cells (RC) and sloughs of root cap cells (SRC) are stained entirely (PC), and hyphae adhere to mucilage and penetrate between sloughs of the root cap cell (SRC). Scale: 23 μm (Jung and Tamai Citation2011).
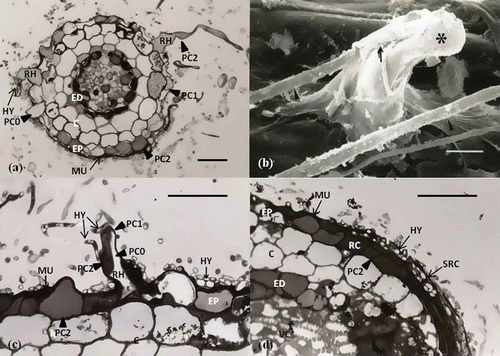
These polyphenolic compound contents began to decline in epidermal cells and root hairs colonised by the hyphae of Sc. verrucosum. This stage involved a dynamic process of polyphenol compound changes resulting in substances that were categorised as not stained, entirely stained, or partially stained. Following mantle formation, polyphenols were not observed in epidermal cells; although some still accumulated in certain epidermal cells and endodermal cells in the proximal zone. Total phenolic content in the root tips at each ECM developmental stage declined with ECM development. Eventually, polyphenolic compounds contained in abundance in the epidermal cells and root hairs of the non-inoculated lateral roots of the host disappeared as the result of the mycorrhization process with the symbiont. These results support the view that cytoplasmic polyphenols induce the colonization of ECM hyphae.
Modification of cell structure and ECM hyphal contact
ECM hyphae
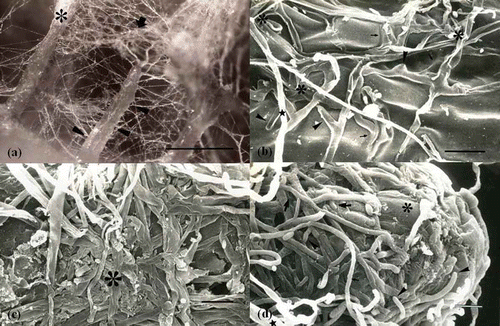
The interplay between hyphae, root hairs and epidermal cells during the early colonizing stage has been reported as root hairs being surrounded by fungal hyphae that penetrated between root hairs (Jung and Tamai Citation2011; Massicotte et al. Citation1988, Citation1989; Thomson et al. Citation1989) (). Colonizing hyphae have been reported in mucilage on the epidermis cells of feeder roots and root hairs (Ashford et al. Citation1996; Jung and Tamai Citation2011; Massicotte et al. Citation1993; Malajczuk et al. Citation1984; Wong et al. Citation1990) (). After first contact with hyphae, their morphology changes with considerable branching and an increase in hyphal diameter (Jung and Tamai Citation2011; Peterson et al. Citation2004) (). Recent studies on the ontogeny of the ECM mantle have shown that the hyphal morphology of the inner mantle becomes tissue-like with compact, flat, linked and repeatedly branched hyphae, increasing the surface area for exchange of nutrients (Jung and Tamai Citation2011; Massicotte et al. Citation1989) (). In addition, the root surface becomes enveloped in loosely organised hyphae or compact hyphae (Jung and Tamai Citation2011; Massicotte et al. Citation1999a, Citation1999b). The mature ECM is commonly the mantle with a cottony outer mantle and extraradical hyphae. Differences in the ECM mantle occur among the inner, middle and outer mantle ().
Figure 3. Structural changes of ECM hyphae occurring during ECM development. (a) Dense extraradical mycelium cluster forms cottony ectomycorrhizal roots (large asterisk). The extraradical mycelium (arrow) and hyphal strands (arrowheads) colonize nearly lateral roots. Scale: 0.5 mm. (b) Hyphae on the elongation zone in the early mantle stage. Hyphae are embedded in mucilage (arrows). Fungal morphology is characterized by greater branching (arrowheads) and greater hyphal diameter (asterisk) than normal hyphae (star). Scale: 10 μm. (c) The inner mantle of mature mantle stage. Hyphal morphology of the inner mantle is modified as tissue-like, with compact, flat and repeatedly branched hyphae (large asterisk). Scale: 10 μm. (d) Root apical zone of the mature mantle stage. Root cap cells (small asterisk) can be observed beneath the branching (arrowhead) and expanding hyphae of the developing inner mantle and loosely organized hyphae (small arrow) of the outer mantle. Scale: 10 μm (Jung and Tamai Citation2011).
Epidermal cell and root hair
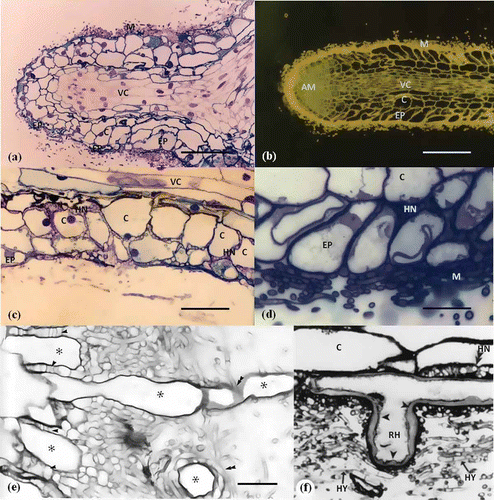
Uninoculated lateral roots tips have numerous root hairs and their root apices became pointed with the slough of root cap cell tissue during root elongation (Jung and Tamai Citation2011; Oh et al. Citation1995; Massicotte et al. Citation1986). ECM hyphal clusters emanating from soil cover epidermis tissue and surround root hairs at first contact. Hence, the root hairs are bent and collapsed when in direct contact with hyphae () and hyphae are embedded in mucilage-like material on the epidermis tissue ( and ). ECM hyphae penetrate between epidermal cells and root hairs () and form a complex highly branched structure, referred to as the Hartig net; inter root cells of the plant interface between the host plant and ECM hyphae (, (c)). Two obvious differences are apparent between angiosperms and conifers in Hartig net formation and structure modification of epidermal cell and cortical cell at the interfacing process. Firstly, the Hartig net in angiosperms is usually confined to the epidermis (, (d)), whereas in conifers it is formed around both epidermal and cortical cells and does not enter the endodermis (, (c)) (Jung and Tamai Citation2011; Massicotte et al. Citation1989; Peterson et al. Citation2004). Secondly, epidermal cells in angiosperms, arising from the interface with the Hartig net, are usually elongated radially in a regular fashion (, (d)) whereas, in conifers, they are modified irregularly in both epidermal and cortical cells (, ). In some case, the root hairs respond by developing thickened cell walls as the interaction progresses (Massicotte et al. Citation2000).
Figure 4. Differences between angiosperms and conifers regarding Hartig net formation and the response of root hair cell to the colonization of ECM hyphae. (a) Longitudinal section of Pinus rigiteada (conifer)–Pisolithus tinctorius ECM root tip. Cortical cells are modified, i.e. axially elongate and irregular in shape due to Hartig net formation. (b) Longitudinal section of Quercus acutissima (angiosperm)–Scleroderma verrucosum ECM root tip. Epidermal cells are modified to a radially elongated shape due to Hartig net formation. (c) Hartig net is formed around inter-cortical cells on Pinus rigiteada (conifer)–Pisolithus tinctorius ECM root tip. (d) Hartig net is formed only between epidermal cells on Quercus acutissima (angiosperm)–Scleroderma verrucosum ECM root tip. They are not interfacing between cortical cells (Jung and Tamai Citation2011). Scale: 20 μm (e) Longitudinal section of root showing root hair (asterisk)–fungal hyphae interaction on Betula alleghaniensis–Laccaria bicolor ECM root tip. Hyphae have penetrated between (arrowheads) and around (double arrowheads) (Massicotte et al. Citation1989). Scale: 20 μm. (f) Response of Pseudotsuga menziesii root hair against the colonization of Rhizopogon parksii ECM hyphae. The cell wall of root hair is thickened (Massicotte et al. Citation2000).
Modification of interface cell structure
Polyphosphate granules of ECM hyphae and Hartig net
The beneficial effects of mycorrhizae on plant growth have been frequently related to an increase in the uptake of immobile nutrients, particularly phosphorus (P) (Bolan Citation1991). The acquisition of phosphates from the soil through fungal hyphae to the plant root is one of the most important roles of ECM association (Schachtman et al. Citation1998).
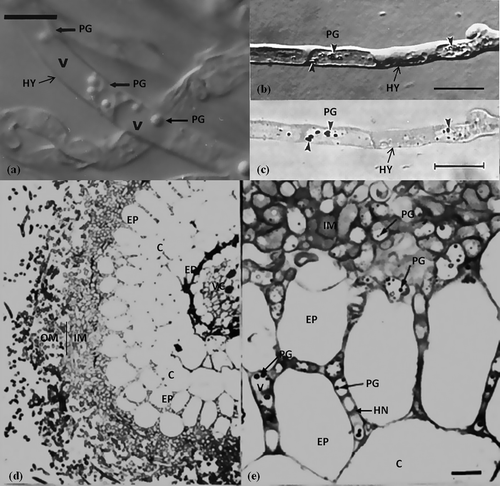
Polyphosphate, a linear condensed polymer of inorganic phosphate (Pi), is present in most microorganisms (Kulaev and Vagabov Citation1983). In fungal cells, a substantial amount of polyphosphate accumulates in vacuoles in the form of electron dense and osmophilic granules (Beever and Burns Citation1980). The capability of mycorrhizal fungi to accumulate phosphate via the formation of polyphosphates under conditions of high external concentrations, and to re-mobilise this storage pool under conditions of low phosphate availability in soil, is believed to be an important factor in the continuous phosphate supply of the host plant (Harley and Smith Citation1983). Polyphosphate granules are present in living ECM hyphae () (Bűcking and Heyser Citation1999). Generally, however, polyphosphate granules are easily precipitated as an artefact of chemical specimen preparation in the vacuoles of the ECM fungal hyphae; the granules are not generated by glutaraldehyde fixation but appear at early stages of ethanol dehydration (, (c)) (Orlovich and Ashford Citation1993). Polyphosphate granules are visible in numerous inner-mantle and Hartig net hyphae cells interfacing with epidermal cells for the exchange of nutrients to host plant (, (e)) (Grellier et al. Citation1989).
Figure 5. Polyphosphate acquisition of ECM hyphae. (a) Polyphosphate granules (PG) in the vacuoles (V) of the living hyphae (HY) of Suillus bovinus (Nomarski DIC microscopy) (Bűcking and Heyser Citation1999). Scale: 10 μm. (b) Polyphosphate granules (PG) are artefacts precipitated in the vacuoles of hyphae due to 2.5% glutaraldehyde and graded ethanol treatment. The hypha after staining with Toluidine Blue O, pH 1.0. Polyphosphate granules (PG) have increased in contrast (Orlovich and Ashford Citation1993). (c) Bright-field image of (b) showing the polyphosphate granules which exhibited γ-metachromasy (Orlovich and Ashford Citation1993). (d) Betula pendula–Paxillus involutus ectomycorrhiza. (e) Polyphosphate granules are visible in numerous inner-mantle (IM) and Hartig net (HN) hyphae cells (Grellier et al. Citation1989).
Hartig net formation
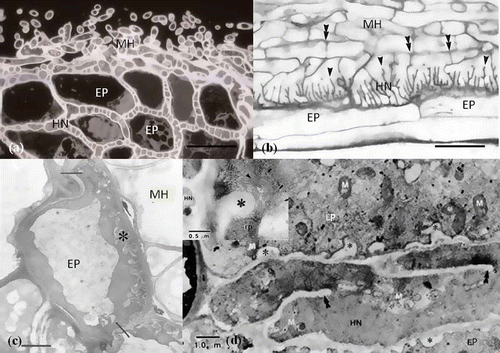
The Hartig net, which is a network of ECM hyphae originating from the inner mantle, is a unique structure of ECM fungi interfacing between epidermal and inter-cortical cells for nutrient exchange with host root tissue. In most angiosperms, the Hartig net develops around epidermal cells, whereas in conifers it is formed around both epidermal and cortical cells. In longitudinal and transversal section, the Hartig net appears as a net divided with several hyphae cellular wall between epidermal and cortical cells (). A well-developed Hartig net branches like a labyrinth, expanding the surface area interface for nutrient exchange, and the modified structure of the hyphae actually surrounds the cell walls of epidermal and cortical cells () (Massicotte et al. Citation1989). This highly branched finger-like or maze-like hyphal system increases the surface area and is the main structure of symbionts for nutrient exchange between interfacing host root cells (Blasius et al. Citation1986)
Figure 6. Structural modification of Hartig net hyphae and epidermal cells for interfacing. (a) Transverse section of Quercus acutissima–Scleroderma verrucosum ECM stained with PAS (Periodic acid Schiff's). Hartig net (HN) hyphae divided by several cell walls are present around epidermal cells (EP). (b) Longitudinal section of Betula alleghaniensis–Laccaria bicolor ECM root tip with the well-developed Hartig net branching like a labyrinth and expanded in surface area. Numerous branches (arrowheads), oriented primarily in a radial direction, and septa (double arrowheads) are evident (Massicotte et al. Citation1989). Scale: 20 μm. (c) Epidermal transfer cell (EP) from a Pisonia grandis mycorrhiza (transverse section) showing a more than average degree of wall ingrowths (asterisk) on the outer tangential wall, abutting fungal mantle hyphae (MF) of the sheath (Allaway et al. Citation1985). Scale: 2 μm. (d) Transmission electron micrograph of the epidermal cells (EP) interfacing with Hartig net (HN) on proximal region of Alnus crispa–Alpova diplophloeus ECM. Numerous wall ingrowths (asterisk) occur on epidermal cells abutting Hartig net hyphae with incomplete septations (double arrowheads). Mitochondria (m) (Massicotte et al. Citation1986).
Ingrowth of epidermal cells
Transfer cell structures are involved in the process of rapid nutrient mobilization. Such cells are formed in a broad variety of healthy plants, in situations in which there is a need for rapid and intensive short distance solute transport. The labyrinthine wall ingrowths in the host can be found in some symbiotic associations, such as legume nodules (Pate et al. Citation1969; Briarty Citation1978). Transfer cells are parenchymal cells modified by the elaboration of wall ingrowths and associated with the plasma membrane. They provide an increased surface area for the short distance transport of nutrients (Gunning and Pate Citation1974). An example is the wall ingrowth phenomenon of host cells in ECM to develop transfer cells. Transfer cells also develop from the outer cortical cells of the ECM of Pisonia grandis R. Br. (Nyctaginaceae) (Allaway et al. Citation1985; Ashford and Allaway Citation1982, Citation1985; Cairney et al. Citation1994) (). Transfer cells also occur at the host–fungus interface in Pinus sylvestris–Suillus bovinus ECM (Duddridge and Read Citation1984), in the tuberculate mycorrhizae of Castanopsis borneensis and Engelhardtia roxburghiana (Haug et al. Citation1991) and in Alnus crispa–Alpova diplophloeus ECM (Massicotte et al. Citation1986) ().
Ecological role of ECM hyphae
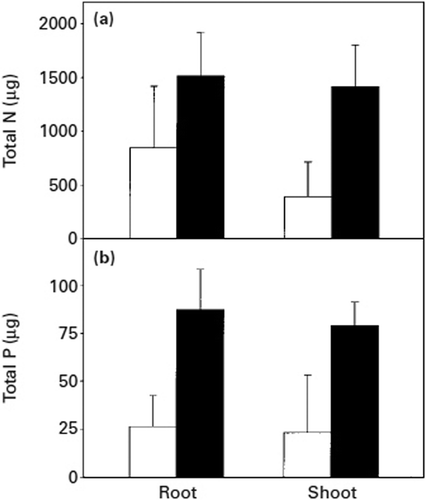
ECM hyphae capture nitrogen and phosphorus ions and facilitate their transfer over a distance of several centimetres to roots under aseptic condition (Melin and Nilsson Citation1950, Citation1953). In symbiotic relationships within natural ecosystems, there are predominantly organic residues from trees, their fungal symbionts and other components of the soil microflora, located below the surface litter layer in the fermentation horizon. Nutrient mobilization from natural organic substrates in the fermentation horizon of forest soils may be a key function of the vegetative mycelium of mycorrhizal systems (Perez-Moreno and Read Citation2000). In the fermentation horizon, material intensively occupied by mycelia of Suillus bovinus and Thelephora terrestris grow from the ECM roots of Pinus sylvestris and their nitrogen (N) and P contents are reduced progressively (Bending and Read Citation1995). The mycelium of Betula pendula–Paxillus involutus ECM proliferate intensively in all three types of litter – beech (Fagus sylvatica), birch (Betula pendula) and pine (Pinus sylvestris) – collected from the fermentation horizon of forest soils. This leads to a significant decline in the P content of litter 90 days (black bars) after colonization by Paxillus involutus compared with the litter before colonization (white bars) (); beech litter can display a >10% loss of N contents (). A major proportion of the phosphorus loss from litter originated in its organic fraction. The yield increases have been associated with gains in whole plant tissue content and concentration of P, but in content only in the case of N ( and ) (Perez-Moreno and Read Citation2000).
Figure 8. Nitrogen (a) and phosphorus (b) concentrations in birch, beech and pine litter before (white bars) and 90 days after (black bars) colonization by Paxillus involutus. Vertical bars indicate ± SE of the mean. Bars with the same letter are not significantly different with Tukey's multiple comparison test, p < 0.05 (Perez-Moreno and Read Citation2000).
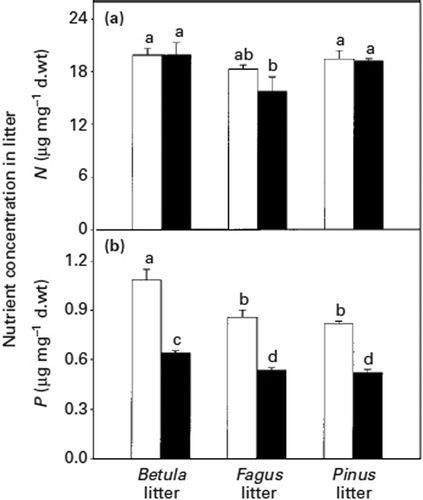
Figure 7. Total nitrogen (a) and phosphorus (b) contents in Betula pendula plants grown in the ectomycorrhizal condition with Paxillus involutus, in chambers with (black bars) or without (white bars) litter additions and harvested 90 days after addition of the litter. Vertical bars indicate ± SE of the mean (Perez-Moreno and Read Citation2000).
The outstanding ecological role of ECM hyphae interfacing with tree root tissue is to increase the exploitation of P to tree from soil. In one study, when the excised ECM roots of beech were soaked in a KH2 32PO4 solution, about 90% of the 32P was found in the Pi on the root tissue (Harley and Loughman Citation1963). The number of polyphosphate granules per hyphal length in Suillus bovinus is increased by an enhanced supply of KH2PO4. In a study with axenic cultures, nearly 80% of the absorbed phosphate accumulates as Pi (orthophosphate) and only 2 ± 9% was found in the polyphosphate pool. However, the phosphate metabolism of a fungus is altered by association with an ECM host. Compared to axenic cultures, approximately 2–5 times as many polyphosphates were formed in the mycorrhizal root, and the proportion of the absorbed phosphate located in the acid-insoluble polyphosphate pool was higher than that observed for the acid-soluble polyphosphate fraction with short-chain polyphosphates (Bűcking and Heyser Citation1999). The amount of polyphosphate in the mycelia of Paxillus involutus associated with Betula pendula was higher than that detected in pure cultures of this fungus (Grellier et al. Citation1989). In ECM of Fagus supplied with KH2PO4 (Harley and McCready Citation1981), nearly 40% of the absorbed phosphates were found in the polyphosphate pool. Polyphosphate content and accumulation were found to be increased more profoundly in the root tissues colonised by Glomus etunicatum at higher levels (400 mg kg−1 dry soil) of Pi (KH2PO4) addition (Takanishi et al. Citation2009).
The collective data supports the view that phosphorus uptake to plants from soil occurs via ECM hyphae. Pi absorbed on the fungal hyphae from external inorganic accumulates as polyphosphate on the fungal sheath. The polyphosphate passes into the hyphal cells of the Hartig net and short chain polyphosphates are transported into the plant root cells by the interfacing between the hyphal cells of the Hartig net and the ECM sheath, and the epidermal and cortical cells of the host plant. Pi passed to the host root is utilised as metabolic organic P for host growth (Harley and Loughman Citation1963; Ezawa et al. Citation2001).
Conclusions
During the pre-symbiotic phase, the host plant releases biochemical metabolites to induce fungal growth and root colonization. Cytoplasmic polyphenols within the epidermal cells and root hairs of host root induce the colonization of ECM hyphae. The root hairs bend and collapse in contact with hyphae, which adhere closely to mucilage and are embedded in the epidermis. Accordingly, root hairs respond by developing thickened cell walls as the interaction progresses. After first contact with hyphae, their morphology changes, with considerable branching and an increase in hyphal diameter. Hyphal morphology of the inner mantle is modified as tissue-like, with compact, flat and repeatedly branched hyphae, increasing the surface area for interfacing. ECM hyphae penetrate the inter-epidermal cells and the Hartig net is associated with inter-epidermal cells (angiosperms) or inter-epidermal and inter-cortical cells (conifers) by a highly branched, finger- or maze-like hyphal system increasing the surface area for interfacing. Hence, the epidermal cells of angiosperms are radially elongated. Polyphosphate granules are precipitated as an artefact of chemical specimen preparation in the vacuoles of the ECM mantle and Hartig net hyphae. Higher proportions of absorbed phosphates are translocated into the polyphosphate pool in the ECM roots. The wall ingrowth phenomenon, which is a response of root cells to mycorrhizal fungi for development as transfer cells, occurs on the epidermal cells adjacent to the mantle hyphae and the Hartig net hyphae interfacing with the epidermal cell wall and exchanging nutrients between plant and fungus in ECM.
The most important role of ECM hyphae interfacing with tree root tissue is to increase the exploitation of P to tree from soil in a forest ecosystem. The plant and fungal cell structures are modified to increase the interaction, i.e. nutritional transportation for mutual benefit, between the host root and ECM hyphae. Nutrient mobilization from natural organic substrates in the fermentation horizon of forest soils is a key role of the vegetative hyphae of ECM. In the fermentation horizon, organic material, intensively occupied by hyphae proliferating from ECM roots of tree species in the forest ecosystem, have their N and P contents reduced progressively.
Acknowledgements
We would like to sincerely thank emeritus Professor Dr Kwang-In Oh of Chonnam National University for the experiment material, Kye-Moon Wie for technical assistance, and Masanori Yasui of the Electron Microscope Center of Hokkaido University. The comments of anonymous reviewers are gratefully acknowledged.
References
- Akiyama , K , Matsuzaki , KI and Hayashi , H. 2005 . Plant sesquiterpenes induce hyphal branching in arbuscular mycorrhizal fungi . Nature , 435 : 824 – 827 .
- Allaway , WG , Carpenter , JL and Ashford , AE. 1985 . Amplification of inter-symbiont surface by root epidermal transfer cells in the Pisonia mycorrhiza . Protoplasma , 128 : 227 – 231 .
- Ashford , AE and Allaway , WG. 1982 . A sheathing mycorriza on Pisonia grandis R. Br. (Nyctaginaceae) with development of transfer cells rather than a Hartig net . New Phytol. , 90 : 511 – 519 .
- Ashford , AE and Allaway , WG. 1985 . Transfer cells and Hartig net in the root epidermis of the sheathing mycorrhiza of Pisonia grandis R.Br. from Seychelles . New Phytol. , 100 : 595 – 612 .
- Ashford , AE , Allaway , WG and Reed , ML. 1996 . A possible role for the thick-walled epidermal cells in the mycorrhizal hair roots of Lysinema ciliatum R. Br. and other Epacridaceae . Ann Bot. , 77 : 375 – 381 .
- Bais , HP , Park , SW , Weir , TL , Callaway , RM and Vivanco , JM. 2004 . How plants communicate using the underground information superhighway . Trends Plant Sci. , 9 : 26 – 32 .
- Bécard , G , Doubs , DD and Pfeffer , PE. 1992 . Extensive in vitro hyphal growth of vesicular–arbuscular mycorrhizal fungi in the presence of CO2 and flavonol . Appl Environ Microbiol. , 58 : 821 – 825 .
- Beever , RE and Burns , DJW. 1980 . Phosphorus uptake, storage and utilization by fungi . Adv Bot Res. , 8 : 127 – 219 .
- Bending , GD and Read , DJ. 1995 . The structure and function of the vegetative mycelium of ectomycorrhizal plants V. Foraging behaviour and translocation of nutrients from exploited litter . New Phytol. , 130 : 401 – 409 .
- Blasius , D , Feil , W , Kottke , I and Oberwinkler , F. 1986 . Hartig net structure and formation in fully ensheathed ectomycorrhizas . Nord J Bot. , 6 : 837 – 842 .
- Bolan , NS. 1991 . A critical review on the role of mycorrhizal fungi in the uptake of phosphorus by plants . Plant Soil , 134 : 189 – 207 .
- Bravo , L. 1998 . Polyphenols: chemistry, dietary sources, metabolism, and nutritional significance . Nutr Rev. , 56 : 317 – 333 .
- Briarty , LG. 1978 . The development of root nodule xylem transfer cells in Trifolium repens . J Exp Bot. , 29 : 735 – 747 .
- Bűcking , H and Heyser , W. 1999 . Elemental composition and function of polyphosphates in ectomycorrhizal fungi: an X-ray microanalytical study . Mycol Res. , 103 : 31 – 39 .
- Buee , M , Rossignol , M , Jauneau , A , Ranjeva , R and Bécard , G. 2000 . The presymbiotic growth of arbuscular mycorrhizal fungi is induced by a branching factor partially purified from plant root exudates . Mol Plant Microbe Interact. , 13 : 693 – 698 .
- Cairney , JWG , Rees , BJ , Allaway , WG and Ashford , AE. 1994 . A basidiomycete isolated from a Pisonia mycorrhiza forms sheathing mycorrhizas with transfer cells on Pisonia grandis R. Br . New Phytol. , 126 : 91 – 98 .
- Cangahuala-Inocente , GC , Steiner , N , Santos , M and Guerra , MP. 2004 . Morphohistological analysis and histochemistry of Feijoa sellowiana somatic embryogenesis . Protoplasma , 224 : 33 – 40 .
- Debeaujon , I , Leon-Kloosterziel , KM and Koornneef , M. 2000 . Influence of the testa on seed dormancy, germination, and longevity in Arabidopsis . Plant Physiol. , 122 : 403 – 413 .
- Debeaujon , I , Nesi , N , Perez , P , Devic , M , Grandjean , O , Caboche , M and Lepiniec , L. 2003 . Proanthocyanidin-accumulating cells in Arabidopsis testa: regulation of differentiation and role in seed development . Plant Cell , 15 : 2514 – 2531 .
- Duddridge , JA and Read , DJ. 1984 . Modification of the host-fungus interface in mycorrhizas synthesised between Suillus bovinus (FR.) O. Kuntz and Pinus sylvestris L . New Phytol. , 96 : 583 – 588 .
- Ezawa , T , Smith , SE and Smith , FA. 2001 . Differentiation of polyphosphate metabolism between the extra- and intraradical hyphae of arbuscular mycorrhizal fungi . New Phytol. , 149 : 555 – 563 .
- Feder , N and O'Brien , TP. 1968 . Plant microtechnique: some principles and new methods . Am J Bot. , 55 : 123 – 142 .
- Fries , N. 1988 . Specific effects of diterpene resin acids on spore germination of ectomycorrhizal basidiomycetes . Experientia , 44 : 1027 – 1030 .
- Fries , N , Serck-Hanssen , K , Dimberg , LH and Theander , O. 1987 . Abietic acid, an activator of basidiospore germination in ectomycorrhizal species of the genus Suillus (Boletaceae) . Exp Mycol. , 11 : 360 – 363 .
- Gianinazzi-Pearson , V , Branzanti , B and Gianinazzi , S. 1989 . In vitro enhancement of spore germination and early hyphal growth of a vesicular–arbuscular mycorrhizal fungus by host root exudates and plant flavonoids . Symbiosis , 7 : 243 – 255 .
- Giovannetti , M , Sbrana , C , Avio , L , Citernesi , AS and Logi , C. 1993 . Differential hyphal morphogenesis in arbuscular mycorrhizal fungi during preinfection stages . New Phytol. , 125 : 587 – 593 .
- Giovannetti , M , Sbrana , C and Logi , C. 1994 . Early process involved in host recognition by arbuscular mycorrhizal fungi . New Phytol. , 127 : 703 – 709 .
- Giovannetti , M , Sbrana , C , Silvia , A and Avio , L. 1996 . Analysis of factors involved in fungal recognition response to host-derived signals by arbuscular mycorrhizal fungi . New Phytol. , 133 : 65 – 71 .
- Grellier , B , Strullu , DG , Martin , F and Renaudin , S. 1989 . Synthesis in vitro, microanalysis and 31P-NMR study of metachromatic granules in birch mycorrhizas . New Phytol. , 112 : 49 – 54 .
- Gunning , BES and Pate , J. 1974 . “ Transfer cells ” . In Dynamic aspects of plant ultrastructure , Edited by: Robards , AW . 441 – 480 . New York : McGraw Hill .
- Gutmann , M. 1995 . Improved staining procedures for photographic documentation of phenolic deposits in semithin sections of plant tissue . J Microsc , 179 : 277 – 281 .
- Gutmann , M , Charpentier , JP , Doumas , P and Jay-Allemand , C. 1996 . Histological investigation of walnut cotyledon fragments for a better understanding of in vitro adventitious root initiation . Plant Cell Rep. , 15 : 345 – 349 .
- Harborne , JB and Mabry , TJ. 1982 . The flavonoids: advances in research , London : Chapman and Hall .
- Harley , JL and Loughman , BC. 1963 . The uptake of phosphate by excised mycorrhizal roots of the beech . New Phytol. , 63 : 350 – 359 .
- Harley , JL and McCready , CC. 1981 . Phosphate accumulation in Fagus mycorrhizas . New Phytol. , 89 : 75 – 80 .
- Harley , JL and Smith , SE. 1983 . Mycorrhizal symbiosis , London : Academic Press .
- Haug , I , Weber , R , Oberwinkler , F and Tsachen , J. 1991 . Tuberculate mycorrhizas of Castanopsis borneensis King and Engellhardtia roxburghiana wall . New Phytol. , 117 : 25 – 35 .
- Jung , HG. 1989 . Forage lignins and their effects on fiber digestibility . Agron J. , 81 : 33 – 38 .
- Jung , NC and Tamai , Y. 2011 . Anatomical observation of polyphenol changes in epidermal cells during the development of Quercus acutissima-Scleroderma verrucosum ectomycorrhizae . Trees , doi: doi10.1007/s00468-011-0592-4
- Kikuchi , K , Matsushita , N , Suzuki , K and Hogetsu , T. 2007 . Flavonoids induce germination of basidiospores of the ectomycorrhizal fungus Suillus bovinus . Mycorrhiza , 17 : 563 – 570 .
- Kulaev , IS. 1979 . “ The localization of inorganic polyphosphates within the cell volutin granules of microorganism. The site of localization of inorganic polyphosphates ” . In The Biochemistry of inorganic polyphosphates , Edited by: Kulaev , I.S. 47 – 66 . Chichester : Wiley .
- Kulaev , IS and Vagabov , VM. 1983 . Polyphosphate metabolism in micro-organisms . Adv Microb Physiol , 24 : 83 – 171 .
- Lagrange , H , Jay-Allgmand , C and Lapeyrie , F. 2001 . Rutin, the phenolglycoside from eucalyptus root exudates, stimulates Pisolithus hyphal growth at picomolar concentrations . New Phytol. , 149 : 349 – 355 .
- Larose , G , Chênevert , R , Moutoglis , P , Gagné , S , Piché , Y and Vierheilig , H. 2002 . Flavonoid levels in roots of Medicago sativa are modulated by the developmental stage of the symbiosis and the root colonizing arbuscular mycorrhizal fungus . J Plant Physiol. , 159 : 1329 – 1339 .
- Ling-lee , M , Chilvers , GA and Ashford , AE. 1977 . A histochemical study of phenolic materials in mycorrhizal and uninfected roots of Eucalyptus fastigata Deane and Maiden . New Phytol. , 78 : 313 – 328 .
- Malajczuk , N , Molina , R and Trappe , JM. 1984 . Ectomycorrhiza formation in Eucalyptus II. the ultrastructure of compatible and incompatible mycorrhizal fungi and associated roots . New Phytol. , 96 : 43 – 53 .
- Massicotte , HB , Peterson , RL , Ackerley , CA and Piché , Y. 1986 . Structure and ontogeny of Alnus crispa–Alpova diplophloeus ectomycorrhizae . Can J Bot. , 64 : 177 – 192 .
- Massicotte , HB , Peterson , RL and Melville , LH. 1988 . Ontogeny of Alnus rubra–Alpova diplophloeus ectomycorrhizae. I. light microscopy and scanning electron microscopy . Can J Bot. , 67 : 191 – 209 .
- Massicotte , HB , Peterson , RL and Melville , LH. 1989 . Hartig net structure of ectomycorrhizae synthesised between Laccaria bicolor (Tricholomaceae) and two hosts: Betula alleghaniensis (Betulaceae) and Pinus resinosa (Pinaceae) . Am J Bot. , 76 : 1654 – 1667 .
- Massicotte , HB , Melville , LH , Molina , R and Peterson , RL. 1993 . Structure and histochemistry of mycorrhizae synthesised between Arbutus menziesii (Ericaceae) and two basidiomycetes, Pisolithus tinctorius (Pisolithaceae) and Piloderma bicolor (Corticiaceae) . Mycorrhiza , 3 : 1 – 11 .
- Massicotte , HB , Melville , LH , Peterson , RL and Molina , R. 1999a . Biology of the ectomycorrhizal fungal genus, Rhizopogon IV. Comparative morphology and anatomy of ectomycorrhizas synthesised between several Rhizopogon species on Ponderosa pine (Pinus ponderosa) . New Phytol. , 142 : 355 – 370 .
- Massicotte , HB , Melville , LH , Peterson , RL and Unestam , T. 1999b . Comparative studies of ectomycorrhiza formation in Alnus glutinosa and Pinus resinosa with Paxillus involutus . Mycorrhiza , 8 : 229 – 240 .
- Massicotte , HB , Melville , LH , Peterson , RL and Molina , R. 2000 . Comparative anatomy of ectomycorrhizas synthesized on Douglas fir by Rhizopogon spp. and the hypogeous relative Truncocolumella citrina . New Phytol. , 147 : 389 – 400 .
- Melin , E and Nilsson , H. 1950 . Transfer of radioactive phosphorus to pine seedlings by means of mycorrhizal hyphae . Physiol Plant. , 3 : 88 – 92 .
- Melin , E and Nilsson , H. 1953 . Transfer of labelled nitrogen from glutamic acid to pine seedlings through the mycelium of Boletus variegatus (S.W.) . Fr. Nature , 171 : 134
- Mosse , B and Hepper , C. 1975 . Vesicular-arbuscular mycorrhizal infections in root organ cultures . Physiol Plant Pathol. , 5 : 215 – 223 .
- Münzenberger , B , Heilemann , J , Strack , D , Kottke , I and Oberwinkler , F. 1990 . Phenolics of mycorrhizas and non-mycorrhizal roots of Norway spruce . Planta , 182 : 142 – 148 .
- Münzenberger , B , Kottke , I and Oberwinkler , F. 1995 . Reduction of phenolics in mycorrhizas of Larix decidua Mill . Tree Physiol. , 15 : 191 – 196 .
- Nair , MG , Safir , GR and Siquiera , JO. 1991 . Isolation and identification of vesicular-arbuscular mycorrhiza stimulatory compounds from clover (Trifolium repens) roots . Appl Environ Microbiol. , 57 : 434 – 439 .
- Niemi , K , Julkunen-Tiitto , R , Häggman , H and Sarjala , T. 2007 . Suillus variegatus causes significant changes in the content of individual polyamines and flavonoids in Scots pine seedlings during mycorrhiza formation in vitro . J Exp Bot. , 58 : 391 – 401 .
- O'Brien , TP , Feder , N and McCully , ME. 1964 . Polychromatic staining of plant cell walls by Toluidine Blue O . Protoplasma , 59 : 367 – 373 .
- Oh , KI , Melville , LH and Peterson , RL. 1995 . Comparative structural study of Quercus serrata and Q. acutissima formed by Pisolithus tinctorius and Hebeloma cylindrosporum . Trees , 9 : 171 – 179 .
- Orlovich , DA and Ashford , AE. 1993 . Polyphosphate granules are an artifact of specimen preparation in the ectomycorrhizal fungus Pisolithus tinctorius . Protoplasma , 173 : 91 – 102 .
- Pate , JS , Gunning , BES and Briarty , LG. 1969 . Ultrastructure and functioning of the transport system of the leguminous root nodule . Planta , 85 : 11 – 34 .
- Perez-Moreno , J and Read , DJ. 2000 . Mobilization and transfer of nutrients from litter to tree seedlings via the vegetative mycelium of ectomycorrhizal plants . New Phytol. , 145 : 301 – 309 .
- Peters , NK , Frost , JW and Long , SR. 1986 . A plant flavone, luteolin, induces expression of Rhizobium meliloti nodulation genes . Science , 233 : 977 – 980 .
- Peters , NK and Long , SR. 1988 . Alfalfa root exudates and compounds which promote or inhibit induction of Rhizobium meliloti nodulation genes . Plant Physiol. , 88 : 396 – 400 .
- Peterson , RL , Massicotte , HB and Melville , LH. 2004 . Mycorrhizas: anatomy and cell biology , Wallingford , , UK : CABI Publishing .
- Piché , Y , Fortin , JA and Lafontaine , JG. 1981 . Cytoplasmic phenols and polysaccharides in ectomycorrhizal and non-mycorrhizal short roots of pine . New Phytol. , 88 : 695 – 703 .
- Poulin , MJ , Bel-Rhlid , R , Piché , Y and Chenevert , R. 1993 . Flavonoids released by carrot (Daucus carrota) seedlings stimulate hyphal development of vesicular-arbuscular mycorrhizal fungi in presence of optimal CO2 enrichment . J Chem Ecol. , 19 : 2317 – 2327 .
- Ranocha , P , Chabannes , M , Chamayou , S , Danoun , S , Jauneau , A , Boudet , AM and Goffner , D. 2002 . Laccase down-regulation causes alterations in phenolic metabolism and cell wall structure in poplar . Plant Physiol. , 129 : 145 – 155 .
- Redmond , JW , Batley , M , Djordjevic , MA , Innes , RW , Kuempel , PL and Rolfe , BG. 1986 . Flavones induce expression of nodulation genes in Rhizobium . Nature , 323 : 632 – 635 .
- Ros Barceló , A , Pedreño , MA , Muñoz , R and Sabater , F . 1989 . Physiological significance of the binding of acidic isoperoxidases to cell wall of lupin . Physiol Plant. , 75 : 267 – 271 .
- Sakai , WS. 1973 . Simple method for differential staining of paraffin embedded plant material using Toluidine Blue O . Stain Technol. , 48 : 247 – 249 .
- Schachtman , DP , Reid , RJ and Ayling , SM. 1998 . Phosphorus uptake by plant: from soil to cell . Plant Physiol. , 116 : 447 – 453 .
- Siqueira , JO , Safir , GR and Nair , MG. 1991 . Stimulation of vesicular-arbuscular mycorrhiza formation and growth of white clover by flavonoid compounds . New Phytol. , 118 : 87 – 93 .
- Takanishi , I , Ohtomo , R , Hayatsu , M and Saito , M. 2009 . Short-chain polyphosphate in arbuscular mycorrhizal roots colonized by Glomus spp.: A possible phosphate pool for host plants . Soil Biol Biochem. , 41 : 1571 – 1573 .
- Thomson , J , Melville , LH and Peterson , RL. 1989 . Interaction between the ectomycorrhizal fungus Pisolithus tinctorius and root hairs of Picea mariana (Pinaceae) . Am J Bot. , 76 : 632 – 636 .
- Tsai , MS and Phillips , AD. 1991 . Flavonoids released naturally from alfalfa promote development of symbiotic Glomus spores in vitro . Appl Environ Microbiol. , 57 : 1485 – 1488 .
- Walker , TS , Bais , HP , Grotewold , E and Vivanco , JM. 2003 . Root exudation and rhizosphere biology . Plant Physiol. , 132 : 44 – 51 .
- Wallace , G , Chesson , A , Lomax , JA and Jarvis , MC. 1991 . Lignin–carbohydrate complexes in graminaceous cell walls in relation to digestibility . Anim Feed Sci Technol. , 32 : 193 – 199 .
- Weiss , M , Mikolajewski , S , Peipp , H , Schmitt , U , Schmidt , J , Wray , V and Strack , D. 1997 . Tissue-specific and development-dependent accumulation of phenylpropanoids in larch mycorrhizas . Plant Physiol. , 114 : 15 – 27 .
- Weiss , M , Schmidt , J , Neumann , D , Wray , V , Christ , R and Strack , D. 1999 . Phenylpropanoids in mycorrhizas of the Pinaceae . Planta , 208 : 491 – 502 .
- Wong , KKY , Montpetit , D , Piché , Y and Lei , J. 1990 . Root colonization by four closely related genotypes of the ectomycorrhizal basidiomycete Laccaria bicolor (Maire) Orton: comparative studies using electron microscopy . New Phytol. , 116 : 669 – 679 .
- Würsch , P , del Vedovo , S , Rosset , J and Smiley , M. 1984 . The tannin granules from ripe carob pod . Lebens Wiss Technol. , 17 : 351 – 354 .