Abstract
The Chinese caterpillar fungus, Ophiocordyceps sinensis (syn. Cordyceps sinensis), is one of the most famous and perhaps the most expensive fungal species in the world. Its biology largely remains a secret, and its commercial cultivation is still a dream. Owing to its medicinal, economic, social and ecological importance, and its limited distribution mainly in China, O. sinensis is herein nominated as the national fungus of China and the fungus of the year (2012) for the journal Mycology. To clarify the confusion, a standard nomenclature is proposed and recommended in this paper for a comprehensive understanding of the terms related to Cordyceps sensu lato and O. sinensis. We also review recent research on the life cycle and distribution of this fungus, parasitism of ghost moths by the fungus, the microbial community of natural Chinese cordyceps and its insect hosts, as well as the secondary metabolites produced by the fungi isolated from natural Chinese cordyceps. By taking advantage of various expertises as well as the government support, we believe that the biological secrets of O. sinensis will be unravelled gradually, and the sustainable development and utilization of this traditional medicine will be fully achieved in the future.
Introduction
Ophiocordyceps sinensis (Berk.) G.H. Sung et al. (syn. Cordyceps sinensis) is one of the most famous and perhaps the most expensive fungal species in the world. O. sinensis parasitizes soil-borne larvae of ghost moths on the Tibetan Plateau (Stone Citation2008). The fungus–caterpillar complex resulting from fungal parasitism has a long history of use in traditional Chinese medicine (TCM) as well as in traditional Tibetan medicine for the treatment of asthma, bronchial and lung inflammation, and other diseases (Zhu et al. Citation1998; Chen, Lee et al. Citation2010). The hand-collected, naturally occurring fungus–caterpillar complex is highly valued by herbalists. Due to huge market demand, limited distribution in nature and the failure of artificial cultivation of this fungus, natural O. sinensis specimens (parasitized caterpillars with fungus fruiting bodies) have been heavily harvested, resulting in the depletion of the natural stock in recent years. O. sinensis has been listed as an endangered species in China (State Council of the People's Republic of China Citation1999).
In addition to being medicinally and economically important, O. sinensis is also ecologically important and considered as a flagship species for its ecosystem (Cannon Citation2010). Consequently, O. sinensis has become the focus of substantial recent research, and its genome is currently being sequenced (An et al. Citation2010). Although there are several recent review articles dealing with this fungus (Paterson Citation2008; Winkler Citation2008; Zhou et al. Citation2009; Guo et al. Citation2010; Shrestha et al. Citation2010), the ecology of the fungus has not been emphasized, and some terms relating to Cordyceps s.l. and O. sinensis are still confusing. In this review, we summarize what is known about the ecology of O. sinensis and suggest standardization of the terms concerning these fungi. Owing to its scientific novelty and social impact in China, we suggest that O. sinensis be selected as the national fungus of China and that it be “fungus of the year” for the journal Mycology.
Ophiocordyceps sinensis as the national fungus of China
Many countries in the world have selected national flowers, birds or trees, with which they have close cultural associations, as a pictorial or cultural icon or symbol. Several fungi in China could be nominated as the “national fungus” in view of their long history of public utilization and social impact. The lingzhi fungus (Ganoderma lucidum, ![]() in Chinese) could be an excellent candidate due to its importance in TCM and Chinese culture. However, G. lucidum is distributed worldwide and is now recognized as a species complex (Hseu et al. Citation1996; Postnova and Skolotneva Citation2010), and hence traditional lingzhi can refer to species other than G. lucidum. Other fungi that are also important in China include Tricholoma matsutake (Ito & Imai) Singer, Monascus purpureus Went, and Lentinus edodes (Berk.) Singer, but these are also distributed worldwide.
in Chinese) could be an excellent candidate due to its importance in TCM and Chinese culture. However, G. lucidum is distributed worldwide and is now recognized as a species complex (Hseu et al. Citation1996; Postnova and Skolotneva Citation2010), and hence traditional lingzhi can refer to species other than G. lucidum. Other fungi that are also important in China include Tricholoma matsutake (Ito & Imai) Singer, Monascus purpureus Went, and Lentinus edodes (Berk.) Singer, but these are also distributed worldwide.
We nominate O. sinensis as the national fungus of China based on the following characteristics:
| 1. | O. sinensis is distributed mainly in China and is even referred to as the “Chinese caterpillar fungus”. According to recent estimates, China accounts for more than 90% of its known production areas (Winkler Citation2008) and more than 95% of its annual yield (Winkler Citation2010). | ||||
| 2. | O. sinensis has close cultural connections to China, and natural O. sinensis specimens have been used as a TCM or an important dietary supplement since ancient times. Its pharmacological properties were discovered 1,500 years ago by herdsmen who observed that their yaks became energized after consuming it (Hollobaugh Citation1993). For a long time, however, the fungus was so difficult to obtain, and expensive, that only nobles and Chinese emperors were able to use it. This fungus first gained worldwide attention when it was credited for the success of Chinese woman athletes at the National Games in Beijing in Citation1993 (Steinkraus and Whitfield Citation1994; Stone Citation2008). Documentation of O. sinensis in China can be traced to the middle 15th century in Tibetan literature or to the late 17th century in Chinese literature (Winkler Citation2008). | ||||
| 3. | Sales of natural O. sinensis specimens represent an important portion of the gross domestic product of local governments and represent most, if not all, of the cash income of many rural families. About 80% of families in the major production areas are involved in collection of natural O. sinensis specimens, and cash income from sales of this resource accounts for 50–80% of their total income (Ma Citation2010). Currently, more than 300,000 Chinese citizens in local regions rely on the collection and sale of this resource (Ma Citation2010). No other fungus in China, or in any other country, plays such an important role in the local economy. The price of natural O. sinensis specimens has been rising in recent years and is currently higher than that of gold owing to its limited distribution and yield. | ||||
| 4. | O. sinensis is considered a flagship species of the Tibetan Plateau (Cannon Citation2010), and over-harvesting of the fungus poses a threat to this fragile ecosystem. The Tibetan Plateau is an especially important ecosystem as it is the source of many important rivers in Asia. It is estimated that in the Nyingchi district of Tibet alone, about 100,000 m2 of grasslands are damaged each year by human activities such as digging natural O. sinensis specimens out of soil, soil compaction by people and vehicles, and destruction from shrub cuttings to establish camps or to use as fuel for campfires (Zhuo et al. Citation2008). These destroyed grasslands are difficult to recover and can even lead to desertification (Yang Citation2008). | ||||
| 5. | O. sinensis is a fascinating organism, and there are still many unanswered questions concerning its biology. These include “Why is its distribution so limited?”, “How has the fungus coevolved with its insect hosts and with the plant hosts of the insects?”, and “Does parasitism of ghost moths by O. sinensis reduce herbivory and thereby alter the plant community?” Recognizing O. sinensis as the national fungus of China will promote research on its biology, ecology, and conservation. | ||||
Terminology related to Cordyceps and O. sinensis
Cordyceps is a traditional genus of ascomycete (sac fungi) that includes about 400 described species (Stensrud et al. Citation2005). All Cordyceps species are endoparasitic, mainly parasitizing insects and other arthropods (thus referred to as entomopathogenic fungi); a few parasitize other fungi. Recent phylogenetic analysis has shown that the traditional genus Cordyceps is not monophyletic and most former species of this genus are now assigned to Cordyceps, Ophiocordyceps, Metacordyceps, and Elaphocordyceps (Sung et al. Citation2007). The most important of these species for TCM are O. sinensis, C. cicada, C. militaris and E. ophioglossoides. Of all Cordyceps s.l. species, the best known is O. sinensis.
The close relationship between species of Cordyceps s.l. and their hosts has resulted in confusing terminology. For example, the fungus–host entities have often been referred to by the Latin name of the fungus, and this has puzzled non-scientists and some researchers. Here, we propose a standard nomenclature (). Because the fungus–host complex is the result of parasitism of a host by a fungus, the complex should be described by a word with general meaning. Analogous to the terms pseudomonad/Pseudomonas or fungi/Fungi, we suggest that the term “cordyceps” (lowercase and without italics) should be used to refer any cordyceps fungus–host complex (i.e. ![]() in Chinese), and the term “cordyceps fungus” (i.e.
in Chinese), and the term “cordyceps fungus” (i.e. ![]() in Chinese) should be used when referring only to the fungus that forms cordyceps. “Cordycipitaceous fungus” (i.e.
in Chinese) should be used when referring only to the fungus that forms cordyceps. “Cordycipitaceous fungus” (i.e. ![]() in Chinese) should be used to represent the fungal species within the family Cordycipitaceae. “Cordyceps s.l.” would refer to any species in the genus Cordyceps s.s. and in the closely related genera Ophiocordyceps, Metacordyceps, and Elaphocordyceps that were traditionally referred to as Cordyceps (i.e.
in Chinese) should be used to represent the fungal species within the family Cordycipitaceae. “Cordyceps s.l.” would refer to any species in the genus Cordyceps s.s. and in the closely related genera Ophiocordyceps, Metacordyceps, and Elaphocordyceps that were traditionally referred to as Cordyceps (i.e. ![]() in Chinese). Two English terms, “vegetable caterpillar” and “vegetable caterpillar fungus”, are found in some publications (Buller Citation1894; Salmon Citation1951) or in Google searches, but these terms are misnomers and should not be used.
in Chinese). Two English terms, “vegetable caterpillar” and “vegetable caterpillar fungus”, are found in some publications (Buller Citation1894; Salmon Citation1951) or in Google searches, but these terms are misnomers and should not be used.
Table 1. Recommended terminology concerning Cordyceps, Ophiocordyceps sinensis, and host insects
Ophiocordyceps sinensis and Chinese caterpillar fungus are normally represented by the terms “dong chong xia cao” (![]() in Chinese) or “dong chong xia cao jun” (
in Chinese) or “dong chong xia cao jun” (![]() in Chinese) (Winkler Citation2008). To differentiate between these terms and in recognition of the importance of this fungus to China, we recommend that the term “Chinese cordyceps” (
in Chinese) (Winkler Citation2008). To differentiate between these terms and in recognition of the importance of this fungus to China, we recommend that the term “Chinese cordyceps” (![]() or
or ![]() in Chinese) (Wang et al. Citation1996) should be used when referring to the O. sinensis–ghost moth caterpillar complex, while “Chinese cordyceps fungus” or “Chinese caterpillar fungus” (
in Chinese) (Wang et al. Citation1996) should be used when referring to the O. sinensis–ghost moth caterpillar complex, while “Chinese cordyceps fungus” or “Chinese caterpillar fungus” (![]() in Chinese) should be used as the common English names for the fungus. The term “natural O. sinensis specimens” has been used in TCM, and we recommend that the term “natural Chinese cordyceps” (
in Chinese) should be used as the common English names for the fungus. The term “natural O. sinensis specimens” has been used in TCM, and we recommend that the term “natural Chinese cordyceps” (![]() in Chinese) should be used for O. sinensis–ghost moth caterpillar complexes collected in nature. The term “winter worm, summer grass” is the literal translation of the Chinese name “
in Chinese) should be used for O. sinensis–ghost moth caterpillar complexes collected in nature. The term “winter worm, summer grass” is the literal translation of the Chinese name “![]() ” and should not be used because it lacks scientific meaning. Some people use the term “aweto” to refer to “
” and should not be used because it lacks scientific meaning. Some people use the term “aweto” to refer to “![]() ”, but aweto is the Māori name for Ophiocordyceps robertsii (syn. Cordyceps robertsii), a species from New Zealand (http://en.wikipedia.org/wiki/Ophiocordyceps_sinensis). The equivalent names for “
”, but aweto is the Māori name for Ophiocordyceps robertsii (syn. Cordyceps robertsii), a species from New Zealand (http://en.wikipedia.org/wiki/Ophiocordyceps_sinensis). The equivalent names for “![]() ” in other countries or regions were summarized by Shrestha et al. (Citation2010). Researchers and others should be aware that in TCM and in a few Chinese publications, “chong cao” (
” in other countries or regions were summarized by Shrestha et al. (Citation2010). Researchers and others should be aware that in TCM and in a few Chinese publications, “chong cao” (![]() in Chinese) represented either “dong chong xia cao” (
in Chinese) represented either “dong chong xia cao” (![]() in Chinese), i.e. Chinese cordyceps, or “chong cao” (
in Chinese), i.e. Chinese cordyceps, or “chong cao” (![]() in Chinese), i.e. cordyceps.
in Chinese), i.e. cordyceps.
Life cycle and distribution of O. sinensis
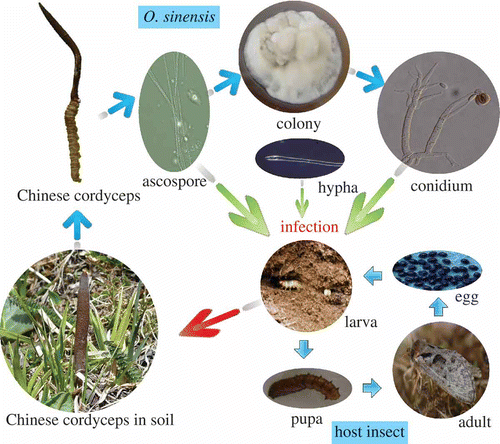
Ophiocordyceps sinensis has an unusual life cycle in nature (). In late autumn, the fungus infects underground larvae of ghost moths within the family Hepialidae. It is not known how the fungus infects the caterpillar; perhaps the caterpillar ingests a fungal spore, perhaps a fungal hypha penetrates a spiracle of the insect, or perhaps an ascospore or a conidium adhering to the insect surface germinates and directly penetrates the insect's cuticle. After entering the caterpillar's body, the fungus grows vegetatively and fills the caterpillar with threadlike hyphae. Even infected by the fungus, the larvae can still move from positions 5(10)–25(35) cm to positions 2–5 cm beneath the soil surface before dying with the head upward. The fungus then grows out from the dead host (usually from the head) and forms a small stroma bud before soil freezing in winter (Buenz et al. Citation2005; Li et al. Citation2006). In the following spring, the stroma bud grows upward, emerging above the soil surface and forming a stalked fruiting body (a sexual, perithecial stroma) (Pu and Li Citation1996; Buenz et al. Citation2005). Thread-like ascospores are released from the perithecia and can presumably infect new caterpillars. In addition to the ascospores, O. sinensis can also produce asexual conidia. The anamorph of the fungus is widely recognized as Hirsutella sinensis (Liu et al. Citation1989). The conidia are produced by conidiogenous cells formed on vegetative hyphae or germinated ascospores.
Growth and reproduction of O. sinensis in nature requires a host insect, but the fungus has a wide host range that includes more than 50 species within the family Hepialidae (Lepidoptera) (Wang and Yao Citation2011). Generally, the life cycle of O. sinensis insect hosts requires 2 or 3 years. The larval stages live underground and feed on roots of plants of more than 19 angiosperm families in alpine meadows on the Tibetan Plateau (Zhu et al. Citation2004).
Natural Chinese cordyceps are a biological community that includes, in addition to the Chinese caterpillar fungus and the insect host, many other microorganisms. Although the effect of those microorganisms on natural Chinese cordyceps is largely unknown, some other fungi can facilitate infection by the Chinese caterpillar fungus into the ghost moth larva (Li et al. Citation2010). In addition to biotic factors, some environment factors, such as temperature, humidity and light, may also affect the infection and sporulation of O. sinensis (Zhang, Yu, et al. Citation2011).
Chinese cordyceps are distributed in alpine regions of the Tibetan Plateau between 3000 and 5000 m asl or up to the snow line, depending on the locality. They are mainly found in China, including eastern Tibet, eastern Qinghai, western Sichuan, northern Yunnan, and southwestern Gansu provinces (Li et al. Citation2010; Yang et al. Citation2010). Tibet and Qinghai are the two major production regions of natural Chinese cordyceps in China, accounting for 80% of the total yields (Ma Citation2010). O. sinensis is also found in other Himalayan countries, including Nepal, Bhutan and India (Shrestha et al. Citation2010). The global distribution area of the fungus is estimated to be about 1 million km2, which represents about 10% of the area of the Chinese mainland (Zhang, Li, et al. Citation2010). Distribution of natural Chinese cordyceps with altitude has varied over time. Compared to 30 years ago, O. sinensis now occurs at higher altitudes in its core distribution regions (Nagqu and Qamdo in Tibet and Yushu in Qinghai) but at lower altitudes in peripheral distribution regions (Yang et al. Citation2010). O. sinensis has not been reported from western regions of the Tibetan Plateau, perhaps due to the dry climate. The distribution of this fungus is very limited probably because it is psychrophilic and must parasitize belowground larvae of ghost moths. Chinese cordyceps reported outside of “High Asia” were erroneously identified and refer to other Cordyceps species, e.g. Cordyceps militaris, Cordyceps gunnii (Shrestha et al. Citation2010; Yang et al. Citation2010).
Although the distribution of natural Chinese cordyceps is limited and its production has decreased (Chen, Zhong et al. Citation2010), the annual yield of this natural resource in China has been as high as 100–200 tons in recent years (Ma Citation2010; Zhang, Li et al. Citation2010). Among the five provinces, Qinghai has the largest production (about 100 tons per year), and Yushu and Guoluo contributed 80% of the total yield of this province (Cai and Sun Citation2010). In Tibet, the total yield is about 40 tons, with 80% contributed by Nagqu and Qamdo (Xu et al. Citation2010).
Parasitism of host insects by O. sinensis and its ecological consequence
Ophiocordyceps sinensis infects insect larvae in soil. A field investigation revealed that the highest infection occurred when 4th to 5th instar larvae were shedding old cuticles and forming new cuticles (Yang et al. Citation1989). In nature, this occurs from early to mid-August and overlaps with the time when O. sinensis releases sexual ascospores (Yang et al. Citation1989). Larvae at less advanced stages are seldom infected probably due to their limited movement and food intake, and those at more advanced stages are seldom infected probably owing to increased resistance. When larvae are infected, they become less active in 6–10 days and die in 15–25 days; infected larvae, however, often move to positions 2–5 cm beneath the soil surface before dying, which facilitates the growth of the fruiting body and its emergence from the soil in the next year (Yang et al. Citation1989). A small stroma bud often grows from the head of infected larvae before the soil freezes in winter. Local people have observed larvae with small stroma buds that were alive and able to move.
O. sinensis hyphal bodies were detected in the hemocoels of 10–20% of the 3rd to 8th instar larvae that were collected in nature (Li et al. Citation1998). Artificially inoculated larvae have also been used to study the growth of O. sinensis within the body of host insect larvae (Zeng et al. Citation2006). Mycelia of O. sinensis enter the hemocoel of the larva and then fragment into fusiform hyphae that multiply by yeast-type budding. After plasmogamy between hyphal fragments occurs, the mycelia of O. sinensis fill the hemocoel (Zeng et al. Citation2006). Two cuticle-degrading serine protease genes (csp1 and csp2) from O. sinensis have been cloned and found to be involved in the degradation of host cuticle proteins (Zhang et al. Citation2008).
Various factors can influence the infection process. The presence of other fungi shortened the time required by O. sinensis to mummify the host larva from 50 days to 3–5 days and increased the infection rate from 3 to 60% (Li et al. Citation2010). Loose soil structure and precipitation facilitate the movement of O. sinensis spores into soil and thereby increase infection (Yang et al. Citation1989). Conditions of generally low temperatures with substantial differences between day and night temperatures can increase the vertical movements of larvae, thus increase the probability that they encounter O. sinensis spores and become infected (Yang et al. Citation1989).
Ghost moths can seriously damage and even kill plants in some ecosystems. For example, parasitism of ghost moths by nematodes in California, USA, greatly affected the abundance of lupine, which would be the dominant plant in the absence of ghost moths (Stock et al. Citation1996). In the tripartite relationship between O. sinensis, insects, and plants, there is significant dependence of higher trophic levels (the fungus is at the top, the plant is at the bottom) on lower trophic levels, and it is possible that the three components together constitute a relatively stable system. Whether overharvest of natural Chinese cordyceps could result in increased herbivory and thereby alter the plant community is largely unknown.
Microbial community of natural Chinese cordyceps and host caterpillars
In attempts to determine the anamorph of O. sinensis (which is now recognized to be Hirsutella sinensis), researchers have isolated many fungi from natural Chinese cordyceps. Jiang and Yao (Citation2002, Citation2003) summarized the fungi associated with natural Chinese cordyceps during 1980s and 1990s, and their list included 22 species belonging to 13 genera (Jiang and Yao Citation2002, Citation2003). The list included seven novel species and also some taxa with nomenclatural problems. After their reviews, an additional fungus, Pseudogymnoascus roseus, was reported (Jiang and Yao Citation2006). Some of these fungi were found to have chemical components and/or pharmaceutical values similar to those of O. sinensis, and fermented mycelial products have been developed and applied in clinical practice (). Among these products, Bailing capsules and Jinshuibao capsules have each achieved annual sales of over RMB 100 million.
Table 2. Fungi that have been isolated from natural Chinese cordyceps and developed into health productsa
To delineate the fungal community of natural Chinese cordyceps, researchers have recently combined culture-dependent and -independent approaches (Zhang YJ, Zhang S, et al. Citation2010). Using a culture-dependent method, the researchers detected 572 fungal strains and 92 putative operational taxonomic units (OTUs) associated with natural Chinese cordyceps. Using a culture-independent method, the researchers detected 490 fungal clones (from approximately 3000 clones of ITS fragments representing the DNA of the entire fungal community), and 118 putative fungal OTUs. Which fungal species are determined to be most abundant depends on the method (culture-dependent or -independent) and on the tissue (stromata, sclerotia, and external mycelial cortices) (Zhang, Sun et al. Citation2010; Zhang YJ, Zhang S, et al. Citation2010). Only 13 OTUs were detected simultaneously by both methods, suggesting that the fungi associated with natural Chinese cordyceps are very diverse. Many potential novel fungal lineages were also detected by each of the two methods. The effects of these fungi on the life cycle of O. sinensis in nature and their potential application in the artificial cultivation of Chinese cordyceps warrant study.
Culture-dependent and -independent methods have also been used to investigate the diversity of fungi and bacteria in intestines of naturally collected or artificially reared host larvae (Yu et al. Citation2008). Insect intestines harbour a diverse microbial community that can affect host nutrition, physiology, and survival. Fungi within the genera Cryptococcus, Geomyces, Trichosporon, and Mortierella were detected in the intestines of different insect hosts (Yu et al. Citation2008). In addition, the frequency of occurrence of bacteria, fungi, and actinomycetes differed among the intestines of wild larvae, laboratory-reared larvae, and mummified larvae (Zhang, Ma et al. Citation2009), and the abundance of bacterial species differed between intestines of wild and laboratory-reared larvae (Zhuo et al. Citation2004; Liu et al. Citation2008). The effect of these microorganisms on larval growth and development requires investigation.
Although most microorganisms associated with insect host larvae are not pathogens, some are like O. sinensis in that they cause host death. These other pathogens, which include many fungi, bacteria and nematodes (Zeng and Yin Citation2003), can interfere with the large-scale rearing of host larvae for the purpose of commercial cultivation of Chinese cordyceps. Paecilomyces spp., Metarhizium spp., and Beauveria spp. are among the fungi that can cause serious diseases in host insect populations (Zeng and Yin Citation2003). These diseases and their possible control methods have been described in several reports (Chen et al. Citation1991; Zeng and Chen Citation2001; Zeng Citation2008).
Secondary metabolites produced by fungi isolated from natural Chinese cordyceps
As noted earlier, Chinese cordyceps is the combination of O. sinensis and a dead caterpillar of a ghost moth. Researchers have suspected that the fungal component could be a valuable source of bioactive metabolites, and extracts prepared from selected species of the Chinese cordyceps-colonizing fungi have been subjected to chemical investigation. Some bioactive secondary metabolites that have been discovered are briefly described in the following paragraphs ().
Figure 2. Structures of the bioactive secondary metabolites identified from the Chinese cordyceps-colonizing fungi.
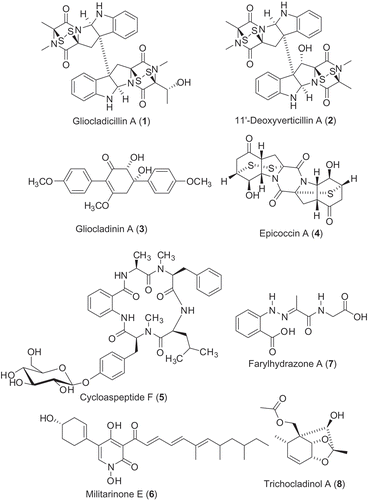
Two new epipolythiodioxopiperazines (ETPs) (Gardiner et al. Citation2005), gliocladicillins A ((1)) and B, along with the known ETPs, 11,11′-dideoxyverticillin A and 11′-deoxyverticillin A ((2)) (Son et al. Citation1999), have been isolated from Gliocladium sp. The two novel compounds, gliocladicillins A and B inhibited the proliferation of HeLa, HepG2, and MCF-7 cells; further study demonstrated that they induced HeLa cell apoptosis via both extrinsic and intrinsic pathways (Chen et al. Citation2009). In addition, they showed profound in vivo inhibitory effects on melanoma B16 cells implanted into immunodeficient mice (Chen et al. Citation2009). The known ETP, 11′-deoxyverticillin A ((2)), was found to enhance the cellular autophagic process, which requires both PARP and RIP-1 participation and which precedes and possibly augments the caspase-dependent apoptotic cell death (Zhang, Chen, et al. Citation2011). Two new p-terphenyl type metabolites, gliocladinins A ((3)) and B, were also identified from the same Gliocladium sp. (Guo et al. Citation2007); they showed modest antimicrobial activity against Staphylococcus aureus (ATCC 6538) and cytotoxicity against HeLa and HCT116 cells.
A group of new diketopiperazines (e.g. epicoccin A; (4)) with modest antimicrobial activity were identified from a strain of Epicoccum nigrum (Guo, Sun, Gao, Chen et al. Citation2009, Zhang et al. Citation2007). Cyclic pentapeptides (e.g. cycloaspeptide F; (5)), N-hydroxypyridones (e.g. militarinones E; (6)), phenylhydrazones, and farylhydrazones ((7)) were all isolated from the crude extract of Isaria farinosa. Cycloaspeptides F ((5)) and G showed cytotoxic effects against HeLa and MCF7 cell lines, whereas militarinone E ((6)) showed significant cytotoxicity against A549 cells (Zhang, Liu et al. Citation2009; Ma et al. Citation2011). New trichocladinols ((8)) were isolated from cultures of Trichocladium opacum; these compounds showed modest cytotoxic effects against the human tumour cell lines HeLa and MCF-7 (Guo, Sun, Gao, Niu et al. Citation2009).
These results demonstrate that Chinese cordyceps-colonizing fungi are a rich source of bioactive compounds. We suspect that these compounds function in the interaction between the fungi, the host and other microorganisms, but determining whether these compounds are produced in vivo and whether they are important in these interactions will require additional research.
Conclusion
The recognition of Ophiocordyceps sinensis as the national fungus of China and “fungus of the year” for the journal Mycology will make scientists in different disciplines more aware of O. sinensis and thereby promote new research on this interesting and important fungus. Our understanding of the biology and ecology of natural Chinese cordyceps is far from complete. For example, the coevolution between O. sinensis and host insects requires investigation. The function of microorganisms associated with natural Chinese cordyceps should be further evaluated. The effect of parasitism of ghost moths by O. sinensis on the natural plant community of the Tibetan Plateau is unknown. Finally, as an endangered species that is endemic to the Tibetan Plateau, O. sinensis requires conservation and has already attracted the attention of international public agencies such as the World Wildlife Fund (WWF) and the Centre Agriculture Bioscience International (CABI). In 2010, the International Center of Ophiocordyceps sinensis Research and Information (http://www.icosri.org/) was established and approved by the Chinese Ministry of Agriculture. We believe that collaboration among scientists, governmental and non-governmental agencies, and other stakeholders can protect O. sinensis from extinction and ensure the sustainable use of this traditional medicine and natural resource.
Acknowledgements
This study was supported by grants from the National Plans of Sciences and Technology (2007BAI32B04), the cooperative project between Guangdong Province and Chinese Academy of Sciences (2009B091300015), the National Science Foundation of China (81102759; 31140013), and the Specialized Research Fund for the Doctoral Program of Higher Education (20101401120007). The authors are grateful to Professor Yongsheng Che for his critical comments on the manuscript. We also thank Professor Bruce Jaffee (University of California at Davis) for serving as a pre-submission technical editor.
References
- An , ZQ , Wang , CS , Liu , XZ and Bennett , JW. 2010 . China's fungal genomics initiative: a whitepaper . Mycology , 1 ( 1 ) : 1 – 8 .
- Buenz , EJ , Bauer , BA , Osmundson , TW and Motley , TJ. 2005 . The traditional Chinese medicine Cordyceps sinensis and its effects on apoptotic homeostasis . J Ethnopharmacol. , 96 ( 1-2 ) : 19 – 29 .
- Buller , WL. 1894 . Note on the vegetable caterpillar (Cordiceps robertsii) . Trans Proc NZ Inst. , 27 : 155 – 156 .
- Cai , PY and Sun , SY. 2010 . Preliminary analysis on Ophiocordyceps sinensis resource sustainable development and utilization in Qinghai Province . Chin J Grassland. , 32 ( Supp. ) : 6 – 9 .
- Cannon , PF. 2010 . The caterpillar fungus, a flagship species for conservation of fungi . Chin J Grassland. , 32 ( Supp. ) : 86 – 88 .
- Chen , J , Gao , ZX , Yu , H and Yu , XM. 1991 . Preliminary study on pathogen and control of black spot disease of Hepialus oblifurcus . Microbiol China , 18 ( 6 ) : 323 – 325 .
- Chen , JY , Lee , SW , Cao , YQ , Peng , YQ , Winkler , D and Yang , DR. 2010 . “ Ethnomycological use of medicinal Chinese caterpillar fungus, Ophiocordyceps sinensis (Berk.) G. H. Sung et al. (Ascomycetes) in Northern Yunnan Province ” . In Int J Med Mushrooms Vol. 12 , 427 – 434 . SW , , China
- Chen , SJ , Zhong , GY and Ma , KS. 2010 . Thinking of the sustainable utilization of Cordyceps sinensis . Chin J Grassland. , 32 ( Supp. ) : 44 – 47 .
- Chen , Y , Guo , H , Du , Z , Liu , XZ , Che , Y and Ye , X. 2009 . Ecology-based screen identifies new metabolites from a Cordyceps-colonizing fungus as cancer cell proliferation inhibitors and apoptosis inducers . Cell Proliferat. , 42 ( 6 ) : 838 – 847 .
- Gardiner , DM , Waring , P and Howlett , B. 2005 . The epipolythiodioxopiperazine (ETP) class of fungal toxins: distribution, mode of action, functions and biosynthesis . Microbiology , 151 ( 4 ) : 1021 – 1032 .
- Guo , HC , Gao , JQ , Xi , QY and Li , XH. 2003 . Research progress on Cordyceps sinensis . J Microbiol. , 23 ( 1 ) : 50 – 55 .
- Guo , HJ , Hu , HJ , Liu , SC , Liu , XZ , Zhou , YG and Che , YS. 2007 . Bioactive p-terphenyl derivatives from a Cordyceps-colonizing isolate of Gliocladium sp . J Nat Prod. , 70 : 1519 – 1521 .
- Guo , HJ , Sun , BD , Gao , H , Chen , XL , Liu , SC , Yao , XS , Liu , XZ and Che , YS. 2009 . Diketopiperazines from the Cordyceps-colonizing fungus Epicoccum nigrum . J Nat Prod. , 72 : 2115 – 2119 .
- Guo , HJ , Sun , BD , Gao , H , Niu , SB , Liu , XZ , Yao , XS and Che , YS. 2009 . Trichocladinols A-C, cytotoxic metabolites from a Cordyceps-colonizing ascomycete Trichocladium opacum . Eur J Org Chem. , : 5525 – 5530 .
- Guo , YL , Xiao , PG and Wei , JC. 2010 . On the biology and sustainable utilization of the Chinese Medicine Treasure Ophiocordyceps sinensis (Berk.) G. H. Sung et al . Mod Chin Med. , 12 ( 11 ) : 3 – 8 .
- Hollobaugh , J. 1993 . A giant awakens . Track Field News , December : 4 – 6 . 24–25
- Hseu , RS , Wang , HH , Wang , HF and Moncalvo , JM. 1996 . Differentiation and grouping of isolates of the Ganoderma lucidum complex by random amplified polymorphic DNA-PCR compared with grouping on the basis of internal transcribed spacer sequences . Appl Environ Microbiol. , 62 ( 4 ) : 1354 – 1363 .
- Jiang , Y and Yao , YJ. 2002 . Names related to Cordyceps sinensis anamorph . Mycotaxon , 84 : 245 – 253 .
- Jiang , Y and Yao , YJ. 2003 . Anamorphic fungi related to Cordyceps sinensis . Mycosystema , 22 : 161 – 176 .
- Jiang , Y and Yao , YJ. 2006 . ITS sequence analysis and ascomatal development of Pseudogymnoascus roseus . Mycotaxon , 94 : 55 – 73 .
- Ke , CK. 2005 . Studies on Cordyceps sinensis in China , Beijing , , China : Science and Technology Press .
- Li , QS , Zeng , W , Yi , DH and Huang , TF. 1998 . Studies on the alternation of generations in Cordyceps sinensis . China J Chin Mater Med. , 23 ( 4 ) : 210 – 212 .
- Li , SP , Yang , FQ and Tsim , KWK. 2006 . Quality control of Cordyceps sinensis, a valued traditional Chinese medicine . J Pharm Biomed Anal. , 41 ( 5 ) : 1571 – 1584 .
- Li , YL , Ma , SL , Liu , X , Xu , HF and Zhang , ZH. 2010 . “ Research status and tendency of Ophiocordyceps sinensis in Qinghai province of China ” . In Ophiocordyceps sinensis resources and environment , 50 – 56 . Lanzhou : Lanzhou University Press; p .
- Liu , L , Wang , ZK , Yu , HW , Chen , SJ , Yan , GF , Xia , YX and Yin , YP. 2008 . Analysis of the bacterial diversity in intestines of Hepialus gonggaensis larvae . Acta Microbiol Sin. , 48 ( 5 ) : 616 – 622 .
- Liu , XJ , Guo , YL , Yu , YX and Zeng , W. 1989 . Isolation and identification of the anamorphic state of Cordyceps sinensis (Berk.) . Sacc. Acta Mycol Sin. , 8 : 35 – 40 .
- Ma , C , Li , Y , Niu , SB , Zhang , H , Liu , XZ and Che , YS. 2011 . N-Hydroxypyridones, phenylhydrazones, and a quinazolinone from Isaria farinosa . J Nat Prod. , 74 ( 1 ) : 32 – 37 .
- Ma , YX. 2010 . “ Ophiocordyceps sinensis resource and its management in China ” . In Ophiocordyceps sinensis resources and environment , 3 – 6 . Lanzhou : Lanzhou University Press. p .
- Paterson , RRM. 2008 . Cordyceps – A traditional Chinese medicine and another fungal therapeutic biofactory? Phytochemistry , 69 ( 7 ) : 1469 – 1495 .
- Postnova , E and Skolotneva , E. 2010 . Ganoderma lucidum complex: Some individual groups of strains Microbiology , 79 ( 2 ) : 270 – 276 .
- Pu , ZL and Li , ZZ. 1996 . Insect Mycology , Aihui : Anhui Science and Technology Press .
- Salmon , JT. 1951 . Vegetable caterpillars . Tuatara , 4 ( 1 ) : 1 – 3 .
- Shrestha , B , Zhang , WM , Zhang , YJ and Liu , XZ. 2010 . What is the Chinese caterpillar fungus Ophiocordyceps sinensis (Ophiocordycipitaceae)? . Mycology , 1 ( 4 ) : 228 – 236 .
- Son , BW , Jensen , PR , Kauffman , CA and Fenical , W. 1999 . New cytotoxic epidithiodioxopiperazines related to verticillin A from a marine isolate of the fungus Penicillium . Nat Prod Lett. , 13 ( 3 ) : 213 – 222 .
- State Council of the People's Republic of China. Order No. 4 of the State Forestry Administration and Ministry of Agriculture: the list of the wild plants under the state emphasized protection [Internet]. 1999; [cited 1999 Sept 9]. http://www.gov.cn/gongbao/content/2000/content_60072.htm (http://www.gov.cn/gongbao/content/2000/content_60072.htm)
- Steinkraus , DC and Whitfield , JB. 1994 . Chinese caterpillar fungus and world record runners . Am Entomol. , 40 ( 4 ) : 235 – 239 .
- Stensrud , O , Hyweljones , N and Schumacher , T. 2005 . Towards a phylogenetic classification of Cordyceps: ITS nrDNA sequence data confirm divergent lineages and paraphyly . Mycol Res. , 109 ( 1 ) : 41 – 56 .
- Stock , SP , Strong , D and Gardner , SL. 1996 . Identification of Heterorhabditis (Nenlatoda: Heterorhabditidae) from California with a new species isolated from the larvae of the ghost moth Hepialis californicus (Lepidoptera : Hepialidae) from the Bodega Bay Natural Reserve . Fundam Appl Nematol. , 19 ( 6 ) : 585 – 592 .
- Stone , R. 2008 . Last stand for the body snatcher of the Himalayas? Science , 322 : 1182
- Sung , GH , Hywel-Jones , NL , Sung , JM , Luangsa-ard , JJ , Shrestha , B and Spatafora , JW. 2007 . Phylogenetic classification of Cordyceps and the clavicipitaceous fungi . Stud Mycol. , 57 ( 1 ) : 5 – 59 .
- Wang , W , Zhong , YC , Zhou , SN and Chen , TC. 1996 . Studies on Cordyceps sinensis I. Confirmations of Chinese name and the insect hosts . Sun Yatsen Univ Forum , : 50 – 54 .
- Wang , XL and Yao , YJ. 2011 . Host insect species of Ophiocordyceps sinensis: a review . ZooKeys , 127 : 43 – 59 .
- Winkler , D. 2008 . Yartsa Gunbu (Cordyceps sinensis) and the fungal commodification of Tibet's rural economy . Econ Bot. , 62 ( 3 ) : 291 – 305 .
- Winkler , D. 2010 . Caterpillar fungus production and sustainability on the Tibetan Plateau and in the Himalayas . Chin J Grassland. , 32 ( Supp. ) : 96 – 108 .
- Xu , BZ , Ma , SJ and Feng , C. 2010 . The situation and management of Ophiocordyceps sinensis in Tibet Autonomous Region . Chin J Grassland. , 32 ( Supp. ) : 10 – 13 .
- Yang , DR. 2008 . Notes on field investigations of Ophiocordyceps sinensis resources in Tibetan plateau . China Nature , : 36 – 39 .
- Yang , DR , Peng , YQ , Chen , JY , Cao , YQ and Yang , P. 2010 . The distribution pattern of Cordyceps sinensis in China and the response to environmental changes . Chin J Grassland , 32 ( Supp. ) : 22 – 27 .
- Yang , YX , Yang , DR , Shen , FR and Dong , DZ. 1989 . Studies on Hepialid larvae for being infected by Chinese “insect herb” fungus (Cordyceps sinensis) . Zool Res. , 10 ( 3 ) : 227 – 231 .
- Yu , HW , Wang , ZK , Liu , L , Xia , YX , Yin , YP , Yuan , Q , Cao , YQ and Peng , GX. 2008 . Analysis of fungal diversity in intestines of Hepialus gonggaensis larvae . Acta Microbiol Sin. , 48 ( 4 ) : 439 – 445 .
- Zeng , W. 2008 . Infection, occurrence, and dissemination of green muscardine of Hepialus gonggaensis Chongqing J Res Chin Drugs Herbs , : 4 – 6 .
- Zeng , W and Chen , SJ. 2001 . Studies on Paecilomyces muscardine of Cordyceps sinensis host insect . China J Chin Mater Med. , 26 ( 7 ) : 21 – 22 .
- Zeng , W , Yin , DH , Li , QS and Li , L. 2006 . The growth of Cordyceps sinensis (Berk.) Sacc. in the infection and parasitic phases . Mycosystema , 25 ( 4 ) : 646 – 650 .
- Zeng , W and Yin , FJ. 2003 . Investigation on death of Hepialus gonggaensis larvae . Chongqing J Res Chin Drugs Herbs , : 5 – 6 .
- Zhang , GR , Yu , JF , Wu , GG and Liu , X. 2011 . Factors influencing the occurrence of Ophiocordyceps sinensis . Acta Ecol Sin. , 31 ( 14 ) : 4117 – 4125 .
- Zhang , LJ , Li , B and Hu , YJ. 2010 . Resource management of Ophiocordyceps sinensis in China . Chin J Grassland , 32 ( Supp. ) : 1 – 5 .
- Zhang , N , Chen , YL , Jiang , RX , Li , EW , Chen , XL , Xi , ZJ , Guo , YL , Liu , XZ , Zhou , YG , Che , YS and Yue , XJ. 2011 . PARP and RIP 1 are required for autophagy induced by 11′-deoxyverticillin A, which precedes caspase-dependent apoptosis . Autophagy , 7 ( 6 ) : 598 – 612 .
- Zhang , YG , Liu , SC , Liu , HW , Liu , XZ and Che , YS. 2009 . Cycloaspeptides F and G, cyclic pentapeptides from a Cordyceps-colonizing isolate of Isaria farinosa . J Nat Prod. , 72 ( 7 ) : 1364 – 1367 .
- Zhang , YG , Liu , SC , Che , YS and Liu , XZ. 2007 . Epicoccins A-D, epipolythiodioxopiperazines from a Cordyceps-colonizing isolate of Epicoccum nigrum . J Nat Prod. , 70 : 1522 – 1525 .
- Zhang , YJ , Liu , XZ and Wang , M. 2008 . Cloning, expression, and characterization of two novel cuticle-degrading serine proteases from the entomopathogenic fungus Cordyceps sinensis . Res Microbiol. , 159 ( 6 ) : 462 – 469 .
- Zhang , YJ , Sun , BD , Zhang , S , Wang , M , Liu , XZ and Gong , WF. 2010 . Mycobiotal investigation of natural Ophiocordyceps sinensis based on culture-dependent investigation . Mycosystema , 29 ( 4 ) : 518 – 527 .
- Zhang , YJ , Zhang , S , Wang , M , Bai , FY and Liu , XZ. 2010 . High diversity of the fungal community structure in naturally-occurring Ophiocordyceps sinensis . PLoS ONE , 5 ( 12 ) : e15570
- Zhang , ZH , Ma , SL , Xu , HF , Liu , X and Li , YL. 2009 . Analysis of change of microbial flora in intestine channel of Hepialus larva which was host of Cordyceps sinensis in Qinghai Province . Chin Qinghai J Anim Vet Sci. , 39 ( 6 ) : 17 – 19 .
- Zhou , XW , Gong , ZH , Su , Y , Lin , J and Tang , KX. 2009 . Cordyceps fungi: natural products, pharmacological functions and developmental products . J Pharm Pharmacol. , 61 ( 3 ) : 279 – 291 .
- Zhu , HF , Wang , LY and Han , HX. 2004 . Fauna Sinica: Insectam, Volume 38: Lepidoptera Hepialidae Epiplemidae , Beijing : Science Press .
- Zhu , JS , Halpern , GM and Jones , K. 1998 . The scientific rediscovery of an ancient Chinese herbal medicine: Cordyceps sinensis Part I . J Altern Complement Med , 4 : 289 – 303 .
- Zhuo , FP , Chen , SJ , Yin , YP , Wang , ZK and Xia , YX. 2004 . Analysis on the Hepialus Gonggaensis’ intestinal bacterial flora . J Chongqing Univ (Nat Sci Ed). , 27 ( 11 ) : 26 – 29 .
- Zhuo , G , Luo , Z and Wang , M. 2008 . The problem and solution of Cordyceps sinensis’s resource sustainable utilization in Tibet . Mod Agric Sci. , 15 ( 5 ) : 29 – 31 .