Abstract
Spore morphologies are a major character in fungal taxonomy, although many isolates are not able to sporulate on common artificial media. This article reviews the effect of nutrition, host tissue, and light on fungal sporulation in artificial media. A trial experiment using 42 strains that failed to sporulate on potato dextrose agar (PDA) and half-strength PDA after 3 months is reported. Five strategies (1/10-strength PDA, CaCO3 water agar, pine needle medium, mulberry agar, and near-ultraviolet light irradiation) were applied to induce these strains to sporulate, with an overall success rate of 62%. Pine needle medium was the most successful method, which induced sporulation of 40% of recalcitrant strains.
Keywords:
Introduction
Fungal spores are specialized cells that function as resting or dispersal propagules, which are important for fungal dissemination and propagation (Kirk et al. Citation2008). Spores and fruiting structures are the most important morphological characters used by mycologists to classify fungi into genera or differentiate closely related species. Although DNA sequence data have been popularized in fungal taxonomy, morphological characters are still essential in taxonomy (Hyde et al. Citation2010). However, numerous isolates fail to sporulate on commonly used media. For example, in endophyte studies, the sterile mycelia account for 4.5–54% of the total isolates (Fisher et al. Citation1994; Guo et al. Citation1998; Sánchez Márquez et al. Citation2008; Sun et al. Citation2012).
Fungal sporulation is a complex process influenced by environmental and endogenous biological rhythms (Wright Citation1979; Timberlake Citation1980). The molecular mechanisms of the induction of conidia in fungi have been intensively studied, mainly in model organisms such as Aspergillus nidulans and Neurospora crassa (Roncal and Ugalde Citation2003; Sun et al. Citation2012). A number of genes have been found to be involved in this process, affecting different steps of the signal transduction pathway (Roncal and Ugalde Citation2003; Xu et al. Citation2012; Zhou et al. Citation2012). In addition, numerous interdependent events must interact, both spatially and temporally, during the course of sporulation, e.g., substrate levels and in vivo enzyme activities (Timberlake Citation1980; Hernandez-Rodriguez et al. Citation2012; Tzima et al. Citation2012). Finding effective environmental factors that induce sporulation is often the first step towards making an accurate identification.
This article summarizes the major factors that induce sporulation, including nutrients, host tissues, and light (Smith and Berry Citation1974; Guo et al. Citation1998). A trial experiment was conducted using five different methodologies and the results are compared and discussed.
Factors related to fungal sporulation
Nutritional control
Many nutritional factors are effective in fungal sporulation, e.g., the carbon source, nitrogen source, and microelements (Timnick et al. Citation1951). Some fungi have specific carbon and nitrogen requirements for sporulation (Engelkes et al. Citation1997; Gao et al. Citation2007). Potato dextrose agar (PDA) is the most widely used medium in fungal isolation and culture. Other commonly used media include potato carrot agar, malt dextrose agar, yeast extract-phosphate medium, Czapek yeast autolysate agar, malt extract agar, cornmeal agar, potato sucrose agar, V8 vegetable juice agar (Booth Citation1971). These media provide favorable growth condition for most endophytic and pathogenic fungi, but are often ineffective in inducing sporulation of sterile isolates (Guo et al. Citation1998; Li et al. Citation2007).
Sporulation usually occurs when growth rate is reduced and is hampered under conditions that favor rapid mycelial growth (Dahlberg and Etten Citation1982). Starvation or nutritional depletion often stimulates sporulation, and some modifications of artificial media with low nutrient have been applied (Wulandari et al. Citation2009; Braun et al. Citation2011). Some typical low nutrient media include water agar media, half- or 1/4-strength PDA (Masangkay et al. Citation2000), and synthetic nutrient-poor agar medium (Nirenberg Citation1976).
Slide culture () (Riddell Citation1950) is often used in the induction of appressoria and conidiophores in Colletotrichum (Cai et al. Citation2009; Liu et al. Citation2011; Su et al. Citation2011). Slide cultures are also a recommended method for conidial induction (). First, the slide was supported by two sticks above one or two layers of filter paper on the bottom of a petri dish, which were autoclaved altogether. Second, the filter paper was soaked with sterilized water to maintain humidity. Third, two 0.5 mm × 0.5 mm pieces of PDA were placed on the slide and inoculated with a small quantity of mycelium at one corner of the agar block. Finally, a sterilized coverslip was placed on the agar block. The assembly was sealed and incubated at the optimum temperature for fungal growth for 7–14 days, after which the coverslip can be removed and observed microscopically.
Figure 1. Slide culture technique to induce the formation of spores. (A) Sterilized filter paper, sticks, and slide. (B) Add sterilized water on the filter paper to maintain humidity. (C) A small amount of mycelia was inoculated on 0.5 mm × 0.5 mm PDA agar. (D) Cover a sterilized coverslip on the agar block, seal the petri dish, and incubate at the fungal growth temperature for 7–14 days. The coverslip was taken out and observed microscopically.
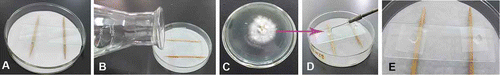
In spite of the fact that nutrient limitation is one of the most widely used strategies to induce conidial formation, its signaling mechanism remains to be understood. One possible explanation is the existence of sensors that signal nutrient status to the cell (Özcan et al. Citation1996; Roncal and Ugalde Citation2003).
Host tissue
Plant pathogenic fungi are often used to induce sporulation on the source host tissues; for example, Photita et al. (Citation2001) used banana petioles to induce sporulation of endophytes isolated from wild banana (Musa acuminata) leaves. Autoclaved leaves of Rhododendron pulchrum cv. Ohmurasaki were also found to induce sporulation of Guignardia endophyllicola (Okane et al. Citation2001). A sterilized petiole fragment of the palm Livistona chinensis was used to induce sporulation of endophytic sterile mycelia isolated from L. chinensis (Guo et al. Citation1998).
Some particular plant tissues are effective in inducing sporulation. Autoclaved pine needles were added onto the surface of agar media to induce pycnidia of some Botryosphaeriaceae spp. (Crous et al. Citation2006). Sterilised branch segments of Sophora japonica were utilized to promote sporulation of the endophytic fungi from lichens (Li et al. Citation2007). Carnation (Dianthus caryophyllus) leaves were found to be a good substrate for sporulation in Fusarium and Pestalotiopsis species (Fisher et al. Citation1982; Wei et al. Citation2006; Liu et al. Citation2010). Leaves of Hydrangea or Gardenia were found to be effective for perithecia and ascospore formation of Glomerella cingulata, Mycosphaerella allicina, Metasphaeria sp., Guignardia sp., and Pleospora herbarum (Furukawa and Kishi Citation2002). Yoshida and Shirata (Citation2000) showed that the leaves of 22 plant species induced sporulation in Colletotrichum dematium. They also tested compounds in mulberry leaves and found that biotin may play a role in inducing sporulation. The biotin in the plant tissues may alter the synthesis of wall polysaccharides and oleic acid and thus trigger the selective expression of genes involved in sporulation (Leaver et al. Citation1947; Tomiyama et al. Citation1962; Misawa Citation1965; Yamaguchi Citation1974; Timberlake Citation1980).
In addition, some chemical supplements also increased sporulation; for example, CaCO3 induced sporulation of some Alternaria species (Shahin and Shepard Citation1979; Masangkay et al. Citation2000). Externally bound calcium has been shown to be directly involved in the induction step (Ugalde and Pitt Citation1986; Ugalde et al. Citation1990).
Light effect
Light has been regarded as an important stimulus for conidial formation. Exposure of mycelia to near-ultraviolet (UV) light and blue light has been widely used (Marsh et al. Citation1959; Dahlberg and Etten Citation1982; Betina Citation1995; Xu et al. Citation2009). For example, spores have been successfully induced by near-UV light irradiation in some species of Ascomycetes (Crous et al. Citation2006), Basidiomycetes (Miller Citation1967), Myxomycetes (Starostzik and Marwan Citation1995), and Zygomycetes (Idnurm et al. Citation2006).
Wavelengths spanning 350–500 nm are most effective in inducing sporulation (Dahlberg and Etten Citation1982). Various isolates showed different threshold dosages under particular wavelengths (Rakoczy Citation1965), and excessive irradiation can inhibit sporulation (Rakoczy Citation1963, 1965). One commonly used method is 12 hours near-UV light irradiation and irradiation in darkness for 12 hours, imitating the diurnal cycles of light and darkness (Marsh et al. Citation1959; Leach Citation1962).
The mechanism by which fungal conidiation is induced by light remains unknown. Dahlberg and Etten (Citation1982) proposed that photoreceptors affect the electron transport system, affecting the pH and ionic balance of the cell as well as glucose and carbohydrate metabolism. Several light-sensing proteins have been identified during the process of fungal conidiation (Corrochano Citation2007; Idnurm et al. Citation2010; Rodriguez-Romero et al. Citation2010; Ruger-Herreros et al. Citation2011). For example, the White Collar-1 and Vivid proteins have been recently identified as the blue light photoreceptor that mediates light induction of the rhythmic conidiation of N. crassa (Froehlich et al. Citation2002; He et al. Citation2002; Schafmeier and Diernfellner Citation2011). In addition, some transcription factors, e.g., BLR-1 and BLR-2, are vital to the photoconidiation process (Sanchez-Arregui et al. Citation2012).
Other factors
Though previous studies focused on factors that affect sporulation, such as temperature (Prasad et al. Citation1973), pH (Yazdany and Lashkari Citation1975), humidity (Paul and Munkvold Citation2005), and mutilation of the mycelium (Rands Citation1917), these studies often focused on spore yield. Generally, combinations of these factors were often adopted, e.g., low nutrient media plus near-UV light irradiation (Crous et al. Citation2006; Wulandari et al. Citation2009; Glienke et al. Citation2011) ().
Table 1. Environmental stimuli for the induction of conidiation in some important pathogenic fungi
A trial experiment for induction of sporulation
Forty-two plant pathogenic fungal strains that did not sporulate on PDA and half-strength PDA after 3 months were selected for the experiment. Four media were chosen, namely 1/10-strength PDA (potato 20 g, boiled for 30 min and filtrated; agar 15 g; distilled water added to constant volume of 1 L), CaCO3 water agar (agar 15 g; CaCO3 30 g; distilled water 1 L, pH 7.4) (Shahin and Shepard Citation1979), pine needle medium (prepared by putting 250 g of fresh pine needles and 20 g of potatoes 20 g into 1 L water, boiling for 30 min, filtrating, keeping the volume to 1 L by adding water, and then adding 20 g of agar), and mulberry agar (mulberry leaf 33 g; sucrose 20 g; agar 18 g; distilled water 1 L) (Yoshida and Shirata Citation2000). The fungi were identified by morphological characters and phylogenetic analysis.
Twenty-one out of the total 42 tested isolates produced spores (). Of these, six isolates sporulated on mulberry agar, fourteen on pine needle medium, nine on 1/10-strengh PDA medium, and six on CaCO3 water agar. The remaining cultures that did not sporulate were exposed to near-UV light, and another five produced spores (). Thus, 26 out of 42 strains (62%) sporulated.
Table 2. Twenty-six successful sporulation-inducing isolates
In this experiment, low nutrient media amended with host tissue induced sporulation in many strains. Pine needle medium was shown to be very effective. Similarly, Crous et al. (Citation2006) used tap water agar with autoclaved pine needles to induce sporulation of Botryosphaeriaceae spp. The media containing ground Austrian pine needles successfully induced pycnidia in Diplodia scrobiculata and D. pinea. One hypothesis is that unidentified compounds in pine needles may stimulate sporulation.
Our experiment also showed that both mulberry leaf and pine needle media were effective in inducing sporulation of Fusarium sp. and Celoporthe sp., which produced abundant conidia ().
Fourteen Colletotrichum strains sporulated in this experiment. Sangeetha and Rawal (Citation2008) tested the best carbon and nitrogen sources required for growth and sporulation of C. gloeosporioides from mango and found heavy sporulation was observed if maltose and ammonium nitrate were used as carbon and nitrogen sources, respectively. Suzaki (Citation2011) compared the conidial mass of C. gloeosporioides on PDA, oatmeal agar (OMA), and carrot juice agar and found that C. gloeosporioides sporulated when the isolates were pre-cultured in potato dextrose broth and then spread on diluted OMA under continuous light. The aims of these studies were to determine the optimal growth and maximal conidial yield of the isolates. Our result indicated that low nutrient is helpful in inducing sporulation in Colletotrichum species.
Onesirosan (Citation1978) studied the factors affecting sporulation of Phomopsis phaeolorum and found that various common media in combination with techniques such as exposure to near-UV light and scraping of the culture surface were ineffective in inducing sporulation, while sterilized plant parts in combination with techniques such as exposure to cool-white fluorescent light were effective in inducing sporulation. In our experiment, three Phomopsis isolates were induced successfully, one only produced spores on CaCO3 water agar, one on pine needle medium and 1/10-strength PDA, and another one on 1/10-strength PDA, pine needle medium, and CaCO3 water agar. The results showed that low nutrients, CaCO3, and pine needle induced sporulation in some Phomopsis strains. Overall, pine needle medium was most effective in inducing sporulation in recalcitrant fungi.
Sterile mycelia are often encountered in taxonomic and ecological studies (Suryanarayanan and Vijaykrishna Citation2001; Su et al. Citation2010; Tejesvi et al. Citation2011; Sun and Guo Citation2012). The induction of sporulation allows confident identification, which results in a better understanding of the fungal communities in nature. Factors such as low nutrient media, host tissue, and light irradiation should be considered in the induction of sporulation in artificial media.
Acknowledgements
This work was financially supported by Chinese Academy of Sciences (KSCX2-YW-Z-1026) and the National Natural Science Foundation of China (NSFC 31110103906).
References
- Aragaki , M. 1961 . Radiation and temperature interaction on the sporulation of Alternaria tomato . Phytopathology , 51 : 803 – 805 .
- Betina , V. 1995 . Photoinduced conidiation in Trichoderma viride . Folia Microbiol , 40 ( 3 ) : 219 – 224 .
- Booth , C. 1971 . Fungal culture media . Methods Microbiol , 4 : 49 – 94 .
- Braun , U , Crous , PW , Groenewald , JZ and Scheuer , C. 2011 . Pseudovirgaria, a fungicolous hyphomycete genus . IMA Fungus , 2 ( 1 ) : 65 – 69 .
- Cai , L , Hyde , KD , Taylor , PWJ , Weir , BS , Waller , J , Abang , MM , Zhang , JZ , Yang , YL , Phoulivong , S Liu , ZY . 2009 . A polyphasic approach for studying Colletotrichum . Fungal Divers , 39 : 183 – 204 .
- Charlton , K. 1953 . The sporulation of Alternaria solani in culture . Trans Br Mycol Soc , 36 ( 4 ) : 349 – 355 .
- Corrochano , LM. 2007 . Fungal photoreceptors: sensory molecules for fungal development and behaviour . Photochem Photobiol Sci , 6 ( 7 ) : 725 – 736 .
- Crous , P , Slippers , B , Wingfield , M , Rheeder , J , Marasas , W , Philips , A , Alves , A , Burgess , T , Barber , P and Groenewald , J. 2006 . Phylogenetic lineages in the Botryosphaeriaceae . Studies Mycol , 55 : 235 – 253 .
- Dahlberg , KR and Etten , J. 1982 . Physiology and biochemistry of fungal sporulation . Annu Rev Phytopathol , 20 ( 1 ) : 281 – 301 .
- Engelkes , C , Nuclo , R and Fravel , D. 1997 . Effect of carbon, nitrogen, and C: N ratio on growth, sporulation, and biocontrol efficacy of Talaromyces flavus . Phytopathology , 87 ( 5 ) : 500 – 505 .
- Fisher , N , Burgess , L , Toussoun , T and Nelson , P. 1982 . Carnation leaves as a substrate and for preserving cultures of Fusarium species . Phytopathology , 72 ( 1 ) : 151 – 153 .
- Fisher , PJ , Petrini , O , Petrini , LE and Sutton , RC. 1994 . Fungal endophytes from the leaves and twigs of Quercus ilex L. from England, Majorca and Switzerland . New Phytol , 127 ( 1 ) : 133 – 137 .
- Froehlich , AC , Liu , Y , Loros , JJ and Dunlap , JC. 2002 . White Collar-1, a circadian blue light photoreceptor, binding to the frequency promoter . Science , 297 ( 5582 ) : 815 – 819 .
- Furukawa , T and Kishi , K. 2002 . Production of perithecia of various ascomycotina on water agar medium emended with leaf pieces . J Phytopathol , 150 ( 11–12 ) : 625 – 628 .
- Gao , L , Sun , MH , Liu , XZ and Che , YS. 2007 . Effects of carbon concentration and carbon to nitrogen ratio on the growth and sporulation of several biocontrol fungi . Mycol Res , 111 ( 1 ) : 87 – 92 .
- Glienke , C , Pereira , OL , Stringari , D , Fabris , J , Kava-Cordeiro , V , Galli-Terasawa , L , Cunnington , J , Shivas , RG , Groenewald , JZ and Crous , PW. 2011 . Endophytic and pathogenic Phyllosticta species, with reference to those associated with Citrus Black Spot . Persoonia , 26 : 47 – 56 .
- Guo , L , Hyde , K and Liew , E. 1998 . A method to promote sporulation in palm endophytic fungi . Fungal Divers , 1 : 109 – 113 .
- He , Q , Cheng , P , Yang , Y , Wang , L , Gardner , KH and Liu , Y. 2002 . White collar-1, a DNA binding transcription factor and a light sensor . Science , 297 ( 5582 ) : 840 – 843 .
- Hernandez-Rodriguez , Y , Hastings , S and Momany , M. 2012 . The septin AspB in Aspergillus nidulans forms bars and filaments and plays roles in growth emergence and conidiation . Eukaryot Cell , 11 ( 3 ) : 311 – 323 .
- Hyde , K , Abd-Elsalam , K and Cai , L. 2010 . Morphology: still essential in a molecular world . Mycotaxon , 114 ( 13 ) : 439 – 451 .
- Idnurm , A , Rodriguez-Romero , J , Corrochano , LM , Sanz , C , Iturriaga , EA , Eslava , AP and Heitman , J. 2006 . The Phycomyces madA gene encodes a blue-light photoreceptor for phototropism and other light responses . Proc Natl Acad Sci USA , 103 ( 12 ) : 4546 – 4551 .
- Idnurm , A , Verma , S and Corrochano , LM. 2010 . A glimpse into the basis of vision in the kingdom Mycota . Fungal Genet Biol , 47 ( 11 ) : 881 – 892 .
- Kirk , MP , Cannon , PF , Minter , DW and Stalpers , JA. 2008 . Dictionary of the fungi. , 10th , 653 Wallingford , (CT) : CABI .
- Larmour , R and Marchant , R. 1977 . The induction of conidiation in Fusarium culmorum grown in continuous culture . J Gen Microbiol , 99 ( 1 ) : 49 – 58 .
- Leach , CM. 1962 . Sporulation of diverse species of fungi under near-ultraviolet radiation . Can J Bot , 40 ( 1 ) : 151 – 161 .
- Leaver , FW , Leal , J and Brewer , CR. 1947 . Nutritional studies on Pyricularia oryzae . J Bacteriol , 54 : 401 – 408 .
- Li , W , Zhou , J , Guo , SY and Guo , LD. 2007 . Endophytic fungi associated with lichens in Baihua mountain of Beijing, China . Fungal Divers , 25 : 69 – 80 .
- Liu , AR , Chen , SC , Wu , SY , Xu , T , Guo , LD , Jeewon , R and Wei , JG. 2010 . Cultural studies coupled with DNA based sequence analyses and its implication on pigmentation as a phylogenetic marker in Pestalotiopsis taxonomy . Mol Phylogenet Evol , 57 ( 2 ) : 528 – 535 .
- Liu , F , Hyde , KD and Cai , L. 2011 . Neotypification of Colletotrichum coccodes, the causal agent of potato black dot disease and tomato anthracnose . Mycology , 2 ( 4 ) : 248 – 254 .
- Marsh , PB , Taylor , EE and Bassler , LM. 1959 . A guide to the literature of certain effects of light on fungi . Plant Dis Rep Suppl , 261 : 251 – 312 .
- Masangkay , RF , Paulitz , TC , Hallett , SG and Watson , AK. 2000 . Characterization of sporulation of Alternaria alternata f. sp. sphenocleae . Biocontrol Sci Technol , 10 ( 4 ) : 385 – 397 .
- Miller , OK. 1967 . The role of light in the fruiting of Panus fragilis . Can J Bot , 45 ( 11 ) : 1939 – 1943 .
- Misawa , T. 1965 . Nutritional factors for phytopathogenic fungi on culture media . Ann Phytopathol SOC Jpn , 31 : 27 – 34 .
- Nirenberg , HI. 1976 . Untersuchungen über die morphologische und iologische Differenzierung in der Fusarium Sektion Liseola . Mitt Biol Bundesanst Land- Forstwirtsch (Berlin–Dahlem) , 169 : 1 – 117 .
- Okane , I , Nakagiri , A and Ito , T. 2001 . Identity of Guignardia sp. inhabiting ericaceous plants . Botany , 79 ( 1 ) : 101 – 109 .
- Onesirosan , PT. 1978 . Factors affecting sporulation by Phomopsis phaeolorum . Mycopathol , 64 : 23 – 27 .
- Özcan , S , Dover , J , Rosenwald , AG , Wölfl , S and Johnston , M. 1996 . Two glucose transporters in S. cerevisiae are glucose sensors that generate a signal for induction of gene expression . Proc Natl Acad Sci USA , 93 ( 22 ) : 12428 – 12432 .
- Paul , P and Munkvold , G. 2005 . Influence of temperature and relative humidity on sporulation of Cercospora zeae-maydis and expansion of gray leaf spot lesions on maize leaves . Plant Dis , 89 ( 6 ) : 624 – 630 .
- Photita , W , Lumyong , S , Lumyong , P and Hyde , KD. 2001 . Endophytic fungi of wild banana (Musa acuminata) at Doi Suthep Pui National Park, Thailand . Mycol Res , 105 ( 12 ) : 1508 – 1513 .
- Prasad , B , Dutt , BL and Nagaich , BB. 1973 . Inducing sporulation in Alternaria solani. I. Effect of water treatment . Mycopathol Mycol Appl , 49 ( 2–3 ) : 141 – 146 .
- Rakoczy , L. 1963 . Influence of monochromatic light on the fructification of Physarum nudum . Acta Soc Bot Pol , 11 : 559 – 562 .
- Rakoczy , L. 1965 . Action spectrum in sporulation of slime mould, Physarum nudum . Acta Soc Bot Pol , 34 : 97 – 112 .
- Rands , RD. 1917 . The production of spores of Alternaria solani in pure culture . Phytopathology , 7 : 316 – 317 .
- Riddell , RW. 1950 . Permanent stained mycological preparations obtained by slide culture . Mycologia , 42 ( 2 ) : 265 – 270 .
- Rodriguez-Romero , J , Hedtke , M , Kastner , C , Muller , S and Fischer , R. 2010 . Fungi, hidden in soil or up in the air: light makes a difference . Annu Rev Microbiol , 64 : 585 – 610 .
- Roncal , T and Ugalde , U. 2003 . Conidiation induction in Penicillium . Res Microbiol , 154 ( 8 ) : 539 – 546 .
- Rotem , J. 1994 . The genus Alternaria: biology, epidemiology and pathogenicity , Saint Paul , (MN) : APS Press .
- Ruger-Herreros , C , Rodríguez-Romero , J , Fernández-Barranco , R , Olmedo , M , Fischer , R , Corrochano , LM and Canovas , D. 2011 . Regulation of conidiation by light in Aspergillus nidulans . Genetics , 188 ( 4 ) : 809 – 897 .
- Sanchez-Arregui , A , Perez-Martinez , AS and Herrera-Estrella , A. 2012 . Proteomic analysis of Trichoderma atroviride reveals independent roles for transcription factors BLR-1 and BLR-2 in light and darkness . Eukaryot Cell , 11 ( 1 ) : 30 – 41 .
- Sangeetha , CG and Rawal , RD. 2008 . Nutritional studies of Colletotrichum gloeosporioides (Penz.) Penz. and Sacc. the incitant of mango anthracnose . World J Agri Sci , 4 ( 1 ) : 717 – 720 .
- Schafmeier , T and Diernfellner , ACR. 2011 . Light input and processing in the circadian clock of Neurospora . FEBS Lett , 585 ( 10 ) : 1467 – 1473 .
- Shahin , EA and Shepard , JF. 1979 . An efficient technique for inducing profuse sporulation of Alternaria species . Phytopathology , 69 : 618 – 620 .
- Singh , B. 1967 . Inducing sporulation in different strains of Alternaria solani . Mycopath Mycol Appl , 32 ( 2 ) : 163 – 171 .
- Smith , IE and Berry , DR. 1974 . An introduction to biochemistry of fungal development , 326 London , , (UK) : Academic Press .
- Starostzik , C and Marwan , W. 1995 . A photoreceptor with characteristics of phytochrome triggers sporulation in the true slime mould Physarum polycephalum . FEBS Lett , 370 ( 1–2 ) : 146 – 148 .
- Su , YY , Guo , LD and Hyde , KD. 2010 . Response of endophytic fungi of Stipa grandis to experimental plant function group removal in Inner Mongolia steppe, China . Fungal Divers , 43 : 93 – 101 .
- Su , YY , Noireung , P , Liu , F , Hyde , KD , Moslem , MA , Bahkali , AH , Abd-Elsalam , KA and Cai , L. 2011 . Epitypification of Colletotrichum musae, the causative agent of banana anthracnose . Mycoscience , 52 ( 6 ) : 376 – 382 .
- Sun , X and Guo , LD. 2012 . Endophytic fungal diversity: review of traditional and molecular techniques . Mycology , 3 ( 1 ) : 65 – 76 .
- Sun , X , Yu , L , Lan , N , Wei , S , Yu , Y , Zhang , H , Zhang , X and Li , S. 2012 . Analysis of the role of transcription factor VAD-5 in conidiation of Neurospora crassa . Fungal Genet Biol , 49 ( 5 ) : 379 – 387 .
- Suryanarayanan , TS and Vijaykrishna , D. 2001 . Fungal endophytes of aerial roots of Ficus benghalensis . Fungal Divers , 8 : 155 – 161 .
- Suzaki , K. 2011 . Improved method to induce sporulation of Colletotrichum gloeosporioides, causal fungus of grape ripe rot . J Gen Plant Pathol , 77 ( 2 ) : 81 – 84 .
- Sánchez Márquez , S , Bills , GF and Zabalgogeazcoa , I. 2008 . Diversity and structure of the fungal endophytic assemblages from two sympatric coastal grasses . Fungal Divers , 33 : 87 – 100 .
- Tejesvi , MV , Kajula , M , Mattila , S and Pirttilä , AM. 2011 . Bioactivity and genetic diversity of endophytic fungi in Rhododendron tomentosum Harmaja . Fungal Divers , 47 : 97 – 107 .
- Timberlake , WE. 1980 . Developmental gene regulation in Aspergillus nidulans . Dev Biol , 78 ( 2 ) : 497 – 510 .
- Timnick , MB , Lilly , VG and Barnett , HL. 1951 . The effect of nutrition on the sporulation of Melanconium fuligineum in culture . Mycologia , 43 ( 6 ) : 625 – 634 .
- Tomiyama , K , Sakai , R and Takakuwa , M. 1962 . “ Physiology of phytopathogenic fungi ” . In Laboratory guide for plant pathologists , Edited by: Asuyama , H , Mukoo , H and Suzuki , N . 335 – 380 . Tokyo , , (Japan) : Japan Plant Protection Association. p .
- Tzima , AK , Paplomatas , EJ , Tsitsigiannis , DI and Kang , S. 2012 . The G protein beta subunit controls virulence and multiple growth- and development-related traits in Verticillium dahliae . Fungal Genet Biol , 49 ( 4 ) : 271 – 283 .
- Ugalde , UO and Pitt , D. 1986 . Calcium uptake kinetics in relation to conidiation in submerged cultures of Penicillium cyclopium . Trans Br Mycol Soc , 87 : 199 – 203 .
- Ugalde , UO , Virto , MD and Pitt , D. 1990 . Calcium binding and induction of conidiation in protoplasts of Penicillium cyclopium . Antonie Leeuwenhoek , 57 ( 1 ) : 43 – 49 .
- van der Aa , HA. 1973 . Studies in Phyllosticta I . Stud Mycol. 5:1–110 ,
- Vakalounakis , DJ and Christias , C. 1981 . Sporulation in Alternaria cichorii is controlled by a blue and near ultraviolet reversible photoreaction . Can J Bot , 59 ( 5 ) : 626 – 628 .
- Wei , JG , Xu , T and Guo , LD. 2006 . Morphological stability and taxonomical significance of the genus Pestalotiopsis . J Laiyang Agric College , 23 : 280 – 284 .
- Wright , BE. 1979 . Causality in biological systems . Trends Biochem Sci , 4 : 110 – 111 .
- Wulandari , N , To-Anun , C , Hyde , K , Duong , L , de Gruyter , J , Meffert , J , Groenewald , J and Crous , P. 2009 . Phyllosticta citriasiana sp. nov., the cause of Citrus tan spot of Citrus maxima in Asia . Fungal Divers , 34 : 23 – 39 .
- Xu , LL , Li , F , Xie , HY and Liu , XZ. 2009 . A novel method for promoting conidial production by a nematophagous fungus, Pochonia chlamydosporia AS6.8 . World J Micro Biotechnol , 25 ( 11 ) : 1989 – 1994 .
- Xu , JW , Zhao , W , Xu , YN and Zhong , JJ. 2012 . Isolation and analysis of differentially expressed genes during asexual sporulation in liquid static culture of Ganoderma lucidum by suppression subtractive hybridization . Mol Biol Rep , 39 ( 4 ) : 3603 – 3610 .
- Yamaguchi , H. 1974 . Mycelial development and chemical alteration of Candida albicans from biotin insufficiency . Sabouraudia , 12 ( 3 ) : 320 – 328 .
- Yazdany , S and Lashkari , K. 1975 . Effect of pH on sporulation of Bacillus stearothermophilus . Appl Microbiol , 30 ( 1 ) : 1 – 3 .
- Yoshida , S and Shirata , A. 2000 . Biotin induces sporulation of mulberry anthracnose fungus, Colletotrichum dematium . J Genl Plant Pathol , 66 ( 2 ) : 117 – 122 .
- Zhou , G , Wang , J , Qiu , L and Feng , MG. 2012 . A Group III histidine kinase (mhk1) upstream of high-osmolarity glycerol pathway regulates sporulation, multi-stress tolerance and virulence of Metarhizium robertsii, a fungal entomopathogen . Environ Microbiol , 14 ( 3 ) : 817 – 829 .