Abstract
A pour-plate dilution method was employed to assess the antagonistic effects of Saccharomyces cerevisiae on the growth of both Aspergillus flavus and Aspergillus parasiticus at 22°C, 25°C, and 32°C. Dilutions of S. cerevisiae were pour plated, and upon solidification, the plates were inoculated with a lawn of either A. flavus or A. parasiticus. Results of this experiment suggest that higher concentrations of S. cerevisiae are effective in inhibiting the growth of both Aspergillus species investigated at all temperatures, but its effects are most pronounced at 22°C. Broader implications of this study include the possibility that S. cerevisiae may be used as a biocontrol agent to protect agricultural products commonly consumed by humans from the toxigenic metabolites produced by A. flavus and A. parasiticus.
Introduction
Yeasts such as Aureobasidium pullulans, Debaryomyces hansenii, Kluyveromyces spp., Pichia anomala, Pichia Guilliermondii, and Saccharomyces cerevisiae have been tested for their ability to suppress mycological growth and limit mycotoxin production on foods such as grapes, coffee beans, cereals, peanuts, and dairy products (Bjornberg & Schnurer Citation1993; Paster et al. Citation1993; Petersson et al. Citation1998, 1999; Masoud & Jakobsen Citation2005; Bleve et al. Citation2006; Masoud & Kaltoft Citation2006; Dimakopoulou et al. Citation2008; Cubaiu et al. Citation2009; Liu & Tsao Citation2009; Serna et al. Citation2009; Prado et al. Citation2011; Somai & Belewa Citation2011; Velmourougane et al. Citation2011). Most of the studies assessing the efficacy of yeast as a biocontrol agent, however, have focused on its antagonistic effects against Aspergillus ochraceus and concomitantly ochratoxin A (OTA) (e.g., Petersson et al. Citation1998; Masoud & Jakobsen Citation2005; Bleve et al. Citation2006; Masoud & Kaltoft Citation2006; Dimakopoulou et al. Citation2008; Cubaiu et al. Citation2009; Serna et al. Citation2009; Velmourougane et al. Citation2011), while fewer studies have examined its potential for biocontrol of aflatoxin-producing species such as Aspergillus flavus and Aspergillus parasiticus (e.g., Paster et al. Citation1993; La Penna et al. Citation2004; Prado et al. Citation2011; Somai & Belewa Citation2011).
Aflatoxins are a potent carcinogen and can contaminate a wide range of agricultural products regularly consumed by humans including corn, tree nuts, and peanuts, and ingestion of aflatoxin-contaminated foods increases the risk of developing hepatocellular carcinoma (Yu & Yuan Citation2004; Varga et al. Citation2011).
Further, it is estimated that annual costs associated with the loss of cotton, peanut, corn, walnut, and almond crops in the United Sates alone approaches $139,000,000 (Robens & Cardwell Citation2003). To combat the colonization of food crops by A. flavus and A. parasiticus, nontoxigenic strains of each have been developed to compete with the toxigenic strains (Pitt & Hocking Citation2006). The nontoxigenic strains are successful in reducing aflatoxin levels, but the recent recognition of sexual reproduction in both A. flavus and A. parasiticus has brought the efficacy and safety of the nontoxigenic strains into question (Pitt & Hocking Citation2006; Olarte et al. Citation2010; Varga et al. Citation2011) as Olarte et al. Citation2010 have shown that one generation of sexual reproduction between a nontoxigenic strain of A. flavus and a toxigenic strain of A. flavus results in an aflatoxin-producing progeny. Continued sexual reproduction between the strains could theoretically lead to successive generations of aflatoxigenic strains, ultimately negating the initial effects of the nontoxigenic strain. These results suggest that additional biocontrol tools are needed to mitigate the growth of A. flavus and A. parasiticus, thus reducing aflatoxin production on food crops. Potential biocontrol agents include the binding of aflatoxin by lactic acid bacteria, the antagonistic effects of nontoxigenic fungi, plant-based antimicrobials, and yeasts.
For example, Lactobacillus rhamnosus can bind aflatoxin B1 to its surface (Haskard et al. Citation2001), while nontoxigenic fungi such as Alternaria and Cladosporuium have antagonistic effects on aflatoxin B1 production (Cvetnic & Pepeljnjak Citation2007). In addition, naturally occurring oils and phenolics derived from spices, plants, and herbs have been shown to inhibit the growth of A. flavus in vitro, but their widespread application to crops is limited by a high minimum inhibitory concentration and the potential addition of unpleasant flavors and aromas to the crops (Rasooli & Abyaneh Citation2004; Lopez-Malo et al. Citation2005). More recent work, however, suggests that the yeasts, S. cerevisiae and Tulbaghia violacea, are antagonists of both A. flavus and A. parasiticus (Joannis-Cassan et al. Citation2011; Prado et al. Citation2011; Somai & Belewa Citation2011), but the antagonistic effects of these yeasts may be temperature dependent. Species of Aspergillus can flourish at temperatures ranging from 25–35°C, while optimal growth of S. cerevisiae occurs in a range of temperatures between 15–30°C (Schindler et al. Citation1967; Sorger & Pelham Citation1988; Serna et al. Citation2009; Sood Citation2011) suggesting that as temperature increases, the efficacy of the yeast to suppress the growth of Aspergillus decreases. To further investigate both the biocontrol potential of yeast and the effect of temperature on its efficacy as a biocontrol tool, a pour-plate dilution method, using a commercially available baker's yeast incubated at three different temperatures, was employed with the expectation that a combination of higher levels of yeast and lower temperatures would lead to a decrease in the growth of both A. flavus and A. parasiticus.
Materials and methods
Individual aflatoxin producing strains of A. flavus (ATCC 26769) and A. parasiticus (ATCC 26690) were cultured on potato dextrose agar and allowed to incubate for 4 days at 25°C. Following the incubation period, 20 g of Fleischmann's® Active Dry Yeast (ACH Foods, Ankeny, IA, USA), which consists of S. cerevisiae and sorbitan monostearate, was added to 180 ml of sterile water and placed in suspension by gently rocking the mixture in an arc 25 times to create a 1:10 dilution. Dilution of the yeast suspension was continued using a standard 10-fold serial dilution method until a 1:1,000,000 dilution was achieved. One milliliter of material from each increment of the serial dilution was placed in a Petri plate, and approximately 20 ml of Sabouraud's dextrose agar (SDA) was added to the plates, and the yeast dilution was gently swirled into the agar. An additional plate consisting of 1 ml of sterile water and 20 ml of SDA served as the control. Following solidification of the agar, plates were inoculated with a lawn of A. flavus. Plates were then divided into three temperature groups (22°C, 25°C, and 32°C) containing seven plates each (six dilution plates and one control plate) and were incubated at their assigned temperature. This process was then repeated using A. parasiticus as the inoculum. The 42 total incubated plates were checked for growth at 48, 96, 168 (1 week), 216, 264, 48, 96, 168 (1 week), 216, 264, and 336 h (2 weeks) and any statistically viable colony-forming units (CFUs) were counted. The experiment was then replicated.
Results
Growth of A. flavus and A. parasiticus was inhibited by S. cerevisiae at all temperatures, but as the concentration of yeast decreased, the inhibitory effects were lessened. Of the six yeast dilutions (1:10, 1:100, 1:1000, 1: 10,000, 1:100,000, and 1:1,000,000) and three temperature regimes (22°C, 25°C, and 32°C), S. cerevisiae was most effective at inhibiting the growth of A. flavus and A. parasiticus at the lowest dilutions (1:10, 1:100, and 1:1000) and at the lowest temperature (22°C).
Aspergillus flavus
A white-colored A. flavus was observed growing on the plates at 48 h. Of the plates incubated at 22°C, growth was noted only on the 1:100,000, 1:1,000,000, and control plates. The control plate was the only countable plate and contained 104 CFUs. Of the plates incubated at 25°C, only the 1:10,000–1:1,000,000 dilution plates and the control had growth. With the exception of the control plate, the growth on these plates was also white. The control plate had already begun to attain the brown hue characteristic of A. flavus. This trend continued for the plates incubated at 32°C, but all growth on these plates was green and uncountable. Only the 1:100,000 plate was countable and contained 5.5 × 105 CFUs/ml ().
Table 1. Presence (+) or absence of Aspergillus flavus growth on SDA pour plates containing differing yeast dilutions over a 2-week period
At 96 h, the 1:10,000 dilution plates incubated at 22 and 25°C were countable. The plate incubated at 22°C had 3.2 × 104 CFUs/ml, while the plate incubated at 25°C had 5.5 × 104 CFUs/ml. In addition, both control plates and the 1:1,000,000 dilution plate incubated at 25°C had attained a brown hue.
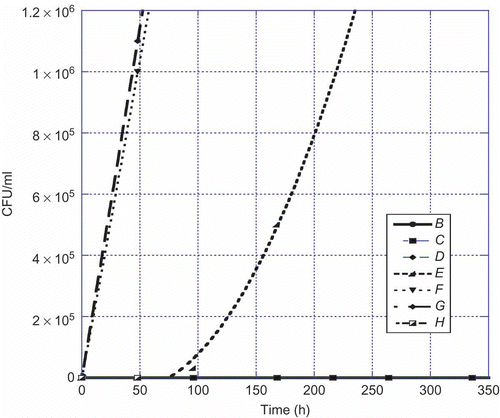
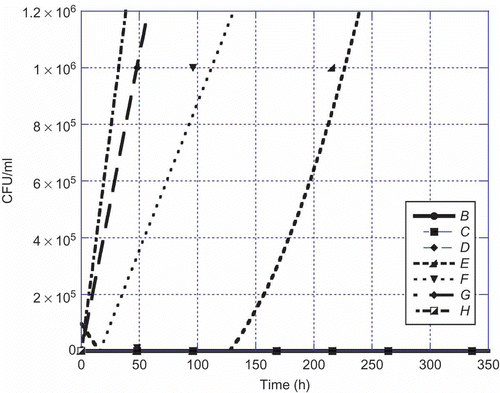
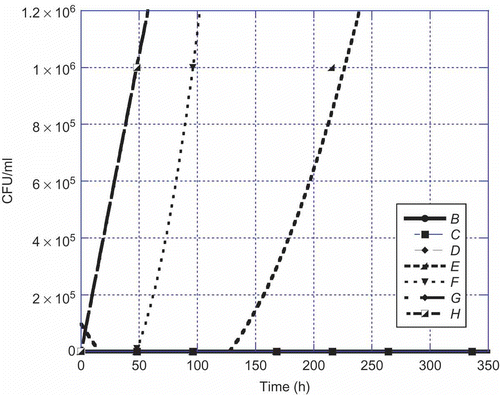
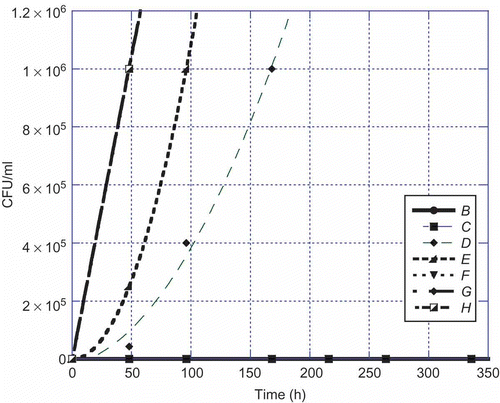
At 32°C, however, all plates had growth, but growth on the 1:10–1:10,000 dilution plates was white, while the remaining plates had brown growth (). Following 168 h (1 week) of incubation, results for the plates incubated at 22°C were constant, but the growth had started to turn brown, and the first white-colored growth was observed on the 1:1000 dilution plate incubated at 25°C. As aforementioned, growth was observed on all plates incubated at 32°C. These growth patterns would remain the same at both the 216 and 264 h marks. At 336 h (2 weeks), all plates incubated at all temperatures had growth; however, the 1:10 and 1:100 dilution plates incubated at 22°C and 25°C, respectively, had only a diffuse, yellow-white-colored growth (). Growth curves for A. flaus at 22°C and 25°C are plotted in and , respectively. At 32°C, the growths were extremely fast for most of the samples and they were not plotted.
Aspergillus parasiticus
Similarly, a white-colored A. parasiticus was observed growing on plates at 48 h. Of the plates incubated at 22°C, only the 1:100,000–1:1,000,000 dilution plates and the control had growth. At 25°C, growth was lacking on the 1:10 and 1:100 dilution plates, and 4.3 × 104 CFUs/ml were counted on the 1:1000 plate. This trend continued for the plates incubated at 32°C, but the control for this temperature group had attained a green hue. Similar results for the plates incubated at 22°C and 25°C were obtained at 96 h, but most of the growth had shifted from white to green in color. With the exception of the 1:100 dilution plate, all plates incubated at 32°C had growth. Both the 1:10 and 1:1000 dilution plates contained 30 CFUs ().
Table 2. Presence (+) or absence (−) of Aspergillus parasiticu s growth on SDA pour plates containing differing yeast dilutions over a 2-week period
At the 168 h mark, the results remained fairly constant, but plates were no longer countable. In addition, the 1:100 dilution plate incubated at 32°C had growth. Following 216 h of incubation, results remained the same for the plates incubated at 22°C, but all plates incubated at 25°C had growth, with the growth on the 1:10–1:10,000 plates having white color. No plates were countable. At 264 h, growth was observed on the 1:1000 dilution plate incubated at 22°C, while growth on the plates incubated at 25°C and 32°C remained the same. This trend continued until the termination of the experiment at 336 h (2 weeks) when, as reported for A. flavus, all plates incubated at all temperatures had growth, but the growth on the 1:10 and 1:100 dilution plates incubated at 22°C and 25°C had only a diffuse, white-colored growth (). Again, we plotted the results for the plates incubated at 22°C and 25°C in and .
Discussion
Purified cell lines of yeasts such as Aureobasidium pullulans, Debaryomyces hansenii, Pichia anomala, P. guilliernondii, and S. cerevisiae have been shown to inhibit the growth of Aspergillus spp. and their concomitant mycotoxin production on various food products (Bjornberg & Schnurer Citation1993; Paster et al. Citation1993; Petersson et al. Citation1999; Dimakopoulou et al. Citation2008; Liu & Tsao Citation2009). More recently, a readily available commercial baker's yeast was reported to reduce the incidence of A. ochraceus and OTA in coffee (Velmourougane et al. Citation2011). Similar results were obtained in this experiment using a commercially available baker's yeast to control the growth of A. flavus and A. parasiticus, but the inhibitory effects of the yeast were moderated by an interaction between the concentration of the yeast, temperature, and time. Some modicom of growth inhibition was achieved at all temperatures, but as expected, the combination of a high concentration of yeast and a lower temperature was most effective in limiting Aspergillus growth. The lower temperature threshold for Aspergillus growth corresponds with the optimal temperature for S. cerevisiae growth (Schindler et al. Citation1967; Sorger & Pelham Citation1988; Serna et al. Citation2009; Sood Citation2011), which suggests that, at ambient temperature (22°C), the yeast may be physiologically more fit to thrive and can outcompete the fungi for resources. Conversely, at the higher temperature (32°C), conditions are optimal for fungal growth but at the upward growth limits for the yeast allowing the fungi to outcompete the yeast. Further, several yeasts, including S. cerevisiae, are known to produce killer toxins, though it was thought that the toxins are only lethal to other yeasts (Marquina et al. Citation2002). Perhaps these toxins are effective in suppressing the growth of A. flavu and A. parasiticus when conditions are optimal for S. cerevisiae. The exact mechanism whereby S. cerevisiae limits the growth of Aspergillus is still poorly understood. Research aimed at identifying this mechanism is ongoing and is focused on how environmental factors such as food availability, population density, and temperature trigger the cues to initiate biological processes such as heat shock protein expression and quorum sensing in the yeast (e.g., Craig & Jacobsen Citation1984; Sprague & Winans Citation2006; Zain et al. Citation2009). An understanding of these underlying mechanisms not only for S. cerevisiae, but also for A. flavus and A. parasiticus provides an opportunity to produce a safe, effective biocontrol tool to protect crops from aflatoxins.
References
- Bjornberg , A and Schnurer , J. 1993 . Inhibition of the growth of grain-storage molds in vitro by the yeast Pichia anomala (Hansen) Kurtzman . Can J Mirobiol. , 39 : 623 – 628 .
- Bleve , G , Grieco , F , Cozzi , G , Logrieco , A and Visconti , A. 2006 . Isolation of epiphytic yeasts with potential for biocontrol of Aspergillus carbonarius and A. Niger on grape . Int J Food Microbiol. , 108 : 204 – 209 .
- Craig , EA and Jacobsen , K. 1984 . Mutations of the heat inducible 70 kilodalton genes of yeast confer temperature sensitive growth . Cell. , 38 : 841 – 849 .
- Cubaiu , L , Abbas , A , Dobson , ADW , Budroni , M and Migheli , Q. 2009 . Effect of antagonistic Saccharomyces cerevisiae on the expression of the PKS gene in ochratoxin A producing Aspergillus spp . J Plant Pathol. , 91 : 56
- Cvetnic , Z and Pepeljnjak , S. 2007 . Interaction between certain moulds and aflatoxin B1 producer Aspergillus flavus NRRL 3251 . Arh Hig Rada. Tokiskol. , 58 : 429 – 434 .
- Dimakopoulou , M , Tjamos , SE , Antoniou , PP , Pietri , A , Battilani , P , Avramidis , N , Markakis , EA and Tjamos , EC. 2008 . Phllyosphere grapevine yeast Aureobasidium pullulans reduces Aspergillis carbonarius (sour rot) incidence in wine-producing vineyards in Greece . Biol Control. , 46 : 158 – 165 .
- Haskard , CA , El-Nezami , HS , Kankaanpaa , PE , Salminen , S and Ahokas , JT. 2001 . Surface binding of aflatoxin B1 by lactic acid bacteria . Appl Env Microbiol. , 67 : 3086 – 3091 .
- Joannis-Cassan , C , Tozlovanu , M , Hadjeba-Medjdoub , K , Ballet , N and Pfohl-Leszkowicz , A. 2011 . Binding of zearalenone, aflatoxin B1 and ochratoxin A by yeast-based products: a method for quantification of adsorption performance . J Food Protect. , 74 : 1175 – 1185 .
- La Penna , M , Nesci , A and Etcheverry , M. 2004 . In vitro studies on the potential for biological control on Aspergillus section Flavi by Kluyveromyces spp . Letters in Appl Microbiol. , 38 : 257 – 264 .
- Liu , S and Tsao , M. 2009 . Biocontrol of dairy moulds by antagonistic dairy yeast Debaryomyces hansenii in yoghurt and cheese at elevated temperatures . Food Contr. , 20 : 852 – 855 .
- Lopez-Malo , A , Alzamora , SM and Palou , E. 2005 . Aspergillus flavus growth in the presence of chemical preservatives and naturally occurring antimicrobial compounds . Int J Food Microbiol. , 99 : 119 – 128 .
- Marquina , D , Santos , A and Peinado , J. 2002 . Biology of killer yeasts . Int Microbiol. , 5 : 65 – 71 .
- Masoud , W and Jakobsen , M. 2005 . Influence of volatile compounds produced by yeasts predominant during processing of Coffea arabica in East Africa on growth and ochratoxin A (OTA) production by Aspergillus ochraceus . Yeast. , 22 : 1133 – 1142 .
- Masoud , W and Kaltoft , CH. 2006 . The effects of yeast involved in the fermentation of Coffea Arabica in East Africa on growth and ochratoxin A (OTA) production by Aspergillus ochraceus . Int J Food Microbiol. , 106 : 229 – 234 .
- Olarte , RA , Horn , BW , Monacell , JT , Stone , EA and Carbone , I. 2010 . Insights into sexual reproduction in Aspergillus flavus from variation in experimental crosses and natural populations . Phytopathol. , 100 : S92
- Paster , N , Droby , S , Chalutz , E , Menasherov , M , Nitzan , R and Wilson , CL. 1993 . Evaluation of the potential of the yeast Pichia guilliermondii as a biocontrol agent against Aspergillus flavus and fungi of stored soya beans . Mycol Res. , 97 : 1201 – 1206 .
- Petersson , S , Hansen , MW , Axberg , K , Hult , K and Schniirer , J. 1998 . Ochratoxin A accumulation in cultures of Penicillium verrucosum with the antagonistic yeast Pichia anomala and Saccharomyces cerevisiae . Mycol Res. , 102 : 1003 – 1008 .
- Petersson , S , Jonsson , N and Schnurer , J. 1999 . Pichia anomala as a biocontrol agent during storage of high-moisture feed grain under airtight conditions . Postharvest Biol Tech. , 15 : 175 – 184 .
- Pitt , JI and Hocking , AD. 2006 . Mycotoxins in Australia: biocontrol of aflatoxin in peanuts . Mycopathol. , 162 : 233 – 243 .
- Prado , G , Madeira , JEG , MoralsVA , D , Oliveira , MS , Souza , RA , Peluzio , JM , Godoy , IJ , Silva , JFM and Pimento , RS. 2011 . Reduction of aflatoxin B1 in stored peanuts (Arachis hypogaea L.) using Saccharomyces cerevisiae . J Food Protect. , 74 : 1003 – 1006 .
- Rasooli , I and Abyaneh , MR. 2004 . Inhibitory effects of thyme oils on growth and aflatoxin production by Aspergillus parasiticus . Food Contr. , 15 : 479 – 483 .
- Robens , J and Cardwell , K. 2003 . The costs of mycotoxin management to the USA: management of aflatoxins in the United States . J Toxicol. , 22 : 139 – 152 .
- Schindler , AF , Palmer , JG and Eisenberg , WV. 1967 . Aflatoxin production by Aspergillus flavus as related to various temperatures . Appl Microbiol. , 15 : 1006 – 1009 .
- Serna , JG , Patino Alvarez , B , Gonzalez-Jaen , MT and Vazquez Estevez , C. 2009 . “ Biocontrol of Aspergillus ochraceus by yeasts ” . In Current research topics in applied microbiology and microbial biotechnology , Edited by: Mendez-Vilas , A . 368 – 372 . Hackensack , NJ : World Scientific; p .
- Somai , BM and Belewa , V. 2011 . Aqueous extract of Tulbaghia violacea inhibit germination of Aspergillus flavus and Aspergillus parasiticus conidia . J Food Protect. , 74 : 1007 – 1011 .
- Sood , M. 2011 . Effect of temperature of incubation on the growth, sporulation and secondary metabolites production of Aspergillus umbrosus . J Phytol. , 3 : 35 – 37 .
- Sorger , PK and Pelham , HRB. 1988 . Yeast heat shock factor is an essential DNA-binding protein that exhibits temperature-dependent phosphorylation . Cell. , 54 : 855 – 864 .
- Sprague , GF Jr and Winans , SC. 2006 . Eukaryotes learn how to count: quorum sensing by yeast . Genes Dev. , 20 : 1045 – 1049 .
- Varga , J , Frisvad , JC and Samson , RA. 2011 . Two new aflatoxin producing species, and an overview of Aspergillus section Flavi . Stud Mycol. , 69 : 57 – 80 .
- Velmourougane , K , Bhat , R , Gopinandhan , TN and Panneerselvam , P. 2011 . Management of Aspergillus ochraceus and ochratoxin-A contamination in coffee during on-farm processing through commercial yeast inoculation . Biol Control. , 57 : 215 – 221 .
- Yu , MC and Yuan , J. 2004 . Environmental factors and risk for hepatocellular carcinoma . Gastroenterol. , 127 : S72 – S78 .
- Zain , ME , Ratak , AA , El-Sheikh , HH , Soliman , HG and Khalil , AM. 2009 . Influence of growth medium on diagnostic characters of Aspergillus and Penicillium species . Afr J Microbial Res. , 3 : 280 – 286 .