Abstract
The distribution of Xylaria endophytes in the leaves of 22 tree species of a dry thorn forest and 27 tree species of a stunted montane evergreen forest of the Western Ghats in southern India was studied. In addition, these endophytes were screened for the production of some bioactive metabolites and extracellular enzymes. All the tree species in both the forest types harboured xylariaceous endophytes. Generally, xylariaceous endophytic infection of the leaves increased during the wet season. Molecular analysis showed that most of the xylariaceous endophytes isolated belonged to Xylaria or Nemania. All endophytes produced cellulase, and most of the isolates produced laccase and lipase enzymes suggesting continuing their life in plant litter as saprotrophs. The culture extracts were inhibitory to fungi, bacteria and algae indicating that they can compete with such organisms in the forest floor while surviving as saprotrophs. Fungi with such dual life strategies appear to be a potential source for biotechnological exploitation.
Introduction
The xylariaceous fungi of the tropics are diverse and colonize several substrata such as dung, ant and termite nests and decayed plant materials; these are phytopathogens and endophytes (Rogers Citation2000; Duong et al. Citation2004; Okane et al. Citation2008). Xylariaceous endophytes are common and have been isolated from tropical plant hosts such as palms, bromeliads, orchids, aroids and forest trees (Whalley Citation1996; Bayman et al. Citation1998; Okane et al. Citation2008; Linnakoski et al. Citation2012). Although their association with angiosperms probably developed along with the Cretaceous radiation of angiosperms (Rogers Citation2000), there are indications that host switching has occurred frequently among these fungi (Davis et al. Citation2003). Identification of xylariaceous endophytes is difficult because the taxonomic systems have largely been ascomata-based and most of them do not produce ascomata in culture (Brunner & Petrini Citation1992; Stadler Citation2011). Stroma characters are variable and hence unreliable (Lee et al. Citation2002; Fournier et al. Citation2011). Molecular data such as internal transcribed spacer (ITS) sequences and other marker sequences have been used for identification or classification of xylariaceous fungi (Lee et al. Citation2000).
Many xylariaceous endophytes produce novel metabolites with unique biological activities (Ondeyka et al. Citation1997; Schlingman et al. Citation2002; Smith et al. Citation2002; Koehn et al. Citation2008). Although there are a few records of Xylaria species from India (Thind & Dargan Citation1978; Dargan Citation1982), little information is available regarding xylariaceous endophytes (Rogers Citation2000; Naik et al. Citation2008; Nath et al. Citation2012) from here. In the present study, we studied the foliar xylariaceous endophytes from two types of forests of the Western Ghats for their distribution, production of bioactive compounds and plant cell wall-degrading enzymes.
Materials and methods
Collection sites
Leaves of dicotyledonous trees from a tropical dry thorn (DT) forest and a stunted montane evergreen (EG) forest were sampled. The DT forest lies in the eastern part of the Mudumalai Wildlife Sanctuary (11° 32′–11° 43′ N lat, 76° 22′–76° 43′ E long), southern India, and receives a mean annual rainfall of 800 ± 265 mm (Suresh & Sukumar Citation1999). The tropical EG forest is located along the southwest of Ootacamund (11° 14′ N lat, 76° 33′ E long), southern India. The mean annual rainfall here is 1300–2500 mm (Suresh & Sukumar Citation1999).
Isolation of endophytes
Leaves of 22 tree species (belonging to 13 families) from DT forest and 27 tree species (belonging to 15 families) from EG forest were sampled for foliar endophytes during the dry and wet seasons ( and ). Three individual trees were sampled for each tree species. From each tree host, 60 healthy, mature, green leaves were collected (20 from each individual), brought to the laboratory in sterile polythene bags and processed within 48 h. The leaves were washed thoroughly in running tap water, and from each leaf, three segments (0.5 cm2 each) were cut from the midrib region including the lamina portion. The one hundred and eighty tissue segments thus obtained for each tree species were then surface-sterilized by dipping them serially in 70% ethanol for 5 s and in 4% NaOCl for 90 s and were finally rinsed in sterile distilled water for 10 s (Suryanarayanan et al. Citation1998). Of these, only one hundred and fifty segments were selected randomly and plated on potato dextrose agar (PDA) medium (supplemented with 150 mg/L chloramphenicol) contained in Petri dishes and were observed for the growth of endophytes. Each 9-cm-diameter Petri dish had 20 ml medium and was plated with 10 leaf segments. Petri dishes were incubated under near ultraviolet lamps (12 h dark: 12 h light cycle) for 21 days at 27°C, as this treatment is known to induce sporulation in fungi (Suryanarayanan Citation1992). The isolation frequency (IF%) of each endophyte species was calculated as follows:
Table 1. Isolation frequency (IF%) of foliar endophytes, xylariaceous endophytes and contribution by xylariaceous endophytes to total IF% in trees of dry thorn forest in dry and wet seasons. Families of tree species given in foot note
Table 2. Isolation frequency (IF%) of foliar endophytes, xylariaceous endophytes and contribution by xylariaceous endophytes to total IF% in trees of evergreen forest in dry and wet seasons. Families of tree species given in foot note
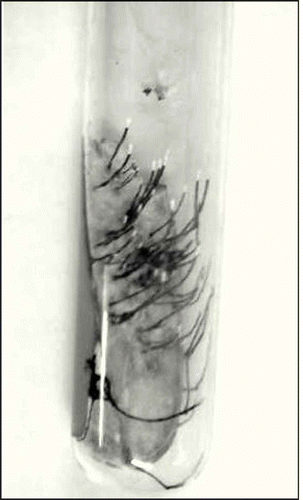
Although different fungal endophytes were isolated from these tree species, present study deals only with xylariaceous fungi. Xylariaceous fungi which shared similar vegetative cultural characteristics () were treated as a single morphospecies and were given code numbers (Suryanarayanan et al. Citation1998). Totally, 29 morphotypes having xylaria-like stroma were isolated. Of these, 16 morphotypes isolated from 11 tree species (DT and EG) belonging to 7 families were selected for a more detailed molecular analysis (). For bioactive compounds and cell wall-degrading enzymes, only 10 isolates representing each group based on molecular phylogeny were selected.
Table 3. List of xylariaceous endophyte isolates used in this study, their locations and accession numbers
DNA extraction, PCR and DNA sequencing
For molecular characterization, the genomic DNA was extracted from the cultures of 29 morphotypes of the Xylaria isolates by the method of Van Kan et al. (Citation1991). The ITS region of nrDNA was amplified by polymerase chain reaction (PCR) with ITS1 (5′-TCCGTAGG TGAACCTGCGG-3′) and ITS4 (5′-TCCTCCGCTTATTGATATGC-3′) primers (White et al. Citation1990). The 50 μl reaction mixture for PCR amplification contained 10 ng DNA, 1x PCR buffer, 1.5 mM MgCl2, 0.2 mM of each dNTPs, 0.5 μM of each primer and 2.5 units of Taq polymerase (Fermentas, Foster City, CA, USA). Amplifications were performed in a thermal cycler (Applied Biosystems, Carlsbad, CA, USA) with an initial denaturation step of 94°C for 3 min followed by 35 cycles of 94°C for 1 min, 50°C for 1 min and 72°C for 1.5 min and a final extension of 72°C for 8 min. ITS products of similar length were digested with different restriction enzymes (TaqI, HinfI, AluI and MboI), as per the instructions of the manufacturer (Fermentas, USA), to study the restriction fragment length polymorphism (RFLP) patterns. Representative ITS/5.8S products of RFLP analysis and different-sized ITS products were used for sequencing. PCR products were purified, quantified and subjected to sequencing by using ITS1 and ITS4 primers. The ITS sequences were submitted to the GenBank and obtained the accession numbers (). Representative cultures were deposited in Microbial Type Culture Collection and Gene Bank, Institute of Microbial Technology, Chandigarh, India, under the accession numbers MTCC10734 (XF10), MTCC10735 (XF16), MTCC10737 (XF5), MTCC10736 (XF4), MTCC11040 (XF15), MTCC10838 (XF8), MTCC10839 (XF2) and MTCC10840 (XF1).
Phylogenetic analysis
A BLASTN search was performed to find similar sequences in the National Center for Biotechnology Information (NCBI) database to find the possible nearest relative of the newly sequenced endophyte strain sequences retrieved from GenBank that were added to the alignments. Sequences were aligned by using the software MAFFT program (http://mafft.cbrc.jp/alignment/server/) and alignment was manually corrected. A phylogenetic tree was reconstructed using MEGA5 (Tamura et al. Citation2011) software. The Kimura two-parameter model (Kimura, Citation1980) was used to estimate evolutionary distance. The phylogenetic reconstruction was done using the neighbor-joining (NJ) algorithm with bootstrap values calculated from 1000 replicate runs.
Assay of extracellular enzymes
Production of extracelluar enzymes such as cellulase, laccase, lipase and pectinase was confirmed by plate assay as described in Rohrmann and Molitoris (Citation1992). Briefly, cellulase activity was determined by growing isolates in yeast extract and peptone medium containing Na-carboxy-methyl cellulose (0.5%). After 5 days, colonies were flooded with 0.2% congo red and destained with 1M NaCl. Yellow areas around the fungal colony indicated cellulase activity. Laccase activity was determined by growing the fungi in glucose, yeast extract and peptone agar medium with 0.05 g α-napthol/l. Appearance of blue colour in the growth medium due to the oxidation of α-naphtol indicated the presence of laccase. Lipase activity was visualized by growing the fungi in peptone agar medium (10.0 g Peptone, 5.0 g NaCl, 0.1 g CaCl2·2H2O, 10 ml Tween 20, 20.0 g agar in 1000 ml distilled water, pH 6.0). Clearing or precipitation around the fungal colony indicated lipolytic activity. For observing pectinase activity, the fungi were grown in pectin agar medium (5.0 g Pectin, 1.0 g yeast extract). After 5 days, the colonies were flooded with 1% aqueous solution of hexadecyl trimethyl ammonium bromide. A clear zone formed around the fungal colony indicated pectinolytic activity. Pectate transeliminase production was detected at pH 7.0 and pectinase activity at pH 5.0 of the medium.
Assay for antimicrobial activity
Three mycelial plugs (0.5 mm diameter) of a fungus cut from a colony growing on PDA medium were inoculated in 100 ml of PD broth and incubated as static culture for 20 days at 27°C. The culture filtrate was then extracted three times with ethyl acetate. The ethyl acetate was evaporated and this crude extract was dissolved in 10% dimethyl sulfoxide (DMSO) (100 mg/ml). It was screened for its antimicrobial activity against the potentially pathogenic bacteria Staphylococcus aureus and Bacillus subtilis using agar diffusion method (Lorian Citation1996). Minimum inhibitory concentrations (MICs) of ethyl acetate extracts were determined according to Ellof (Citation1998) using Müller–Hinton broth on a 96-well microtiter plate. The stock solutions of the culture extracts were diluted and transferred into the first well, and serial dilutions were performed in order to have concentrations ranging from 1.5 to 100 μg/ml. The inoculum was added to all wells and the plates were incubated at 37°C for 48 h. Antimicrobial activity was detected by adding 20 μl of 0.5% triphenyl tetrazolium chloride (TTC; Merck, NJ, USA) aqueous solution. MIC was defined as the lowest concentration of the extract that inhibited visible growth as indicated by the TTC-staining (dead cells are not stained by TTC). Uninoculated medium extracted and treated in a similar way was used as control.
Assay for other bioactive compounds
Endophytic isolates were grown in PD broth (static culture) for 20 days at 27°C. The culture filtrate (200 ml) was extracted with methanol, concentrated to 1.5 ml volume and 50 μl of this was spotted on a TLC (Thin Layer Chromatography) aluminium sheet (precoated silica gel, layer thickness 0.1 mm; Merck) and developed with butanol, acetic acid and distilled water (3:1:1) solvent system up to 10 cm length. Such TLC plates were used for bioautogram to detect antialgal and antifungal activities (Schulz et al. Citation1995; Thirunavukkarasu et al. Citation2012) Chlorella fusca and Cladosporium cucumerinum were used as assay organisms. Uninoculated medium extracted and treated in a similar way was used as control.
Results
Xylariaceous endophytes were isolated from leaves of all tree species of DT and EG forests, during dry and wet season. In DT tree hosts, the IF% of Xylariaceae when present ranged from 0.7 to 20.0 during the dry season and from 0.7 to 59.3 in the wet season (); in EG forest, this was 0.7–22.0 in the dry season and 0.7–51.3 in the wet season (). Colonization of leaf tissues by endophytes as reflected by the total isolation frequency was not influenced by seasons ( and ). In general, the contribution of Xylariaceae to the total endophytic colonization frequency increased during the wet season. For instance, in the DT forest, during wet season, Xylariaceae contributed to 49% and 60% to the total IF% of the endophytes in the leaves of Acacia sundra and Pongamia pinnata (); in EG forest, the contribution by Xylariaceae during the wet season was 88% in the leaves of Eurya nitida (). Totally, 29 morphotypes having xylaria-like stroma were isolated and these cultures were subjected for ITS–RFLP analysis. Based on the RFLP analyses, 16 morphospecies of Xylaria endophytes isolated from 11 tree species (DT and EG) belonging to 7 families were selected for further molecular analysis ().
Molecular diversity
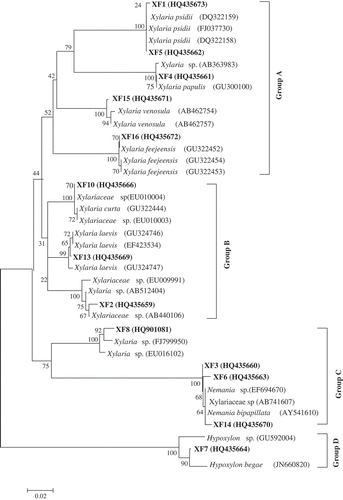
BLASTN analysis revealed that except XF9, XF11 and XF12, other sequences showed similarity with Xylaria species. XF9 showed similarity with Sordariomycetes spp., XF11 with Thielavia sp. and XF12 with Sarocladium spp. Hence these isolates were not included for phylogenetic analysis. Phylogenetic relationships inferred from ITS1-5.8S-ITS2 region sequences of species of Xylaria and related genera are shown in . Phylogenetic analysis clustered all the sequences into four groups. Group A consisted of Xylaria psidii, Xylaria papulis, Xylaria venosula and Xylaria feejeensis. XF1, XF4, XF5, XF15 and XF16 were clustered with these isolates with strong bootstrap values. Group B included Xylaria curta, Xylaria laevis and Xylariaceae sp., and XF2, XF10 and XF13 were included in this group. XF3, XF6, XF8 and XF14 constituted group C along with Xylaria sp. and Nemania spp. XF7 formed as separate group (group D) along with Hypoxylon spp.
Bioactive compounds and extracellular enzymes
All the tested isolates except XF10 produced antialgal and antifungal metabolites (). For antibacterial activity, XF1 was most active against B. subtilis and XF10 was active against S. aureus. All isolates except XF1 had a MIC value more than 2.5 μg/ml against B. Subtilis, whereas XF1 showed MIC value of 1.30 μg/ml against B. subtilis. However, except XF10, all other isolates tested showed MIC value of >2.5 μg/ml against S. aureus while XF10 showed MIC value of 1.28 μg/ml (). All the 10 isolates tested produced cellulase, while nine of them produced lipase (). A few of them produced pectinase, pectate transeliminase and laccase ().
Table 4. Antialgal, antifungal activities, MICs against Staphylococcus aureus and Bacillus subtilis and extracellular enzyme production by xylariaceous endophytes
Discussion
Although xylariaceous endophytes are known to be common in tropical plants, this is the first study that records their occurrence in tropical dicotyledonous woody trees belonging to 49 species in 24 families attesting to the wide distribution of these endophytes. We isolated more than one morphospecies of endophytic Xylariaceae from the leaves of the same host plant. Since the different morphotypes proved to be distinct species based on molecular diagnosis, it again reflected the wide host range of endophytic Xylariaceae. Earlier studies have shown that xylariaceous endophytes are generalists displaying loose host affiliation (Brunner & Petrini Citation1992; Bayman et al. Citation1998; Okane et al. Citation2008). Our observation showed that they colonize trees of disparate families irrespective of the season and the environment (forest type), which is an indication of their adaptation as cosmopolitan endophytes. Certain xylariaceous taxa have wide geographic distribution (Rogers Citation2000). Indeed, Davis et al. (Citation2003) showed that endophytic Xylaria infecting liverworts from Jamaica and North Carolina are closely related to each other and to the endophytes colonizing angiosperms in China, Europe and Puerto Rico highlighting the wide geographic range and host acquisition capacity of this group. Termite-associated Xylaria also show no host specificity (Guedegbe et al. Citation2009). All these results taken together confirm that xylariaceous members are generalists with reference to the substrata they utilize.
In the present study, except XF7, XF9, XF11 and XF12, all other isolates were either Xylaria or Nemania species and were isolated from different tree species. Similar results were reported by Okane et al. (Citation2008) where they showed that Xylaria and Nemania occur more commonly as endophytes of angiosperms than Hypoxylon and related fungi. Furthermore, in our study the DNA sequence analysis showed that XF14 clustered with the wood decaying fungus N. bipapillata (); Okane et al. (Citation2008), who isolated this fungus as an endophyte, opined that xylariaceous endophytes can also survive as saprotrophs. It is important to note that some of the species names assigned to the DNA sequences obtained from Xylaria spp. may be incorrect (Okane et al. Citation2008). This may be largely due to the fact that the species concept in Xylaria is not concrete (Fournier et al. Citation2011). Although ITS and 18S rDNA sequence analyses have been used to characterize Xylaria endophytes (Liu et al. Citation2010), Fournier et al. (Citation2011) believe that apart from rDNA sequences, application of multigene DNA sequence data including those of β-tublin, α-actin and RPB2 genes (Hsieh et al. Citation2010) can more accurately approximate a phylogenetic assessment of xylariaceous fungi. Thus, more focused studies using different DNA sequence data as well as information regarding the teleomorph states (Osono et al. Citation2013) are essential to discern the distribution of tropical xylariaceous endophytes and recognize how these fungi alternate their life between living in the plant tissues as endophytes and as saprotrophs in senescent plant tissues and wood (Stadler Citation2011; Suryanarayanan Citation2011).
It has been hypothesized that xylariaceous endophytes become active saprobes once the tissues which they colonize as endophytes become senescent (Petrini et al. Citation1995; Whalley Citation1996; Collado et al. Citation2001). A recent study by Osono et al. (Citation2013) on xylariaceous endophytes in a cool temperate forest in Japan also supports this view. Endophyte colonization of living tissues could help them overcome competition with other saprobic fungi owing to their presence in the tissue even before senescence (Suryanarayanan Citation2011). The xylariaceous endophytes produce extracellular enzymes needed for degrading dead plant tissues substantiating the view that these fungi are transient saprobes (Whalley Citation1996). Recent studies indicate that fungal endophytes including members of Xylariaceae are a repository of novel industrial enzymes (Suryanarayanan et al. Citation2012). The present study indicates that it would be worthwhile investigating xylariaceous endophytes for their plant biomass-degrading enzymes as well.
Endophytic fungi of tropical plants are a potential source of diverse natural products such as alkaloids, benzopyranones, coumarins, chromones, cytochalasins, enniatins, quinones, peptides, phenols, phenolic acids, semiquinones, steroids, terpenoids, xanthones and lactones with novel bioactivities (Suryanarayanan et al. Citation2010; Tejesvi & Pirttilä Citation2011). Xylariaceous endophytes produce antimalarial (Jiménez-Romero et al. Citation2008), antibacterial (Liu et al. Citation2008), antifungal (Boonphong et al. Citation2001; Oliveira et al. Citation2011), antiviral (Pittayakhajonwut et al. Citation2005) and other novel bioactive compounds (Schueffler & Anke Citation2011). Our study also indicated the production of bioactive compounds by the xylariaceous endophytes of the Western Ghats forests. In the present study, 9 of the 10 isolates tested produced antialgal and antifungal metabolites () and similar type of results were also reported by Dai et al. (Citation2006). Endophytic Xylariaceae have been reported to produce antibacterial metabolites active against S. aureus, Escherichia coli, Salmonella typhimurium and Shigella sp. (Liu et al. Citation2008).
The wide host range of xylariaceous endophytes and their possible continued survival as saprobes in plant litter and wood explain their cosmopolitan occurrence. The production of several antagonistic substances and plant cell wall-degrading enzymes could be strategies evolved for such a biphasic life aiding in ecological success and nutrient acquisition. In this context, the report of Zuccaro et al. (Citation2011) that different genes are expressed during the plant-dependant and saprotrophic life of an endophyte is significant. Although the survival of tropical xylariaceous endophytes as plant litter degraders is known, it is yet to be firmly established if the endophytic phase orchestrates plant tissue senescence to facilitate a switch to the saprobic phase.
In conclusion, many tree species of the forest of Western Ghats harboured xylariaceous endophytes and their infection of the leaves increased during the wet season. Most of the xylariaceous endophytes belonged to Xylaria or Nemania. Many of these fungi produced extracellular enzymes and metabolites inhibitory to fungi, bacteria and algae indicating that they can compete with such organisms in the forest floor while surviving as saprophytes. More detailed studies on tropical xylariaceous fungi both as endophytes and as litter degraders are needed to clearly understand their role in the ecosystem such that their metabolic versatility can be effectively harnessed technologically.
Acknowledgements
TSS thanks the Department of Biotechnology, Govt. of India, for funding the Indo-German project BT/IN/German/11/TSS/2010 and Swami Shukadevananda, Chairman, VINSTROM for facilities and Prof. R. Sukumar, Centre for Ecological Science, Indian Institute of Science, Bengaluru for helping in the collection of samples from the forests.
References
- Bayman , P , Angulo-Sandoval , P , Báez-Ortiz , Z and Lodge , DJ. 1998 . Distribution and dispersal of Xylaria endophytes in two tree species in Puerto Rico . Mycol Res. , 102 : 944 – 948 .
- Boonphong , S , Kittakoop , P , Isaka , M , Pittayakhajonwut , D , Tanticharoen , M and Thebtaranonth , Y. 2001 . Multiplolides A and B, new antifungal 10-membered lactones from Xylaria multiplex . J Nat Prod. , 64 : 965 – 967 .
- Brunner , F and Petrini , O. 1992 . Taxonomy of some Xylaria species and xylariaceous endophytes by isozyme electrophoresis . Mycol Res. , 96 : 723 – 733 .
- Collado , J , Platas , G and Pelaez , F. 2001 . Identification of an endophytic Nodulisporium sp. from Quercus ilex in central Spain as the anamorph of Biscogniauxia mediterranea by rDNA sequence analysis and effect of different ecological factors on distribution of the fungus . Mycologia. , 93 : 875 – 886 .
- Dai , J , Krohn , K , Flörke , U , Draeger , S , Schulz , B , Kiss-Szikszai , A , Antus , S , Kurtán , T and van Ree , T. 2006 . Metabolites from the endophytic fungus Nodulisporium sp. from Juniperus cedre . Eur J Org Chem. , 15 : 3498 – 3506 .
- Dargan , JS. 1982 . Xylaria musooriensis – a new species from India . Mycologia. , 74 : 523
- Davis , EC , Franklin , JB , Shaw , AJ and Vilgalys , R. 2003 . Endophytic Xylaria (Xylariaceae) among liverworts and angiosperms: phylogenetics, distribution and symbiosis . American J Bot. , 90 : 1661 – 1667 .
- Duong , LM , Lumyong , S , Hyde , KD and Jeewon , R. 2004 . Emarcea castanopsidicola gen. et sp. nov. from Thailand, a new xylariaceous taxon based on morphology and DNA sequences . Studies Mycol. , 50 : 253 – 260 .
- Eloff , JN. 1998 . A sensitive and quick microplate method to determine the minimal inhibitory concentration of the plant extracts bacteria . Plant Medica. , 64 : 711 – 713 .
- Fournier , J , Flessa , F , Peršoh , D and Stadler , M. 2011 . Three new Xylaria species from southwestern Europe . Mycol Progress. , 10 : 33 – 52 .
- Guedegbe , HJ , Miambi , E , Pando , A , Houngnandan , P and Rouland-Lefevre , C. 2009 . Molecular diversity and host specificity of termite-associated Xylaria . Mycologia. , 101 : 686 – 691 .
- Hsieh , H-M , Lin , C-R , Fang , M-J , Rogers , JD , Fournier , J , Lechat , C and Ju , Y-M. 2010 . Phylogenetic status of Xylaria subgenus Pseudoxylaria among taxa of the subfamily Xylarioidea (Xylariaceae) and phylogeny of the taxa involved in the subfamily . Mol Phylogen Evol. , 54 : 957 – 969 .
- Jiménez-Romero , C , Ortega-Barria , E , Arnold , AE and Cubilla-Rios , L. 2008 . Activity against Plasmodium falciparum of lactones isolated from the endophytic fungus Xylaria sp . Pharm Biol. , 46 : 1 – 4 .
- Kimura , M. 1980 . A simple method for estimating evolutionary rates of base substitutions through comparative studies of nucleotide sequences . J Mol Evol. , 16 : 111 – 120 .
- Koehn , FE , Kirsch , DR , Feng , X , Janso , J and Young , M. 2008 . A cell wall-active lipopeptide from the fungus Pochonia bulbillosa . J Nat Prod. , 71 : 2045 – 2048 .
- Lee , JS , Kwan , SK and Jung , HS. 2000 . Phylogenetic analysis of Xylaria based on nuclear ribosomal ITS–5.8S–ITS2 sequences . FEMS Microbiol Lett. , 187 : 89 – 93 .
- Lee , NS , Dohjima , T , Bauer , G , Li , H , Li , MJ , Ehsani , A , Salvaterra , P and Rossi , J. 2002 . Expression of small interfering RNAs targeted against HIV-1 rev transcripts in human cells . Nat Biotechnol. , 20 : 500 – 505 .
- Linnakoski , R , Puhakka-tarvainen , H and Pappinen , A. 2012 . Endophytic fungi isolated from Khaya anthotheca in Ghana . Fungal Ecol. , 5 : 298 – 308 .
- Liu , K , Ding , X , Deng , B and Chen , W. 2010 . 10-Hydroxycampothecin produced by a new endophytic Xylaria sp., M 20, from Camptotheca acuminata . Biotechnol Lett. , 32 : 689 – 693 .
- Liu , X , Dong , M , Chen , X , Jiang , M , Lv , X and Zou , J. 2008 . Antimicrobial activity of an endophytic Xylaria sp. YX-28 and identification of its antimicrobial compound 7-amino-4-methylcoumarin . Appl Microbiol Biotechnol. , 78 : 241 – 247 .
- Lorian , V. 1996 . Antibiotics in Laboratory Medicine. , 3rd , Baltimore , MD : Williams and Wilkins .
- Naik , BS , Shashikala , J and Krishnamurthy , YL. 2008 . Diversity of fungal endophytes in shrubby medicinal plants of Malnad region, Western Ghats, Southern India . Fungal Ecol. , 1 : 89 – 93 .
- Nath , A , Raghunatha , P and Joshi , SR. 2012 . Diversity and Biological activities of endophytic fungi of Emblica officinalis, an ethnomedicinal plant of India . Mycobiology. , 40 : 8 – 13 .
- Okane , I , Srikitikulchai , P , Toyama , K , Læssøe , T , Sivichai , S , Hywel-Jones , N , Nakagiri , A , Potacharoen , W and Suzuki , K. 2008 . Study of endophytic Xylariaceae in Thailand: diversity and taxonomy inferred from rDNA sequence analyses with saprobes forming fruit bodies in the field . Mycoscience. , 49 : 359 – 372 .
- Oliveira , C , Regasini , LO , Silva , GH , Pfenning , LH , Young , MCM , Berlinck , RGS , Bolzani , VS and Araujo , AR. 2011 . Dihydroisocoumarins produced by Xylaria sp. and Penicillium sp. endophytic fungi associated with Piper aduncum and Alibertia macrophylla . Phytochem Lett. , 4 : 93 – 96 .
- Ondeyka , JG , Helms , GL , Hensens , OD , Goetz , MA , Zink , DL , Tsipouras , A , Shoop , WL and Slayton , L. 1997 . Nodulisporic acid A, a novel and potent insecticide from a Nodulisporium sp., isolation, structure determination and chemical transformations . J Amer Chem Soc. , 119 : 8809 – 8816 .
- Osono , T , Tateno , O and Masuya , H. 2013 . Diversity and ubiquity of xylariaceous endophytes in live dead leaves of temperate forest trees . Mycoscience. , 54 : 54 – 61 .
- Petrini , O , Petrini , LE and Rodrigues , K. 1995 . Xylariaceaous endophytes: an exercise in biodiversity . Fitopatol Bras. , 20 : 531 – 539 .
- Pittayakhajonwut , P , Suvannakad , R , Thienhirun , S , Prabpai , S , Kongsaereec , P and Tanticharoen , M. 2005 . An anti-herpes simplex virus-type 1 agent from Xylaria mellisii (BCC 1005) . Tetrahedron Lett. , 46 : 1341 – 1344 .
- Rogers , JD. 2000 . Thoughts and musings on tropical Xylariaceae . Mycol Res. , 104 : 1412 – 1420 .
- Rohrmann , S and Molitoris , HP. 1992 . Screening for wood-degrading enzymes in marine fungi . Can J Bot. , 70 : 2116 – 2123 .
- Schlingmann , G , Milne , L and Carter , GT. 2002 . Isolation and identification of antifungal polyesters from the marine fungus Hypoxylon oceanicum LL-15G256 . Tetrahedron. , 58 : 6825 – 6835 .
- Schueffler , A and Anke , T. 2011 . Antimicrobial compounds from tree endophytes , Edited by: Pirttilä , AM and Frank , AC . 265 – 294 . New York: Springer : Endophytes of forest trees: biology and applications .
- Schulz , B , Sucker , J , Aust , HJ , Krohn , K , Ludewig , K , Jones , PG and Döring , D. 1995 . Biologically active secondary metabolites of endophytic Pezicula species . Mycol Res. , 99 : 1007 – 1015 .
- Smith , CJ , Morin , NR , Bills , GF , Dombrowski , AW , Salituro , GM , Smith , SK , Zhao , A and MacNeil , DJ. 2002 . Novel sesquiterpenoids from the fermentation of Xylaria persicaria are selective ligands for the NPYY5 receptor . J Org Chem. , 67 : 5001 – 5004 .
- Stadler , M. 2011 . Importance of secondary metabolites in the Xylariaceae as parameters for assessment of their taxonomy, phylogeny, and functional biodiversity . Curr Res Envion Appl Mycol. , 1 : 75 – 133 .
- Suresh , HS and Sukumar , R. 1999 . Phytogeographical affinities of flora of Nilgiri Biosphere Reserve . Rheedea. , 9 : 1 – 21 .
- Suryanarayanan , TS. 1992 . Light-incubation: a neglected procedure in mycology . Mycologist. , 6 : 144
- Suryanarayanan , TS. 2011 . Diversity of fungal endophytes in tropical trees , Edited by: Pirttilä , AM and Frank , AC . 67 – 80 . New York: Springer : Endophytes of forest trees: biology and applications .
- Suryanarayanan , TS , Kumaresan , V and Johnson , JA. 1998 . Foliar fungal endophytes from two species of the mangrove Rhizophora . Can J Microbiol. , 44 : 1003 – 1006 .
- Suryanarayanan , TS , Thirunavukkarasu , N , Govindarajulu , MB and Gopalan , V. 2012 . Fungal endophytes: an untapped source of biocatalysts . Fungal Divers. , 54 : 19 – 30 .
- Suryanarayanan , TS , Venkatachalam , A , Thirunavukkarasu , N , Ravishankar , JP , Doble , M and Geetha , V. 2010 . Internal mycobiota of marine macroalgae from the Tamilnadu coast: distribution, diversity and biotechnological potential . Bot Mar. , 53 : 457 – 468 .
- Tamura , K , Peterson , D , Peterson , N , Stecher , G , Nei , M and Kumar , S. 2011 . MEGA5: molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods . Mol Biol Evol. , 28 : 2731 – 2739 .
- Tejesvi , MV and Pirttilä , AM. 2011 . Potential of tree endophytes as sources for new drug compounds , Edited by: Pirttilä , AM and Frank , AC . 295 – 311 . Endophytes of forest trees: biology and applications. New York: Springer; p .
- Thind , KS and Dargan , JS. 1978 . Xylariaceae of India – IV. The genus Daldinia . Kavaka. , 6 : 15 – 24 .
- Thirunavukkarasu , N , Suryanarayanan , TS , Girivasan , KP , Venkatachalam , A , Geetha , V , Ravishankar , JP and Doble , M. 2012 . Fungal symbionts of marine sponges from Rameswaram, southern India: species composition and bioactive metabolites . Fungal Divers. , 55 : 37 – 46 .
- Van Kan , JAL , van den Ackerveken , GFJM and de Wit , PJGM. 1991 . Cloning and characterization of the cDNA of avirulence avr9 of the fungal pathogen Cladosporium fulvum, the casual agent of tomato leaf mold . Mol Plant Microbe Interact. , 4 : 52 – 59 .
- Whalley , AJS. 1996 . The xylariaceous way of life . Mycol Res. , 100 : 897 – 922 .
- White , TJ , Bruns , T , Lee , S and Taylor , J. 1990 . “ Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics ” . In PCR protocols: a guide to methods and applications , Edited by: Innis , MA , Gelfand , DH , Sninsky , JJ and White , TJ . 315 – 322 . New York : Academic Press; p .
- Zuccaro , A , Lahrmann , U , Güldener , U , Langen , G , Pfiffi , S , Biedenkopf , D , Wong , P , Samans , B , Grimm , C and Basiewicz , M . 2011 . Endophytic life strategies decoded by genome and transcriptome analyses of the mutualistic root symbiont Piriformospora indica . PLoS Pathog. , 7 ( 10 ) : e1002290 doi: 10.1371/journal.ppat.1002290