Abstract
Both yeasts and nematodes are significant components of the soil biomass and biodiversity and fulfil a wide variety of ecological functions. However, relatively little is known about the interactions between yeasts and nematodes, including the potential use of yeasts by nematodes as a food source and potential diseases that these yeasts can cause in nematodes. To begin investigating their ecological relationships, we tested the in vitro attractive ability of representative yeast species on nematodes. A total of 15 yeast strains belonging to six species were assayed for their attraction abilities towards two nematode species. Our results suggest that nematodes are able to distinguish between their microbial food source and yeast pathogens. Furthermore, our analyses demonstrated that host nematodes, yeast species, and in some cases yeast strains all contributed to the variation in attraction abilities. We hypothesize that volatile and/or diffusible organic compounds released from the yeasts are involved in attracting the nematodes. These results suggest the attraction and consumption interaction between soil yeasts and nematodes may be common in the environment. These interactions may be significant in regulating the populations of both the yeasts and their nematode hosts in natural soil ecosystems. The data presented here could also help to develop nematode-based model systems for studying fungal pathogenesis.
Introduction
Yeasts are a significant component of the global biodiversity, fulfilling various functions in a variety of ecosystems (Botha Citation2011). Within the soil ecosystem, there are diverse microenvironments that are conducive for different yeasts to grow and persist. Yeasts are usually present at a density of between 10−2 and 10−5 cells per gram of soil (Vazquez et al. Citation1993; Haridy Citation2002). These unicellular fungi generally obtain their nutrients from decomposing organic plant compounds via respiration or fermentation. Thus, most known environmental yeasts have been found to be associated with plants. The environmental yeasts may be consumed by organisms at higher trophic levels such as insects, nematodes, and vertebrates, including humans. Indeed, yeasts have been used by humans for food conversion and preservation for hundreds of years. Several yeast species are a significant component of the human diet in brewing, bread-making, and the productions of vitamins. However, many types of yeasts can cause diseases in a variety of animals and have the potential to regulate animal host populations. These interactions may be key components of interaction networks between soil predators, yeasts and other microbes, and plants in natural environments. In agriculture and forestry, these types of interactions are of particular importance since the quality and efficiency of crop and wood production can be severely impacted by these relationships.
Similar to yeasts, nematodes play a pivotal role in the soil environment and can significantly impact vegetables, crops, and forestry products. Since soil nematodes typically require a thin coat of water in order to navigate through the soil, the water content of the soil environment has a significant influence on nematode community structures (Neher Citation2010). Similar to yeasts, the diversity of nematodes found in soil is likely related to the diversity of microenvironments, and the biotic and abiotic factors of these niches. Indeed, nematodes are among the most abundant organisms and have some of the most diverse roles at multiple trophic levels in a soil ecosystem. Examples of these nematodes include fungal hyphae-feeding nematodes, bacterivorous nematodes, plant and animal pathogenic nematodes, and nematodes that feed on unicellular eukaryotes such as yeasts (Neher Citation2010). Fungivorous nematodes are commonly found in forested areas and their interactions with various yeasts are likely prevalent in this type of ecosystem. Their abundance and broad distributions should generate opportunities for interactions between nematodes and yeasts in a diversity of ecological niches.
Table 1. Strains of yeasts used in this study
For soil nematodes, their environmental awareness can result from their ability to sense chemical compounds in their immediate environments. Since soil nematodes generally occupy an environment with contacts to both water and air, they are able to sense both volatile compounds and dissolved compounds in the water (Troemel Citation1999). The chemosensory ability of nematodes could be a key to their survival as it could guide them towards safe food sources, away from pathogen sources, and allowing them to sense other nematodes for mating purposes. Thus, a better understanding of a nematode’s ability to differentiate between a pathogenic microorganism from a harmless one would be very important for their survival.
The population dynamics in the interaction between nematodes and yeasts is likely influenced by both interacting partners and their environments. This dynamics can be regulated through either the top-down or the bottom-up approach (Neher Citation2010). The top-down control occurs when nematodes regulate the population dynamics of their yeast food source, whereas bottom-up control describes dynamics in which nematode populations are regulated by yeasts. An example of top-down control can be seen in a standard culture of Caenorhabditis elegans feeding on a non-pathogenic microbial food source such as Escherichia coli. Bottom-up control can be seen when a pathogenic microbial prey such as Cryptococcus neoformans kills the host nematodes after being infected (Mylonakis et al. Citation2002).
At present, our understanding of the interactions between nematodes and yeasts is very rudimentary (Neher Citation2010). For example, it is not known whether yeast species differ in their ability to attract or repel nematodes or whether different yeast strains within the same species would have the same attraction or repelling abilities. In addition, it is not known whether different nematode species would respond similarly to the same yeast strains or species.
The objective of this study is to examine the potential differences in nematode attraction ability among strains and species belonging to six different pathogenic yeasts: Candida albicans, Candida glabrata, Candida parapsilosis, Candida tropicalis, C. neoformans, and Cryptococcus gattii. These yeast species were chosen because they are either geographically and/or ecologically broadly distributed or have been shown in previous studies to possess killing ability towards nematodes. In addition, these yeast species are also of considerable medical significance as they can cause human diseases. In this study, two model nematode species were used, C. elegans and Caenorhabditis briggsae, to elucidate the yeasts’ attractive abilities and to investigate whether these two nematode species could be differentially susceptible to the examined yeasts. We discuss the implications of our results on yeast–nematode interactions and on fungal pathogenesis research.
Materials and methods
A total of 15 strains belonging to six yeast species were examined in this study (). These include three strains of C. tropicalis, two strains of C. albicans, one strain each of C. glabrata and C. parapsilosis, and four strains each of C. neoformans and C. gattii. The details of strain information can be found in . The Candida strains were either from the bloodstream of patients with candidemia or from the environment around Hamilton, Ontario, Canada. However, strains of C. neoformans and C. gattii were from diverse geographic origins and represented divergent lineages (Kidd et al. Citation2005; Sun & Xu Citation2007). These strains were identified based on both standard mycological tests and/or their sequences at the internal transcribed spacer (ITS) regions of the nuclear ribosomal RNA gene cluster. Cryptococcus neoformans strains JEC20 and JEC21 are isogenic except at the mating-type locus (Sun & Xu Citation2007). These strains and species were chosen to examine the potential differences between species and strains of common yeasts in their nematode-attraction abilities.
In this study, wild-type nematodes C. elegans strain N2 and C. briggsae AF16 were used as hosts for the attraction assays. These two model nematode species were used to determine if there was any host specificity in attractions to yeasts. Nematodes were cultured and maintained on standard nematode growth medium (NGM) agar with E. coli OP50 lawn as food source. Yeasts were cultured on standard yeast extract-peptone-dextrose (YEPD) agar.
Attraction assays
To prepare microbial cells for the attraction assay, actively growing yeast cells were collected and suspended in 400 μl of sterile ddH2O and then vortexed to ensure homogeneous distribution and no obvious cell clumping. Sixty microlitres of the yeast suspension representing about 108 cells is then transferred and spread evenly with a glass spreader onto one side of each 9-cm plate containing the YEPD agar medium. The inoculated plates are incubated at room temperature (∼23°C) to allow the yeasts to grow overnight. For the positive control plates, 60 μl of E. coli cells suspension (representing about 108 cells) was prepared and spread evenly onto one side of a YEPD plate. The plate with E. coli cells was then incubated at 37°C overnight.
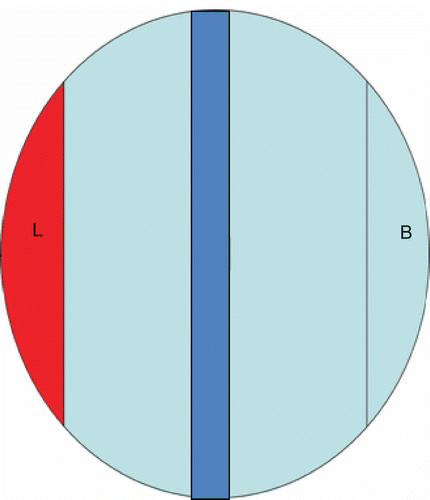
The nematodes were prepared by transferring onto fresh NGM agar with E. coli lawn and allowed to grow for 3 days. After 3 days, most of the E. coli lawn had been consumed and the nematodes were washed off the culture plate using sterile ddH2O into suspension. The prepared nematodes and microbes were assayed for the nematode attraction using a recently described method (Srinivasan et al. Citation2012) but with modifications shown in Briefly, each 9-cm petri dish containing microbial cells on the YEPD agar was divided according to the dimensions shown in A suspension of approximately 1000 nematodes was placed in the central square area along the central vertical axis to facilitate liquid absorption and to prevent nematode clumping. After 7 hours, the nematodes were counted for each region on the plate using a stereomicroscope at 250× magnification. At least six repeats were done for each isolate. For each set of assay, we included both the negative (no microbial cell) and the positive (E. coli OP50; Caenorhabditis Genetics Center at the University of Minnesota, St. Paul, MN, USA) controls.
Data analysis
Each nematode present in the microbial cell lawn (L) and the corresponding no-microbial cell blank (B) area was counted and the difference in nematode counts between the two areas was calculated (). This number represents the raw attraction score of the yeast strain to the specific host nematode strain used. Potential differences among batches for the E. coli lawns were also calculated and used to minimize any potential batch effects. Specifically, we standardized the E. coli attraction value as 1.00 in each batch assay and the attraction value of each yeast strain was then scaled to its corresponding E. coli control in the batch. Thus, the final attraction value for each yeast strain in each test was a ratio of the raw attraction score of the yeast strain over the raw attraction score of the corresponding E. coli treatment.
SPSS v20 (IBM; http://ibm-spss-statistics.soft32.com) was used to conduct paired Student t-tests to determine if there were statistically significant differences between strains within the same species in their nematode-attraction abilities. Inter-species differences were similarly identified via paired Student t-tests. Each strain of each yeast species was treated as an individual repeat for inter-species analysis. For both intra- and inter-species analyses, the results were also compared to the positive (E. coli) and negative (blank) control plates.
Similarly, the potential differences between the two nematode species were also examined for each yeast species using the paired Student t-test. Furthermore, ANOVA analysis was used to reveal the overall contributions of yeast strains, yeast species, nematode host, and the interaction effects between yeast and nematodes for the overall observed variations in yeast–nematode attraction.
Results
Attraction of nematodes towards yeasts
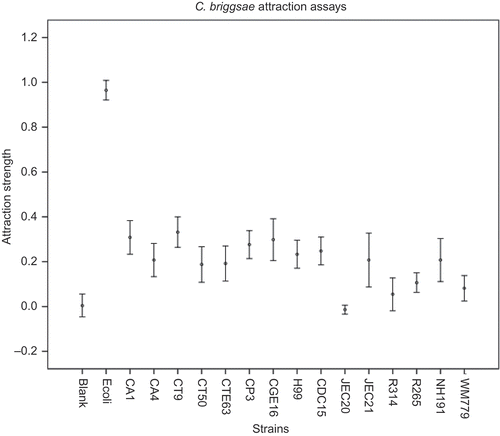
Figure 2 shows the relative attraction values of each yeast strain when tested against the C. briggsae nematode host. Our assay showed clear differences between E. coli and the blank negative control as well as between E. coli and all 15 yeast strains in their attractions to C. briggsae (). Thirteen of the 15 yeast strains tested demonstrated statistically significant higher (p < 0.05) attractions than that of the blank controls. The two yeast strains that failed to show significant attraction ability were C. neoformans strain JEC20 and C. gattii strain R314. For nematode C. briggsae, the most attractive yeast in the genus Candida that we tested was the clinical isolate C. tropicalis CT9. CT9 attracted about one-third as many nematodes as did the non-pathogenic food E. coli OP50. The most attractive Cryptococcus yeast for C. briggsae was C. neoformans strain CDC15. It attracted about 25% as many nematodes as E. coli OP50. Among the 15 strains, JEC20 was the only strain that showed a lower attraction value than the blank control (–0.014), suggesting that JEC20 might be repulsive for C. briggsae. However, the attraction values between JEC20 and blank control were not significantly different from each other (p > 0.5).
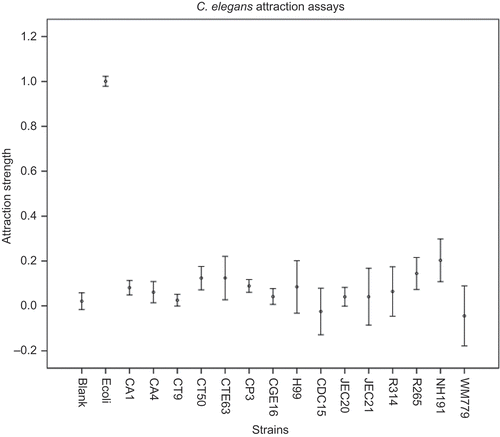
Figure 3 shows the relative attraction values of each yeast strain when paired with C. elegans. Our assay showed a clear difference between E. coli and negative control as well as between E. coli and all the 15 tested yeast strains (). However, several differences were observed when compared to results obtained using C. briggsae as nematode host. First, C. elegans was overall less attracted towards Candida and Cryptococcus yeasts than C. briggsae. For example, with C. elegans as the host, only seven (instead of 13 for C. briggsae) out of the 15 tested Candida and Cryptococcus strains showed a significantly greater attraction than the blank control plates (p < 0.05). Second, unlike treatments using C. briggsae as hosts, the highest attraction value for C. elegans was observed for strain NIH191, which showed about one-fifth of the attraction value of E. coli OP50. In addition, while C. neoformans CDC15 was the most attractive Cryptococcus yeasts for C. briggsae, it was slightly repulsive for C. elegans, exhibiting an attraction value lower than the blank control. Another Cryptococcus strain WM779 also showed a negative attraction value. Below we describe the effects of host species, yeast species, and yeast strains separately.
Host effect
A significant host-specific effect was clearly observed when comparing the attraction ability of these yeasts. In general, yeasts were able to attract a greater proportion of C. briggsae worms than C. elegans worms. At the species level, even the least attractive yeast species to C. briggsae had a higher attraction value than the most attractive yeast species to C. elegans. Another interesting observation is that C. gattii was the least attractive yeast species for C. briggsae but the most attractive for C. elegans. This is just one of several differences between the two nematode’s preference of food sources which indicate that there are key differences in which C. briggsae and C. elegans respond to the stimuli from yeast lawns.
Intra-species differences among yeast strains in attraction abilities
For C. parapsilosis and C. glabrata, we tested only one strain for each of the two species. Both strains were only weakly attractive (mean = 0.0887 and 0.0414, respectively) for C. elegans, but were more attractive (mean = 0.276 and 0.298, respectively) for C. briggsae. While both strains showed significantly higher attraction ability than the blank control for C. briggsae (p < 0.05), for C. elegans, only C. parapsilosis strain CP3 showed an attraction ability different from the blank control.
Candida albicans
Compared to E. coli OP50, the two C. albicans strains CA1 and CA4 were both only weakly attractive (mean = 0.0807 and 0.0611, respectively) for C. elegans and C. briggsae (mean = 0.308 and 0.207, respectively). However, both strains showed significantly greater attraction ability for the two nematodes than the negative blank controls. Between the two C. albicans strains, CA1 was more attractive for C. briggsae than CA4. However, these two strains did not show significant difference in their attraction abilities for C. elegans.
Candida tropicalis
All three tested C. tropicalis strains (CT9, CT50, and CTE63) were attractive for both C. elegans (mean = 0.0253, 0.124, and 0.124, respectively) and C. briggsae (mean = 0.332, 0.187, and 0.192, respectively). For C. elegans, strain CT9 showed significantly lower attraction ability than the other two. However, the three strains showed no significant differences in their attraction abilities for C. briggsae.
Cryptococcus gattii
All four strains of Cryptococcus gatti (R314, R265, NIH191, and WM779) were attractive for C. elegans (mean = 0.0544, 0.106, 0.207, and 0.0811, respectively). For C. briggsae, however, while three strains (R314, R65, and NIH191) were attractive (mean = 0.0640, 0.144, and 0.203, respectively), strain WM779 was weakly repulsive (mean = –0.0447). All four yeast strains showed significantly lower attraction abilities than E. coli cells for both C. elegans and C. briggsae. Though strain NIH191 was more attractive than other three C. gattii strains for both C. elegans and C. briggsae, pairwise tests revealed that less than half of the comparisons were statistically significant.
Table 2. ANOVA analysis of the effect of Cryptococcus yeast strain and nematode species on yeast-attraction abilities to nematodes
Table 3. ANOVA analysis of the effect of Candida yeast strain and nematode species on yeast-attraction abilities to nematodes
Cryptococcus neoformans
Of the four C. neoformans strains, three (H99, JEC20, and JEC21) were weakly attractive (mean = 0.0845, 0.0405, and 0.0410) for C. elegans, while the fourth CDC15 was weakly repulsive (mean = –0.0253). For the C. briggsae nematodes, three strains (H99, CDC15, and JEC21) were also attractive (mean = 0.233, 0.247, and 0.207, respectively), while JEC20 was weakly repulsive (mean = –0.0139). All four strains showed significantly lower attraction ability than E. coli for both host nematodes. For C. elegans, none of the four strains showed significantly greater attraction ability than the blank negative control or than other three strains. However, while no significant difference in attraction ability was observed among strains H99, CDC15, and JEC21 for C. briggsae, they all showed significantly greater attraction abilities than both strain JEC20 and the blank negative control.
Mating-type effect
We observed a significant mating-type-specific effect in the attraction ability of C. neoformans for C. briggsae. The C. neoformans strains JEC20 and JEC21 differed at the mating-type locus but were isogenic for the rest of their genomes. Interestingly, they showed significant differences in attraction values for C. briggsae (strain JEC20: mean = –0.0139, SD = 0.0312; strain JEC21: mean = 0.2075, SD = 0.1144). However, this mating-type-specific effect was not found for a different nematode host C. elegans, where strains JEC20 and JEC21 showed similar and weak attractions (JEC20: mean = 0.0404, SD = 0.0337; JEC21: mean = 0.0410, SD = 0.1019).
Comparisons between yeast species
Although our data suggested that C. neoformans and C. gattii were generally less attractive for the two nematodes than Candida species, none of the pairwise species comparisons showed that the differences were statistically significant. This was mainly due to the significant variations among strains from within most individual yeast species. Among the 15 yeast strains, a range of attraction abilities were observed, from weakly attractive to no attraction and to weakly repellent ( and ).
Factorial ANOVA
Three factorial ANOVA tests were done in order to identify the relative contributions of the nematode species, yeast species, and yeast strain to the variance in attraction values. Also, we are interested in any potential interaction effects between these factors in their contributions to the variations. In these analyses, due to the large evolutionary divergence between Candida (in phylum Ascomycota) and Cryptococcus (in phylum Basidiomycota), the yeast species were separated into two groups for analyses for strain-specific effects. The results from the factorial ANOVA are summarized in –. Depending on the groups of yeasts analysed, host nematode species contributed 19% and 35% to the total attraction variation, respectively, for Cryptococcus and Candida yeasts. Similarly, a strong yeast-strain-specific effect was also seen in the Cryptococcus and Candida assays. Our analyses revealed that strain-specific effect contributed to 41% of the variation in the Cryptococcus attraction values and to 36% of the variation in the Candida attraction values. In addition, there were significant yeast strain and host nematode interaction effects, contributing about 35% to the total variance in the Cryptococcus attraction values and about 12% in the Candida assays. The yeast species-specific effect and the nematode–yeast species interaction effects were similarly significant (p = 0.000; p = 0.003; ).
Table 4. ANOVA analysis of the effect of Candida yeast species and nematode species on yeast-attraction abilities to nematodes
Discussion
In this study, we identified that the attraction of nematode to yeasts depended on several factors: the host nematode, yeast species, and yeast strains within each species. In addition, there were strong interaction effects for both nematode–yeast species combinations and nematode–yeast strain combinations. The modified method presented here provided an effective means to reveal the potential attractions of nematodes towards yeasts and allowed us to dissect the specific effect of individual factors and their combinations.
Strain-specific attraction
Our study found a large variation in attraction ability among strains of the same yeast species. The results suggest a strong strain-specific effect in the production and release of nematode attraction signals such as volatile organic compounds (VOCs). Previous studies have shown great variability among strains within and between fungal species in their production of VOCs (Imhof et al. Citation1995; Gao et al. Citation2002; Buzzini et al. Citation2003; Scotter et al. Citation2005). Some VOCs have long been known to attract nematodes (Bargmann et al. Citation1993). Gao, Martin, and Virginia’s work with the Stachybotrys chartarum mould indicated that VOC production was not only strain specific, but also dependent on environmental factors such as the type of medium used. Their findings were also corroborated by Buzzini et al. (Citation2003) who tested various species of Candida, among other tropical ascomycetous yeasts, and noted many differences in ester production among strains within and between species. It is likely that the strain-specific effects observed in our experiments can be partly attributed to the wide variability in the types and amounts of VOCs among strains within species. Some VOCs are known to repel nematodes at certain concentrations, while at another concentration, they may be an attractant (Rumbaugh Citation2010). Indeed, such strong strain-specific effects could have masked potential yeast-species-specific effects in nematode attractions.
In addition to VOCs, diffusible water-soluble metabolites may also play a role in nematode attraction. Depending on the chemicals, these water soluble and diffusible metabolites might take a longer time than VOCs to establish a concentration gradient to impact nematode behaviour (Bargmann et al. Citation1993).
Reduced attraction to pathogenic yeasts
The noticeably lower attraction values of Cryptococcus and Candida yeasts as compared to food E. coli suggests that nematodes are, to a certain degree, capable of differentiating between different types of microbes. Some Candida (Pukkila-Worley et al. Citation2009) and Cryptococcus (Mylonakis et al. Citation2002) species are known to be pathogenic towards C. elegans, and this lower degree of attraction might be the result of an interplay between co-evolutionary forces between the host nematode and the fungal pathogens. As described by one model for C. neoformans virulence, the pathogenicity of C. neoformans towards nematodes and humans were likely developed as a response to nematode and amoeba predation. That natural antagonism could have led the host nematodes to evolve a mechanism to recognize and avoid some of the VOCs or diffusible metabolites produced by these harmful microbes. However, the yeasts may also evolve mechanisms to counter the hosts’ avoidance by emitting VOCs or metabolites that are shared with nematode food microbes, as shown in the nematocidal bacterium Bacillus nematocida (Niu et al. Citation2010).
Strong host-specific effects
As revealed by ANOVA analysis, host nematodes had a big contribution to the overall variance in attraction ability of yeasts. This strong host-specific effect could be a result of the significant genetic divergence between the two hosts, leading to a difference in their ability of detecting and reacting to the various diffusible compounds produced by the yeasts. Indeed, these two nematode species have diverged approximately 80–110 million years from each other and less than 65% of the genes in their genomes can be assigned to orthologous pairs (Stein et al. Citation2003). Between their orthologous pairs, an average of 80% amino acid sequence identity was found. Significant variability in response to a chemosensory stimulus among species across the genus Caenorhabditis has been previously observed in a study by Jovelin et al. (Citation2003). A greater understanding of the molecular differences between the chemosensory systems of C. elegans and C. briggsae is necessary in order to elucidate the underlying mechanism that produces this strong host-specific effect observed in our study.
Both nematodes strains used in the experiment, N2 for C. elegans and AF16 for C. briggsae, are standard laboratory strains first isolated in 1966 and 1972, respectively. Strain N2 was isolated from a mushroom compost in England, whereas strain AF16 was isolated from a soil sample in India (WormBase Citation2009). Mushroom composts are known to contain a higher concentration of VOCs than most typical soil (Derikx et al. Citation1990). Their original environments may affect the sensitivities of the two strains to detect and respond to VOC stimulus. However, given the large number of generations that both of these nematodes have been propagated in the lab, and given their constant diet based on non-pathogenic E. coli food, their differential attractions to yeasts revealed here are remarkable and likely represent their innate genetic differences in response to attractants produced by these yeasts.
Mating-type effect
In C. neoformans, we observed mating-type-specific effect of attractions to C. briggsae but not to C. elegans. While the mechanism(s) for these differences are unknown, there are several possibilities. First, mating-type alpha in C. neoformans (represented by strain JEC21) is known to be more prevalent in the environment and thus the nematodes are more likely to have encountered and adapted to these yeasts more often than those of mating type a strains (represented by strain JEC20). Second, the two mating types produce different pheromones characteristic to their mating types which may also have a significant effect on their nematode-attraction abilities. Third, the differential host responses between C. briggsae and C. elegans might be related to their native ecological distributions and historical encounters with C. neoformans. Regardless of the mechanism(s) for the observed mating-type effect, this result again re-enforces the important roles of yeast strains and host nematodes in the interaction.
Conclusion
Our results indicates that nematodes are able to distinguish pathogenic yeasts from their standard food source as all yeast species tested showed significantly lower attraction than the non-pathogenic E. coli OP50. The attraction ability of yeasts from the Candida and Cryptococcus genera varied from positive attraction, to no attraction, and to slightly repulsive. ANOVA analysis revealed that the attraction was heavily influenced by the host nematode species, yeast strains, and yeast species. We postulate that the observed yeast strain-specific effect are due to strain-specific VOCs and/or diffusible metabolite profiles and the host effect may be due to inherent differences in the sensory systems between C. elegans and C. briggsae. In addition, we developed a simple and sensitive assay to measure the attractiveness of yeast species to nematodes. This type of assay will be useful in future studies to help differentiate small species- and strain-specific differences in microbial attractiveness to nematodes.
The observed host- and yeast-strain-specific effects on attraction form a strong basis for future studies to address questions that are environmentally, agriculturally, and medically relevant. For example, will environmental yeasts and nematode isolates from the same ecological niches show greater or lower attractions? What are the strain-specific compounds that mediate the interactions between nematodes and microbes? What happens to the nematodes and yeasts after their initial contact? A more thorough understanding of nematode-yeast interactions can be beneficial for the agricultural and forestry industries that suffer heavily from harmful nematode pests. A highly attractive and pathogenic yeast could be effectively used in a controlled environment to reduce the effect of plant-parasitic nematodes. In addition, our results suggest that nematodes could be powerful hosts for understanding the initial phases of yeast-host interaction during the pathogenesis of human yeast pathogens.
Acknowledgements
The authors would like to thank Dr. Bhagwati Gupta and his lab and for their assistance with providing nematode strains and advice. This research was supported by a grant from the Natural Sciences and Engineering Research Council (NSERC) of Canada and by McMaster University.
References
- Bargmann CI, Hartwieg E, Horvitz HR. 1993. Odorant-selective genes and neurons mediate olfaction in C. elegans. Cell. 74:515–527. doi:10.1016/0092–8674(93)80053-H
- Botha A. 2011. The importance and ecology of yeasts in soil. Soil Biol Biochem. 43:1–8. doi:10.1016/j.soilbio.2010.10.001
- Buzzini P, Martini A, Cappelli F, Pagnoni UM, Davoli P. 2003. A study on volatile organic compounds (VOCs) produced by tropical ascomycetous yeasts. Antonie Leeuwenhoek. 84:301–311.
- Derikx PJL, Den Camp HO, Van der Drift C, Van Griensven LJLD, Vogels GD. 1990. Odorous sulfur compounds emitted during production of compost used as a substrate in mushroom cultivation. Appl Environ Microb. 56:176–180.
- Gao P, Martin J, Virginia W. 2002. Volatile metabolites produced by three strains of Stachybotrys chartarum cultivated on rice and gypsum board. Appl Occup Environ Hyg. 17:430–436. doi:10.1080/1047322029003546
- Haridy MSA. 2002. Occurrence of yeasts in cultivated soils in El-Minia City, Egypt. Mycobiology. 30:27–30. doi:10.4489/MYCO.2002.30.1.027
- Imhof R, Glättli H, Bosset J. 1995. Volatile organic compounds produced by thermophilic and mesophilic single strain dairy starter cultures. LWT-Food Sci Technol. 28:78–86.
- Jovelin R, Ajie BC, Phillips PC. 2003. Molecular evolution and quantitative variation for chemosensory behaviour in the nematode genus Caenorhabditis. Mol Ecol. 12:1325–1337.
- Kidd SE, Guo H, Bartlett KH, Xu J, Kronstad JW. 2005. Comparative gene genealogies indicate that two clonal lineages of Cryptococcus gattii in British Columbia resemble strains from other geographical areas. Eukaryot Cell. 4:1629–1638. doi:10.1128/EC.4.10.1629
- Maganti H, Bartfai D, Xu J. 2012. Ecological structuring of yeasts associated with trees around Hamilton, Ontario, Canada. FEMS Yeast Res. 12:9–19. doi:10.1111/j.1567-1364.2011.00756.x
- Maganti H, Yamamura D, Xu J. 2011. Prevalent nosocomial clusters among causative agents for candidemia in Hamilton, Canada. Medical Mycol. 49:530–538. doi:10.3109/13693786.2010.547880
- Mylonakis E, Ausubel FM, Perfect JR, Heitman J, Calderwood SB. 2002. Killing of Caenorhabditis elegans by Cryptococcus neoformans as a model of yeast pathogenesis. Proc Natl Acad Sci USA. 99:15675–15680. doi:10.1073/pnas.232568599
- Neher DA. 2010. Ecology of plant and free-living nematodes in natural and agricultural soil. Ann Rev Phytopathol. 48:371–394. doi:10.1146/annurev-phyto-073009-114439
- Niu Q, Huang X, Zhang L, Xu J, Yang D, Wei K, Niu X, An Z, Bennet JW, Zou C, et al. 2010. A Trojan horse mechanism of bacterial pathogenesis against nematodes. Proc Natl Acad Sci USA. 107:16631–16636. doi:10.1073/pnas.1007276107
- Pukkila-Worley R, Peleg AY, Tampakakis E, Mylonakis E. 2009. Candida albicans hyphal formation and virulence assessed using a Caenorhabditis elegans infection model. Eukaryot Cell. 8:1750–1758. doi:10.1128/EC.00163-09
- Rumbaugh KP. 2010. Wnt signaling. Proc Natl Acad Sci USA. 107:1–17. doi:10.1895/wormbook.1.7.1
- Scotter JM, Langford VS, Wilson PF, McEwan MJ, Chambers ST. 2005. Real-time detection of common microbial volatile organic compounds from medically important fungi by selected ion flow tube-mass spectrometry (SIFT-MS). J Microbiol Meth. 63:127–134. doi:10.1016/j.mimet.2005.02.022
- Srinivasan J, von Reuss SH, Bose N, Zaslaver A, Mahanti P, Ho MC, O’Doherty OG, Edison AS, Sternberg PW, Schroeder FC. 2012. A modular library of small molecule signals regulates social behaviors in Caenorhabditis elegans. PLoS Biol. 10:e1001237. doi:10.1371/journal.pbio.1001237
- Stein LD, Bao Z, Blasiar D, Blumenthal T, Brent MR, Chen N, Chinwalla A, Clarke L, Clee C, Coghlan A, et al. 2003. The genome sequence of Caenorhabditis briggsae: a platform for comparative genomics. PLoS Biol. 1:E45. doi:10.1371/journal.pbio.0000045
- Sun S, Xu J. 2007. Genetic analyses of a hybrid cross between serotypes A and D strains of the human pathogenic fungus Cryptococcus neoformans. Genetics. 177:1475–1486. doi:10.1534/genetics.107.078923
- Troemel ER. 1999. Chemosensory signaling in C. elegans. Bioessays. 21:1011–1020. doi:10.1002/(SICI)1521-1878(199912)22:13.0.CO;2-V
- Vazquez FJ, Acea MJ, Carballas T. 1993. Soil microbial populations after wildfire. FEMS Microbiol Ecol. 13:93–104. doi:10.1016/0168-6496(93)90027-5
- WormBase. 2009. Release 204 [Internet] [cited 2009 Jul 29]. Available from: http://www.wormbase.org.