Abstract
This study investigated several collections of the genus Cantharellus (Cantharellaceae) from the northwestern Himalayas, India, on the basis of morphology and molecular data. Phylogenetic relationships and species limits were investigated by using nuclear ribosomal large subunit sequences (LSU). We recognized 13 species: Cantharellus appalachiensis Petersen, C. cibarius Fries, C. lateritius (Berk) Singer, C. miniatescens Heinem, C. minor Peck, C. pseudoformosus and seven species, C. applanatus sp. nov., C. elongatipes sp. nov., C. fibrillosus sp. nov., C. himalayensis sp. nov., C. indicus sp. nov., C. natarajanii sp. nov., and C. umbonatus sp. nov., as new to science. All these species are described and their taxonomy and ecology are discussed. In addition, a key is provided to all the recognized species. The phylogenetic analysis recovered 10 major supported clades of Cantharellus species.
Introduction
Cantharellus is one of the important genera of wild edible mushrooms, collected from Europe, Africa, Asia and North America. The economic importance of the Cantharellaceae has resulted in considerable research on their ecology, physiology and phylogenetics (Danell Citation1994; Dunham et al. Citation2003; Eyssartier et al. Citation2009; Buyck and Hofstetter Citation2011; Tian et al. Citation2012; Buyck et al., Citation2013). The significant levels of proteins, lipids, minerals, vitamins and some neutraceuticals in their basidiocarps are of considerable value (Pilz et al. Citation2003; Kumari et al. Citation2011a). Noteworthy taxonomic works on Cantharellaceae include those of Fries (Citation1821–1832), Persoon (Citation1825), Smith and Morse (Citation1947), Corner (Citation1966, 1969), Bigelow (Citation1978), Petersen (Citation1979), Feibelman et al. (Citation1994, Citation1996), Dahlman et al. (Citation2000), Moncalvo et al. (Citation2006), Eyssartier et al. (Citation2009), Buyck and Hofstetter (Citation2011) and Buyck et al. (Citation2013) who have made it possible to deal quite effectively with cantharelloid mycoflora.
Although the taxonomy of Cantharellaceae has been well studied in other parts of the world, only preliminary work has been done in India. The species reported from India to date include: C. appalachiensis Peterson, Cantharellus cibarius Fries, C. cinnabarinus Schw., C. friesii Quel., C. lateritius (Berk) Singer, Lilloa, C. luteocomus Bigelow and C. minor Peck (Bhatt and Lakhanpal Citation1988; Dhancholia et al. Citation1991; Abraham et al. Citation1995). The diversity of Cantharellus in particular and the fungal diversity in general are understudied in the northwestern Himalayan region. Hence, repeated collection efforts have been made to investigate the diversity of Cantharellales occurring in this region. Earlier, we reported C. miniatescens and C. pseudoformosus from the Himalayan region (Kumari et al. Citation2009, Citation2011b). In the present study, we describe seven species of Cantharellus new to science.
Materials and methods
Fungal collections
Basidiomes were collected from the forests of northwestern Himalayas, India, which includes Himachal Pradesh (30° 22′–33° 12′ N and 70° 47′–74° 04′ E) and Uttarakhand (29° 37′–30° 15′ N and 77° 53′–79° 15′ E) (, ). Both these states have arctic, alpine, sub-alpine and temperate biomes. These regions have diverse floras because of their varied climate zones and wide range of altitudes (Kumar et al. Citation1990). The distribution of rainfall is uneven and varies from 600 to 3200 mm annually. Of the total annual rainfall, 75% of the rainfall is during the rainy season (June to September) and the rest in the winter season (December to March). The majority of our collections were located in temperate forests, at elevations between 1600 and 2400 m amsl, associated with Quercus and Rhododendron in hardwood and Pinus, Cedrus, Picea and Abies in coniferous forests.
Figure 1. Collection sites for the Cantharellaceae from the forest of the northwestern Himalayas, India.
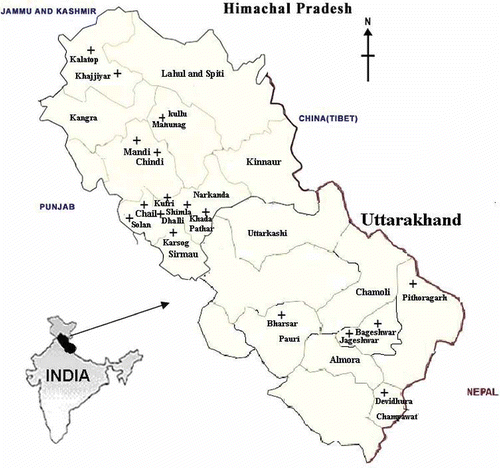
Table 1. Data of Cantharellus species examined from the northwestern Himalayas of India.
Morphological analysis
In the forest forays of Himachal Pradesh, during the rainy seasons of 2005–2009, several taxa of Cantharellus were collected. The macroscopic and microscopic characteristics of all the collections were studied and documented by using standard techniques (Singer Citation1986). Colour terminology was noted from Mearz and Paul (Citation1930). Micromorphological features of dried specimens were examined with a Leica DM LS2 microscope with light and phase contrast optics. For basidiospores, basidia and other anatomical structures (at least 45 individuals per collection) were measured. Rehydrated fungal tissue was mounted in water and 3% KOH, 2% Congo red, 2% phloxine and Melzer’s reagent. The basidiospores were studied from the spore deposits and from fresh material. Voucher specimens have been deposited in the Punjabi University Herbarium (PUN), India ().
DNA extraction, PCR and DNA sequencing
For molecular characterization, genomic DNA was extracted from basidiomes by the method of Van Kan et al. (Citation1991). Primer pairs ITS4R (5′-GCATATCAATAAGCGGAG GA-3′) (White et al. Citation1990) and LR5 (5′-ATCCTGAGGGAAACTTC-3′) (Vilgalys and Hester Citation1990) were used to amplify a portion of the LSU gene. The ITS region of nrDNA was amplified by PCR with ITS1 (5′-TCCGTAGGTGAACCTGCGG-3′) and ITS4 (5′-TCCTCCGCTTATTGATATGC-3′) primers (White et al. Citation1990). The 50 µL reaction mixture for PCR amplification contained: 10 ng DNA, 1× PCR buffer, 1.5 mM MgCl2, 0.2 mM of each dNTPs, 0.5 µM of each primer and 2.5 units of Taq polymerase (Fermentas, USA). Amplifications were performed in a thermal cycler (Perkin Elmer, USA) with an initial denaturation step of 94°C for 3 min followed by 35 cycles of 94°C for 1 min, 50°C for 1 min and 72°C for 1.5 min and a final extension of 72°C for 8 min. PCR products were purified and cloned in Ins TA clone PCR cloning kit (Fermentas, USA) as per the manufacturer’s instructions and transformed into E. coli DH5α cells. Screening of clones was done using promoter specific (M13F and M13R) primers. Primers used for sequencing were ITS4R and LR5 for the LSU region and ITS1 and ITS4 for the ITS region.
Phylogenetic analysis
Sequences were aligned by using the MAFFT software (Katoh et al. Citation2002) and adjusted manually using the data set editor in PAUP (Swofford Citation2002). The sequences have been deposited in GenBank and accession numbers are listed in . The alignments have been submitted to TreeBase (http://www.treebase.org) under the accession number S11503. The LSU data set assembled for the phylogenetic analysis consisted of 40 sequences. Additionally, the phylogenetic tree was reconstructed using 26 ITS sequences of Cantharellus spp., Craterellus species were used as an outgroup taxon for rooting purposes.
Maximum parsimony (MP), maximum likelihood (ML) and Bayesian analysis (BA) were performed with the following parameters. (i) MP: equally weighed parsimony analysis was performed using PAUP (Swofford Citation2002) with 1000 heuristic search replicates performed with starting trees generated by stepwise addition with random addition sequences followed by Tree Bisection Reconnection (TBR) branch swapping. Gaps were treated as missing data. To assess the relative support for each clade, bootstrap (BS) values were calculated from 1000 replicates. Optimal models of DNA substitution was inferred using the Akaike information criterion (AIC) (Akaike Citation1981) as implemented in the MrModelTest ver. 2.3 (Nylander Citation2008). (ii) ML: The analysis was performed in PAUP* with a GTR+I+G model of nucleotide substitution, starting trees obtained via stepwise addition, random sequence addition, TBR branch swapping and MaxTrees set to one million generations. (iii) BA: Bayesian analysis utilized the Metropolis-coupled Markov Chains Monte Carlo search algorithm as implemented in the program MrBayes v 3.1.2 (Ronquist and Huelsenbeck Citation2003). Two simultaneous independent replicates of six were run for 5 million generations with sampling at every 100th generation, and the convergence of the runs visualized using Tracer ver. 1.4 (Rambaut et al. Citation2013). Those tree sampled prior to searches reaching a split deviation frequency value of 0.01 were discarded as the burn-in, and the remaining trees were used to calculate Bayesian posterior probabilities (PP) of the individual clades. Only Bayesian posterior probabilities (PP) greater than or equal to 95% are considered significant. To compare topologies resulting from the different search criteria, unconstrained trees (MP, ML) were compared in PAUP* using the Kishino–Hasegawa test (Kishino and Hasegawa Citation1989) in order to determine whether trees were significantly different. Trees were figured in Treeview (Page Citation1996).
Results
Analysis of the LSU data set
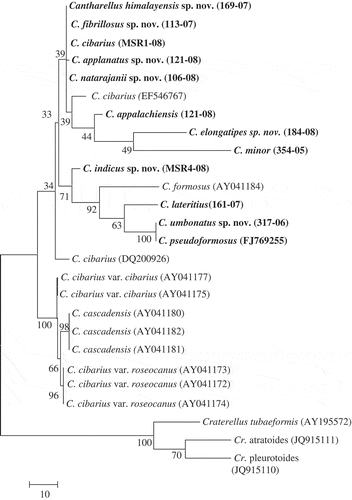
The final data set consisted of 40 sequences of 792 characters, of which 224 were parsimony informative, 487 and 81 were constant and parsimony-uninformative, respectively. Maximum parsimony analysis resulted in nine equally parsimonious trees with the branch-and-bound search (TL = 229, CI = 0.84, RI = 0.94). Maximum likelihood analysis recovered a single topology (−ln L = 3564.5978). The resulting MP and ML topologies did not differ significantly. The Kishino–Hasegawa tests among the topologies obtained from ML and MP indicated that the ML tree was significantly better and one of the maximum likelihood trees is shown in . Most single species clades received moderate to strong support (65–100% BS, 56–100% PP).
Figure 2. Phylogeny of Cantharellus generated from maximum likelihood of LSU sequences, rooted with Craterellus species. Parsimony bootstrap support (BS) and Bayesian posterior probability (PP) values >50% are given at the internodes (BS/PP). The bold species represent the Indian collections.
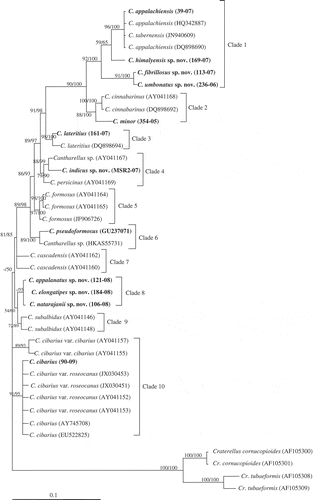
The phylogenetic tree, based on LSU sequences was clustered into 10 clades. Cantharellus appalachiensis, C. fibrillosus, C. himalayensis and C. umbonatus are clustered together as clade 1. Cantharellus cinnabarinus and C. minor are grouped in clade 2, while Cantharellus lateritius formed as a distinct monophyletic clade (clade 3). Clade 4 consisted of Cantharellus indicus, C. persicinus and C. formosus, formed as a separate clade (clade 5). C. pseudoformosus and C. cascadensis are clustered as clade 6 and clade 7, respectively. Sequences of C. appalanatus, C. natarajanii and C. elongatipes, which are new to science are grouped as clade 8 and C. subalbidus sequences formed into clade 9. Canthareullus cibarius sequences are clustered into clade 10.
Analysis of the ITS data set
Sequences generated from ITS contained 406 bp from the 5′ end of the ITS 1 and 560 from the 3′ end of the ITS 2 including the 5.8S nrDNA gene. The aligned data set contained 1105 characters, of which 521, 226 and 358 were constant, parsimony-uninformative and parsimony-informative, respectively. In MP analysis, five equally parsimonious trees resulted with the branch-and-bound search (TL = 314, CI = 0.70, RI = 0.80). Maximum likelihood analysis recovered a single topology (−ln L = 8812.2). The resulting MP and ML topologies did not differ significantly ().
Taxonomy
Cantharellus appalachiensis Petersen, Svensk Bot, Tidsker. 65:402. 1971. , –B
Figure 4. Basidiocarps of A. C. appalachiensis; B. C. applanatus; C. C. cibarius; D. C. elongatipes; E. C. fibrillosus; F. C. himalayensis; G. C. indicus; H. C lateritius; I. C. minor; J. C. natarajanii and K. C. umbonatus. (All the photographs are the holotype and the bar represents 2 cm.)

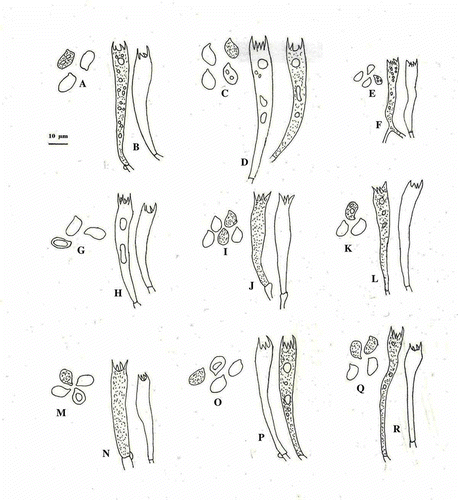
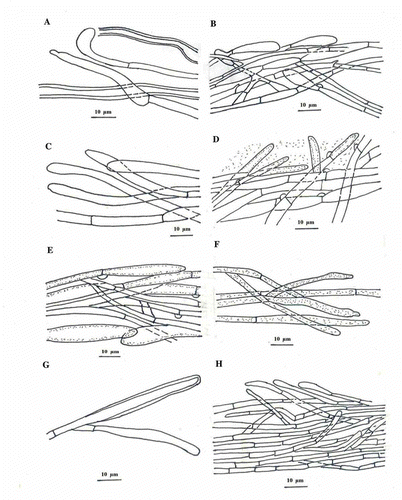
Figure 5. Basidiospores and basidia of A & B, C. appalachiensis; C & D, C. applanatus; E & F, C. elongatipes; G & H, C. fibrillosus; I & J, C. himalayensis; K & L, C. indicus; M & N, C. lateritius; O & P, C. natarajanii and Q & R, C. umbonatus. (All the elements are drawn from the holotype and the bar represents 10 µm.)
Pileus 3–7 cm wide, plano convex to shallow depressed, pinkish yellow (10I-4) to yellowish (10G-2), appressed to faintly squarose, surface glutinous, non-hygrophanous, covered with indistinct scales; margin irregular, non-striate, lobed, split, uplifted with age. Context up to 1-cm thick, yellowish. Lamellae decurrent, folded, interveined, hymeniform folded up to 2-mm broad, Apricot yellow (9K-5). Stipe 3.5–7 × 0.5–1.1 cm, terete with slightly expanded apex, lacunose, stipe surface fibrous pinkish yellow to citron yellow (10J-2); taste very pleasant (like other chanterelles). Spore deposit creamish white Basidiospores [45/2/2], 7.5–9.5 × 4.5–6 µm, L = 8.4 µm, W = 5.2, Q = 1.62, ellipsoid to elongate, inamyloid, smooth, faintly yellowish in 3% KOH, contents monoguttulate to granulated. Basidia ±52–77 × 7.5–9 µm, cylindrical to narrowly clavate, strerigmata 4.5–7 µm long, cornuted 4–6 per basidium, developing basidia with evenly granulose, light yellow contents, basal septa with clamps. Basidioles numerous with opaque light yellow contents in 3% KOH. Pileipellis epicutis made up of subclavate to clavte cells with projecting cystidiod end, ±70–100 µm long, made up of radially to sub-radially arranged hyphae, 4–12 µm diam, granulated wall thin to slightly thick and pale yellowish in 3% KOH, context hyphae cylindric ±3–17 µm diam, slightly thick walled. Hymenophoral trama interwoven with cylindric hyphae ±2.5–7 µm wide. Pleurocystidia and Cheilocystidia absent. Stipe cuticle made up of subclavate to clavate hyphae, thin to slightly thick walled, ±3–11-µm wide, contents similar to suprapellis, septa frequently clamped.
Habitat. gregarious to caespitose; on soil under Cedrus deodara.
Specimens examined. India, Himachal Pradesh, Shimla, Chail forest, N31°06′ E77°.10′ 1800 m, 30 July 2007, 39-07; same location, 12 August 2008, 84-08; Uttarakhand, Jageshwar forest N29°00′ E79°.17′ 1646 m, 24 August 2008, 95-08. Accession No.: PUN 3959 (95-08).
Commentary. Based on the taxonomic characters of the present specimen with a previously described type specimen (Bigelow Citation1978), it was identified as Cantharellus appalachiensis. The morphological features such as matted cap with appressed fibrils, non-hygrophanous, similar colour tones, folded lamellae, intervenose, subacute edges, similar spore size, and pileipellis hyphae non-encrusted with cystidiod end cells indicated similarity with C. appalachiensis. Further, phylogenetic analysis of LSU sequences supported its identity with C. appalachiensis.
Cantharellus applanatus Deepika, Upadhyay & Reddy, sp. nov. , –D,
MycoBank MB519516
Figure 6. Pileipellis of, A. C. applanatus; B. C. elongatipes; C. C. fibrillosus; D. C. himalayensis; E. C. indicus; F. C. lateritius; G. C. natarajanii and H. C. umbonatus.
Pileus upto 3–6.5 cm, applanate to shallow depressed; golden yellow (9L-6), smooth; margin regular, split. Context 3–7-mm thick, yellowish, confluent, unchanging on exposure to air, surface smooth, glabrous. Lamellae decurrent, folded, anastomosing, forked up to 2-mm broad, golden yellow (9L-6). Stipe central, 2.5–5 × 0.4–0.7 cm, cream (9D-2), equal in the diameter throughout, surface glabrous to thin hairy. Spore deposit white. Basidiospores [55/2/2], 7–8.5 (–9) × 4.5–5.5 µm, L = 7.6 µm, W = 4.5, Q = 1.54, ellipsoid, inamyloid, smooth, contents monoguttulate to granulated. Basidia ±55–78 × 6.0–7.5 µm, clavate, sterigmata 2.5–4 µm diam, cornuted 4–5 per basidium, developing basidia with evenly granulose, light yellow contents, basal septa with clamps. Basidioles numerous with opaque light yellow contents in 3% KOH. Hymenophoral trama irregular to interwoven, branched, hyaline to faint yellowish, slightly constricted at septa which are frequently clamped, 3–7.5 µm wide. Pileipellis consisting of compactly arranged projecting end hyphae, cylindric to filamentous, yellowish to yellowish brown, branched, thin to thick walled, frequently clamped, non-amyloid hyphae, 3–8 µm-wide, contents granulose. Pleurocystidia and cheilocystidia absent. Stipe cuticle made up of yellowish brown, cylindric to filamentous, 2.5–7-µm wide, branched, hyphae frequently clamped.
Habitat. caespitose to gregarious, on soil among green grass with P. roxburghii.
Specimens examined. India, Himachal Pradesh, Shimla, Karsog forest, N31°24′ E77°.12′ 1900 m, 5 August 2007, 43-07; same location, 21 August 2008, 121-08. Holotype: PUN 3964 (121-08).
Commentary. C. applanatus formed same clade as C. elongatipes and C. natarajanii, however was differentiated by its pileus and stipe features. Some of the morphological characters, such as colour, spore range and Q values of C. applanatus match well with the characters described for C. viscosus Berk (Corner Citation1966). However, the present specimen can be distinguished from C. viscosus by its applanate pileus, compared to the prominent infundibuliform in C. viscosus. It can be further distinguished from C. cascadensis and C. subalbidus (white Cantharellus) by pileus and stipe colour, hymenium surface and basidiospores, basidia and the width of hymenium.
Etymology: from the Latin word applanatus–applanate, referring to the shape of the cap.
Cantharellus cibarius Fries, Syst. Mycol. I: 318. Sohi et al. (Citation1964).
Commentary. This is a common species of Cantharellus, frequently found in the Indian Himalayas and easily recognized by field characters, especially the fruit-like smell. Fries described the type specimen in detail from the Kashmir region, India. Colour of pilei and stipe is ochraceous brown to yellowish brown; the decurrent hymenium forms blunt ridges or veins; basidia is frequently six spored, slender clavate with basal clamp-connections. The basidiospores are 8.5–10.5 (–11) × 4.5–6 µm, L = 8.5 µm, W = 5.5 µm, Q = 1.54, ellipsoid, smooth, non-amyloid, wall hyaline, contents monoguttulate to multiguttulate, that coincide with previously described C. cibarius. The distinctness of C. cibarius was also supported by phylogenetic analysis of LSU sequences, which formed same clade with other similar species.
Habitat. On soil, gregarious to caespitose; under Quercus incana.
Specimens examined. India, Uttarakhand – Bageshwar forest, N29°45′ E79°.04′ 1696 m, 15 July 2008, MSR1-08; Devidhura forest N29°05′ E78°.00′ 1615 m, 12 August 2009, MSR3-09; Himachal Pradesh – Karol forest N30°92′ E77°.15′ 2100 m, 22 September 2009, 90-09. Accession No. PUN 3973 (90-09).
Cantharellus elongatipes Deepika, Upadhyay & Reddy, sp. nov. , –F,
MycoBank MB519521
Pileus upto 1.5 cm, convex to plano convex with slightly depressed in the centre; Mirabelle (10J-7), orangish yellow, smooth; margin regular to irregular, split. Context thin. Lamellae strongly decurrent, anastomosing to subdistant, hymeniform folded up to 1-mm high, orange. Stipe 3–3.5 × 0.4–0.7 cm, equal in diameter, lacunose, stipe surface glabrous, Mirabella (10J-7) to dirty orange. Spores deposit white. Basidiospores [55/2/2] 6–7.5 × 4.5–5.5 µm, L = 6.6 µm, W = 4.9 µm, Q = 1.35, broadly ellipsoid to ellipsoid, non-amyloid, smooth, wall hyline, contents monoguttulate. Basidia ±52–70 × 7–10 µm, narrowly clavate to clavate, strerigmata 2.5–4.5 µm long, cornuted 4–5 per basidium, developing basidia with evenly granulose, light yellow contents, basal septa with clamps. Basidioles numerous with opaque light yellow contents in 3% KOH. Subhymenium made up of non-inflated hyphal segments, branched, septate with frequently clamped. Hymenophoral trama interwoven with cylindric hyphae ±2.5–6-µm wide. Pileipellis made up of sub parallel arranged filamentous hyphae, septate, clamped, hyaline to pale yellowish, 2–3.5-µm wide, followed by clavate to subclavate or irregularly arranged cylindrical elements, 9–18-µm wide, granulated. Pleurocystidia and Cheilocystidia absent. Stipe cuticle made up of hyaline to pale yellow, filamentous to cylindrical, followed by cystoid end cells, branched, frequently clamped hyphae, 2.5–8-µm wide.
Habitat. gregarious to caespitose; on soil under Cedrus deodara.
Specimens examined. India, Uttarakhand, Pauri Garhwal, Bharsar forest, N29°45′ E78°.55′ 2350 m, 29 September 2008, 184-08; same location, 8 September 2009, 295-09. Holotype: PUN 3966 (184-08).
Commentary. Presence of clamp connections throughout the basidiomes, cantharelloid sterigmata and the almost cylindrical basidia placed the present specimen in the genus Cantharellus. When young, C. elongatipes can be mistaken with basidiomes of C. minor but can be differentiated by its less fleshy stipe and decurrent hymenium. The name C. elongatipes has been assigned due to the presence of small pileus as compared to stipe length (pileus diam 1–1.5 cm, stipe length 2–4.5 cm). This new species forms a distinct lineage in phylogenetic tree generated from LSU sequences ().
Etymology: elongatipes – refers to the long stipe.
Cantharellus fibrillosus Deepika, Upadhyay and Reddy, sp. nov. , –H,
MycoBank MB519517
Pileus up to 5–8.20-cm wide, infundibuliform, Mustard brown (14D-10) in the centre, golden wheat (11D-7) outwards, fibrillose, dense in the centre, surface dry, hygrophanous; margin irregular, non-striate, decurved. Context 2–3-mm thick, creamish, confluent, unchanging on exposure to air, surface smooth but not glabrous. Lamellae abundantly decurrent to sub-decurrent, anastomosing, separable easily from the flesh. Stipe 6–8 × 0.7–1.0-cm thick, yellowish orange (10C-4), terete with slightly swollen at base, surface smooth to hairy. Spore deposit white. Basidiospores [45/2/2] (8–) 8.5–12 (–12.5) × 5–6 (–6.5) µm, L = 9.7 µm, W = 5.9 µm, Q = 1.64, ellipsoid to cylindrical, non-amyloid, non-cyanophilic, wall hyaline, contents monoguttulate to multiguttulate with greenish referective oil droplets; spores deposit white. Basidia ±60–90 × 9–11.5 µm, clavate, sterigmata 3.5–6.5 × 1–2-µm diam., cornuted 2–6 per basidium, developing basidia with evenly granulose, light yellow contents, basal septa with clamps. Basidioles numerous with opaque light yellow contents in 3% KOH. Hymenophoral trama irregular to interwoven, branched, hyaline to faint yellowish, constricted slightly at septa, septa frequently clamped. Pileipellis consisting of filamentous and interwovenly arranged hyphae, hyaline, branched, thin- to thick-walled, frequently clamped, non-amyloid hyphae, 3–12-µm wide, contents granulose. Pleurocystidia and Cheilocystidia absent. Stipe cuticle made up of hyaline to yellowish, branched, frequently clamped hyphae, 1.5–5-µm wide.
Habitat. caespitose to gregarious, on soil under Cedrus deodara and P. wallichiana.
Specimens examined. India, Himachal Pradesh, Shimla, Khada Pathar forest, N30°72′ E78°.63.2′ 1950 m, 30 September 2006, 236-06; Dhalli forest N 30°17′ E78°.45′ 1700 m, 18 September 2007, 113-07; same location, 11 August 2008, 17-08, Holotype: PUN 3957 (113-07).
Commentary. C. fibrillosus clustered with C. umbonatus in LSU, however their morphology was distinct. Also, this species can be differentiated from C. ianthinoxanthus Kuhner (Corner Citation1966) by its stipe colour and smaller basidia. The stipe colour of C. ianthinoxanthus is clear yellow, while that of C. fibrillosus is light orange, the stipe becoming brown towards the apex and lighter near the base; C. fibrillosus also has smaller basidia with 4–6 sterigmata.
Etymology: from the Latin word fibrillosus – fibrillose, referring to the pileus surface.
Cantharellus himalayensis Deepika, Upadhyay & Reddy, sp. nov. , –J,
MycoBank MB519518
Pileus up to 3–7-cm wide, infundibuliform, margin wavy, irregular, faintly striate to non-striate, concolour yellowish, Pecan brown (14A-9) in the centre due to scales, Champagne (11B-3) to Beige (11B-4) outwards, surface dry, non-hygrophanous. Context fleshy upto 1.3-cm wide, yellowish. Lamellae folded, hymeniform, interveined to bifurcate. Sugarcane (10B-6) to Cornhusk (10E-6) or Capuccine buff (9E-5), edges subacute. Stipe up to 4–7.5 × 0.8–1.1 cm, tapering downwards, stipe surface glabrous to thin hairy at below. Beige (11B-4) to Pecan brown (14A-9), darker at the base as leather brown. Smell of apricot; taste indistinct. Spore deposit creamish white. Basidiospores [45/1/1] 6–8 × 4.5–6 µm; L = 5.91 µm, W = 5.16 µm; Q = 1.34, ellipsoid to broadly ellipsoid, inamyloid, smooth, hyaline, apiculus upto 0.5-µm long, contents monogutullate to multigutullate and frequently granulated. Basidia 60–85 × 8–11-µm long, narrow clavate to subcylindric, sterigmata 4 per basidium, 6–9.5-µm long, contents granulated with multi oil droplets, basal septa with clamps. Basidioles numerous with opaque light yellow contents in 3% KOH. Pileipellis made up of parallel to repent filamentous hyphae, 3–9-µm wide, followed by cylindric hyphae, thin to thick walled yellowish, septate, branched, context hyphae compactly arranged. Hymenophoral trama irregular to interwoven, made up of thin walled, clamped, branched hyphae, 3–6-µm wide. Subhymenium made up of non-inflated hyphal segments, septate, branched, clamped. Pleurocystidia and Cheilocystidia absent. Stipe cuticle made up of longitudinally arranged thin walled septate, branched, clamped hyphae, 3–12-µm wide, contents similar to pileipellis hyphae.
Habitat. Caespitose to gregarious; among mosses on soil in mixed forest dominated by Cedrus deodara.
Specimens examined. India, Himachal Pradesh, Shimla, Kadha Pathar forest, N30°72′ E78°.63.2′ 1950 m, 30 September 2006, 43-06; Kufri forest, N30°00′ E77°.15′ 1700 m, 6 September 2007, 169-07; same location, 23 August 2009, 32-09. Holotype: PUN 3972 (169-07).
Commentary. This species can be distinguished from C. cibarius by the presence of basidia with four spores, long sterigmata (up to 9.5 µm), smaller spores (6–8 × 4.5–6 µm) and partially gelatinized pileipellis. C. himalayensis was grouped with C. appalachiensis in LSU, however greatly distinguished from C. appalachiensis, which has larger ellipsoid to elongate spores (7.5–9 × 4.5–6 µm), and basidia bearing 4–6 curved sterigmata (4.5–7 µm long). The larger basidiomes of this species differentiate it from C. minor.
Etymology: from the Latin word Himalayensis – Himalaya, referring to locality.
Cantharellus indicus Deepika, Upadhyay & Reddy, sp. nov. , –L,
MycoBank MB519519
Pileus 3–7 cm broad, shallow depressed, infundibuliform to applanate; egg yellow to pale ochraceous yellow; margin invoute, split, irregular, lobed. Context 3–7-mm thick, pale yellow to creamish, confluent, unchanging on exposure to air, surface smooth but not glabrous. Lamellae decurrent, folded to anastomosing, crowded, separable easily from the flesh. Stipe 4–9 × 0.5–1 cm, Sunset (10C-4), slightly swollen at the base, surface smooth to hairy. Spores deposit white. Basidiospores [35/2/2] 7–10 × 4.5–5.5 µm, L = 7.9 µm, W = 5.2 µm, Q = 1.5, ellipsoid, smooth, non-amyloid, non-cyanophilic, wall hyaline, contents monoguttulate to multiguttulate with greenish referective oil droplets; spore deposit white. Basidia ±65–90 × 9.5–11 µm, clavate, sterigmata 3.5–4.5 × 1.2–2-µm diam., cornuted 4–6 per basidium, developing basidia with evenly granulose, light yellow contents, basal septa with clamps. Hymenophoral trama irregular to interwoven, branched, hyaline to faint yellowish, constricted slightly at septa, septa frequently clamped. Pileipellis consisting of filamentous and interwovenly arranged hyphae, hyaline, branched, thin to thick walled, frequently clamped, non-amyloid hyphae, 3–8-µm wide, contents granulose. Pleurocystidia and Cheilocystidia absent. Stipe cuticle made up of hyaline to yellowish, cylindric, branched, frequently clamped hyphae, 1.8–3.5-µm wide.
Habitat. caespitose to gregarious, on soil under Quercus leucotrichophora.
Specimens examined. India, Himachal Pradesh, Shimla, Kufri forest, N30°00′ E77°.15′ 1700 m, 3 July 2007, MSR2-07; same location, 25 July 2008, MSR4-08; same location, 14 August 2009, 45-09. Holotype: PUN 3962 (MSR2-07).
Commentary. This species shares some common morphological features (long stem compared to the width of the pileus) with C. cibarius var. longipes Peck as described by Corner (Citation1966). It can be distinguished from C. cibarius var. longipes which has a smooth pileus, more or less yellow hymenium, and yellowish-white branched stem and from C. persicinus which has a peach or pink colour pileus 2–4.5-cm wide; convex, becoming broadly convex or nearly flat, and spores 10.5–11.5 × 6–7 µm and basidia with four sterigmata. The distinctness of C. indicus from other species was supported by phylogenetic analysis of LSU sequences.
Etymology: from the latin word Indicus – India, referring to the region.
Cantharellus lateritius (Berk) Singer, Lilloa 22: 729. 1951. , –N,
Pileus upto 3–8-cm broad depressed in the middle to sub-infundibuliform, surface hygrophanous, moist, light yellow (10G-5) to golden yellow (9I-6), sometime appressed to fibrillose; margin inrolled, irregular, split, sometime lobed. Hymenium smooth, without ridges or folds, Golden corn (9I-5). Stipe 3–7 × 0.4–1 cm, central to eccentric, tapering downwards, Raffia (11E-5), lacunose, glabrous, consistency cartilaginous, stuffed then hollow, context yellowish; taste mild pleasant when fresh. Spore deposits white. Basidiospores (45/2/2) 7–9 × 4.5–5.5 (–6) µm, L = 7.9 µm, W = 5.02 µm, Q′ = 1.57, broadly ellipsoid to ellipsoid, smooth, non-amyloid, non-cyanophillic, wall hyline, contents monogutullate to multigutullate. Basidia ±29–60 × 4.5–6 µm, cylindro-clavate to clavate, sterigmata 2–4 per basidium, developing basidia with evenly granulose, basal septa with clamps. Basidioles numerous with opaque light yellow contents in 3% KOH. Pileipellis made up of repent to interwovenly arranged, 2–6 µm wide, embedded in partially gelatinized matrix, branched, septa, clamped, contents granulated, context hyphae comparatively broader. Hymenophoral trama subparallel to interwovenly arranged, partially gelatinized at top, pale yellowish in 3% KOH, branched, clamped, 2–6-μm wide. Pleurocystidia and Cheilocystidia absent. Stipe cuticle made up of hyaline, branched, thin to thick walled, granulated contents, 1.5–3.5-μm wide, clamp connections present.
Habitat. caespitose to gregarious, on soil under Cedrus deodara.
Specimens examined. India, Himachal Pradesh, Shimla, Kadha Pathar forest, N30°72′ E78°.632′ 1950 m, 13 September 2005, 119-05; same location, 28 September 2006, 333-06; same location, 11 August 2007, 161-07. Accession No.: PUN 3958 (161-07).
Commentary. The specimen showed similarities with C. lateritius as described by Bigelow (Citation1978), with completely smooth hymenophore, sweet smell and clamped hyphae, except that the Q values are slightly smaller. This species was reported from Jageshwar forest, Uttarakhand by Dhancholia et al. (Citation1991), but they did not mention any taxonomic details to support their identification. This species can be distinguished from other reported Cantharellus by its smooth hymenium, lacking ridges or folds. Phylogenetic analysis of LSU sequences also supported identity of the specimen as C. lateritius, which formed a separate clade ().
Cantharellus minor Peck, Annual Rep. New York State Cab 23: 122. 1872.
Commentary. Cantharellus minor is easily recognized by its field characters: small basidiomata, yellow to orange-yellow pileus with decurrent hymenophore. Sohi et al. (Citation1964) described the type specimen in detail from Kashmir region, India. In the present study, this specimen was also collected from forests of Chail and Khada Pathar. After documentation and microscopic examination this specimen easily agrees with the description of C. minor described by Sohi et al. (Citation1964). C. minor clustered with C. cinnabarinus in LSU, however differed mainly based on pileus size and colour. Colour of pilei and stipe is yellow to yellow-orange, gills are crowed, not distant, basidiospores are 6.5–9.5 × 4.5–5.5 µm, L = 8.2 µm, W = 5.5 µm, Q = 1.49, ellipsoid, smooth, non-amyloid, wall hyaline, contents monoguttulate to multiguttulate.
Habitat. On soil, gregarious to caespitose; under Cedrus deodara and Quercus dilatata.
Specimens examined. India, Himachal Pradesh, Shimla, Kadha Pathar forest, N30°72′ E78°.63.2′ 1950 m, 15 July 2005, 354-05; same location, 22 July 2008, 251-09. Accession No.: PUN 3971 (354-05).
Cantharellus miniatescens Heinem., BULL. Jard. Bot. Etat Brux. 28, 393, Heinemann Citation1958, f.36; Fl. Ic. Champ.
Commentary. Cantharellus miniatescens is recognized in the field by its campanulate pileus, folded decurrent lamellae, stipe surface smooth but not glabrous, slightly swollen at base, colour dull ochre to dull ochraceous orange. Distinctive microscopic characters are ellipsoid spores, frequently clamped pileipellis hyphae and subclavate to lanceolate terminal cells. However, the spore Q value is comparatively smaller in the present described specimen (Q = 1.74) which suggests that the spores are more cylindrical than the type specimen (Q = 1.66), described by Heinemann (Citation1958). The overall macroscopic and microscopic details of the present specimen are in conformity with C. miniatescens Heinem except for the slight variation in spore shape.
This species was described by authors in detail from the northwestern Himalayas, India (Kumari et al. Citation2009). This specimen was collected from Dhalli Reserve forest, Shimla. After documentation and microscopic examination this specimen agrees with the description of type specimen of C. miniatescens (Heinemann Citation1958).
Habitat. Solitary to gregarious; on soil under the mixed forest dominated by Cedrus deodara.
Specimens examined. India, Himachal Pradesh, Shimla, Dhalli Reserve forest. N31°20′ E76°.11′ 1700 m, 25 July 2007, Accession No. RCU 65/07.
Cantharellus natarajanii Deepika, Upadhyay & Reddy, sp. nov. , –P,
MycoBank MB519522
Pileus 5–10-cm wide, hemispherical, plano-convex to finally depressed, Golden yellow (9K-4) to Chinese yellow (10K-6), smooth, margin irregular, wavy, lobed, non-striate. Context 3–5-mm thick, lemon yellow to yellow, confluent, unchanging on exposure to air, surface smooth, glabrous. Lamellae distinctly hymeniform, decurrent, anastomosing to distinctly interveined, golden yellow (9L-6). Stipe central, 3.5–6 × 0.4–1 cm, Sunset, slightly expanded at apex, surface glabrous to appressed fibrils. Spore deposit white. Basidiospores [54/2/2], 6.5–9 × 5.–6.5 µm, L = 7.6 µm, W = 5.67, Q = 1.34, broadly ellipsoid to ellipsoid, thin walled, smooth, faintly yellowish in 3% KOH, contents monoguttulate to multi oil refractive gutullate, inamyloid. Basidia ±57–85 × 6.5–10.5 µm, clavate, sterigmata 4.5–8 × 1.2–2.5 µm diam., cornuted 4–5 per basidium, developing basidia with evenly granulose, light yellow contents, basal septa with clamps. Basidioles numerous with opaque light yellow contents in 3% KOH. Hymenophoral trama irregular to interwoven, branched, hyaline to faint yellowish, slightly constricted at septa, septa frequently clamped. Pileipellis consisting of cylindric to filamentous and interwovenly arranged hyphae, end-cells are disctintly subclavate to subventricose, yellowish to yellowish brown, branched, thin to thick walled, frequently clamped, non-amyloid hyphae, 3–10-µm wide, followed by cystoids end hyphae, contents granulose. Pleurocystidia and Cheilocystidia absent. Stipe cuticle made up of yellowish brown, cylindric to filamentous, branched, frequently clamped hyphae, 2.5–10-µm wide.
Habitat. caespitose to gregarious, on soil among moss under Cedrus deodara and Quercus dilatata.
Specimens examined. India, Himachal Pradesh, Shimla, Chail forest, N31°06′ E77°.10′ 1700 m, 30 July 2008, 106-08; same location, 22 August 2009, 35-09; same collection, Uttarakhand – Jageshwar forest N29°00′ E79°.17′, 29 August 2009, 93-09. Holotype: PUN 3963(106-08).
Commentary. This species can be distinguished from C. cibarius which is much larger (pileus 15 cm broad, spores range 8.5–11.5 × 4.5–5.5 µm), moreover the colour morphology is dissimilar, Important character of C. natarajanii is “pileus hyphal end-cells which is distinctly subclavate to subventricose” which clearly differentiates from C. cibarius. Phylogenetic analysis of LSU sequences also confirmed its distinction with other species of Cantharellus ().
Etymology: Is named in honour of Professor K. Natarajan on the account of his contribution towards Indian mycology.
Cantharellus pseudoformosus Deepika, Upadhyay & Reddy, Mycoscience 52: 147–151. 2011
Commentary This species was described from the northwestern Himalayas, India (Kumari et al. Citation2011b), who reported that this collection is morphologically similar to C. formosus Corner, as described by Pilz et al. (Citation2003). However, the molecular analyses of LSU and ITS sequences showed C. pseudoformosus is distinct from C. formosus.
Habitat. Gregarious to caespitose; on soil under trees of Cedrus deodara.
Specimens examined. India, Himachal Pradesh, District-Chamba, Khajjiyar, N32°10′ E75°.45′ 6400 m, 28 September 2007, 281-07; Suala (25 km away from the Khajjiyar guest house); same collection, 28 September 2007, 272-07; Bharmour (5 km away from the Bharmour bus stand), same collection, 12 September 2009, 282-09. Holotype: PUN 3883 (281-07).
Cantharellus umbonatus Deepika, Upadhyay & Reddy, sp. nov. , –R,
MycoBank MB519523
Pileus 4–5 cm, applanate then umbilicate, Capuccine orange to yellow, smooth; margin irregular, hygrophanous, nonstriate, lobed. Context 2–3 mm thick, straw (10F-2), confluent, unchanging on exposure to air, surface smooth but not glabrous. Lamellae abundantly decurrent, interveined to anastomosing, white reaching up to the half of the stipe. Stipe 9–11.5 × 1–1.5 cm thick, central, Margurite yellow (10C-1), terete with slightly swollen apex, radicate, surface fibrous, stipe trama colonial buff (10G-2). Spore deposit white. Basidiospores [55/2/2] (8–) 8.5–10 (–10) × 5–5.5 (–6) µm, L = 8.99 µm, W = 5.08 µm, Q = 1.76, ellipsoid to cylindrical, non-amyloid, non-cyanophilic, wall hyaline, contents granulated to multiguttulate with greenish referective oil droplets. Basidia ±67–99 × 7–9.7 µm, clubed shaped to sub clavate, sterigmata 3.5–6.5 × 1.2–2 µm diam., cornuted 4–6 per basidium, developing basidia with evenly granulose, light yellow contents, basal septa with clamps. Basidioles numerous with opaque light yellow contents in 3% KOH. Subhymenium made up of narrow to non-inflated hyphal end cells. Hymenophoral trama irregular to interwoven, branched, hyaline to faint yellowish, constricted slightly at septa, septa frequently clamped. Pileipellis consisting of filamentous and interwovenly arranged, hyaline, branched, thin to thick walled, frequently clamped, non-amyloid hyphae, 3–12 µm wide, followed by protruding end cells, contents granulose. Pleurocystidia and Cheilocystidia absent. Stipe cuticle made up of hyaline to yellowish, branched, frequently clamped hyphae, 2.5–6 µm wide.
Habitat. caespitose to gregarious, on soil under the trees of Cedrus deodara.
Specimens examined. India, Himachal Pradesh, Shimla, Khada Pathar forest, N30°72′ E78°.63.2′ 1950 m, 25 July 2006, 316-06; same location, 29 July 2007, 348-07; same location, 28 August 2009, 217-09. Holotype: PUN 3968 (316-06).
Commentary. C. umbonatus clustered with C. fibrillosus sequence of LSU whereas clustered with C. pseudoformosus with ITS sequences. However, morphologically distinct characters separated this species as new to the world from C. fibrillosus and P. pseudoformosus. C. umbonatus is readily distinguished by its slightly umbonate pileus and umber hymenium that reaches up to half of the stipe. The species is closely related to C. cyanoxanthus Heim, Corner (Citation1966), but differs in pileus, stem and hymenium colour. Morphologically, it was also compared with C. appalachiensis and C. cinnabarinus and found that the C. umbonatus differs from C. appalachiensis by its dark orange to yellow, with smooth pileus surface, and ellipsoid to elongate basidiospores (Q = 1.62). On the other hand C. cinnabarinus is smaller with pileus up to 1.5 cm broad and with distinctive flamingo-pink colours. Etymology: From the latin word umbonatus – umbilicate, referring to the shape of the cap.
Hymenophore smoothC. lateritius
1.* Hymenophore with prominent folds2
Lamellae yellow, yellow orange or reddish orange when mature3
2.* Lamellae whitish to off-whiteC. umbonatus sp. nov.
Fruit-bodies infundibuliform, ocharaceous brown to yellowish brown4
3.* Fruit-bodies planoconvex to convex, hemispherical to applanate, egg yellow to yellowish range7
Pileus up to 7-cm broad, surface with prominent fibrils, squamules at the center, usually surface uneven and rugose C. fibrillosus sp. nov.
4.* Pileus <7 cm broad, surface with matted fibrils or glabrous5
Pileus ocharaceous yellow with gelatinizes pileipellis hyphae, four basidiospores per basidiumC. himalayensis sp. nov.
5.* Pileus ocharaceous yellow without gelatinized hyphae, 4–6 basidiospores per basidium6
Pileipellis epicutis with projecting cystidiod end subclavate to clavate cells, spore 7–9 × 5–6 µmC. pseudoformosus
6.* Pileipellis epicutis with elongate to broad clavate end cells, spores 8.5–11.5 × 4.5–5.8 µmC. cibarius
Stipe more than the width of pileus8
7.* Stipe equal to width of pileus or smaller9
Fruit bodies plum yellow to organgish, pileus 1.5 cm broad, stipe up to 4.5 cmC. elongatipes sp. nov.
8.* Fruit bodies egg yellow to pale ochraceous yellow, pileus 7-cm broad, stipe up to 12 cmC. indicus sp. nov.
Pileus <3 cm broad10
9.* Pileus >3 cm broad11
Pileus yellowish orange to orange; context concolorous with pileus; spores 7–11.5 µm longC. minor
10.* Pileus deep yellow, context pale yellow to yellow; spore 5–6 (–7) µm longC. friessi
Lamellae decurrent, folded, interveined, hymeniform folded, yellowish, stipe surface fibrous, pinkish yellow to citron yellow12
11.* Stipe surface glabrous to thin hairy, yellowish13
Pileus and stipe dull brown, becoming dingy yellowish to dingy orangish yellow with brownish tones remaining on the pileus disc and stipe baseC. appalachiensis
12.* Pileus and stipes colour not as in C. appalachiensis14
Pileus appalanate to shallow depressed; spore 7–8.5 × 4.5–5.5 µm C. applanatus sp. nov.
13.* Pileus convex to shallow depressed; spore 10–13 × 6–8.5 µm C. luteocomus
Pileus cuticle made up of repent hyphae, context cells are distinctly subclavate to subventricoseC. natarajanii sp. nov.
14.* Pileus cuticle made up of parallel to suberectely arranged hyphaeC. miniatescens
Key to the Indian species of Cantharellus
Discussion
All the species described above are morphologically well delimited. The majority of the species can be unambiguously recognized based on their spores, although the size and colour of the basidiome, the nature of the pileus, pileipellis hyphae and ecology are also useful characteristics in species identification. Most of the species are common and widespread in northwestern Himalayan coniferous forests; however, Cantharellus miniatescens seems to be very rare and found only Dhalli Reserve forest, Shimla, under Cedrus deodara. Although, C. friessi and C. luteocomus were reported from western Himalayan region by Bhatt and Lakhanpal (Citation1988), we did not find either in our collections. All the collected specimens were critically examined and also compared with previously reported C. friessi and C. luteocomus and found to differ from these two species based on various uncommon characters. We have summarized the morphological characters of all the species collected from the northwestern Himalayas of India in . Although Cantharellus species are reported from all over the world, only few species have either complete ITS sequences or LSU sequences in public databases, indicating poor coverage of these species. We could not achieve congruence in the LSU and ITS phylogenies because of the limited number of ITS sequences in the databases. The species having LSU sequences in the databases are higher, hence; in the present study we have reconstructed the phylogenetic trees using these sequences for further analysis.
Table 2. Comparison of morphological characters of Cantharellus species found in the northwestern Himalayas region of India.
Phylogenetic analysis of LSU sequences yielded consistent topologies in different taxa of Cantharellaceae. The phylogenetic tree, based on limited species of Cantharellus, indicated that the present infrageneric classification is composed of non-monophyletic taxa. A revised infrageneric classification of Cantharellus is an issue for future studies with more species included and data from additional genes added. Our results are consistent with the monophyletic character of the species of Cantharellus, providing support to some taxonomic questions. Nevertheless, delimitation of some of those species requires further studies. In addition to its contribution to the study of the taxonomy of Cantharellus, this work would be useful to design the probes in the identification of new species, or as a database for comparing sequences of unidentified species.
In conclusion, phylogenetic analysis of LSU sequences revealed clades with statistical support corresponding to circumscribed morphological characters and identified seven new species within Cantharellus. This study suggests that the Indian subcontinent and the Himalayas are likely to harbour a considerable part of the still undiscovered fungal diversity, and possibly many endemic species. We analysed two loci and tried to avoid erroneous identification but it cannot be ruled out that multiloci analysis comparing type sequences would further corroborate this identification.
Acknowledgements
The authors are thankful to Prof. Jeffrey K. Stone, Oregon State University, USA, for correcting the manuscript and TIFAC-CORE, Thapar University, Patiala, for the experimental facilities.
References
- Abraham TK, Vrinda KB, Pradeep CK, Joseph AV. 1995. Edible Cantherelle from southern India. Mushroom Res. 4:73–76.
- Akaike H. 1981. Likelihood of a model and information criteria. J Econom. 16:3–14. doi:10.1016/0304-4076(81)90071-3
- Bhatt RP, Lakhanpal TN. 1988. New records of fleshy fungi for India. Ind J Mycol Plant Pathol. 18:140–142.
- Bigelow HE. 1978. The cantharelloid fungi of New England and adjacent areas. Mycologia. 70:707–755. doi:10.2307/3759354
- Buyck B, Hofstetter V. 2011. The contribution of tef-1 sequences to species delimitation in the Cantharellus cibarius complex in the southeastern USA. Fungal Divers. 49:35–46. doi:10.1007/s13225-011-0095-z
- Buyck B, Kauff F, Cruaud C, Hofstetter V. 2013. Molecular evidence for novel Cantharellus (Cantharellales, Basidiomycota) from tropical African miombo woodland and a key to all tropical African chanterelles. Fungal Divers. 58:281–298. doi:10.1007/s13225-012-0215-4
- Corner EJH. 1957. Craterellus. Pers. Cantharellus. Fr. and Pseudocraterellus gen. nov. Sydowia. 1:266–276.
- Corner EJH. 1966. A monograph of cantharelloid fungi. England: Oxford University Press; p. 255.
- Corner EJH. 1969. Notes on cantharelloid fungi. Nova Hedwig. 18:783–818.
- Dahlman M, Danell E, Spatafora JW. 2000. Molecular systematics of Craterellus: cladistic analysis of nuclear LSU rDNA sequence data. Mycol Res. 104:388–394. doi:10.1017/S0953756299001380
- Danell E. 1994. Formation and growth of the ectomycorrhiza of Cantharellus cibarius. Mycorrhiza. 5:89–97. doi:10.1007/BF00202339
- Dhancholia S, Bhatt JC, Pant SK. 1991. Studies on some Himalayan agarics. Acta Bot Indica. 19:104–109.
- Dunham SM, O’Dell TE, Molina R. 2003. Analysis of nrDNA sequences and microsatellite allele frequencies reveals a cryptic chanterelle species Cantharellus cascadensis sp. nov. from the American Pacific Northwest. Mycol Res. 107:1163–1177.
- Eyssartier G, Stubbe D, Walleyn R, Verbeken A. 2009. New records of Cantharellus speceis (Basidiomycota, Cantharellaceae) from Malaysian depterocarp rainforest. Fungal Divers. 36:57–67.
- Feibelman T, Bayman P, Cibula WG. 1994. Length variation in the internal transcribed spacer of ribosomal DNA in chanterelles. Mycol Res. 98:614–618. doi:10.1016/S0953-7562(09)80407-3
- Feibelman T, Bennett JW, Cibula WG. 1996. Cantharellus tabernensis: a new species from the southeastern United States. Mycologia. 88:295–301. doi:10.2307/3760934
- Fries E. 1821–1832. Systema mycologicum, sistens fungorum ordines, genera et species. Vols. I–II, Berlingiana, Lund, Sweden; Vols. III and Index. Greifswald: Ernesti Mauritiji; 520 p.
- Heinemann P. 1958. Champignons recoltes au Congo belge par Madame M. Goossens-Fontana. III. Cantharellineae. Bull Jard Bot Bruxelles. 28:385–438. doi:10.2307/3667153
- Katoh K, Misawa K, Kuma K, Miyata T. 2002. MAFFT: a novel method for rapid multiple sequence alignment based on Fourier transform. Nucleic Acids Res. 30:3059–3066. doi:10.1093/nar/gkf436
- Kishino H, Hasegawa M. 1989. Evaluation of the maximum likelihood estimate of the evolutionary tree topologies from DNA sequence data, and the branching order in hominoidea. J Mol Evol. 29:170–179. doi:10.1007/BF02100115
- Kumar A, Bhatt RP, Lakanpal TN. 1990. The Amanitaceae of India. Dehradun: Bishen Singh Mahendra Pal Singh; p. 160.
- Kumari D, Reddy MS, Upadhyay RC. 2011a. Cantharellus pseudoformosus, a new species associated with Cedrus deodara from India. Mycoscience. 52:147–151. doi:10.1007/S10267-010-0080-5
- Kumari D, Upadhyay RC, Reddy MS. 2009. New records of family Cantharellaceae from India. Mushroom Res. 18:47–50.
- Kumari D, Upadhyay RC, Reddy MS. 2011b. Antioxidant activity of three species of wild mushroom genus Cantharellus collected from north-western Himalaya, India. Int J Agric Biol. 13:415–418.
- Mearz A, Paul MA. 1930. A dictionary of colour. London: Mc Graw-Hill Book Company Inc; p. 207.
- Moncalvo JM, Nilsson RH, Koster B, Dunham SM, Bernauer T, Matheny PB, Porter TM, Margaritescu S, Weiß M, Garnica S. 2006. The cantharelloid clade: dealing with incongruent gene trees and phylogenetic reconstruction methods. Mycologia. 98:937–948. doi:10.3852/mycologia.98.6.937
- Nylander JAA. 2008. MrModeltest, version 2.3 README [Internet]. [cited 2008 May 22]. Available from: http://www.abc.se/~nylander/mrmodeltest2/mrmodeltest2.html
- Page RD. 1996. TreeView: an application to display phylogenetic trees on personal computers. Comput Appl Biosci. 12:357–358.
- Persoon CH. 1825. Mycologia Europaea. Vol. 2. Erlangen: Palm; p. 4–17.
- Petersen RH. 1979. Notes on cantharelloid fungi. IX. Illustrations of new or poorly understood taxa. Nova Hedwig. 31:1–23.
- Pilz D, Norvell L, Danell E, Molina R. 2003. Ecology and management of commercially harvested chanterelle mushrooms. USDA Forest Service General Technical Report PNW-576. Washington (DC): USDA Forest Service.
- Rambaut A, Suchard MA, Xie W, Drummond AJ. 2013. Tracer – MCMC trace analysis tool version v1.6 [Internet]. [cited 2013 August 4]. Available from: http://beast.bio.ed.ac.uk/Tracer
- Ronquist F, Huelsenbeck JP. 2003. MrBayes 3: Bayesian phylogenetic inference under mixed models. Bioinformatics. 19:1572–1574. doi:10.1093/bioinformatics/btg180
- Singer R. 1986. The agaricales in modern taxonomy. 4th ed. Koenigstein: Koeltz Scientific Books; p. 981.
- Smith AH, Morse EE. 1947. The genus Cantharellus in the Western United States. Mycologia. 39:497–534. doi:10.2307/3755192
- Sohi HS, Kumar S, Seth PK. 1964. Some interesting fleshy fungi from Himachal Pradesh; India. Indian Phytopathol. 17:317–322.
- Swofford DL. 2002. PAUP*: phylogenetic analysis using parsimony (*and other methods). Version 4.0b10. Sunderland (MA): Sinauer Associates.
- Tian XF, Buyck B, Shao SC, Liu PG, Fang Y. 2012. Cantharellus zangii, a new subalpine basidiomycete from southwestern China. Mycotaxon. 120:99–103. doi:10.5248/120.99
- Van Kan JAL, van den Ackerveken GFJM, de Wit PJGM. 1991. Cloning and characterization of cDNA of avirulence avr9 of the fungal pathogen Cladosporium fulvum, causal agent of tomato leaf mold. Mol Plant Microbe Interact. 4:52–59. doi:10.1094/MPMI-4-052
- Vilgalys R, Hester M. 1990. Rapid genetic identification and mapping of enzymatically amplified ribosomal DNA from several Cryptococcus species. J Bacteriol. 172:4238–4246.
- White TJ, Bruns T, Lee S, Taylor J. 1990. Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In: Innis MA, Gelfand DH, Sninsky JJ, White TJ, editors. PCR protocols: a guide to methods and applications. San Diego (CA): Academic Press; p. 315–322.