ABSTRACT
Here, we review the current state of knowledge concerning high-elevation members of the extremophilic Cryptococcus albidus clade (now classified as the genus Naganishia). These fungi dominate eukaryotic microbial communities across the highest elevation, soil-like material (tephra) on volcanoes such as Llullaillaco, Socompa, and Saírecabur in the Atacama region of Chile, Argentina, and Bolivia. Recent studies indicate that Naganishia species are among the most resistant organisms to UV radiation, and a strain of N. friedmannii from Volcán Llullaillaco is the first organism that is known to grow during the extreme, diurnal freeze-thaw cycles that occur on a continuous basis at elevations above 6000 m.a.s.l. in the Atacama region. These and other extremophilic traits discussed in this review may serve a dual purpose of allowing Naganishia species to survive long-distance transport through the atmosphere and to survive the extreme conditions found at high elevations. Current evidence indicates that there are frequent dispersal events between high-elevation volcanoes of Atacama region and the Dry Valleys of Antarctica via “Rossby Wave” merging of the polar and sub-tropical jet streams. This dispersal hypothesis needs further verification, as does the hypothesis that Naganishia species are flexible “opportunitrophs” that can grow during rare periods of water (from melting snow) and nutrient availability (from Aeolian inputs) in one of the most extreme terrestrial habitats on Earth.
Introduction
Recent work in the Atacama Desert Region of Chile and Argentina has shown that some of the highest elevation (>6000 m.a.s.l.) soil-like environments on Earth are dominated by basidiomycetous yeasts in the former Cryptococcus albidus clade most closely related to Naganishia friedmannii (Costello et al. Citation2009; Lynch et al. Citation2012; Solon et al. Citation2017). In addition, these same yeasts are fairly easy to isolate from soils at elevations from 5000 (Pulschen et al. Citation2015) to over 6000 m.a.s.l. (Vimercati et al. Citation2016) in the Atacama region. These findings are somewhat surprising given the extreme dryness of these sites and the fact that they contain some of the lowest levels of total carbon (<0.03%) of any soil environment yet studied on Earth (Schmidt et al. Citation2012). In addition, high elevation sites in the Atacama region have a thin atmosphere, extreme aridity, high levels of UV radiation, low pH values, and extreme diurnal freeze-thaw cycles (Schmidt Citation1999; Lynch et al. Citation2012; Cabrol et al. Citation2014; Pulschen et al. Citation2015). This unique combination of environmental stressors makes these high-elevation sites ideal for studying the dry-cold limits to life on Earth, as well as offering one of the best Earth analogues for the surface and near sub-surface of Mars (Lynch et al. Citation2012; Pulschen et al. Citation2015).
The extreme nature of high-elevation sites in the Atacama region brings into question whether Naganishia species can grow in this environment or if they are just dormant propagules that fall onto these remote slopes from the atmosphere. The goal of this paper is to present evidence for and against a functional role for Naganishia species in extreme high-elevation and high-latitude ecosystems. More specifically, it is argued that fungi in the genus Naganishia have adapted to be transiently functional during brief periods of water and nutrient availability in extremely dry, oligotrophic volcanic soils. Their unique adaptations include a resistance to high doses of UV radiation, adaptation to low pH values, the ability to grow at temperatures below 0°C, and most importantly their ability to grow during the extreme freeze-thaw cycles that occur on a daily, year-round basis at elevations above 6000 m.a.s.l.. This paper is a review of the current state of knowledge concerning the distribution and ecological tolerances of members of the genus Naganishia found at elevations from 5000 to over 6000 m.a.s.l. in the Atacama region, and compares them to other extreme high-elevation sites and to the well-studied Naganishia species from the Dry Valleys of Antarctica. Broader and more general discussions of the biogeography of yeasts found in cold environments are available in the literature (e.g. Vishniac Citation2006; Buzzini et al. Citation2012).
Phylogeny of the Naganishia clade
Determining precise phylogenetic relationships is crucial to our understanding of the ecology of widely dispersed organisms such as Naganishia species. The present study benefits greatly from multiple phylogenetically relevant genes having been sequenced for the extremophilic isolates and environmental samples discussed in the present paper. The unofficial type species for extremophilic Naganishia species is N. friedmannii (Vishniac Citation1985a) which was originally isolated from Antarctic cryptoendolithic samples described by Friedmann (Citation1982). Later sequencing efforts showed that N. friedmannii and other Antarctic (e.g. N. antarcticus, N. consortionis, and N. vishniacii) and Himalayan (N. bhutanensis) cryptococci grouped in a clade with N. albidus (Takashima and Nakase Citation1999). This grouping has been confirmed by numerous other studies including the recent work of Liu et al. (Citation2015a, Citation2015b) who showed that N. friedmannii, N. antarcticus, N. vishniacii and N. bhutanensis are in the albidus clade of the Filobasidiales based on a seven-gene (RPB1, RPB2, TEF1, CYTB, and 3 rDNA genes) phylogeny. Also relevant to the present study, Vimercati et al. (Citation2016) showed that the isolate from Volcán Llullaillaco (discussed below) forms a monophyletic clade with N. friedmannii based on long-read 18S environmental sequences (1739 bp, JX099190) from Llullaillaco (Lynch et al. Citation2012). Likewise, the Naganishia isolate from Volcán Saírecabur studied by Pulschen et al. (Citation2015) showed 100% identity with N. friedmannii over a 599 bp region of the LSU rRNA gene.
The environmental and biogeochemical setting
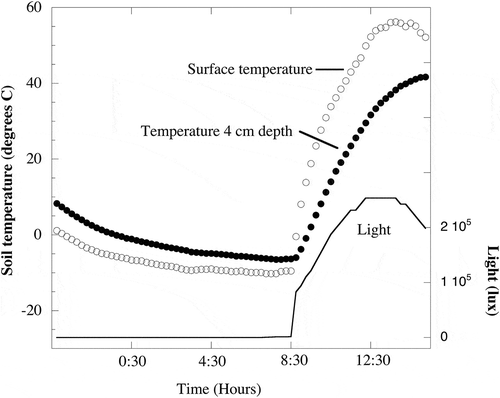
This paper focuses on recent work conducted in what is perhaps the most extreme terrestrial environment on Earth, that is, the highest reaches of massive stratovolcanoes in the Atacama region of Chile, Bolivia, and Argentina (). Many studies have focused on the low-elevation areas of the Atacama Desert (e.g. Arroyo et al. Citation1988; Richter and Schmidt Citation2002; Drees et al. Citation2006; Demergasso et al. Citation2010; Neilson et al. Citation2012). However, very little biological work has been done in the remote, high-elevation, unvegetated zone that exists above 5100 m.a.s.l.. The highest reaches of these volcanoes receive limited snowfall, most of which sublimates back to the atmosphere resulting in some of the driest soil-like material (tephra) yet studied on Earth (, ). In addition, the thin atmosphere combined with intense solar radiation impinging on volcanic tephra creates daily freeze-thaw cycles at the soil surface (−10 to +56°C on a single summer day, ). Levels of UV radiation are very high at these sites; UVA and UVB levels at 5091 m.a.s.l. are 25–33% higher than levels measured at lower elevation sites in the Atacama (Yungay, 948 m.a.s.l.; Pulschen et al. Citation2015). Also of relevance to this paper is the fact that the tephra soils on these high volcanoes are among the most oligotrophic soils or sediments yet studied. Concentrations of carbon and nitrogen are at or below detection limits (), even though deep sequencing of DNA in soils from 6030 m.a.s.l. indicates that there is foreign (Aeolian) plant DNA from lower elevations being deposited there (Vimercati et al. Citation2016). In addition, these sites are all acidic, with pH values of 4.1–5.4 (), adding yet another dimension of stress to organisms living in these soils.
Table 1. Biogeochemical characteristic of soils from the three volcanoes and the Colorado site (Navajo Peak) discussed in this paper. Note the low pH values at these sites.
Figure 1. Map showing the location of the three volcanoes discussed in this paper. From south to north, microbial communities on Llullaillaco (6723 m) and Socompa (6051 m) have been studied extensively via culture-independent and culturing approaches (Costello et al. Citation2009; Lynch et al. Citation2012, Vimercati et al. Citation2016; Solon et al. Citation2017), whereas Naganishia and other yeasts have been cultured (Pulschen et al. Citation2015) from moderately high slopes of Saírecabur (5971 m). Map is redrawn from Costello et al. (Citation2009).
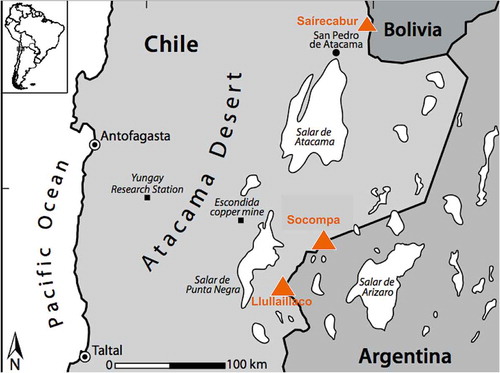
Figure 2. Photograph taken on Volcán Llullaillaco in February, 2009 looking down from 5800 m.a.s.l. to the landscape between Llullaillaco and Socompa. The immediate foreground shows the Mars-like tephra material (at 5800 m.a.s.l.) dominated by N. friedmannii OTUs.

Figure 3. Soil temperatures and light measured at 5500 m.a.s.l. on Volcán Socompa during a summer night and day (Feb. 10 and 11, 2009). Soil surface temperatures ranged from −10.2°C at 7:30 h to a high of 56.2°C at 13:45 h. Temperatures at 4 cm depth ranged from −6.5°C at 7:45 h to 41.7°C at 15:15 h. No light penetrated to 4 cm depth and light at the surface reached the maximum measurable levels (253,512 lx) for a period of 1.75 h starting at 12:15 h. Temperature data were originally reported in Lynch et al. (Citation2012) and light data are previously unpublished.
Global aerial dispersal of Naganishia species
The presence of foreign plant DNA in the plant-free areas on these volcanoes (Vimercati et al. Citation2016) suggests that even the microbes in these soils may just be dormant propagules from far off locations. Many microbes can be transported over global distances (Kellogg and Griffin Citation2006; Schlesinger et al. Citation2006), and biogeographic patterns of microbial community structure suggest widespread long-distance dispersal at global scales (Barberán et al. Citation2007, Darcy et al. Citation2011; Itani and Smith Citation2016). Mathematical models of long-range transport also show that smaller particles – like spores – are likely to easily attain global distribution (Norros et al. Citation2014, Wilkinson et al. Citation2012). But not all microbes can survive aerial transport, since the atmosphere itself is an extreme environment with stressors including high UV radiation, extreme temperatures, and high potential for desiccation (Griffin et al. Citation2001).
There is evidence for the survivability of members of the genus Naganishia in the upper trophosphere and perhaps even higher in the atmosphere. For example, N. albida has been isolated from filtered air samples from 2,700 m.a.s.l. in the Northwest USA (Smith et al. Citation2012) and from 1,464 m.a.s.l. in Western Europe (Amato et al. Citation2007), showing that these fungi are still viable after aerial transport. From a culture-independent perspective, N. albida and other Naganishia OTUs were detected in Israeli dust storms from 2012 and 2013 (Katra et al. Citation2014). Similar dust events have been tracked from Asia to North America indicating a potential route for dispersal of N. albida through the atmosphere (Griffin et al. Citation2001; Smith et al. Citation2012). Whether or not these organisms are active in the air column is unknown, but there is the potential for global dispersal of members of Naganishia species via wind and cloud formations in the troposphere. Members of the N. albida clade have also been found in the International Space Station where high levels of ionising radiation are a persistent stressor (Dadachova and Casadevall Citation2008).
Other yeast in the Tremellomycetes (including human pathogens) also show the potential for global atmospheric dispersal. For example, Cryptococcus gatti was traditionally considered to be restricted to tropical and subtropical climates; however, there is increasing evidence that it can be dispersed via the atmosphere even to colder areas such as the Northwest USA (Kidd et al. Citation2004; Byrnes et al. Citation2010). Other investigations into the ecological niches of these opportunistic pathogens have revealed a global distribution (Chen et al. Citation2014) often associated with reservoirs such as guano (Nielsen et al. Citation2007), Eucalyptus trees (Ellis and Pfeiffer Citation1990a), and Cassia trees (Lazera et al. Citation2000; Granados and Castañeda Citation2005). Furthermore, air sampling in Australia during the flowering season of Eucalyptus camaldulensis contained C. gattii (Ellis and Pfeiffer Citation1990b) as did air samples collected after tree-cutting activities in Canada (Kidd et al. Citation2007). Finally, Casadevall et al. (Citation2003) identified several virulence factors of C. neoformans that are “dual use” with both an environmental survival function and a pathogenetic function: a capsule that provides desiccation resistance as well as production of melanin for UV shielding, heat tolerance, and cold tolerance. All three factors would enhance survival during atmospheric transport.
There is also an open question of whether the small (1–2 µm in diameter), easily dispersed sexual spores are able to survive long-distance dispersal or whether it is only whole cells that are being dispersed long distances. For most pathogenic Cryptococcus species, sexual spores are only known to be produced in the laboratory and have not been found in nature or in clinical samples (Velagapudi et al. Citation2009); however, Nielsen et al. (Citation2007) found that Cryptococcus neoformans can mate on pigeon guano. To our knowledge, no extremophilic members of the genus Naganishia are known to have a sexual stage (Barnett et al. Citation2000), but this could just be because the source habitat (reservoir) for this group has not yet been discovered. There is a chance that the extremophilic members of the genus Naganishia are being dispersed from some as yet undiscovered source habitat where sexual reproduction can occur. It is intriguing in this regard that there is a recent report of a pathogenic strain of N. friedmannii (Ekhtiaria et al. Citation2017) – perhaps indicating that we are only just starting to understand the global reservoirs for extremophilic members of the genus Naganishia.
The search for a possible global reservoir for Naganishia species is an important goal for future studies. Our working model is that the reservoir is the high elevation volcanoes discussed in this paper, because that is where we find the highest relative abundance of members of this group (see next section) on a global scale. The relative abundance of Naganishia species in microbial communities of the Dry Valleys of Antarctica is less clear because most studies have been based on culture-dependent approaches focusing solely on specific species (Vishniac and Hempfling Citation1979; Vishniac Citation1985a, Citation1985b, Citation2006). Two recent culture-independent studies of microbial communities indicated that Naganishia are rare in many Dry Valley soils (Fell et al. Citation2006), but are quite common in several higher elevation sites (Dreesens et al. Citation2014). It is possible that the source for the Naganishia species found in Antarctica is the continuous dispersal of dust and particles from the Southern Andes via meandering Rossby waves in the troposphere (Madden Citation1979). These waves result from the confluence of the polar and subtropical jet streams which join and separate at periodic intervals (Ambrizzi et al., Citation1995; James Citation1988; Polvani and Saravanan Citation2000) and could drive the aerial dispersal of microbes in both directions between the Andes and Antarctica. Visualisation of Rossby waves can be found online (http://squall.sfsu.edu/crws/archive/jetstream_archive.html).
Landscape patterns of Naganishia distribution
Environmental sequencing efforts using three different primer sets and two different sequencing technologies all indicate that high-elevation tephra on Socompa and Llullaillaco are dominated by phylotypes closely related to N. friedmannii. The first report showing this dominance came from the culture-independent study of Volcán Socompa conducted by Costello et al. (Citation2009). They collected tephra samples (0 to 10 cm depth) in March, 2005 along a 1-kilometre transect at 5,235 m.a.s.l. and combined the samples to make a clone library using Sanger sequencing of the 18S rRNA gene (primer pair 515F and 1391R). This library was overwhelmingly dominated (61%) by Naganishia friedmannii and included only six other 18S OTUs (Costello et al. Citation2009). On a subsequent expedition in February 2009, samples (0 to 4 cm depth) were collected at six locations spanning elevations of 6030 to 6330 m.a.s.l. on the Argentinian side of Volcán Llullaillaco, and elevations of 5820 to 6031 m.a.s.l. on Volcán Socompa. The Llullaillaco samples were Sanger sequenced using primer pair 4Fa-short and 1492R and yielded high-quality, long-read sequences of which 92% fell into a single OTU (97% identity) most closely related to N. friedmannii (Lynch et al. Citation2012). The 2009 Socompa samples were sequenced using Ilumina MiSeq (primer set 1391F/EukBr) and showed overwhelming dominance of Naganishia in barren soils at 5820 and 6031 m.a.s.l. on Socompa (Solon et al. Citation2017). Finally, numerous samples were collected in 2016 across a range of elevations (5100 to 5600 m.a.s.l.) on the Chilean side of Llullaillaco and again showed an overwhelming dominance of all dry sites by Naganishia (Solon et al. Citation2017). In addition to culture-independent studies, N. friedmannii has been isolated into pure culture from sites at 6030 m.a.s.l. on Llullaillaco (Vimercati et al. Citation2016) and sites at 3981 and 5047 m.a.s.l. on Volcán Saírecabur (Pulschen et al. Citation2015). The ecological tolerances of these isolates are discussed in the next section (below).
The complete dominance of N. friedmannii at all dry sites on Llullaillaco and Socompa led us to examine environmental sequence data sets from a number of other extreme high-elevation sites throughout the world. These included 18S data sets from unvegetated sites in the high Himalayas (Schmidt et al. Citation2011), the high Andes of Peru (Nemergut et al. Citation2007), Denali National Park, Alaska (Darcy and Schmidt Citation2016), Mt Kilimanjaro (Vimercati et al. unpubl.), and along the continental divide in Colorado. Out of these environmental sequencing surveys significant numbers of Naganishia species were only found at the highest sites sampled in Colorado (Freeman et al. Citation2009). Importantly, these sites were also the only sampled sites (among the global sites listed above) that had soil pH values (pH 4.5) similar to those found on the three volcanoes (). All the other sites (Alaska, Peru and Nepal) had soil pH values above 7.5 (Nemergut et al. Citation2007; Schmidt et al. Citation2011; Darcy and Schmidt Citation2016). On-going work at the Niwot Ridge LTER site in Colorado and at sites on Volcán Llullaillaco are attempting to parse out the effects of elevation versus pH on the landscape distribution patterns of N. friedmannii (Gendron et al., unpublished data). It is also possible that many of the Naganishia species isolated from alkaline sites across the Antarctic Dry Valley landscape actually originated in acidic microsites. De Los Ríos et al. (Citation2003) showed that endolithic cyanobacterial communities contain acidic microsites and many Dry Valley Naganishia species were isolated from soils downhill from endolithic sites (Vishniac Citation1985b) or directly from endolithic samples (Friedmann Citation1982; Vishniac Citation1985a). Obviously more work is needed to understand the factors controlling the landscape and global distribution of extremophilic Naganishia species.
Ecological tolerances of Naganishia
The fact that the genus Naganishia dominates culture-independent surveys of high-elevation volcanic sites and that these yeasts are easy to isolate from such soils, does not prove that they are actually functioning in situ. An alternative hypothesis is that they are just dormant propagules that are being globally distributed to high elevations (like many bacteria) and are extremely adept at surviving in a dormant state (see discussion above). The sequencing and culturing approaches used so far to study these volcanoes cannot distinguish between dormant and active cells; that is, dormant cells are sequenced in environmental DNA libraries and also can germinate when exposed to rich laboratory media, even if they are not active in the environment they are isolated from. One approach to resolving this issue would be to use transcriptomic approaches, but such methods are very hard to implement in extremely remote, oligotrophic, low-biomass systems. A less direct approach is to determine whether the organisms that are cultured from these systems are able to function and grow under realistic extreme conditions in the laboratory.
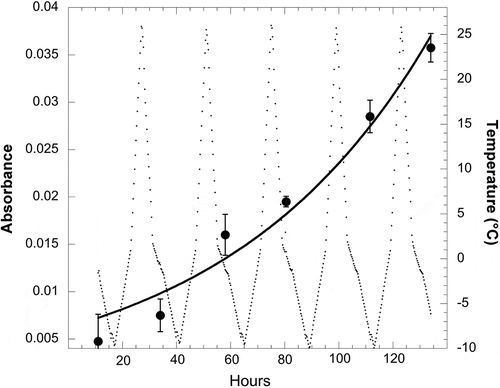
So far, studies of eco-physiological tolerances of the genus Naganishia have addressed their ability to grow at low temperatures (), at moderately high salinity levels, and during extreme freeze-thaw cycles, as well as to withstand high levels of UV radiation. summarises data from a number of sources showing the ability of Naganishia to grow at temperatures as low as −6°C. It is also evident from that all Naganishia isolates have Tmax values of 27°C or lower (i.e. they cannot grow at temperatures above 27°C). These Tmax values () indicate that all of these isolates are psychrophilic or psychrotrophic and are well adapted to function at the cold temperatures that predominate at high elevations. The only study to examine the growth of N. friedmannii (or any microorganism) during extreme freeze-thaw cycles was recently carried out by Vimercati et al. (Citation2016). Vimercati et al. (Citation2016) conducted a series of experiments with tephra soils from 6030 m.a.s.l. on Volcán Llullaillaco and showed that N. friedmannii was the only species to increase in relative abundance during a two-month incubation of soils at temperatures fluctuating between −10°C every night and +27°C every day. The increase in N. friedmannii relative abundance was even more pronounced if supplemental water was applied to the soils, but it also increased when soils were at extremely dry field levels (0.24% water). At the end of this experiment, N. friedmannii was isolated from these soils and then shown to be able to grow at −6°C with an optimum growth temperature of 17°C (). Even more extraordinarily, this N. friedmannii isolate was able to grow in liquid culture during extreme freeze-thaw cycles in the lab (), exhibiting an overall growth rate (µ) of 0.013 h−1 (doubling time of 50 h), a rate equivalent to its growth rate at a constant 0°C (Vimercati et al. Citation2016). The experiment shown in was done in low volumes of growth media so that they froze every night and completely melted every day. To our knowledge, this is the first demonstration of the growth of any microbe during extreme freeze-thaw cycles, although activity of microbes during such cycles has long been known (reviewed in Schmidt et al. Citation2009a). The ability to survive and thrive within a very broad temperature range may be a characteristic widespread within the Tremellomycetes; even pathogenic Cryptococcus gatti and C. neoformans exhibit broad tolerance to a range of temperatures (Nichols et al. Citation2007, Garcia-Solache and Casadevall Citation2010), but have not been shown to be able to grow at subfreezing or fluctuating temperatures like members of the genus Naganishia.
Table 2. Growth rates, optimal temperatures (Opt), and maximum temperature (Tmax) for growth of N. friedmannii isolates from Llullaillaco, Socompa and Antarctic sites, and N. vishniacii and N. antarcticus from the Dry Valleys of Antarctica.
Figure 4. Growth of the N. friedmannii isolate from Volcán Llullaillaco during five consecutive freeze-thaw cycles in a specially designed freeze-thaw chamber (Vimercati et al. Citation2016). Growth of the cultures (4 replicates, large dots) was measured using absorbance at 630 nm and verified by microscopic observations. Temperature of the growth medium (small dots, background) was measured using data loggers and verified by thermocouples in the actual growth media. The exponential growth curve (black line) was fit to the data by non-linear regression and the exponential growth model: at = a0 eµt, where µ is the exponential growth rate with units of h−1, and a0 and at are absorbance at time 0 and time t, respectively (Schmidt et al. Citation2009b). Error bars are standard deviation of measurements from four replicate cultures. Data are redrawn (with permission from the authors) from Vimercati et al. (Citation2016).
N. friedmannii isolates have also been shown to withstand high levels of UV radiation and relatively high salt concentrations. For example, Pulschen et al. (Citation2015) showed that an N. friedmannii strain could withstand levels of UV radiation on a par with the extremely radiation-resistant bacterium, Deinococcus radiodurans. It has also been shown recently that even non-extremophilic species in the Tremellomycetes such as the human pathogen Cryptococcus neoformans is among the most resistant species yet tested to gamma radiation (Khajo et al. Citation2011). Therefore, radiation resistance may be a wide-spread trait in the Tremellomycetes, not necessarily limited to organisms that can grow and survive in high-elevation environments.
Likewise, members of the genus Naganishia have been shown to be somewhat halotolerant, with growth ceasing at sodium chloride levels of 1.2–1.8 M for various strains of N. antarticus, N. friedmannii, and N. vishniacii (Vishniac and Kurtzman Citation1992; Pulschen et al. Citation2015). While these values are respectable, they do not indicate that they are halophilic and put these strains at the lower range of halotolerant yeasts. For example, Corte et al. (Citation2006) screened 27 halotolerant yeast species that can tolerate 10% NaCl and found that growth ceased at concentrations of between 1.7 and 3.8 M, with an average of 2.5 M. In addition, none of the Antarctic Naganishia species can grow in the presence of 10% NaCl, whereas about 30% of the non-Antarctic species listed in Barnett et al. (Citation2000) could grow in the presence of 10% NaCl.
Ecophysiological studies of Naganishia isolates indicate that some of them are metabolically versatile in terms of how many different organic compounds they can use for growth. Of particular interest is the work of Vimercati et al. (Citation2016) who showed that the N. friedmannii isolate from Volcán Llullaillaco could metabolise 74% of 27 organic compounds commonly used for classifying yeast. In contrast, N. friedmannii and N. consortionis from Antarctica used 48 and 30% of the same 27 organic compounds, respectively (Barnett et al. Citation2000), perhaps indicating that the Antarctic isolates have a narrower metabolic niche than the Llullaillaco isolate. The metabolic versatility of the Llullaillaco isolate may indicate that this organism is an opportunitroph as defined by Polz et al. (Citation2006), that is, an organism capable of “exploiting spatially and temporally variable resources”. Many of the substrates used for growth by this organism are plant-derived compounds (e.g. cellobiose, arbutin, salicin) indicating that it can take advantage of Aeolian transported plant material that is likely the main source of organic matter to extreme high-elevation ecosystems (Ley et al. Citation2004; Mladenov et al. Citation2012; Vimercati et al. Citation2016). Our working hypothesis is that N. friedmannii on Llullaillaco and other volcanoes are dormant during long periods of dryness and only come out of dormancy and opportunistically metabolise Aeolian organic debris following the melting of rare snow events. This sort of opportunistic strategy has also been suggested for other cold-desert microbes, especially phototrophs (Pointing Citation2016; Schmidt et al. Citation2017). More work is needed to test the opportunitroph hypothesis for N. friedmannii and to compare the ecological strategies of N. friedmannii strains from the Andes and Antarctica.
Taken together, ecophysiological studies of N. friedmanniii and other members of the genus Naganishia indicate that these are very resilient and resistant organisms, but that more work is needed to definitively prove that they can grow and function under the extreme conditions found at elevations above 6000 m.a.s.l. on volcanoes such as Llullaillaco and Socompa.
Conclusions and future directions
Evidence presented in this review supports the hypothesis that members of the genus Naganishia can function as self-sustaining populations in what is probably the most extreme terrestrial environment on Earth. Their ability to withstand high levels of UV radiation and low pH values combined with their unique ability to grow during extreme freeze-thaw cycles indicates that they are adapted to live where these three stressors are continuously in play. Also, given that they are halotolerant (rather than halophilic), psychrotolerant (rather than psychrophilic), and metabolically versatile indicates that they are flexible in their response to water potential, temperature, and nutrient availability. Therefore, given the evidence available so far, our working model for why Naganishia species are so prevalent on these high elevation volcanoes is that they are flexible “opportunitrophs” (cf Polz et al. Citation2006; Westrich et al. Citation2016) that can grow during rare periods of water (from melting snow) and nutrient availability (from Aeolian inputs). However, much more work is needed to verify that Naganishia species are actually functioning in these high elevation soils (and at similar sites in Antarctica) and to understand how they are dispersed through the atmosphere to remote sites throughout the cryosphere.
Acknowledgements
The authors thank E.K. Costello, A. Seimon, S.R.P. Halloy, M. Farias, C. Vitry, and P. Sowell for help in the field. Funding was provided by NSF grants DEB-1258160 and DEB-1457827 and grants from the National Geographic Society.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Amato P, Parazols M, Sancelme M, Laj P, Mailhot G, Delort AM. 2007. Microorganisms isolated from the water phase of tropospheric clouds at the Puy de Dome: major groups and growth abilities at low temperatures. FEMS Microbiol Ecol. 59:242–254.
- Ambrizzi T, Hoskins BJ, Hsu HH. 1995. Rossby wave propagation and teleconnection patterns in the austral winter. J Atmos Sci. 52:3661–3672.
- Arroyo MTK, Squeo FA, Armesto JJ, Villagran C. 1988. Effects of aridity on plant diversity in the northern Chilean Andes - results of a natural experiment. Ann Mo Bot Gard. 75:55–78.
- Barberán A, Henley J, Fierer N, Casamayor EO. 2007. Structure, inter-annual recurrence, and global-scale connectivity of airborne microbial communities. Sci Total Environ. 487:187–195.
- Barnett JA, Payne RW, Yarrow D. 2000. Yeasts, characteristics and identification. Cambridge: Cambridge University Press; p. 1139.
- Buzzini P, Branda E, Goretti M, Turchetti B. 2012. Psychrophilic yeasts from worldwide glacial habitats: diversity, adaptation strategies and biotechnological potential. FEMS Microbiol Ecol. 82:217–241.
- Byrnes EJ, Li W, Lewit Y, Ma H, Voelz K, Ren P, Carter DA, Chaturvedi V, Bildfell RJ, May RC, et al. 2010. Emergence and pathogenicity of highly virulent Cryptococcus gattii genotypes in the northwest United States. PLoS Pathog. 6:e1000850.
- Cabrol NA, Feister U, Häder D, Piazena H, Grin EA, Klein A. 2014. Record solar UV irradiance in the tropical Andes. Front Environ Sci. doi:10.3389/fenvs.2014.00019
- Casadevall A, Steenbergen JN, Nosanchuk JD. 2003. ‘Ready-made’ virulence and ‘dual-use’ virulence factors in pathogenic environmental fungi - the Cryptococcus neoformans paradigm. Curr Opin Microbiol. 6:332–337.
- Chen SCA, Meyer W, Sorrell TC. 2014. Cryptococcus gattii infections. Clin Microbiol Rev. 27:980–1024.
- Corte L, Rellini P, Lattanzi M, Picchetta C, Fatichenti F, Cardinali G. 2006. Diversity of salt response among yeasts. Ann Microbiol. 56:363–376.
- Costello EK, Halloy SRP, Reed SC, Sowell P, Schmidt SK. 2009. Fumarole-supported islands of biodiversity within a hyperarid, high-elevation landscape on Socompa volcano, Puna de Atacama, Andes. Appl Environ Microbiol. 75:735–747.
- Dadachova E, Casadevall A. 2008. Ionizing radiation: how fungi cope, adapt, and exploit with the help of melanin. Curr Opin Microbiol. 11:525–531.
- Darcy JL, Lynch R, King AJ, Robeson MS, Schmidt SK. 2011. Global distribution of Polaromonas phylotypes - evidence for a highly successful dispersal capacity. PLoS One. 6:e23742.
- Darcy JL, Schmidt SK. 2016. Nutrient limitation of microbial phototrophs on a debris-covered glacier. Soil Biol Biochem. 95:156–163.
- de los Ríos A, Wierzchos J, Sancho LG, Ascaso C. 2003. Acid microenvironments in microbial biofilms of Antarctic endolithic microecosystems. Environ Microbiol. 5:231–237.
- Demergasso C, Dorador C, Meneses D, Blamey J, Cabrol N, Escudero L, Chong G. 2010. Prokaryotic diversity patterns in high‐altitude ecosystems of the Chilean Altiplano. J Geophys Res. 115:G00D09.
- Drees KP, Neilson JW, Betancourt JL, Quade J, Henderson DA, Pryor BM, Maier RM. 2006. Bacterial community structure in the hyperarid core of the Atacama Desert, Chile. Appl Environ Microbiol. 72:7902–7908.
- Dreesens LL, Lee CK, Cary SC. 2014. The distribution and identity of edaphic fungi in the McMurdo Dry Valleys. Biology. 3:466–483.
- Ekhtiaria M, Farahyara S, Falahatia M, Razmjoua E, Ashrafi-Khozania M, Ghasemib Z, Abbasi-Nejat Z. 2017. The first report of onychomycosis caused by Cryptococcus friedmannii (Naganishia friedmannii) a basidiomycetous yeast. Medical Mycol Case Rep. 15:25–27.
- Ellis DH, Pfeiffer TJ. 1990a. Natural habitat of Cryptococcus neoformans var. Gattii J Clin Microbiol. 28:1642–1644.
- Ellis DH, Pfeiffer TJ. 1990b. Ecology, life cycle, and infectious propagule of Cryptococcus neoformans. The Lancet. 336:923–925.
- Fell JW, Scorzetti G, Connell L, Craig S. 2006. Biodiversity of micro-eukaryotes in Antarctic Dry Valley soils with < 5% soil moisture. Soil Biol Biochem. 38:3107–3119.
- Freeman KR, Martin AP, Karki D, Mitter M, Meyer AF, Longcore JE, Simmons DR, Schmidt SK. 2009. Evidence that chytrids dominate fungal communities in high-elevation soils. Proc Natl Acad Sci USA. 106:18315–18320.
- Friedmann EI. 1982. Endolithic microorganisms in the Antarctic cold desert. Science. 215:1045–1053.
- Garcia-Solache MA, Casadevall A. 2010. Global warming will bring new fungal diseases for mammals. MBio. 1:e00061–10.
- Granados DP, Castañeda E. 2005. Isolation and characterization of Cryptococcus neoformans varieties recovered from natural sources in Bogotá, Colombia, and study of ecological conditions in the area. Microb Ecol. 49:282–290.
- Griffin DW, Kellogg CA, Shinn EA. 2001. Dust in the wind: long range transport of dust in the atmosphere and its implications for global public and ecosystem health. Glob Change Hum Health. 2:20–33.
- Itani GN, Smith CA. 2016. Dust rains deliver diverse assemblages of microorganisms to the Eastern Mediterranean. Sci Rep. 6:22657.
- James IN. 1988. On the forcing of planetary‐scale Rossby waves by Antarctica. Quart J Roy Meteorol Soc. 114:619–637.
- Katra I, Arotsker L, Krasnov H, Zaritsky A, Kushmaro A, Ben-Dov E. 2014. Richness and diversity in dust storm borne biomes at the Southeast Mediterranean. Sci Rep. 4:7537–7541.
- Kellogg CA, Griffin DW. 2006. Aerobiology and the global transport of desert dust. Trends Ecol Evol. 21:638–644.
- Khajo A, Bryan RA, Friedman M, Burger RM, Levitsky Y. 2011. Protection of melanized Cryptococcus neoformans from lethal dose gamma irradiation involves changes in melanin’s chemical structure and paramagnetism. PLoS One. 6:e25092.
- Kidd SE, Bach PJ, Hingston AO, Mak S, Chow Y, MacDougall L, Kronstad JW, Bartlett KH. 2007. Cryptococcus gattii dispersal mechanisms, British Columbia, Canada. Emerg Infect Dis. 13:51–57.
- Kidd SE, Hagen F, Tscharke RL, Huynh M, Bartlett KH. 2004. A rare genotype of Cryptococcus gattii caused the cryptococcosis outbreak on Vancouver Island (British Columbia, Canada). Proc Natl Acad Sci USA. 101:17258–17263.
- King AJ, Freeman KR, Lozupone CA, Knight R, Schmidt SK. 2010. Biogeography and habitat modelling of high-alpine bacteria. Nature Commun. 1:53, doi: 10.1038/ncomms.
- Lazera MS, Cavalcanti MS, Londero AT, Trilles L, Nishikawa MM, Wanke B. 2000. Possible primary ecological niche of Cryptococcus neoformans. Med Mycol. 38:379–383
- Ley RE, Williams MW, Schmidt SK. 2004. Microbial population dynamics in an extreme environment: controlling factors in talus soils at 3750m in the Colorado Rocky Mountains. Biogeochemistry. 68:313–335.
- Liu X-Z, Wang Q-M, Göker M, Groenewald M, Kachalkin AV, Lumbsch HT, Millanes AM, Wedin M, Yurkov AM, Boekhout T, et al. 2015b. Towards an integrated phylogenetic classification of the Tremellomycetes. Studies Mycol. 81:85–147.
- Liu X-Z, Wang Q-M, Theelen B, Groenewald M, Bai F-Y, Boekhout T. 2015a. Phylogeny of tremellomycetous yeasts and related dimorphic and filamentous basidiomycetes reconstructed from multiple gene sequence analyses. Studies Mycol. 81:1–26.
- Lynch R, King AJ, Farías ME, Sowell P, Vitry C, Schmidt SK. 2012. The potential for microbial life in the highest elevation (>6000 m.a.s.l.) mineral soils of the Atacama region. J Geophys Res. 117:G02028.
- Madden RA. 1979. Observations of large‐scale traveling Rossby waves. Rev Geophys. 17:1935–1949.
- Mladenov N, Williams MW, Schmidt SK, Cawley K. 2012. Atmospheric deposition as a source of carbon and nutrients to an alpine catchment of the Colorado Rocky Mountains. Biogeosciences. 9:3337–3355.
- Neilson JW, Quade J, Ortiz M, Nelson WM, Legatzki A, Tian F, LaComb M, Betancourt JL, Wing RA, Soderlund CA, et al. 2012. Life at the hyperarid margin: novel bacterial diversity in arid soils of the Atacama Desert, Chile. Extremophiles. 16:553–566.
- Nemergut DR, Anderson S, Cleveland CC, Martin AP, Miller AE, Seimon A, Schmidt SK. 2007. Microbial community succession in unvegetated, recently-deglaciated soils. Microb Ecol. 53:110–122.
- Nichols CB, Perfect ZH, Alspaugh JA. 2007. A Ras1‐Cdc24 signal transduction pathway mediates thermotolerance in the fungal pathogen Cryptococcus neoformans. Molecular Microbiol. 63:1118–1130.
- Nielsen K, De Obaldia AL, Heitman J. 2007. Cryptococcus neoformans mates on pigeon guano: implications for the realized ecological niche and globalization. Eukaryot Cell. 6:949–959.
- Norros V, Rannik Ü, Hussein T, Petäjä T, Vesala T, Ovaskainen O. 2014. Do Small Spores Disperse Further than Large Spores?. Ecology. 95:1612–1621.
- Pointing SB. 2016. Hypolithic communities. In: Weber B, Büdel B, Belnap J, eds. Biological soil crusts: an organizing principle in drylands. Switzerland: Springer International; p. 199–213.
- Polvani LM, Saravanan R. 2000. The three-dimensional structure of breaking Rossby waves in the polar wintertime stratosphere. J Atmospheric Sci. 57:3663–3685.
- Polz MF, Hunt DE, Preheim SP, Weinreich DM. 2006. Patterns and mechanisms of genetic and phenotypic differentiation in marine microbes. Philos Trans R Soc B. 361:2009–2021.
- Pulschen AA, Rodrigues F, Duarte RTD, Araujo GG, Santiago IF, Paulino-Lima IG, Rosa CA, Kato MJ, Pellizari VH, Galante D. 2015. UV-resistant yeasts isolated from a high-altitude volcanic area on the Atacama Desert as eukaryotic models for astrobiology. MicrobiologyOpen. 4:574–588.
- Richter M, Schmidt D. 2002. Cordillera de la Atacama. Das trockenste Hochgebirge der Welt. Petermanns Geogr Mitt. 146:48–57.
- Schlesinger P, Mamane Y, Grishkan I. 2006. Transport of microorganisms to Israel during Saharan dust events. Aerobiologia. 22:259–273.
- Schmidt D. 1999. Das Extremklima der Nordchilenischen Hochatacama Unter Besonderer Berücksichtigung der Höhengradienten. Dresdener Geographische Beiträge. 4:1–122.
- Schmidt SK, Darcy JL, Sommers P, Gunawan E, Knelman JE, Jager K. 2017. Freeze–thaw revival of rotifers and algae in a desiccated, high-elevation (5500 meters) microbial mat, high Andes, Perú. Extremophiles. 21:573–580.
- Schmidt SK, King AJ, Karki D, Robeson MS, Nagy L, Williams M, Mitter M, Freeman KR. 2011. Phylogeography of microbial phototrophs in the dry valleys of the high Himalayas and Antarctica. Proc Roy Soc B. 278:702–708.
- Schmidt SK, Naff C, Lynch R. 2012. Fungal communities at the edge: ecological lessons from high alpine fungi. Fungal Ecol. 5:443–452.
- Schmidt SK, Nemergut DR, Miller AE, Freeman KR, King AJ, Seimon A. 2009a. Microbial activity and diversity during extreme freeze-thaw cycles in periglacial soils, 5400 m Elevation, Cordillera Vilcanota, Perú. Extremophiles. 13:807–816.
- Schmidt SK, Wilson KL, Monson RK, Lipson DA. 2009b. Exponential growth of “snow molds” at sub-zero temperatures: an explanation for high beneath-snow respiration rates and Q10 values. Biogeochemistry. 95:13–21.
- Smith DJ, Jaffe DA, Birmele MN, Griffin DW, Schuerger AC, Hee J, Roberts MS. 2012. Free tropospheric transport of microorganisms from Asia to North America. Microb Ecol. 64:973–985.
- Solon AJ, Vimercati L, Darcy JL, Arán P, Porazinska D, Dorador C, Farias ME, Schmidt SK. 2017. Landscape patterns of microbial communities on Volcáns Socompa and Llullaillaco. Microbial Ecol. (In Review).
- Takashima M, Nakase T. 1999. Molecular phylogeny of the genus Cryptococcus and related species based on the sequences of SSU rDNA and internal transcribed spacer regions. Microbiol Culture Collections. 15:35–47.
- Velagapudi R, Hsueh YP, Geunes-Boyer S, Wright JR, Heitman J. 2009. Spores as infectious propagules of Cryptococcus neoformans. Infection Immun. 77:4345–4355.
- Vimercati L, Hamsher S, Schubert Z, Schmidt SK. 2016. Growth of a high-elevation Cryptococcus sp. during extreme freeze-thaw cycles. Extremophiles. 20:579–588.
- Vishniac HS. 1985a. Cryptococcus friedmannii, a new species of yeast from the Antarctic. Mycologia. 77:149–153.
- Vishniac HS. 1985b. Cryptococcus socialis sp. nov. and Cryptococcus consortionis sp. nov., antarctic Basidioblastomycetes. Int J Syst Bacteriol. 35:119–122.
- Vishniac HS. 2006. A multivariate analysis of soil yeasts isolated from a latitudinal gradient. Microb Ecol. 52:90–103.
- Vishniac HS, Hempfling WP. 1979. Cryptooccus vishniacii sp. nov., an Antarctic Yeast. Int J Syst Bacteriol. 29:153–158.
- Vishniac HS, Kurtzman CP. 1992. Cryptococcus antarcticus sp. nov, and Cryptococcus albidosimilis sp. nov, Basidioblastomycetes from Antarctic soils. Int J Syst Bacteriol. 42:547–553.
- Westrich JR, Ebling AM, Landing WM, Joyner JL, Kemp KM, Griffin DW, Lipp EK. 2016. Saharan dust nutrients promote Vibrio bloom formation in marine surface waters. Proc Natl Acad Sci USA. 113:5964–5969.
- Wilkinson DM, Koumoutsaris S, Mitchell EAD, Bey I. 2012. Modelling the effect of size on the aerial dispersal of microorganisms. J Biogeogr. 39:89–97.
