ABSTRACT
Mycotoxins are toxic secondary metabolites produced by various filamentous fungi, of which Fusarium, Aspergillus and Penicillium are the three main genera. Fusarium verticillioides is one of the most dominant toxigenic fungal species, associated with fumonisin contamination in grain-based feeds, such as compound abalone feed. Mycotoxin production is influenced by temperature and available nutrients. In this study the aims were: to determine if abalone feed as growth substrate favours mycotoxin production for toxigenic fungi; to determine the most effective temperature for fumonisin production by F. verticillioides on abalone feed; and to assess the effect of the aquatic environment on fumonisin-contaminated abalone feed. A total of 93 fungal isolates were inoculated onto abalone feed, including species belonging to the genera Fusarium, Aspergillus and Penicillium. Feed inoculated with F. verticillioides were incubated at two different temperatures and fumonisin-contaminated feed was submerged into seawater for 24 h. Results showed that mycotoxins were produced when abalone feed was inoculated with toxigenic fungi, and that F. verticillioides produced higher concentrations of fumonisins at a lower temperature. Submerging fumonisin-contaminated feed in seawater showed that this toxin leached into the seawater, lowering the risk of fumonisins to be consumed by abalone.
Introduction
Mycotoxins are low-weight molecular secondary metabolites mainly produced in the mycelial structures of certain filamentous fungi, during fungal growth (D’Mello and Macdonald Citation1997; Placinta et al. Citation1999; Leslie and Summerell Citation2006; Bhat et al. Citation2010). These secondary metabolites are toxic to humans and animals when consumed and can even be carcinogenic, neurotoxic, nephrotoxic or immunosuppressive – especially when chronic exposure occurs (Gelderblom et al. Citation1988; Hussein and Brasel Citation2001; Lazicka and Orzechowski Citation2010; Feijó Corrêa et al. Citation2018). The most common type of exposure in humans and animals is by consumption of contaminated feedstuffs or foods (Rheeder et al. Citation1992; Bhat et al. Citation2010; Mwanza Citation2011). Fungi known to produce mycotoxins are referred to as toxigenic fungi. This group is dominated by three genera, namely Aspergillus, Penicillium and Fusarium, and to a lesser extent other genera that include Alternaria, Claviceps and Stachybotrys (Bennett and Klich Citation2003; Wambacq et al. Citation2016). Fusarium species represent a number of plant pathogens, and are responsible for infection before and during harvesting, while Penicillium and Aspergillus more commonly colonise commodities and foods during drying and storage (Sweeney and Dobson Citation1998; Placinta et al. Citation1999; Miller Citation2008; Osibona et al. Citation2018).
Aspergillus species are responsible for the production of aflatoxins. Aflatoxins are made up of several toxic compounds, of which B1 (AFB1) is the most abundantly produced. The optimum growth rate of these species and the optimum temperature for aflatoxin production are almost identical, with 10–43°C and 12–40°C, respectively (Sweeney and Dobson Citation1998). Severe liver and brain damage has been linked to aflatoxin-contaminated feed, along with other clinical symptoms, such as skin lesions, yellowing of the body surface and eye cataracts (El-Sayed and Khalil Citation2009; Baldissera et al. Citation2018). The susceptibility to aflatoxins varies between species. While warm water fish such as channel catfish are less susceptible, rainbow trout are highly susceptible (Lee et al. Citation1968; Schoenhard et al. Citation1981; Manning Citation2005; Santacroce et al. Citation2008).
Ochratoxins are produced by storage fungi, such as certain species of Penicillium and Aspergillus, with ochratoxin A (OTA) being the most common. Production of OTA by species like P. verrucosum takes place at more temperate climates (4–31°C), while production of OTA by A. ochraceaus takes place at higher temperatures up to 37°C (Moss Citation2002a; Manning Citation2010). Not only does the temperature differ between these two species for optimal OTA production, but they also have different substrate requirements. Aspergillus ochraceus produce OTA optimally when grown on oil seeds such as peanuts and soybeans while, P. verrucosum produce OTA optimally when grown on cereal grains like maize, barley and wheat (Klich Citation2007; Manning Citation2010). Ochratoxin A caused necrosis of kidney tubular and liver cells, along with a reduction in weight gain in rainbow trout (Doster et al. Citation1974; Manning Citation2005).
Mycotoxins produced by Fusarium are widespread contaminants of animal feed, and have been shown to cause biological side effects even when present at low concentrations (Marasas et al. Citation1980; Gelderblom et al. Citation1988; Rheeder et al. Citation1992; Moss Citation2002b; Tuan et al. Citation2003; Adeyemo et al. Citation2018). Trichothecenes and fumonisins are the major mycotoxin groups associated with Fusarium spp. (Sweeney and Dobson Citation1998; Hussein and Brasel Citation2001; Moss Citation2002b; Desjardins and Proctor Citation2007; Miller Citation2008). Optimal temperature for mycotoxin production (25 ± 1°C) falls within the optimal growth temperature for Fusarium spp. Fumonisins are mainly produced by F. verticillioides and are heat stable, water soluble and consist of four series, the B series being the most abundant naturally occurring group (Musser et al. Citation1996; Seo and Lee Citation1999; Yildirim et al. Citation2000; Rheeder et al. Citation2002). The metabolic disruptions caused by fumonisins in fish can lead to cellular deregulation, cell death and a decrease in weight gain (Goel et al. Citation1994; Yildirim et al. Citation2000; Tuan et al. Citation2003; Adeyemo et al. Citation2018). Reduction in weight gain of any cultured animals has a negative impact on farm production. Fumonisins are water soluble (Alberts et al. Citation1990), and it is possible that when aquatic animals are fed under normal conditions they will consume very little to no fumonisins, especially when feed is not consumed immediately, but left in the water for the animals to eat on demand. Associated risks of mycotoxins in abalone are unknown.
In South Africa, the abalone industry makes use of commercially produced feed, mainly produced from a protein source and locally sourced grains. This is a favourable medium for fungal growth (Greeff-Laubscher et al. Citation2018). The feeding ratio for these cultured abalone is ±1 g feed/952.38 mL. However, feed is not always consumed immediately and can stay in the water for up to 32 h before it is consumed or flushed by the water system. Most farms have their water completely replaced every two hours, which can potentially remove contaminants from the system.
Mycotoxin production is influenced by several factors, including environmental conditions, fungicides, fertilisers and the interactions between toxigenic fungal species. In addition, strain specificity, variation and instability can also affect mycotoxin production (Luchese and Harrigan Citation1993; Placinta et al. Citation1999; Brzonkalik et al. Citation2011). The first aim of this study was to determine the ability of previously isolated and identified toxigenic fungal species to produce mycotoxins on abalone feed, under storage conditions. The second aim was to determine the impact of two different average temperatures – 16°C and 26°C (representing winter and summer temperatures in South Africa along the coastline) on fumonisin production by F. verticillioides, previously isolated from abalone feed. The final objective was to assess the effect of the aquatic environment on fumonisin contaminated abalone feed. This is important to determine the fumonisin concentration in feed at the time of consumption. Understanding the changes in mycotoxin levels when feed contaminated with fumonisins is exposed to water, can assist in future risk assessments to determine allowable limits in compound feed intended for aquatic animals.
Materials and methods
Sample preparation
Different toxigenic genera
Twenty one (21) Fusarium, 36 Aspergillus and 36 Penicillium strains previously isolated from abalone feed, were inoculated onto PDA (Potato dextrose agar), MEA (Malt extract agar) and CYA (Czapek yeast agar) respectively, from 15% glycerol stocks that were kept at 4°C. Cultures were incubated at 26 ± 1°C for 10 days. Fusarium verticillioides MRC0826 was included to serve as a positive control for fumonisin production (Rheeder et al. Citation2002). Aspergillus flavus MRC3951 (Thembo et al. Citation2010) was included as a positive control for the production of aflatoxins and P. viridicatum MRC0356 as an ochratoxin producing strain. Negative controls were set up containing sterile feed and water with no fungal inoculum, and natural controls containing sterile water and non-sterile feed, in which no inoculum was included.
Different temperatures and water submerging
Three different isolates (MRL117, MRL124, MRL336), all identified as Fusarium verticillioides, were used in this part of the study. The selected isolates produced the highest amounts of fumonisins on abalone feed as growth substrate (previous experiment). Cultures were stored in 15% glycerol at 4°C. Cultures were first inoculated on water agar to confirm viability. Throughout the experiment cultures were kept on water agar. Seven days before inoculation, cultures were transferred onto Potato dextrose agar (PDA) and incubated at 26°C.
Inoculation and incubation conditions
Erlenmeyer flasks were prepared with abalone feed (Production date 24/04/2015; Batch #114/15) and MilliQ water in a 1 g: 1.2 mL ratio (). Flasks were covered with cotton wool and tin foil, left overnight at room temperature and autoclaved the following day. Overnight incubation was done to allow the feed to absorb the water. Sterile flasks were aseptically inoculum with agar plugs (2 mm) from one of the tester strains. Negative controls with no inoculant were included as well as natural controls. Natural controls were represented by feed left to rot naturally, resulting in a natural fungal community rather than a pure culture colonising the feed. All flasks were incubated (See for respective times and temperatures), followed by mycotoxin analyses.
Table 1. Inoculation and incubation information for three different experiments to achieve three different objectives.
In preparation for water exposure, sufficient flasks were prepared to perform fumonisin extractions on feed before water exposure, and after 24 h of water exposure. Experiments were done in triplicate. After the incubation period, flasks were randomly divided into three groups with three flasks each. One group was labelled as “before water exposure” and the second group was labelled as “water exposure”. The same protocol was followed for both the control groups.
Water exposure
Sterile plastic containers were prepared with 4 L autoclaved seawater and plastic pipes (5 mm thick) for aeration. Flasks labelled as “before water exposure” were set aside for fumonisin extractions and the contents of the flasks labelled as “water exposure” were added into the seawater directly from the Erlenmeyer flasks. Feed was left in the water for 24 h. Water samples were taken before feed was added (T0). Thereafter, water samples were taken 2 h (T1), 8 h (T2) and 24 h (T3) after the feed was added into the water. Water samples were taken with a sterile syringe and filtered through a Minisart (National Separations (Pty) Ltd., South Africa) 0.02 μm (regenerated cellulose) RC syringe filter, into a 2 mL screw neck amber glass vial and sent to the Central Analytical Facility (CAF), Stellenbosch University for fumonisin quantification, using liquid chromatography tandem mass spectrometry LC-MS/MS.
Mycotoxin analyses
A toxin extraction protocol adapted from Szécsi et al. (Citation2005) was used to extract the various mycotoxins. Extractions were done by adding 70% analytical grade methanol extraction buffer (4 mL: 1 g feed) into the flasks. Feed was crushed and stirred with a stainless steel spatula and then incubated on a shaker at 180 rpm, for 60 min at 25°C. Following incubation, 15 mL of the slurry was filtered through single layer cheesecloth into a 50 mL centrifuge tube. Tubes were centrifuged for 10 min at 4°C at 500 rpm. Thereafter, 2 mL supernatant was filtered into a 2 mL microcentrifuge tube through a Minisart (National Separations (Pty) Ltd., South Africa) 0.02 μm (regenerated cellulose) RC syringe filter. After overnight incubation at 4°C, samples were centrifuged for 10 min at 14,000 rpm. After centrifugation, 1800 μL supernatant was transferred to a 2 mL screw neck amber glass vial (Rose et al. Citation2013). The analytes, together with the freshly prepared standard dilution series of known concentration, were sent to the Central Analytical Facility (CAF), Stellenbosch University for quantification of fumonisins (FB1, FB2 and FB3), trichothecenes (DON, NIV, 15ADON, Fx) ZEA, aflatoxins (AFB1, AFG1, AFB2 and AFG2), and OTA, using liquid chromatography tandem mass spectrometry (LC-MS/MS). The analytical standards, used to prepare the standard dilution series, were obtained from Sigma- Aldrich (South Africa), and were all guaranteed >98% pure. See for the concentrations measured.
Table 2. Mycotoxin measuring limits (mg/L) for this study.
Mycotoxins were detected in the sterile control samples. These mycotoxins were not produced under laboratory conditions and were present in the feed when collected from the supplier. Values from analysis of the sterile control samples were considered as being the mycotoxin concentration of feed without any fungal growth. These values served as a baseline and were deducted from the mycotoxin values measured in inoculated samples to achieve a true value of the mycotoxin concentration produced by a specific isolate. Provision was made for potential sample carry-over, by deducting 0.05% (% specified by the auto-sampler) from values following readings greater than 0.5 mg/L. Sample carry-over occurs when the liquid of a previous sample elutes upon subsequent samples due to the chemical/physical characteristics of the sample, analysis system or both. This takes place especially when very high concentrations of the measuring substance, in this case the mycotoxins, are present in a single sample. As a result, a subsequent sample is contaminated and does not reflect the true concentration of the specific sample. Samples that were exposed to water for 24 h were centrifuged at 800 rpm for 10 min prior to fumonisin extractions, to get rid of the excess water.
Statistical analysis
STATISTICA 13 software was used to perform statistical analyses. To determine the effect of temperature on fumonisin production over 10 weeks, factorial analysis of variance (ANOVA), was performed. Tests for equal variance failed, due to the high amount of zero values. Logarithmic (Ln) transformations were used as follows to transform data closer to normality: Ln(x + 1), where x is the fumonisin concentration measured. Bonferroni method was used for post hoc multiple comparisons between time intervals. Significance was assigned to p-values <0.05.
Results
Different toxigenic genera
Aspergillus
Only AFB1 and AFB2 () were detected in the samples and no AFG1 and AFG2. One A. oryzae isolate produced more than 60 mg/L AFB1 and more than 20 mg/L AFB2, which were the highest aflatoxin levels detected in this study. Aspergillus flavus MRC3951 produced both AFB1 and AFB2 on the feed. Seven out of the nine A. flavus isolates from this study produced aflatoxins, ranging from 0.3 to 45.7 mg/L AFB1 and from 0.02 to 8.8 mg/L AFB2. Other aflatoxin-producing species from this study included A. effusus and A. tennesseensis.
Table 3. Measured aflatoxin concentration (mg/L) produced by Aspergillus spp. inoculated on abalone feed.
Penicillium
Ochratoxin A (OTA) was not detected in any of the feed samples inoculated with Penicillium isolates in this study. No OTA was detected in either the sterile control or the natural control. Penicillium viridicatum MRC356 produced OTA as high as 536.4 mg/L on abalone feed. However, this value exceeded the highest measurable concentration, and could therefore be regarded as an inaccurate value, but is still regarded as positive. Isolates used in this study included a number of species, namely P. crustosum, P. chrysogenum, P. polonicum, P. melanoconidium, P. aethiopicum, P. griseofulvum, P. novae-zeelandiae and P. corylophilum. These isolates were unable to produce OTA when colonising abalone feed, in contrast to the strains used as a positive control, P. viridicatum MRC356, which were able to produce high levels of OTA.
Fusarium
Fusarium verticillioides MRC826 along with 15 isolates in this study produced FB1, FB2 and FB3 when growing on abalone feed (). No trichothecenes (TCT) were detected in any of the isolates. Feed (sterile control) used in this experiment showed low levels of natural fumonisin contamination with no TCT’s present (). All F. verticillioides isolates produced all three groups of fumonisins, with isolate MRL124 producing the highest concentrations. Only some of the F. subglutinans isolates produced low levels of fumonisins. Single isolates of F. chlamydosporum and F. oxysporum produced low levels and no fumonisins, respectively.
Table 4. Measured fumonisin concentration (mg/L) produced by Fusarium spp. inoculated on abalone feed.
Fumonisin production at different temperatures
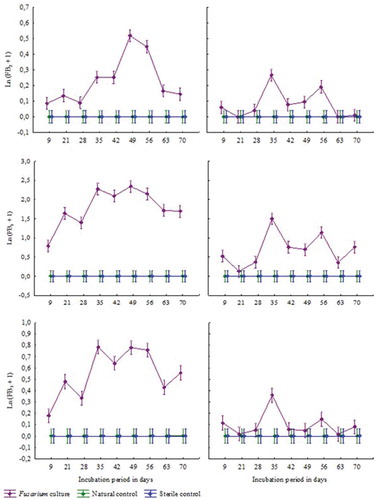
Over the 70-day incubation period fungal growth could be seen at both incubation temperatures on feed inoculated with Fusarium verticillioides, and natural controls, while no fungal growth took place on sterile control samples. No fumonisins were detected in the sterile controls. Although growth was visible on the natural controls from as early as 9 days, no fumonisins were detected in any of these samples (). Fumonisin levels stayed stable in both the sterile and natural controls over 70 days. In contrast, fumonisin production could be measured in samples inoculated with F. verticillioides from day 9. A significant increase in production of FB1, FB2 and FB3 took place at day 35 for both the incubation temperatures (). The highest total fumonisin levels were measured at 16°C after 49 days (). The measured concentration was 11.7533 mg/L while the highest levels at 26°C were 4.3225 mg/L, measured after 35 days (). Overall, fumonisin production was higher at 16°C than at 26°C. Fumonisin B1 was the most abundantly produced fumonisin at both temperatures by the Fusarium isolate.
Table 5. Fumonisin production on abalone feed, by Fusarium verticillioides, at 16°C and 26°C, over a 70 day incubation period.
Water exposure
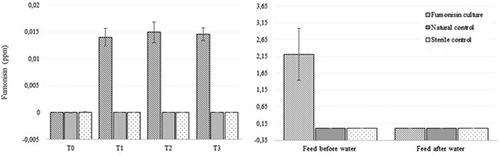
Results showed a significant increase in total fumonisins measured in the water at the first measurement taken after contaminated feed was placed in the water and a significant decrease in fumonisin concentrations in feed (no fumonisins detected) that was soaked in seawater for 24 h ().
Discussion
South African abalone farms are located along the coastline where the climate is known to be humid, creating the optimum conditions for fungal growth when compound feeds are stored (Whitlow & Hagler Jr. Citation2005). Some fungal genera such as Fusarium, Aspergillus and Penicillium produce toxic secondary metabolites better known as mycotoxins (Bezuidenhout et al. Citation1988; Cole Citation1986; Wei and Jong Citation1986; Gelderblom et al. Citation1988; Cutuli et al. Citation1991; Rheeder et al. Citation1992; Cabañes et al. Citation2010). Mycotoxin production is species- and strain-dependent, and is influenced by the growth substrate and environmental conditions (D’Mello and Macdonald Citation1997; Melcion et al. Citation1997; Brzonkalik et al. Citation2011; Garcia et al. Citation2012).
The first part of this study aimed to assess the potential of fungal isolates to produce mycotoxins when re-inoculated onto abalone feed. Isolates used during this study were previously isolated from abalone feed and feed ingredients (Greeff-Laubscher et al. Citation2018). In total 94 isolates were inoculated onto abalone feed to assess potential mycotoxin production at standardised temperatures over 6 weeks. Formulated abalone feeds are made up of a mixture of grains together with protein, making it an ideal substrate, not only for fungal growth but also for mycotoxin production (Diener and Davis Citation1969; Burgess et al. Citation1981; Cassini Citation1981; Greeff-Laubscher et al. Citation2018). This was indeed the finding for the positive control strains in this study. All three strains that were used as positive controls from each genus were able to produce their respective mycotoxins.
Most Aspergillus flavus strains are known aflatoxin producers (D’Mello and Macdonald Citation1997; Dutta and Das Citation2001; Moss Citation2002a; Bennett and Klich Citation2003; Johnson et al. Citation2004; Manning Citation2005; Klich Citation2007). Even though all the isolates in this study were subjected to the same environmental conditions during incubation, two A. flavus isolates did not produce aflatoxins while the others produced varied concentrations of aflatoxins, with the highest total aflatoxin level of 54.5 mg/L (MRL332) detected on abalone feed. This is relatively low compared to previous studies where aflatoxin production ranged from less than 10 mg/L to >100 mg/L in sucrose based liquid growth medium (Horn and Dorner Citation1999; Geiser et al. Citation2000). Even though A. flavus and A. oryzae have several homologues of aflatoxin biosynthesis pathway genes, genetic defects have led to the silencing of the aflatoxin pathway in A. oryzae (Watson et al. Citation1999; Takahashi et al. Citation2002). This has been supported by many studies (Wei and Jong Citation1986; Barbesgaard et al. Citation1992; Geiser et al. Citation2000; Bennett and Klich Citation2003; Klich Citation2007). Blumenthal (Citation2004) lists a few other toxic secondary metabolites produced by A. oryzae, but supports the theory that A. oryzae does not produce aflatoxins (Geiser et al. Citation2000). In contrast, two different studies found that A. oryzae originally isolated from wheat grains and meat products, produced aflatoxins when re-inoculated onto wheat and liquid media respectively (El-Kady et al. Citation1994; Atalla et al. Citation2003). Despite these findings, the overall agreement is still that A. oryzae is non-toxigenic, and that isolates could have been wrongly identified, such as A. oryzae NRRL1988, which was reported to produce aflatoxins but was later re-identified as A. parasiticus (Fennell and Morse Citation1976; Blumenthal Citation2004). This possibility is not unlikely as A. oryzae falls within the A. flavi clade, and is closely related to toxigenic species (Samson et al. Citation2014). Considering the effect that the growth substrate and other environmental conditions have on mycotoxin production (D’Mello and Macdonald Citation1997), we suggest that some A. oryzae isolates are toxigenic but that they are dependent on the substrate for mycotoxin formation. From this study, one of the two A. oryzae isolates tested produced both AFB1 and AFB2. Aflatoxin production by A. oryzae MRL304 was exceptionally high, the highest of all the isolates tested. Thus it is suggested that more research is conducted on this specific isolate to confirm identification and toxin production on a variety of growth substrates.
No OTA was detected in abalone feed inoculated and incubated with Penicillium species. Isolates used in this study were unable to produce detectable limits of OTA when colonising abalone feed, in contrast to the strain used as a positive control, P. viridicatum MRC356, which produced high levels of OTA. However, P. viridicatum MRC356 produced OTA concentrations exceeding the maximum level of detection. This is an indication that if toxigenic Penicillium species colonise abalone feed, ochratoxins can be produced – but because the isolates tested in this study did not produce detectable levels of OTA, they are potentially non-toxigenic and therefore not a concern to the abalone industry.
Fusarium verticillioides is a species well known to produce fumonisins on many different substrates (Bezuidenhout et al. Citation1988; Marasas et al. Citation1988; Rheeder et al. Citation1992; Beukes Citation2015; Janse van Rensburg et al. Citation2017). All the F. verticillioides strains in this study produced mycotoxins, ranging from 0.33 mg/L to 12.43 mg/L. The strains with the highest levels were almost 10 times higher than the positive control. The isolate used as a positive control, F. verticillioides MRC826, produced surprisingly low levels of fumonisins on abalone feed, with a total fumonisin concentration of only 1.19 mg/L, of which 1.03 mg/L were FB1. This is contrary to a study by Gelderblom et al. (Citation1988) who inoculated the same strain (F. verticillioides MRC826) onto maize and incubated at the same temperature for a shorter period of time. They were able to extract up to 2 g FB1 from 1 kg culture material (2000 mg/L), indicating that although formulated abalone feed favours fumonisin production, it may not be the best substrate to promote fumonisin production specifically for F. verticillioides MRC826. However, the higher production levels of the other F. verticillioides isolates tested in this study indicate that compound abalone feed is a suitable substrate for fumonisin production, and that their ability varies between strains. Fumonisin B1 was the most abundantly produced toxin throughout this study. Formulated abalone feed and shrimp feed consists of similar ingredients, thus it would be expected that isolates from these two feeds would perform similarly in terms of mycotoxin productions (Anukul et al. Citation2014). However, FB1 production by isolates in this study ranged from similar to higher levels than those reported for F. verticillioides strains isolated from shrimp feed (Anukul et al. Citation2014). Isolates from that study (Anukul et al. Citation2014) produced FB1 ranging between 1600 and 3600 ng/g (1.6–3.6 mg/L). The difference in results could be because toxin production of isolates from Anukul et al. (Citation2014) were tested on sterile maize and not formulated feed, which is therefore not a true reflection of the anticipated FB1 production when growing on formulated feed. Furthermore, even though incubation temperatures for the two studies were similar, incubation time in the current study was 4 weeks longer, giving the fungal isolates more time to produce mycotoxins (D’Mello and Macdonald Citation1997).
Many authors have stated that fumonisin production is influenced by environmental factors such as substrate, pH, temperature and humidity (D’Mello and Macdonald Citation1997; Melcion et al. Citation1997; Parsons Citation2008; Garcia et al. Citation2012; Janse van Rensburg et al. Citation2017). In the first part of this study, it was concluded that F. verticillioides isolates, originally isolated from abalone feed, can produce fumonisins when re-inoculated onto abalone feed and left to colonise. This should be of concern, because abalone farms in South Africa are mainly located along the coast where humidity is high with average temperatures of 16°C ± 2°C and 26°C ± 2°C during winter and summer months, respectively. These temperatures fall well within the range required for fungal growth and fumonisin production by Fusarium species (Alberts et al. Citation1990; Janse van Rensburg et al. Citation2017).
The second part of the current study evaluated fumonisin production by F. verticillioides, isolated from abalone feed, on the feed at two different temperatures, over a period of 10 weeks. Conditions in this study simulated storage temperatures of abalone feed on abalone farms in South Africa. This fungus was able to grow at both temperatures without difference in macro-growth characteristics, except for a slight colour change from white to beige. These results support the findings of Cahagnier et al. (Citation1995), that fumonisin production can be influenced by environmental factors without influencing the fungal growth. Therefore, when growth is visible, regardless of the amount of biomass, it is possible that fumonisin production is in progress, but mycotoxin analyses is needed to confirm the level of contamination (Melcion et al. Citation1997). Although previous studies reported 25°C ± 2°C to be optimal temperatures for fumonisin production (Alberts et al. Citation1990; Melcion et al. Citation1997), this study showed that fumonisin production was higher at 16°C than at 26°C (). However, the fumonisin production per unit in this study was not even a fraction of the amount reported by previous studies, where fumonisin production on maize measured >14,000 mg/L and 850 mg/L, after 7 and 10 days respectively (Alberts et al. Citation1990; Melcion et al. Citation1997). In contrast, Garcia et al. (Citation2012) reported less than 0.02 mg/L fumonisin after F. verticillioides was incubated on sterile soybeans for 21 days at different temperatures ranging between 15°C and 30°C. Although the fumonisin levels measured in the latter study were lower than the levels measured in our study, it is closer to this study and more comparable. One of the differences between these studies is the growth substrate, which is categorised as a biological factor that influences mycotoxin production. There appears to be a link between the protein content of these substrates and the fumonisin production reported by the different authors, including the results found in this study. Soybeans have the highest protein content of 48–50%, but low levels of fumonisin production were reported on this substrate (Garcia et al. Citation1997, Citation2012). Abalone feed used in this study contains 35% protein, and fumonisin levels were measured between 0.157 and 11.753 mg/L, while maize contains only 8–11% protein, but had the highest fumonisin production reported (Alberts et al. Citation1990; Melcion et al. Citation1997; South African Grain Laboratory Citation2011; FAO Citation2016; Marifeed Citation2016). Although more research is needed, it could be hypothesised that protein content in the growth substrate influences the fumonisin production, and that higher protein content reduces fumonisin production. This could possibly be because of the difference in solubility of soybean protein compared to maize protein (Castro-Rubio et al. Citation2006). Therefore, the amount and source of protein in abalone feed should be taken into consideration when formulating feed. This is especially important as agricultural commodities used in processing can also be a source for fumonisin contamination (Greeff-Laubscher et al. Citation2018).
In contrast to the Penicillium isolates from this study that appear to be non-toxigenic, results showed that some of the Fusarium and Aspergillus isolates are toxigenic and can produce potent mycotoxins when grown on compound abalone feed under certain conditions. Although Aspergillus spp. isolated from abalone feed were able to produce aflatoxins when re-inoculated onto abalone feed, no detectable levels of aflatoxins were measured on abalone feed and ingredients in a previous study (Greeff-Laubscher et al. Citation2018). Therefore, when feed is kept fresh and stored correctly it lowers the risk of aflatoxin contamination. This was confirmed by the absence of detectable toxins in the natural control samples. These samples were not inoculated with a pure culture but rather left to mould naturally, in order to reflect feed that could be left in storerooms to mould.
With more species present and representing many different genera, interactions between species, such as competition for nutrients, takes place. When naturally-occurring fungal species colonised the feed it was likely that either none of the mycotoxins tested in this study were produced above detectable limits; or it is possible that these interactions had a detoxifying effect on the feed, as mycotoxin concentrations measured on sterile feed were higher than on the natural controls. Mycotoxin production in this study was shown to be strain-dependent, thus it is recommended that the industry make use of routine screening for possible toxigenic fungal contamination in parallel with mycotoxin contamination on feed. To date, mycotoxin risk assessments to establish allowable mycotoxin contamination levels in abalone feed and ingredients, have not been conducted for the South African market. It is expected that high levels of mycotoxins can decrease the growth rate in abalone, similar to other aquatic animals. However, fumonisin levels measured in this study were substantially lower than any current international maximum allowable limits (CitationFood standards agency; FDA Citation2001; National Department of Agriculture Citation2006). Abalone feed used in this study had a moisture content of 8.5–11.5%.
The abundant FB1 production ( and ) is in agreement with current available literature (Schumacher et al. Citation1995; Melcion et al. Citation1997; Rheeder et al. Citation2002; Moss Citation2002b; Waśkiewicz et al. Citation2015). While a number of studies investigated the effect of fumonisins on aquatic animals such as catfish and Nile tilapia (Goel et al. Citation1994; Tuan et al. Citation2003; Manning Citation2005; Soriano et al. Citation2005), this is the first study to investigate the effect of seawater on fumonisin-contaminated abalone feed, and showed that FB1 was the only fumonisin that could be detected in the water even after 24 h. This is especially important in understanding the risks involved when feed is not consumed immediately. During this study, fumonisin levels in the abalone feed decreased significantly when submerged into the water, while FB1 concentration in the water increased significantly (). This is a strong indication that fumonisins leach from the feed into water, in less than 2 h. Although this reduces the concern of abalone consuming fumonisin-contaminated feed, it increases the potential risk to the direct surrounding environment. If fumonisin-contaminated water gets in contact with soil at any stage, fumonisins could stay behind posing a threat to the surrounding ecosystem (Williams et al. Citation2003). It is worth noting that the fumonisin concentrations measured in the water were below 1 mg/L due to the high dilution factor. The same feed to water ratio used on abalone farms were used in this study. Considering the high dilution factor, the environmental risk is considered to be exceedingly small and unlikely to be problematic. However, it was previously suggested that more studies should be conducted on a wider scale to provide detailed explanation of fumonisin migration in the environment and the risk for the ecosystem and wild fish in their natural habitat (Waśkiewicz et al. Citation2015). Therefore, it is recommended that the presence of fumonisins and other mycotoxins be monitored, not only on abalone farms but on any animal farm that uses compound feed, until the effect that migrating fumonisins or any mycotoxins have on the environment and wild fish, is fully understood and proven to have no damaging effect.
Acknowledegments
This research was supported by the Stellenbosch University. The first author was supported by the National Research Foundation (NRF) and the Agricultural sector education training authority (AgriSETA). We thank the anonymous abalone feed supplier for supplying abalone feed and the South African Medical Research Council (MRC) for the provision of toxigenic fungal strains used in this analysis.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Adeyemo BT, Tiamiyu LO, Ayuba VO, Musa S, Odo J. 2018. Effects of dietary mixed aflatoxin B1 and fumonisin B1 on growth performance and haematology of juvenile Clarias gariepinus catfish. Aquaculture. 491:190–196.
- Alberts JF, Gelderblom WCA, Thiel PG, Marasas WFO, Van Schalkwyk DJ, Behrend Y. 1990. Effects of Temperature and incubation period on production of Fumonisin B1 by Fusarium moniliforme. Appl Environ Microbiol. 56:1729–1733.
- Anukul N, Maneeboon T, Roopkham C, Chuaysrinule C, Mahakarnchanakul W. 2014. Fumonisin and T-2 toxin production of Fusarium spp. isolated from complete feed and individual agricultural commodities used in shrimp farming. Mycotoxin Res. 30:9–16.
- Atalla MM, Hassanein NM, El-Beih AA, Youssef YAG. 2003. Mycotoxin production in wheat grains by different Aspergilli in relation to different relative humidities and storage periods. Nahrung - Food. 47:6–10.
- Baldissera MD, Souza CF, Zeppenfeld CC, Descovi SN, Moreira KLS, da Rocha MIUM, da Veiga ML, da Silva AS, Baldisserotto B. 2018. Aflatoxin B1-contaminated diet disrupts the blood–brain barrier and affects fish behavior: involvement of neurotransmitters in brain synaptosomes. Environ Toxicol Pharmacol. 60:45–51.
- Barbesgaard P, Heldt-Hansen HP, Diderichsen B. 1992. On the safety of Aspergillus oryzae: a review. Appl Microbiol Biotechnol. 36:569–572.
- Bennett JW, Klich M. 2003. Mycotoxins. Clin Microbiol Rev. 16:497–516.
- Beukes I. 2015. Pathogenicity and mycotoxin production of the Fusarium graminearum species complex in South African grains. [place unknown]: Stellenbosch university.
- Bezuidenhout SC, Gelderblom WCA, Gorstallman CP, Horak RM, Marasas WFO, Spiteller G, Vleggaar R. 1988. Structure elucidation of the fumonisins, mycotoxins from Fusarium moniliforme. J Chem Soc Commun. 1730:743–745.
- Bhat R, Rai RV, Karim AA. 2010. Mycotoxins in food and feed. Present status and future concerns. Compr Rev Food Sci Food Saf. 9:57–81.
- Blumenthal CZ. 2004. Production of toxic metabolites in Aspergillus niger, Aspergillus oryzae and Trichoderma reesi: justification of mycotoxin testing in food grade enzyme preparations derived from the three fungi. Regul Toxicol Pharmacol [Internet]. 39:214–228. Available from: http://linkinghub.elsevier.com/retrieve/pii/S0273230003001247.
- Brzonkalik K, Herrling T, Syldatk C, Neumann A. 2011. The influence of different nitrogen and carbon sources on mycotoxin production in Alternaria alternata. Int J Food Microbiol [Internet]. 147:120–126.
- Burgess LW, Dodman RL, Pont W, Mayers P. 1981. Fusarium diseases of wheat, maize and grain sorghum in Eastern Australia. In: Nelson PE, Toussoun TA, Cook RJ, editors. Fusarium Dis Biol Taxon. University Park: The Pennsylvania State University Press; p. 64–76.
- Cabañes FJ, Bragulat MR, Castellá G. 2010. Ochratoxin A producing species in the genus Penicillium. Toxins (Basel). 2:1111–1120.
- Cahagnier B, Melcion D, Richard-Molard D. 1995. Growth of Fusarium moniliforme and its biosynthesis of fumonisin B1 on maize grain as a function of different water activities. Lett Appl Microbiol. 20:247–251.
- Cassini R. 1981. Fusarium diseases of wheat and corn in Western Europe. In: Nelson PE, Toussoun TA, Cook RJ, editors. Fusarium Dis Biol Taxon. University Park and Lonon: The Pennsylvania State University Press; p. 56–63.
- Castro-Rubio A, García MC, Marina ML. 2006. Rapid separation of soybean and cereal (wheat, corn, and rice) proteins in complex mixtures: application to the selective determination of the soybean protein content in commercial cereal-based products. Anal Chim Acta. 558:28–34.
- Cole RJ. 1986. Etiology of turkey ’X’disease in retrospect: a case for the involvement of cyclopiazonic acid. Mycotoxin Res. 2:3–7.
- Cutuli MT, Cuellar A, Camara JM, Mateos A, Suarez G. 1991. Different media and methodologies for the detection of aflatoxin production by Aspergillus flavus strain isolated from trout feed. Mycopathologia. 113:121–125.
- D’Mello JPF, Macdonald AMC. 1997. Mycotoxins. Anim Feed Sci Technol. 69:155–166.
- Desjardins AE, Proctor RH. 2007. Molecular biology of Fusarium mycotoxins. Int J Food Microbiol [Internet]. 119:47–50; [accessed 2013 Mar 12. http://www.ncbi.nlm.nih.gov/pubmed/17707105.
- Diener UL, Davis ND. 1969. Aflatoxin formation by Aspergillus flavus. In: Goldblatt LA, editor. Aflatoxin Sci background, control implic. New York: Academic press; p. 13–54.
- Doster RC, Sinnhuber RO, Pawlowski NE. 1974. Acute intraperitoneal toxicity of ochratoxin a and B derivatives in rainbow trout (Salmo gairdneri). Food Cosmet Toxicol [Internet]. 12:499–505.http://www.sciencedirect.com/science/article/pii/0015626474900637.
- Dutta TK, Das P. 2001. Isolation of aflatoxigenic strains of Aspergillus and detection of aflatoxin B1 from feeds in India. Mycopathologia. 151:29–33.
- El-Kady I, El-Maraghy S, Zohri A. 1994. Mycotoxin producing potential of some isolates of Aspergillus flavus and Eurotium groups from meat products. Microbiol Res. 149:297–307.
- El-Sayed YS, Khalil RH. 2009. Toxicity, biochemical effects and residue of aflatoxin B(1) in marine water-reared sea bass (Dicentrarchus labrax L.). Food Chem Toxicol [Internet]. 47:1606–1609. [accessed 2013 Apr 17]. http://www.ncbi.nlm.nih.gov/pubmed/19375478.
- FAO. 2016. Maize in human nutrition. Agric Consum Prot Dep [Internet]. [accessed 2016 Apr 3]. http://www.fao.org/agriculture-consumer-protection-department/en/.
- FDA. 2001. Background Paper in Support of Fumonisin Levels in Animal Feed: Executive Summary of this Scientific Support Document [Internet]. [accessed 2016 Mar 2]. http://www.fda.gov/Food/FoodborneIllnessContaminants/NaturalToxins/ucm212900.htm
- Feijó Corrêa JA, Orso PB, Bordin K, Hara RV, Luciano FB. 2018. Toxicological effects of fumonisin B1in combination with other Fusarium toxins. Food Chem Toxicol [Internet]. 121:483–494.
- Fennell I, Morse RE. 1976. Aspergillus oryzae (NRRL strain 1988): a clarification. Am Assoc Adv Sci. 194:1188.
- Food standards agency. Mycotoxins in animal feed [Internet]. [accessed 2016 Apr 14]. http://www.food.gov.uk/business-industry/farmingfood/crops/mycotoxinsguidance/animalfeed
- Garcia D, Barros G, Chulze S, Ramos AJ, Sanchis V, Marín S. 2012. Impact of cycling temperatures on Fusarium verticillioides and Fusarium graminearum growth and mycotoxins production in soybean. J Sci Food Agric. 92:2952–2959.
- Garcia MC, Torre M, Marina ML, Laborda F, Rodriquez AR. 1997. Composition and characterization of soyabean and related products. Crit Rev Food Sci Nutr. 37:361–391.
- Geiser DM, Dorner JW, Horn BW, Taylor JW. 2000. The phylogenetics of mycotoxin and sclerotium production in Aspergillus flavus and Aspergillus oryzae. Fungal Genet Biol. 31:169–179.
- Gelderblom WC, Jaskiewicz K, Marasas WF, Thiel PG, Horak RM, Vleggaar R, Kriek NP. 1988. Fumonisins-novel mycotoxins with cancer-promoting activity produced by Fusarium moniliforme. Appl Environ Microbiol [Internet]. 54:1806–1811. http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=202749&tool=pmcentrez&rendertype=abstract.
- Goel S, Lenz SD, Lumlertdacha S, Lovell RT, Shelby RA, Li M, Riley RT, Kemp BW. 1994. Sphingolipid levels in catfish consuming Fusarium moniliforme corn culture containing fumonisins. Aquat Toxicol. 30:285–294.
- Greeff-Laubscher M, Beukes I, Marais GJ, Jacobs K. 2018. The occurrence of mycotoxigenic fungi in abalone feed in South Africa. African J Mar Sci. 40:383–394.
- Horn BW, Dorner JW. 1999. Regional differences in production of aflatoxin B1 and cyclopiazonic acid by soil isolates of Aspergillus flavus along a transect within the United States. Appl Environ Microbiol. 65:1444–1449.
- Hussein HS, Brasel J. 2001. Toxicity, metabolism, and impact of mycotoxins on humans and animals. Toxicology. 167:101–134.
- Janse van Rensburg B, McLaren NW, Flett BC. 2017. Grain colonization by fumonisin-producing Fusarium spp. and fumonisin synthesis in South African commercial maize in relation to prevailing weather conditions. Crop Prot [Internet]. 102:129–136.
- Johnson RA, Zabrecky J, Kiryu Y, Shields JD. 2004. Infection experiments with Aphanomyces invadans in four species of estuarine fish. J Fish Dis. 27:287–295.
- Klich MA. 2007. Aspergillus flavus: the major producer of aflatoxin. Mol Plant Pathol. 8:713–722.
- Lazicka K, Orzechowski O. 2010. The characteristics of the chosen mycotoxins and their toxic influence on the human and animal metabolism. Nat Sci [Internet]. 2:544–550. http://www.scirp.org/journals/NS.
- Lee DJ, Wales JH, Ayres JL. 1968. Synergism between cyclopropenoid fatty acids and chemical carcinogens in rainbow trout (Salmo gairdneri). Cancer Res. 28:2312–2318.
- Leslie JF, Summerell BA. 2006. The Fusarium laboratory manual. First. [place unknown]: Blackwell Publishing
- Luchese RH, Harrigan WF. 1993. Biosynthesis of aflatoxin-the role of nutritional factors. J Appl Bacteriol. 74:5–14.
- Manning BB. 2005. Mycotoxins in aquaculture. In: Diaz D, editor. mycotoxin blue B. 1st ed. Nottingham: Nottingham University press; p. 139–154.
- Manning BB. 2010. Mycotoxins in aquaculture feeds. South Reg Aquac Cent. https://srac.tamu.edu/serveFactSheet/221.
- Marasas WFO, Kellerman TS, Gelderblom WC, Coetzer JA, Thiel PG, van der Lugt JJ. 1988. Leukoencephalomalacia in a horse induced by fumonisin B1 isolated from Fusarium moniliforme. Onderstepoort J Vet Res. 55:197–203.
- Marasas WFO, Wehner FC, van Rensburg SJ, Van Schalkwyk DJ. 1980. Mycoflora of corn produced in human esophageal cancer areas in Transkei, Southern Africa. Phytopathology. 71:792–796.
- Marifeed. 2016. Abfeed [Internet]. [accessed 2016 Apr 3]. http://www.marifeed.com/abfeed/.
- Melcion D, Cahagnier B, RichardMolard D. 1997. Study of the biosynthesis of fumonisins B1, B2 and B3 by different strains of Fusarium moniliforme. Lett Appl Microbiol [Internet]. 24:301–305. //a1997wv22400016.
- Miller JD. 2008. Mycotoxins in small grains and maize: old problems, new challenges. Food Addit Contam. 25:219–230.
- Moss MO. 2002a. Mycotoxin review - 1. Aspergillus and Penicillium. Mycologist [Internet]. 16:1–4. http://www.journals.cambridge.org/abstract_S0269915X02003014.
- Moss MO. 2002b. Mycotoxin review - 2. Fusarium. Mycologist. 16:2–5.
- Musser SM, Gay ML, Mazzola EP. 1996. Identification of a new series of fumonisins containing 3-hydroxypyridine. J Nat Prod. 59:970–972.
- Mwanza M. 2011. A comparative study of fungi and mycotoxin contamination in animal products from selected rural and urban areas of South Africa with particular reference to the impact of this on the health of rural black people. [place unknown]: University of Johannesburg.
- National Department of Agriculture. 2006. Fertilizers, Farm Feeds, Agricultural Remedies and Stock Remedies Act 36 of 1947; p. 3–98.
- Osibona A, Ogunyebi O, Samual T. 2018. Storage fungi and mycotoxins associated with stored smoked Catfish (Clarias gariepinus). J Appl Sci Environ Manag. 22:643–646.
- Parsons MW. 2008. Biotic and abiotic factors associated with Fusarium ear rot of maize caused by Fusarium verticillioides [Internet]. [place unknown]: Iowa State University. http://lib.dr.iastate.edu/etd/11603/.
- Placinta CM, D’Mello JPF, Macdonald AMC. 1999. A review of worldwide contamination of cereal grains and animal feed with Fusarium mycotoxins. Anim Feed Sci Technol. 78:21–37.
- Rheeder JP, Marasas WFO, Thiel PG, Sydenham EW, Shephard GS, Van Schalkwyk DJ. 1992. Fusarium moniliforme and fumonisins in corn in relation to human esophageal cancer in Transkei. Postharvest Pathol mycotoxins. 82:353–357.
- Rheeder JP, Marasas WFO, Vismer HF. 2002. Production of fumonisin analogs by Fusarium species. Appl Environ Microbiol. 68:2101–2105.
- Rose LJ, Mouton M, Beukes I, Flett BC, van der Vyver C, Viljoen A. 2013. Multi-environment evaluation of Maize Inbred lines for resistance to Fusarium Ear Rot and Fumonisins. Plant Dis. 100:2134–2144.
- Samson RA, Visagie CM, Houbraken J, Hong SB, Hubka V, Klaassen CHW, Perrone G, Seifert KA, Susca A, Tanney JB, et al. 2014. Phylogeny, identification and nomenclature of the genus Aspergillus. Stud Mycol [Internet]. 78:141–173. http://linkinghub.elsevier.com/retrieve/pii/S0166061614000050.
- Santacroce MP, Conversano MC, Casalino E, Lai O, Zizzadoro C, Centoducati G, Crescenzo G. 2008. Aflatoxins in aquatic species: metabolism, toxicity and perspectives. Rev Fish Biol Fish. 18:99–130.
- Schoenhard GL, Hendricks JD, Nixon JE, Lee DJ, Wales JH, Russell O, Pawlowski NE. 1981. Aflatoxicol-induced Hepatocellular carcinoma in Rainbow Trout (Salmo gairdneri) and the synergistic effects of cyclopropenoid fatty acids aflatoxicol-induced. Cancer Res. 41:1011–1014.
- Schumacher J, Mullen J, Shelby R, Lenz S, Ruffin DC, Kemppainen BW. 1995. An investigation of the role of Fusarium moniliforme in duodenitis/proximal jejunitis of horses. Vet Hum Toxicol. 37:39–45.
- Seo J, Lee Y. 1999. Natural occurence of the C series of fumonisins in moldy corn. Appl Environ Microbiol. 65:1331.
- Soriano JM, Gonzalez L, Catala AI. 2005. Mechanism of action of sphingolipids and their metabolites in the toxicity of fumonisin B1. Prog Lipid Res. 44:345–356.
- South African Grain Laboratory. 2011. The South African Grain Laboratory NPC [Internet]. [accessed 2016 Apr 3]. http://www.sagl.co.za/Maize/SAAverages.aspx.
- Sweeney MJ, Dobson ADW. 1998. Mycotoxin production by Aspergillus, Fusarium and Penicillium species. Int J Food Microbiol. 43:141–158.
- Szécsi Á, Bartók T, Varga M, Magyar D, Mesterházy Á. 2005. Determination of trichothecene chemotypes of Fusarium graminearum strains isolated in Hungary. J Phytopathol. 153:445–448.
- Takahashi T, Chang PK, Matsushima K, Yu J, Abe K, Bhatnagar D, Cleveland TE, Koyama Y. 2002. Nonfunctionality of Aspergillus sojae aflR in a strain of Aspergillus parasiticus with a disrupted aflR gene. Appl Environ Microbiol. 68:3737–3743.
- Thembo KM, Vismer HF, Nyazema NZ, Gelderblom WCA, Katerere DR. 2010. Antifungal activity of four weedy plant extracts against selected mycotoxigenic fungi. J Appl Microbiol. 109:1479–1486.
- Tuan NA, Manning BB, Lovell RT, Rottinghaus GE. 2003. Responses of Nile tilapia (Oreochromis niloticus) fed diets containing different concentrations of moniliformin or fumonisin B1. Aquaculture. 217:515–528.
- Wambacq E, Vanhoutte I, Audenaert K, De Gelder L, Haesaert G. 2016. Occurrence, prevention and remediation of toxigenic fungi and mycotoxins in silage: A review. J Sci Food Agric. 96:2284–2302.
- Waśkiewicz A, Bocianowski J, Perczak A, Goliński P. 2015. Occurrence of fungal metabolites - fumonisins at the ng/L level in aqueous environmental samples. Sci Total Environ. 524–525:394–399.
- Watson AJ, Fuller LJ, Jeenes DJ, Archer DB. 1999. Homologs of aflatoxin biosynthesis genes and sequence of aflR in Aspergillus oryzae and Aspergillus sojae. Appl Environ Microbiol. 65:307–310.
- Wei D-L, Jong S-C. 1986. Production of aflatoxins by strains of the Aspergillus flavus group maintained in ATCC. Mycopathologia. 93:19–24.
- Whitlow LW, Hagler WM Jr. 2005. Mycotoxins in feed. Feedstuffs. [accessed Sep]; p. 69–79.
- Williams LD, Bacon CW, Meredith FI, Franzluebbers AJ, Wyatt RD, Smith MA, Riley RT. 2003. Leaching and binding of fumonisins in soil microcosms. J Agric Food Chem. 51:685–690.
- Yildirim M, Manning BB, Lovell RT, Grizzle JM. 2000. Toxicity of Moniliformin and Fumonisin B1 Fed singly and in combination in diets for young Channel Catfish Ictalurus punctatus. J World Aquac Soc. 31:599–608.