ABSTRACT
Endophytic fungi became an attractive source for the discovery of new leads, because of the complexity and the structural diversity of their secondary metabolites. The genus Fusarium comprising about 70 species is extremely variable in terms of genetics, biology, ecology, and consequently, secondary metabolism and have been isolated from countless plants genera from diverse habitats. These endophytic microbes may provide protection and survival strategies in their host plants with production of a repertoire of chemically diverse and structurally unprecedented secondary metabolites reported to exhibit an incredible array of biological activities including antimicrobial, anticancer, antiviral, antioxidants, antiparasitics, immunosuppressants, immunomodulatory, antithrombotic, and biocontrol ability against plants pathogens and nematodes. This review comprehensively highlights over the period 1981–2019, the bioactive potential of metabolites produced by endophytes from Fusarium genus.
Abbreviations: AIDS: Acquired immune deficiency syndrome; BAPT: C-13 phenylpropanoid side chain-CoA acyltransferase; CaBr2: Calcium bromide; DBAT: 10-deacetylbaccatin III-10-O-acetyl transferase; DNA: Deoxyribonucleic acid; EI-MS: Electron ionization mass spectrometer; EN: Enniatin; ERK: Extracellular regulated protein kinase; EtOAc: Ethyl acetate; FDA: Food and Drug Administration; GAE/g: Gallic acid equivalent per gram; GC-MS: Gas chromatography–mass spectrometry; HA: Hyperactivation; HCV: Hepatitis C Virus; HCVPR: Hepatitis C Virus protease; HeLa: Human cervical cancer cell line; HIV: Human immunodeficiency viruses; HPLC: High Performance Liquid Chromatography; IAA: Indole‐3‐acetic acid; IARC: International Agency for Research on Cancer; IC50: Half maximal inhibitory concentration; LC50: Concentration of the compound that is lethal for 50% of exposed population; LC-MS: Liquid chromatography–mass spectrometry; MCF-7: Human breast cancer cell line; MDR: Multidrug-resistant; MDRSA: Multidrug-resistant S. aureus; MFC: Minimum fungicidal concentration; MIC: Minimum inhibitory concentration; MRSA: Multidrug-resistant S. aureus; MTCC: Microbial type culture collection; PBMCs: Peripheral blood mononuclear cells; PCR: Polymerase chain reaction; TB: Tuberculosis; TLC: Thin layer chromatography; TNF: Tumor necrosis factor; WHO: World Health Organization
http://www.zoobank.org/urn:lsid:zoobank.org:pub:D0A7B2D8-5952-436D-85C8-C79EAAD1013C
Introduction
The enormous impact of infectious diseases on our world today cannot be dramatized and neither underrated (Fauci and Morens Citation2012). In fact, today’s world is facing the high-impact of some infectious diseases such as Zika, Ebola, West Nile, influenza, food-borne illness, and global pandemics like HIV, TB, and malaria (Tacconelli et al. Citation2018) without leaving aside others diseases such as cancers (Plummer et al. Citation2016) and neurodegenerative disorders (Gitler et al. Citation2017). The threats from neglected tropical diseases, healthcare-associated infections, and invasive fungal infections, not to mention the continued discovery of new and emerging pathogens is constant (Woolhouse and Dye Citation2001; Woolhouse and Gowtage-Sequeria Citation2005; Chiller Citation2017). To worsen the situation, the antimicrobial resistance (AMR) is increasingly recognized as a public health crisis requiring a global action (Looke et al. Citation2015). In fact, WHO identified more than 20 of the most important resistant pathogens at a global level for which there is an urgent need for new treatments (Tacconelli et al. Citation2018).
The recent report of the Food and Drug Administration (FDA) shows that from the 38% of drugs discovered from natural products, microbes contributed to about 25%. These findings have really highlight the critical role of microorganisms as a sustainable pipeline for new drug discovery (Newman and Cragg Citation2016). Indeed, natural product-derived compounds provided impressive and continuous pools for medicinal chemistry applications and encouraged most of the leading pharmaceutical companies in screening microbial natural extracts for the development of high-throughput libraries (Hung and Lin Citation2017). Since it is estimated to only 1% the proportion of microbial pathogens (Woolhouse and Gowtage-Sequeria Citation2005), more than 90% of the existing non-pathogenic microbial communities can be fully explored to identify potential active compounds needed to cure diseases affecting mankind. Microbes are extraordinary organisms with the ability to lives in unusual habitats, including extreme temperature, pressure, acidity, or basicity, or within higher organisms which consequently affect their secondary metabolism (Schulze-Makuch et al. Citation2018). Living inside the tissues of higher plants, endophytic fungi have gained incredible attention over the past decades because of their great diversity and their exceptional ability to produce structurally novel and complex bioactive metabolites (Strobel Citation2018). Overall, almost all genera of fungi have been isolated as endophytes of plants, including Fusarium species.
With their ability to colonize a large variety of plants species (Imazaki and Kadota Citation2015), Fusarium represents a large cosmopolitan genus comprising more than 70 species capable of producing a wide array of active metabolites (Summerell and Leslie Citation2011). The extraordinary discrepancy within the genus in terms of genetics, which affect not only their biology and interaction with their surrounding organisms, but also their secondary metabolism has made Fusarium species one of the most important group of fungi (Stępień et al. Citation2018). The facility of colonizing diverse hosts has been credited to their outstanding biosynthetic abilility allowing them to occupied their ecological niche (Bills and Gloer Citation2016). Any species belonging to Fusarium genus, can be isolated as an endophyte of plants. This endophytic lifestyle has therefore given them an ability to produce diverse chemical scaffolds (Kaul et al. Citation2016). Some of their metabolites are produced by many species, while others are limited to a few or only a single species (Thrane Citation2001). This secondary metabolism is believed to be a chemical defence mechanism initiated by endophytes to assist the host plant under attack by insects and pathogens (Ji et al. Citation2009). Overall, these metabolites have been found to display an incredible spectrum of biological activities. As a matter of fact, some of the most important therapeutic agents and lead compounds implicated in the development of effective therapies against diseases such as cancer, malaria, neurological, cardiovascular diseases, and autoimmune disorders have been identified in the metabolome of several Fusarium species. Moreover, fungi from this genus have demonstrated their great agricultural importance not only by acting as biocontrol agents, but also by producing fungicidal and nematicidal chemicals. Altogether, this genus is a prolific source of bioactive secondary metabolites and can contribute in a spectacular and indispensable way to improve human health. To shed light upon the ability of endophytic Fusarium species to produce such useful metabolites applicable in pharmaceutical and agricultural industries, we summarized in this review the data available in past and recent (1981–2019) literature reports on the bioactive potential of metabolites produced by endophytic belonging to this genus.
1. Medicines from Fusarium species
The concept of bioprospecting natural products is well established and recognized, thanks to the number of well-known drugs originated from natural sources. For decades, the drive to investigate microorganisms for potential medical application have been nurtured by the discovery of Penicillium sp.-producing penicillin (Fleming Citation1929). This led to the recognition of plant-derived endophytic fungi as capable of producing such life-changing molecules (Strobel and Daisy Citation2003; Strobel Citation2018). Undeniably, endophytic Fusarium species living inside host plant tissues without causing any symptoms of disease have proven over the years an outstanding potential by producing compounds actually approved for the treatment of several diseases including cancer, malaria, oxidative stress-related diseases, and inflammatory disorders. In fact, medicines such as vinblastine and vincristine (Zhang et al. Citation2000; Tung et al. Citation2002; Kumar et al. Citation2013; Ashuthosh et al. Citation2013), podophyllotoxin (Kour et al. Citation2008; Nadeem et al. Citation2012), camptothecin and its analogues (Kusari et al. Citation2009; Shweta et al. Citation2010; Venugopalan et al. Citation2016; Ran et al. Citation2017), taxol (Li et al. Citation2009; Gurudatt et al. Citation2010; Elavarasi et al. Citation2012; Xiong et al. Citation2013), rohitukine (Kumara et al. Citation2014), ginkgolide B (Cui et al. Citation2012), quinine and cinchonidine (Hidayat et al. Citation2015) have been reported as products of in vitro fermentation of several Fusarium species. These findings have been over the years a catalyst to continue the exploration of this genus with a goal to sustain the production of such pharmacologically important drugs via microbial fermentation but have also encouraged the investigation of these species as sources of a potential drug candidates against diverse diseases. The is summarizing the important drugs identified in the metabolome of Fusarium spp., the fungal-producing strain and the yield of production.
Table 1. Well-known Drugs produced by Fusarium species through in vitro fermentation.
2. Exploring fusarium species as sources of antimicrobial agents
Today, the healthcare system is currently being challenged by the emergence and re-emergence of multidrug-resistant (MDR) pathogens. Infections caused by multiresistant pathogens are difficult to cure and the patient must undergo multiple treatments regimen with broad-spectrum antibiotics, known to be less efficient, more toxic and more expensive (Humberto et al. Citation2010). To address this issue, new antimicrobial compounds are urgently needed in this modern era to fill the drug development pipeline. Several studies have indicated the possible prospect of endophytes from Fusarium genus as a promising resource of antimicrobial compounds. In the quest of the potential starting points for the development of new antibiotics, various research groups have investigated the activity of extracts prepared from endophytic fungi belonging to Fusarium genus against a broad range of bacteria and fungi pathogens. These studies mostly aimed at identifying potent extracts that could be progressed for chemical analysis in order to isolate and characterise potential active principles. In fact, crude extracts from several fungi including F. solani endophyte of Taxus baccata (Tayung et al. Citation2011), F. equiseti and Fusarium sp. Dzf18 isolated from Garcinia parvifolia (Sim et al. Citation2010), endophytic F. oxysporum (Musavi and Balakrishnan Citation2013), F. oxysporum isolated from C.odorata (Toghueo et al. Citation2016a), F. oxysporum isolated from R. apiculate (Moron et al. Citation2018) and F. lateritium endophyte of Rhizophora mucronata (Hamzah et al. Citation2018) were reported to exhibit a broad antimicrobial spectrum against various pathogenic microorganisms indicating the conceivability of potential bioactive metabolites in their crude mixture. These findings fits well with the general claim that fungi can produce a complex mixture of myriad compounds capable of broad antimicrobial activity. This give rise to further chemical investigation using different chromatographic and spectroscopic tools for both purification and identification of the active ingredients.
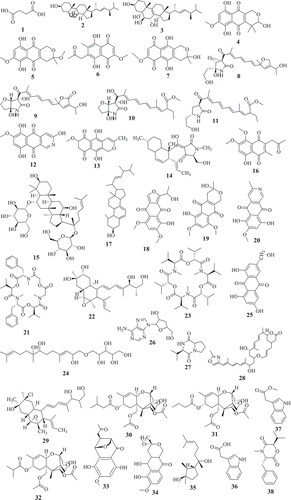
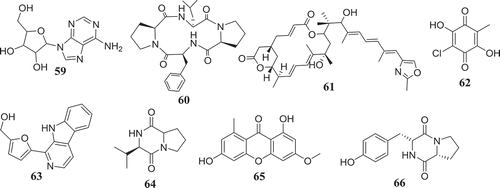
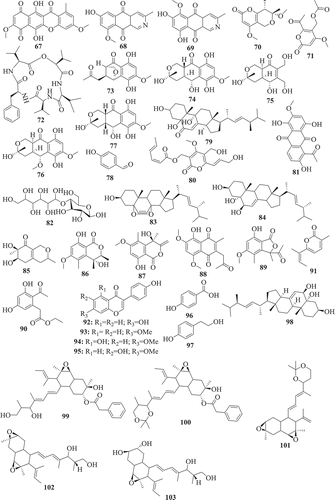
This approach so-called bioguided fractionation, has been used to investigate several active extracts from Fusarium species and dozens of active compounds were identified (). In fact, butanedioic acid (1), 5alpha, 8alpha-epidioxyergosta-6, 22-dien-3beta-ol (2), and, 22-dien-3beta, 5alpha, 6beta, 7alpha-tetraol (3), displaying antimicrobial activity were isolated from active petroleum extract of Fusarium sp. Ppf4 endophyte of P. polyphylla (Huang et al. Citation2009). Compounds 3,6,9-trihydroxy-7-methoxy-4,4-dimethyl-3,4-dihydro-1H-benzo[g]isochromene-5,10-dione (4), 3-O-methylfusarubin (5), javanicin (6) and fusarubin (7) exhibiting activity against various bacterial strains (MIC <1 to 256 μg/mL) and Mycobacterium tuberculosis strain H37Rv (MIC 8–256 μg/mL) were also isolated from anti-bacterial extract from endophyte F. solani (Shah et al. Citation2017). The extensive chemical investigation of extract from F. solani JK10, an endophyte of Chlorophora regia, resulted in the isolation of seven new compounds among which compounds 7, 8, 9, 10 and 11 demonstrated antibacterial activity against gram-negative and positive bacteria with MIC 5–10μg/mL (Kyekyeku et al. Citation2017). Antibacterial compounds, fusarubin (7), bostrycoidin (12), and anhydrofusarubin (13) were also isolated from extract of F. solani, endophyte from the root of C. alata (Khan et al. Citation2018). Equisetin (14), the antibacterial compound with MIC 8–16 μg/mL was purified from extract of Fusarium sp. isolated from O. dillenii by Ratnaweera et al. (Citation2015). Similarly, Jin et al. (Citation2017) reported the activity of ginsenoside (15) (MIC 1.6–3.2 mg/mL) isolated from extract of endophyte Fusarium sp. PN8 from P. notoginseng.
Seven active metabolites, including 8-hydroxy-5,6-dimethoxy-2-methyl-3-(2-oxo-propyl)-1,4-naphthoquinone (16), ergosta-5,7,22-trien-3β-ol (17), nectriafurone-8-methyl ether (18), 9-O-methyl fusarubin (19), bostrycoidin (12), and bostrycoidin-9-methyl ether (20) were isolated from potent extract of F. proliferatum, endophyte of Syzygium cordatum (Dame et al. Citation2016). Beauvericin (21) isolated from extracts of endophytes, F. oxysporum (C. kanehirae) and F. redolens Dzf2 (D. zingiberensis) were reported to exhibited broad antibacterial activity (Xu et al. Citation2010; Wang et al. Citation2011). The bioassay-guided fractionation of F. tricinctum extract, an endophyte of Salicornia bigelovii led to fusarielin B (22) and enniatin B (23) along with a new sesquiterpenoid, fusartricin (24). All the three compounds were potents against a wide range of pathogens (Zhang et al. Citation2015a). Among the 20 compounds isolated from ethyl acetate extract of F. equiseti, an endophytic fungus isolated from Padina pavonica, compounds w-hydroxyemodin (25) and cordycepin (26) were potent against B. subtilis and S. aureus. Cyclo (D-cis-Hyp-L-Leu) (27) was the most potent against B. megaterium while, 17-demethyl-2,11-dideoxy-rhizoxin (28) and w-hydroxyemodin (25) were active against C. albicans (Hawas et al. Citation2016).
The investigation of antifungal extract from Fusarium sp. (strain 05JANF165) led to the isolation of a new antifungal compound, fusarielin E (29) (Gai et al. Citation2007). Similarly, a new pentaketide antifungal agent, CR377, showing potency against C. albicans was purified from the extract of Fusarium sp. isolated from Selaginella pallescens by Brady and Clardy (Citation2000). Another study by Campos et al. (Citation2011) reported the purification of T2‐toxin (30) and a mixture of 8‐n‐butyrylneosolaniol (31) and 8‐isobutyrylsolaniol (32) exhibiting antifungal activity (MIC 75–640μM) against eleven clinical strains of Paracoccidioides brasiliensis. Kornsakulkarn et al. (Citation2011) reported that while Javanicin (6) exhibited antifungal activity (IC50 6.16 μg/mL), compounds 5, 6, (1S,4S,10S)-6,9-dihydroxy-8-methoxy-10-(2-oxopropyl)-3,4-dihydro-1,4-methanobenzo[c]oxepin-5(1H)-one (33) and (3R)-5,6-dihydroxy-3,7-dimethoxy-3-methyl-1,3,4,4a,5,10a-hexahydro-10H-benzo[g]isochromen-10-one (34) were active against Mycobacterium tuberculosis. Although not highly potent, compounds 33 and 34 are small molecules with structure relatively easy to synthesize through medicinal chemistry efforts. These compounds may offer an opportunity to generate starting points to create a library of semisynthetic compounds needed to help accelerate the discovery of the new antimycobacterial drugs. This can be very useful for drug discovery against tuberculosis since most of the commercial libraries available have already been screen against M. tuberculosis. These natural products can provide new scaffolds to boost drug discovery against this infectious disease and many others neglected tropical diseases. Overall, these studies revealed that the exploration of active and extremely complex crude extracts can lead to the purification of unknown compounds exhibiting broad to a narrow spectrum of activity. Dozens of unknown compounds with interesting activity profile were identified through a bioguided exploration of crude metabolites from Fusarium spp. This effect-related analytical approach has and continues to be reported as an ideal strategy for exploring natural products for drug discovery. This approach considered as a powerful tool to explore complex mixture to identify a target compounds (Weller Citation2012) has directed the discovery of countless active compounds from diverse natural sources (Peters et al. Citation2010; Kildgaard et al. Citation2017; Bayrami et al. Citation2018).
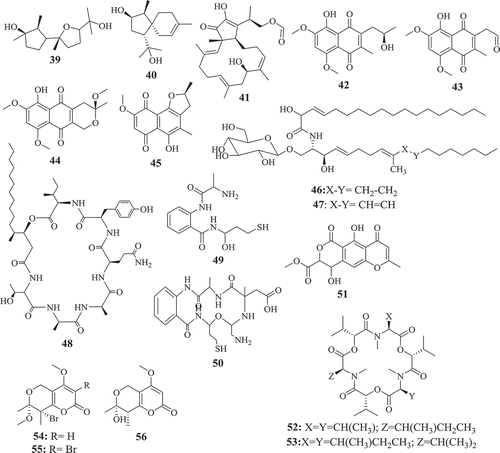
Although the bio-guided fractionation is a successful approach to identify potent compounds from crude mixture, several compounds exhibiting interesting activity have also been reported from a chemical investigation of an extract with unknown activity (). Therefore, antibacterial (MIC 12.5–100μg/mL) compounds, (1R,2S,3R)-3-((S)-2-hydroxy-6-methylhept-5-en-2-yl)-1,2-dimethylcyclopentan-1-ol (35), indol-3-acetic acid (36), methyl indolyl-3-acetate (37), bassiatin (38), beauvericin (21), epicyclonerodiol oxide (39), 3β-hydroxy-β-acorenol (40), fusaproliferin (41), 5-O-methylsolaniol (42), 5-O-methyljavanicin (43), methyl ether fusarubin (44), and anhydrojavanicin (45) were isolated from extract of F. proliferatum AF-04, endophyte of the green Chinese onion (Jiang et al. Citation2019). Similarly, fusaruside (46), and a known metabolite (2S,2ʹR,3R,3ʹE,4E,8E)-1-O-beta-D-glucopyranosyl-2-N-(2ʹ-hydroxy-3ʹ-octadecenoyl)-3-hydroxy-9-methyl-4,8-sphingadienine (47) both showing strong antibacterial (MIC 1.9–7.8μg/mL) activity were isolated from a chloroform-methanol extract of Fusarium sp. IFB-121, an endophytic fungus in Quercus variabilis (Shu et al. Citation2004). Ibrahim et al. (Citation2018a) also reported the strong (MIC 0.11–0.24 μM) antifungal activity of fusaripeptide A (48), a new cyclodepsipeptide isolated from Fusarium sp., an endophyte from the roots of Mentha longifolia L.
A new benzamide derivative, namely fusarithioamide A (49) with strong antibacterial (MIC ranging from 3.1 to 6.9 μg/mL) was purified from the EtOAc extract of F. chlamydosporium isolated from the leaves of Anvillea garcinii (Ibrahim et al. Citation2016a). This finding encourages the same authors to continue the investigation of this extract which led to the identification of fusarithioamide B (50), a new aminobenzamide derivative, exhibiting antimicrobial activity towards C. albicans, E. coli, B. cereus, and S. aureus with MIC of 1.9 μg/mL (Ibrahim et al. Citation2018b). The liquid chromatography-mass spectrometry analysis of methanol extract from endophyte F. tricinctum isolated from the fruits of Hordeum sativum revealed the presence of six antibiotic compounds, enniatins A, A1, B, B1, B2, and Q (Zaher et al. Citation2014). Even though some active compounds were reported from this approach, this blind investigation of crude extract often led to the isolation of inactive compounds. For instance, Xiao et al. (Citation2018) reported the purification of a novel pyrone derivative bearing two fused five-member rings, together with two new naphthalenone derivatives from an extract of Fusarium sp. HP-2, endophytic fungus isolated from “Qi-Nan” agarwood but none of the isolated compounds were activity against a panel of pathogens tested. Likewise, inactive fusarimine, a novel polyketide-derived isoquinoline alkaloid, was isolated from cultures of Fusarium sp. LN-12, an endophytic fungus isolated from the leaves of Melia azedarach (Yang et al. Citation2012). F. sporotrichioides strain M-1–1 isolated from bean bull was reported to 4 beta, 8 alpha-diacetoxy-12,13-epoxytrichothec-9-ene-3 alpha, 15-diol, and 4 beta-acetoxy-12,13-epoxy-trichothec-9-ene-3 alpha,8 alpha,15-triol (Ishii and Ueno Citation1981). Similarly, 5-hydroxy-7-methoxy-40-O-(3-methylbut-2-enyl) isoflavone, along with known compounds, eriodictyol, vittarin-B, 3,6,7-trihydroxy-1-methoxyxanthone, 1,3,6-Trihydroxy-8-methylxanthine and cyclo (Phe–Tyr) were isolated from Fusarium sp. ZZF60, a mangrove endophytic fungus (Huang et al. Citation2012). Although, these compounds couldn’t exhibit antimicrobial activity, these data are not enough to classify them as inactive. Further investigation of these compounds against others pathogens (parasites, virus) could reveals outstanding potency.
Apart from the two approaches mentioned above, other strategies such as the coculture or the addition of chemicals to culture medium have been successfully applied to induce the production of active antimicrobial compounds by Fusarium spp (). In fact, the co-cultivation of endophytic fungal F. tricinctum with the B. subtilis 168 trpC2 increased by about 78-fold the accumulation of lateropyrone, enniatins, and fusaristatin A. Additional compounds such as (−)-citreoisocoumarin, macrocarpon C, 2-(carboxymethylamino) benzoic acid, and (−)-citreoisocoumarinol were identified. Among these compounds, lateropyrone (51), enniatins B1 (52) and A1 (53), exhibited broad antibacterial activity (Ola et al. Citation2013). Co-culturing F. tricinctum with S. lividans also led to the production of new compounds including fusatricinones A–D, and dihydrolateropyrone. In addition, antibiotic compounds such as lateropyrone, enniatins B, B1 and A1, and fusaristatin A, were also upregulated (Moussa et al. Citation2019).
In addition to co-cultivation, supplementing the culture medium with chemicals has resulted in a production of active compounds (). Adding CaBr2 to the culture medium of a marine-endophyte F. tricinctum resulted in the production of two new compounds, bromomethylchlamydosporols A (54) and B (55), along with chlamydosporol (56) and fusarielin A, all exhibiting activity against sensitive and resistant strains of S. aureus (Nenkep et al. Citation2010). These strategies of inducing new bioactive metabolites are of interest since many secondary metabolism gene clusters are silent under standard laboratory conditions (Bergmann et al. Citation2007). Therefore, methods such as genetic engineering (Bergmann et al. Citation2007), mutagenesis, the OSMAC approach (Bode et al. Citation2002), treatment with epigenetic modifiers (Cichewicz Citation2010; Brakhage Citation2013), co-cultivation (Marmann et al. Citation2014; Bertrand et al. Citation2014a; Netzker et al. Citation2015) or addition of small chemicals (Toghueo et al. Citation2016b, Citation2018) have been successfully applied during the past years to activate these silent gene clusters in filamentous fungi and induce the formation of many new active metabolites. These strategies have opened new avenues and will certainly continue to play a significant role in the elucidation of cryptic natural products from microbial sources.
3. Antiparasitic agents from fusarium species
Among diseases caused by parasites, malaria is the most life-threatening with an estimated number of morbidity cases of 219 million and 435 000 of deaths occurred in 2017, worldwide (WHO Citation2018). Even though this infection is curable, the global malaria elimination is being challenged by the continued emergence of parasite resistance to antimalarial medicines (WHO Citation2018). To fill the antimalarial drug development pipeline, new antiplasmodial agents are urgently needed. In the past, the exploration of natural products have provided antimalarial drugs. Therefore, the screening natural sources could lead to the discovery of such lead compounds. In this respect, fusaripeptide A (48) was reported by Ibrahim et al. (Citation2018a) to display significant antiplasmodial activity toward P. falciparum (D6 clone) with an IC50 value of 0.34 μM (). This compound active in the nanomolar range can constitute a great starting point for new drug discovery against malaria.
Although, not many compounds isolated from Fusarium spp. have been tested for their antiplasmodial activity, extracts from different Fusarium species exhibited very good potency against resistant P. falciparum strains. This finding suggests that exploring these species may offer a chance for the discovery of compounds with the potential to cure an infection caused by resistant strains. Indeed, crude metabolites produced by F. oxysporum isolated from Symphonia globulifera was found to exhibit very good activity (IC50 of 1.7µg/mL) against the chloroquine-resistant P. falciparum INDO (Ateba et al. Citation2018). Also, the potency (IC50 1.62–4.38 μg/mL) of extracts from Fusarium sp. N240 isolated from C. odorata against both resistant and sensitive strains of P. falciparum was reported (Toghueo et al. Citation2018). The similar observation was made while investigating ethyl acetate extract from Fusarium sp. AMst1 endophyte of Annona muricata against P. falciparum Pf3D7, PfDd2, and PfIndo (IC50 1.16–1.43 μg/mL). This extract was also found to inhibit the transition of the ring to the trophozoite stage (Toghueo et al. Citation2019). Another study by Kaushik et al. (Citation2014) shows that extract and fractions from endophytic fungus Fusarium sp. from a marine alga were very potent against P. falciparum 3D7. These studies suggest that deeper bioguided investigations are needed to fully explore the metabolome of these fungi for new antiplasmodial drug discovery.
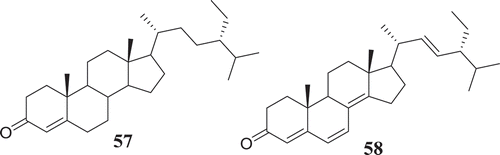
The potential of endophytic Fusarium species to produce compounds active against Trypanosoma and Leishmania parasites, causative agents of chagas diseases and leishmaniasis, respectively, have been investigated as well (). The results reported till now suggest an interesting activity profile and encourage continuing the investigation. As a matter fact, beauvericin (21) isolated from Fusarium sp. WC9 was reported to inhibit T. cruzi with IC50 2.43 μM (Campos et al. Citation2015). Against L. donovani, the moderate activity of methanol extract of endophyte F. tricinctum isolated from the fruits of Hordeum sativum was reported by Zaher et al. (Citation2014). The two new compounds, Integracides F (57) and G (58) isolated from Fusarium sp., an endophytic fungus of Mentha longifolia were found to exhibit anti-leishmanial activity with IC50 values of 3.74 and 2.53 μg/mL, respectively (Ibrahim et al. Citation2016b). Compounds anhydrofusarubin (13) and beauvericin (21) isolated from F. oxysporum SS46 endophyte of Smallanthus sonchifolius also exhibited promising activity against Leishmania braziliensis (Nascimento et al. Citation2012). Although isolated compounds may be considered as moderate inhibitors, their small molecular-like structure offers the possibility for medicinal chemistry that could improve their drug-likeness and may lead to the discovery of potential drug candidates.
4. Endophytic fungi from fusarium species and their antiviral agents
Over the course of human civilization, viral infections have caused millions of human deaths worldwide, driving the development of antiviral drugs in a pressing need (De Clercq Citation2004). Endophytic fungi represent a vast reservoir of bioactive molecules, which could potentially be used as antivirals in the future. In this regard, Fusarium species have been reported to produce metabolites capable of antiviral activity (). The bioguided fractionation of extracts from the endophytic fungus F. equiseti led the isolation of several compounds among which cordycepin (36) and ara-A (59) showed less potency, cyclic tetrapeptidecyclo-[Phenylalanyl-pro-leu-pro] (60), 17-demethyl-2,11-dideoxy-rhizoxin (61), 5-chloro-3,6-dihydroxy-2-methyl-1,4-benzoquinone (62) and perlolyrine (63) were moderately potent. Cyclo (L-Pro-L-Val) (64) and griseoxanthone C (65) showed good potency against HCV NS3/4A protease while, ω-hydroxyemodin (25) and cyclo (L-Tyr-L-Pro) (66) were the most potent HCVPR inhibitors (Hawas et al. Citation2016). Compounds with similar core structure including stachybogrisephenone B, grisephenone A, and 3,6,8-Trihydroxy-1-methylxanthine, isolated from the cultures of sponge-derived fungus Stachybotry sp. HH1 ZDDS1F1–2, were also reported to inhibit in vitro the replication of EV-71 with IC50 values of 30.1, 50.0 and 40.3 μM, respectively (Qin et al. Citation2014). Similarly, AGI-B4 isolated from marine-derived fungus Neosartorya fischeri strain 1008F1 showed strong inhibitory effect on the replication of tobacco mosaic virus (TMV), with IC50 values of 260 μM (Tan et al. Citation2012). The broad antiviral spectrum of these compounds, with similar structure, suggests that further medicinal chemistry efforts may lead to the synthesis of several derivatives among which potential candidates for further studies towards drug discovery against HCV, EV-71, TMV and other related viruses could be identified.
5. Endophytic fungi from fusarium species as sources of novel anticancer compounds
Since the declaration of the “war on cancer” in 1971 in the United States, only a few battles have been won and the war is still ongoing (Michelakis et al. Citation2008) and seems endless. With the global cancer burden estimated to have risen to 18.1 million new cases and 9.6 million deaths in 2018, cancer is increasingly risen (IARC Global Cancer Observatory 2018). Despite the seriously challenged breakthrough in anticancer chemotherapy and the endless search for newer anticancer agents, the discovery of endophytic fungi-producing anticancer compounds has given hope to the scientific community (Stierle et al. Citation1993; Kharwar et al. Citation2011). Endophytic fungi producing interesting and unmatchable secondary metabolites are very useful in providing chemical scaffolds for anticancer drugs discovery (Tan and Zou Citation2001). In a continuation of the search for secondary metabolites with anticancer activity, several endophytic fungi strains from Fusarium genus have been investigated with promising results ().
Using the bioassay-guided investigation, several cytotoxic compounds were identified from endophytic fungi from Fusarium species. As a matter of fact, beauvericin (21) and bikaverin (67) among the compounds isolated from EtOAc extract of F. oxysporum, endophyte of Cylindropuntia echinocarpus were cytotoxic against a panel of four sentinel cancer cell lines, NCI-H460 (non-small-cell lung), MIA Pa Ca-2 (pancreatic), MCF-7 (breast), and SF-268 (CNS glioma). Similarly, Zhan et al. (Citation2007) also showed that among all the compounds isolated from F. oxysporum endophyte of Ephedra fasciculate, only beauvericin (21) inhibited the metastatic prostate cancer (PC-3M) and breast cancer (MDA-MB-231) cells and also showed antiangiogenic activity in HUVEC-2 cells at sub-lethal concentrations. According to Chowdhury et al. (Citation2017), only 7-desmethylscorpinone (68) and 7-desmethyl-6-methylbostrycoidin (69) among compounds produced by F. solani isolated from Aponogeton undulates were strongly potent against four human tumour cell lines, MDA MB 231, MIA PaCa2, HeLa, and NCI H1975 (IC50 0.34–30.72µM). Fusarilactone A (70) and fusarilactone B (71) isolated from an extract of endophytic fungus Fusarium sp. displayed moderate activity against SMMC-7721, A-549, and MCF-7 cell lines (Chen et al. Citation2014). Sansalvamide (72), isolated from Fusarium sp. collected from Halodule wrightii was also found to have significant anticancer activity against the National Cancer Institute’s 60-cell-line panel (Belofsky et al. Citation1999) and was later identified as an inhibitor of the topoisomerase I by Hwang et al. (Citation1999). Several cytotoxic compounds including dihydronaphthalenone (73), 5-hydroxydihydrofusarubins A (74), 5-hydroxydihydrofusarubins B (75), 5-methoxydihydrofusarubin B (76), and 5-hydroxydihydrofusarubin C (77) were also reported by Kornsakulkarn et al. (Citation2011). Nascimento et al. (Citation2012) after the fractionation of cytotoxic ethyl acetate extract from F. oxysporum SS46 identified anhydrofusarubin (13) and beauvericin exhibiting activity against different cancer cell lines. Similarly, compounds 4-hydroxybenzaldehyde (78), bostrycoidin (12), anhydrofusarubin (13) and 3,5,9-trihydroxyergosta-7,22-diene-6-one (79) with significant cytotoxicity against vero cells were isolated from ethyl acetate extract of endophytic fungus F. solani by Khan et al. (Citation2018).
Unlike bioguided fractionation, the random investigation of extracts can also lead to the isolation of compounds with potency. In fact, based only on the assumption that fungi belonging to the genus Fusarium can produce a cytotoxic metabolites, several compounds were isolated and then tested for their anticancer activity. Allantopyrone A (80) isolated from Fusarium sp. IM-37, an endophytic fungus from R. mucronata was tested against HL60 cells line and showed activity with IC50 of 0.32 mM (Shiono et al. Citation2014). Integracides F (57) and G (58) was also found to display cytotoxicity towards BT-549 and SKOV-3 (IC50 0.12–1.97µg/mL) (Ibrahim et al. Citation2016b). Likewise, 5-acetyl-2-methoxy-1,4,6-trihydroxy-anthraquinone (81), isolated from the culture of the endophytic fungus Fusarium sp. (No. b77) was cytotoxic against Hep G2 and Hep2 cells (Shao et al. Citation2010). Several compounds were isolated from extract of F. equiseti, an endophyte of Salicornia bigelovii and tested for their anticancer activity. Diglucotol (82) and ergosterol peroxide (83) showed weak potency (IC50 52.4–99.39 μM) and cerevisterol (84) was moderately active (IC50 32.4–41.5 μM) against MCF-7, MDA-MB-231 and Caco-2 cancer cells (Wang et al. Citation2014). Compounds, fusaraisochromenone (85), fusaraisochromanone (86), (R)-3,4-dihydro-4,8-dihydroxy-6-methoxy-4,5-dimethyl-3-methyleneisochromen-1-one (87), 8-O-methyljavanicin (88), 3-acetyl-7-hydroxy-5-methoxyl-3H-isobenzofuran-l-one (89), curvulin (90), fusalanipyrone (91), daidzein (92), formononetin (93), 7-O-methyl genistein (94), kakkatin (95), p-hydroxy benzoic acid (96), and tyrosol (97) isolated from endophytic fungus Fusarium sp. PDB51F5 were reported to exhibit cytotoxic activity against various cell line with IC50 ranging from 148–162µM (Boonyaketgoson et al. Citation2015).
A new oxysporidinone analogue and a new 3-hydroxyl-2-piperidinone derivative, along with (−)-4,6′-anhydrooxysporidinone, (+)-fusarinolic acid, gibepyrone D, beauvercin, cerevisterol, fusaruside, and (2S,2′R,3R,3′E,4E,8E)-1-O-D-glucopyranosyl-2-N-(2′-hydroxy-3′-octadecenoyl)-3-hydroxy-9-methyl-4,8-sphingadienine isolated from F. oxysporum were evaluated for cytotoxicity against PC-3, PANC-1, and A549 cancer cell lines and only beauvericin (21) showed potency with IC50 ranging from 10.4 to 49.5μM (Wang et al. Citation2011). Fusarithioamide A (49) and ergosta-7,22-diene-3β,5α,6β-triol (98) produced by F. chlamydosporium endophyte of Anvillea garcinii were evaluated for their in vitro cytotoxic activity against KB, BT-549, SK-MEL, and SKOV-3 cell lines. The results showed that compound 98 was active towards all tested cell lines while, compound 49 possessed potent and selective activity towards BT-549 and SKOV-3 cell lines (Ibrahim et al. Citation2016a). Fusarithioamide B (50) isolated from the same extract was strongly potent and selective towards BT-549, MCF-7, SKOV-3, and HCT-116 cell lines with IC50 values of 0.09, 0.21, 1.23, and 0.59µM, respectively (Ibrahim et al. Citation2018b). These data suggest that fusarithioamide A and B could be a good starting point to provide promising anticancer candidate molecules for drug development.
The antibiotic compounds enniatins A1 (53), B (23) and B1 (52) isolated from F. tricinctum, an endophyte of Aristolochia paucinervis were reported to exhibit moderate cytotoxic activity against HepG2 and C6 cells (IC50 10–25 µM), and high toxicicity against H4IIE cells (IC50 1–2.5 µM). Additional mechanism of action study showed that all enniatins increased caspase 3/7 activity and nuclear fragmentation as markers for apoptotic cell death in H4IIE cells. Specifically, enniatin A1, B1, and, also enniatin B decreased the activation of extracellular regulated protein kinase (ERK) (p44/p42). Enniatins A1 and B1, was also able to inhibit moderately the activation of tumour necrosis factor a (TNF-a)-induced NF-jB (Wätjen et al. Citation2009). In a similar study, Vasundhara et al. (Citation2016) showed that extract of F. tricinctum, an endophytic fungus of Taxus baccata was cytotoxic (IC50 225–220μg/mL) against human breast cancer cell line (MCF-7), human cervical cancer cell line (HeLa) and peripheral blood mononuclear cells (PBMCs). Interestingly, this extract inhibited the proliferation of concanavalin A-stimulated PBMCs and the tumour necrosis factor (TNF)-α production in concanavalin A-stimulated PBMCs and MCF-7. These results are indicating that the antiproliferative activity observed could be associated with TNF-α and suggest that the chemical investigation of this extract could lead to the characterisation of potent anticancer compounds with the potential to inhibit the TNF-α.
Hemphill et al. (Citation2017) by using the OSMAC (One Strain Many Compounds) approach, significantly increased (up to 80-fold) the production of fusarielin J (99), in addition to inducing the production of fusarielin K (100), fusarielin L (101), fusarielins A (102) and B (103) by F. tricinctum, endophyte from Aristolochia paucinervis. More interestingly, these compounds were active (IC50 12.5–84.6μM) against the human ovarian cancer cell line A2780. This method consisting on manipulating culture conditions such as carbon, salts and minerals sources, temperature, pH, incubation time and many other parameters have been previously applied with success to induce the production of several bioactive metabolites (Bode et al. Citation2002).
6. Antioxidant and antiaging activities of metabolites from fusarium species
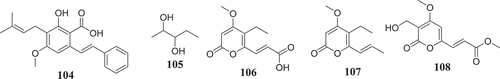
Gradually, the ability of endophytic fungi to produce potent antioxidant metabolites is been recognized. These bioactive compounds are often identified from screening using various antiradical and antioxidant assays (). For instance, using the DPPH radical scavenging assay, Hamzah et al. (Citation2018) reported the free radical scavenging potential of methanol-extract of F. lateritium endophyte of Rhizophora mucronata. Similarly, the phenolic content (160.51 mg of GAE/g of extract) and the free radical scavenging activity (IC50 89.61 µg/mL) of ethyl acetate extract of F. oxysporum isolated from the flower part of Dendrobium lindleyi was also reported (Bungtongdee et al. Citation2019). Likewise, the free radical scavenging activity (IC50 482 μg/mL) of crude metabolites from endophytic F. tricinctum was reported by Vasundhara et al. (Citation2016). According to Khan et al. (Citation2018), bostrycoidin (12), anhydrofusarubin (13), 4-hydroxybenzaldehyde (78) and fusarubin (7) exhibited significant antioxidant activity with IC50 values of 1.6, 12.4, 28.9 and 34.8 μg/mL, respectively. Another natural antioxidant compound named Cajaninstilbene acid (104) was reported from extracts of F. solani (ERP-07), F. oxysporum (ERP-10), and F. proliferatum endophytes of Cajanus cajan (Zhao et al. Citation2012). These studies have demonstrated the potential of Fusarium spp. to produce antioxidant compounds and could serve as encouragement for further investigations in order to identify more compounds that could be developed as novel antioxidant drugs.
According to Bungtongdee et al. (Citation2019), extract from F. oxysporum with strong antimutagenic activity was found to be composed of gibepyrone A, pyrrolo [1, 2-a] pyrazine-1,4-dione, hexahydro-3-(2-methylpropyl) and indoleacetic acid as major components. Another study by Tiwari et al. (Citation2014) reveals that as compared to 1,5-pentanediol, 2,3-pentanediol (105) isolated from F. oxysporum endophyte of Curcuma amada was found to improve antiaging properties against Caenorhabditis elegans. This compound structurally like the commercially available 1,5-pentanediol can be further developed as a new antiaging agent (). This finding also suggests that more investigation into extracts from Fusarium species might lead to the identification of more compounds with antiaging potential.
Sperm motility and hyperactivation (HA) are important for fertility. Recently, there has been significant progress in understanding factors controlling these events such as the generation, and regulation of calcium signals. Both pH-regulated membrane Ca2+channels and Ca2+ stores have been implicated in controlling HA (Alasmari et al. Citation2013). Impressively, endophytic fungi from Fusarium spp. have been reported to produce compounds () such as (E)-3-(5-ethyl-4-methoxy-2-oxo-2H-pyran-6-yl) acrylic acid (106), cladobotrin V (107), allantopyrone A (80) and islandic acid-II methyl ester (108) with the ability to restore fertility (Shiono et al. Citation2014).
7. Immunosuppressive and immunomodulator metabolites from fusarium species
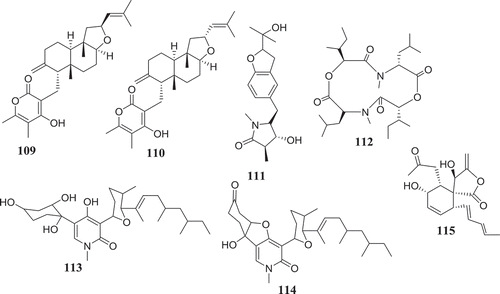
Endophytic Fusarium species can also produce substances that can influence the immune system of animals (). In reference to subglutinol A (109) and B (110), two well-known immunosuppressive agents produced by F. subglutinans, an endophyte of T. wilfordii (Lee et al. Citation1995), we may hypothetized that further investigation of extracts from Fusarium spp. might lead to the identification of compounds useful in the treatment of patients undergoing organs transplantation to avoid rejection.
Four compounds including, rigidiusculamide E (111), [-(α-oxyisohexanoyl-N-methyl-leucyl)2-] (112), (−)-oxysporidinone (113) and (−)-4,6′-anhydrooxysporidinone (114) isolated from F. tricinctum SYPF 7082, an endophyte of root of P. notoginseng were reported by Sun et al. (Citation2018) to exhibit immunomodulatory activity. The similar potential was observed with Fusaspirol A (115) a new compound isolated from F. solani B-18 by Ariefta et al. (Citation2019).
8. Antithrombotic agents from fusarium species
Cardiovascular diseases, such as acute myocardial infarction, ischaemic heart disease, and high blood pressure, are the leading causes of death in the world (Mine et al. Citation2005). Thrombin-mediated fibrinogen conversion to fibrin and fibrin monomer cross-linking result in the formation of a clot and the thrombosis occurs when the clots are not lysed. A variety of plasminogen activators have been widely studied and used as thrombolytic agents. However, these agents are very expensive and have side effects including gastrointestinal bleeding, and immunogenicity (Shao Citation2012). Therefore, new thrombolytic agents are in urgent demand. Although, several potential thrombolytic agents confirmed in clinical trials (Han et al. Citation2010), there is still an urgent need to explore new sources of fibrinolytic agents which could have better efficacy, specificity, and fewer side effects. Microbes are the most preferred source of such enzymes due to their diversity, feasibility in mass culture and ease in genetic manipulation (Mander et al. Citation2011). For the continuing search for antithrombotic agents from microbes, Wu et al. (Citation2009) screened extracts from 1,075 endophytic fungi and identified extract from Fusarium sp. as the most potent. From that extract, an antithrombotic agent was identified as a 28-kDa single-chain fibrinolytic enzyme with no homology with other known fibrinolytic enzymes. From another study, a strongly potent fibrinolytic enzyme exhibiting not only protease activity between pH 2.5 and 11.5 but was also stability at high temperature (up to 50°C) was isolated from Fusarium sp. BLB isolated from leaves of Hibiscus. Additionally, the N-terminal amino acid sequence of this enzyme was identical to previously reported proteases (Ueda et al. Citation2007). These data are supporting the claims that fungi can be a good source for a fibrinolytic enzyme with potential for application in the treatment of thrombosis (Peng et al. Citation2005; Rovati et al. Citation2010; Zhang et al. Citation2015b).
9. Biocontrol ability of endophytic fusarium species
9.1 Induction of plant defence system
The potential of Fusarium endophytes to impact plant physiology and improve plant defence system have been intensively investigated. For instance, in tomato plant infected by Tetranychus urticae, the inoculation of endophyte F. solani strain K (FsK) isolated from the root tissues of tomato altered the plant responses by instigating the defence-related genes (Pappas et al. Citation2018). Additionally, Garantonakis et al. (Citation2018) reveals that all plants colonized by FsK were more resistant to damage caused by Nesidiocoris tenuis. Likewise, another strain of F. solani was able to induce systemic resistance in tomato plants infected by Septoria lycopersici via the induction of pathogenesis-related genes (Kavroulakis et al. Citation2007). In Hordeum vulgare, the presence of endophyte F. oxysporum was able to confer disease resistance against virulent pathogens (Schulz et al. Citation1999). This resistance was positively correlated to the increasing concentrations of phenolic metabolites in the plant. This observation was supported by Yong et al. (Citation2009) who correlated the enhancement in terpenoids concentration to the growth and resistance of Euphorbia pekinensis after inoculation of endophytic fungi Fusarium spp. Conjointly, these studies suggest that the presence of endophytes in host tissues can definitely help the plant to resist against invading pathogens not only by eliciting the host response mechanism, but also stimulating the production of antagonistic metabolites (Kloepper and Ryu Citation2006; Van Bael et al. Citation2012). These positive results may suggest that Fusarium species can effectively be harnessed as an effective tool to improve the resistance of crop plants to pathogens attack. Far more investigations in this area are needed to turn these species into biocontrol agents to fight against plant pathogenic microorganisms affecting the agricultural crop production industry.
9.2 Improve plant resistance against abiotic stresses
In addition to their ability to maintain the health of plants, Fusarium endophytes also play an imperative role in preparing the plant against abiotic stresses as well as enhancing the growth and the production yields (Lata et al. Citation2018). For instance, the presence of F. culmorum in Leymus mollis was found to increase his ability to tolerate the stress associated with high salinity (Rodriguez et al. Citation2008). In more recent study, Shah et al. (Citation2019) demonstrated that endophytic Fusarium sp. from the root of Dendrobium moniliforme was able to promote the growth and development of Rhynchostylis retusa. This supported the finding of Cui et al. (Citation2018) who reported earlier the ability of three endophytes isolates of F. redolens KY379544, F. nematophilum KY379572 and F. nematophilum KY379764 to promote the germination of C. songaricum seeds. Moreover, metabolites produced by several Fusarium spp. were also reported to promote the growth of plants. To name but a few, study by Zhong et al. (Citation2016) showed that polysaccharides obtained from endophytic F. oxysporum stimulated the sprout growth, flavonoid accumulation, and antioxidant capacity of tartary buckwheat. Another F. oxysporium Dzf17 endophyte of Dioscorea zingiberensis was reported to induce the diosgenin accumulation in cell suspension culture of the host plant (Li et al. Citation2011).
It is clear that endophytes are an integral part of plant biology and served as important components of plant micro-ecosystems (Christian et al. Citation2016; Jia et al. Citation2016). Therefore, the concept of “mycovitalism” introduced by Vujanovic and Vujanovic (Citation2007) after observing the positive effect of F. semitectum on orchid seed germination in increasingly accepted. Indeed, recent research data are leaning towards the holobionts nature of plants (Vandenkoornhuyse et al. Citation2015) and increasingly providing evidence that plants are dependent on the microbes inhabiting inside them (Selosse et al. Citation2014). All the evidence are showing today that endophytes, especially fungal endophytes play important beneficial roles in host plant development and physiology including increased stress tolerance, enhanced root growth and provision for special nutrition and water (Wani et al. Citation2015). This is also true for Fusarium species frequently isolated as an endophyte in a wide-varied host (Rather et al. Citation2018; Strobel Citation2018). Data presented above are supporting the ability of Fusarium species as an important component of the host plant and confirmed the potentiality of using these endophytic species to limit the effect of abiotic stresses on crop plants and improve the production yield.
9.3 Nematicidal activity
In order to replace biohazardous nematocides, there is a strong drive of finding natural product-based alternatives to contain nematode pests affecting agricultural crops. The use of microorganisms in controlling plant-parasitic nematodes is receiving increasing attention as an important alternative for toxic chemicals. In this respect, endophytic fungi from Fusarium genus were investigated for their nematicidal potential and the data collected so far shows that these species may constitute a potential source for new nematicidal agents. In fact, several studies were reported describing the potential of endophytic F. oxysporum isolates to inhibit various nematodes parasites at different stages of their development (Hallman and Sikora Citation1994; Vu et al. Citation2004; Dubois et al. Citation2004; Mennan et al. Citation2005; Athman et al. Citation2006; Shahasi et al. Citation2006; Dababat and Sikora Citation2007; Mwaura et al. Citation2009; Mwaura et al. Citation2010; Yang et al. Citation2012; Van Dessel et al. Citation2011; Martinuz et al. Citation2012; Waweru et al. Citation2014). Another endophyte species, F. moniliforme strain Fe14 was also reported for his activity against M. graminicola by Le et al. (Citation2016). This antagonist activity is often suspected to be achieved via the production of antinemic metabolites ().
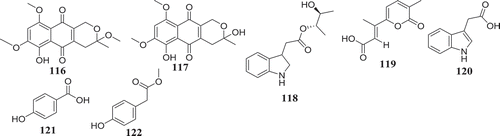
In fact, endophytic F. oxysporum exhibiting nematocidal activity was found to produce compounds bikaverin (67), 3-O-methyl-8-O-methyl fusarubin (116), 8-O-methyl fusarubin (117), anhydrofusarubin (13), and fusarubin (7), with activity against M. incognita (Kundu et al. Citation2016). Similarly, chlamydosporin (118) produced by F. chlamydosporum, isolated from the root of Suaeda glauca exhibited significant phytotoxic activity against the radicle growth of Echinochloa crusgalli (Wang et al. Citation2018). Four other compounds including gibepyrone D (119), indole‐3‐acetic acid (120), 4‐hydroxybenzoic acid (121) and methyl 2‐(4‐hydroxyphenyl) acetate (122), produced by F. oxysporum 162 (Fo162) displayed good nematocidal activities. Moreover, compound 120 was also found to trigger the plant resistance mechanism (Bogner et al. Citation2016). The overall presentation of data is demonstrating the outstanding biocontrol potential of Fusarium spp. and support the continue investigation of these fungi as sources of potential biocontrol agents.
Conclusion and perspectives
Fungi belonging to Fusarium genus represent one of the most important groups of fungi because of their implication as plant pathogens. Fortunately, the non-pathogenic species from this genus, particularly endophytes are of equal importance because of their outstanding biosynthetic ability. In fact, our study demonstrates that endophytic Fusarium species are untapped bioresource with novel and interesting biological and chemical diversity. As a matter of fact, more than 100 structurally unique chemical compounds exhibiting a wide range of biological activities including antimicrobial, anticancer, antiviral, antioxidants, antimalarial, antiparasitics, immunosuppressants, immunomodulators, antithrombotic, and biocontrol have been reported from these species. However, this study also showed that only a few species from this large genus have been investigated so far, suggesting that a significant number of strains remain unexplored. Therefore, systematic bioguided investigations are needed to explore the full potential and the diversity of bioactive metabolites produced by Fusarium species. Investigating metabolites produced by these microorganisms can offer an opportunity to discover novel natural products with unique chemical scaffolds as starting points for lead discovery for the treatment of human and plant diseases.
Acknowledgements
Rufin Marie Kouipou Toghueo is a postdoctoral researcher at the Drug Discovery Unit, University of Dundee. This integrated programme is developed by the Drug Discovery Unit with the support of the Wellcome Centre for Anti-Infective Research as a special programme for leadership research, capacity building and training in drug discovery against tropical diseases.
Disclosure statement
No potential conflict of interest was reported by the author.
References
- Alasmari W, Barratt CL, Publicover SJ, Whalley KM, Foster E, Kay V, Martins Da Silva S, Oxenham SK. 2013. The clinical significance of calcium-signalling pathways mediating human sperm hyperactivation. Hum Reprod. 28(4):866–876.
- Ariefta NR, Nikmawahda HT, Aboshi T, Murayama T, Tawaraya K, Koseki T, Katagi G, Kakihara Y, Shiono Y. 2019. Fusaspirols A-D, novel oxaspirol derivatives isolated from Fusarium solani B-18. Tetrahedron. 10(8):1371–1377.
- Ashuthosh K, Prasad A, Absar A. 2013. Cultural, morphological and molecular characterization of vinca alkaloids producing endophytic fungus Fusarium solani isolated from Catharanthus roseus. Int J Bot Res. 3:1–12.
- Ateba JET, Toghueo RMK, Awantu AF, Mba’ning BM, Gohlke S, Sahal D, Rodrigues-Filho E, Tsamo E, Boyom FF, Sewald N, et al. 2018. Antiplasmodial Properties and Cytotoxicity of Endophytic Fungi from Symphonia globulifera Clusiaceae). J Fungi. 4:70.
- Athman SY, Dubois T, Viljoen A, Labuschagne N, Coyne D, Ragama P, Gold C, Niere B. 2006. In vitro antagonism of endophytic Fusarium oxysporum isolates against the burrowing nematode Radopholus similis. Nematology. 8:627–636.
- Bayrami Z, Hajiaghaee R, Khalighi-Sigaroodi F, Rahimi R, Farzaei MH, Hodjat M, Baeeri M, Rahimifard M, Navaei-Nigjeh M, Abdollahi M. 2018. Bio-guided fractionation and isolation of active component from Tragopogon graminifolius based on its wound healing property. J Ethnopharmacol. 226. 48–55.doi:10.1016/j.jep.2018.08.002.
- Belofsky GN, Jensen PR, Fenical W. 1999. Sansalvamide: A new cytotoxic cyclic depsipeptide produced by a marine Fungus of the Genus Fusarium. Tetrahedron Lett. 40:2913–2916.
- Bergmann S, Schümann J, Scherlach K, Lange C, Brakhage AA, Hertweck C. 2007. Genomics-driven discovery of PKS-NRPS hybrid metabolites from Aspergillus nidulans. Nat Chem Biol. 3:213–217. doi:10.1038/nchembio869.
- Bertrand S, Azzollini A, Schumpp O, Bohni N, Schrenzel J, Monod M. 2014a. Multi-well fungal co-culture for de novo metabolite-induction in time-series studies based on untargeted metabolomics. Mol Biosyst. 10:2289–2298. doi:10.1039/C4MB00223G.
- Bills GF, Gloer JB. 2016. Biologically active secondary metabolites from the fungi. Microbiol Spectr. 4(6): FUNK-0009-2016. doi: 10.1128/microbiolspec.FUNK-0009-2016.
- Bode HB, Bethe B, Höfs R, Zeeck A. 2002. Big effects from small changes: possible ways to explore nature’s chemical diversity. Chembiochem. 3:619–627.
- Bogner CW, Kamdem RS, Sichtermann G, Matthäus C, Hölscher D, Popp J, Proksch P, Grundler FMW, Schouten A. 2016. Bioactive secondary metabolites with multiple activities from a fungal endophyte. Microbial Biotech. 10(1):175–188.
- Boonyaketgoson S, Trisuwan K, Bussaban B, Rukachaisirikul V, Phongpaichit S. 2015. Isochromanone derivatives from the endophytic fungus Fusarium sp. PDB51F5. Tetrahedron Lett. 56:5076–5078.
- Brady SF, Clardy J. 2000. CR377, a new pentaketide antifungal agent isolated from an endophytic fungus. J Nat Prod. 63:1447–1448.
- Brakhage AA. 2013. Regulation of fungal secondary metabolism. Nat Rev Microbiol. 11:21–32. doi:10.1038/nrmicro2916.
- Bungtongdee N, Sopalun K, Laosripaiboon W, Iamtham S. 2019. The chemical composition, antifungal, antioxidant and antimutagenicity properties of bioactive compounds from fungal endophytes associated with Thai orchids. J Phytopathol. 167:56–64.
- Campos FF, Johann S, Cota BB, Alves TMA, Rosa LH, Caligiorne RB, Cisalpino PS, Rosa CA, Zani CL. 2011. Antifungal activity of trichothecenes from Fusarium sp. against clinical isolates of Paracoccidioides brasiliensis. Mycoses. 54:122–129.
- Campos FF, Junior PAS, Romanha AJ, Araújo MSS, Siqueira EP, Resende JM, Alves TMA, Martins-Filho OA, Dos Santos VL, Rosa CA, et al. 2015. Bioactive endophytic fungi isolated from Caesalpinia echinata Lam. (Brazilwood) and identification of beauvericin as a trypanocidal metabolite from Fusarium sp. Mem Inst Oswaldo Cruz. Rio de Janeiro. 110(1):65–74.
- Chakravarthi BVSK, Das P, Surendranath K, Karande AA, Jayabaskaran C. 2008. Production of paclitaxel by Fusarium solani isolated from Taxus celebica. J Bios. 33:259–267.
- Chen Z-M, Dong W-B, Li Z-H, Feng T, Liu J-K. 2014. New chlamydosporol derivatives from the endophytic fungus Fusarium sp. #001. J Asian Nat Prod Res. 16(5):465–470.
- Cheng L, Ma QM, Tao GJ, Tao WY, Wang RM, Yang J, Guo XL. 2007. Systemic identification of a paclitaxel producing endophytic fungus. Ind Microbiol. 37:23–30.
- Chiller T. 2017. The unexpected and troubling rise of Candida auris. Centers for Disease Control and Prevention, National Center for Emerging and Zoonotic Infectious Diseases (NCEZID), Division of Foodborne, Waterborne, and Environmental Diseases (DFWED). https://www.medscape.com/viewarticle/884470?src=par_cdc_stm_mscpedt&faf=1
- Chowdhury NS, Sohrab MH, Rana MS, Hasan CM, Jamshidi S, Rahman KM. 2017. Cytotoxic Naphthoquinone and Azaanthraquinone Derivatives from an Endophytic Fusarium solani. J Nat Prod. 80(4):1173–1177.
- Christian N, Sullivan C, Visser ND, Clay K. 2016. Plant host and geographic location drive endophyte community composition in the face of perturbation. Microb Ecol. 72:621–632.
- Cichewicz RH. 2010. Epigenome manipulation as a pathway to new natural product scaffolds and their congeners. Nat Prod Re. 27:11–22. doi:10.1039/B920860G.
- Cui J-L, Vijayakumar V, Zhang G. 2018. Partitioning of fungal endophyte assemblages in root-parasitic plant Cynomorium songaricum and Its Host Nitraria tangutorum. Front Microbiol. 9:666. doi:10.3389/fmicb.2018.00666.
- Cui Y, Yi D, Bai X, Sun B, Zhao Y, Zhang Y. 2012. Ginkgolide B produced endophytic fungus (Fusarium oxysporum) isolated from Ginkgo biloba. Fitoterapia. 5:913–920.
- Dababat AA, Sikora RA. 2007. Influence of the mutualistic endophyte Fusarium oxysporum162 on Meloidogyne incognita attraction and invasion. Nematology. 9:771–776.
- Dai W, Tao W. 2008. Preliminarly study on fermentation conditions of taxol-producing endophytic fungus. Chem Ind Eng Prog. 27:883–886.
- Dame ZT, Silima B, Gryzenhout M, Van Ree T. 2016. Bioactive compounds from the endophytic fungus Fusarium proliferatum. Nat Prod Res. 30(11):1301–1304.
- De Clercq E. 2004. Antivirals and antiviral strategies. Nat Rev Microbiol. 2:704–720. doi:10.1038/nrmicro975.
- Deng BW, Liu KH, Chen WQ, Ding XW, Xie XC. 2009. Fusarium solani, Tax-3, a new endophytic taxol-producing fungus from Taxus chinensis. World J Microbiol Biotech. 25:139–143.
- Dubois T, Gold CS, Coyne D, Paparu P, Mukwaba E, Athman S, Kapindu S, Adipala E. 2004. Merging biotechnology with biological control: banana Musa tissue culture plants enhanced by endophytic fungi. Uganda J Agric Sci. 9:445–451.
- Elavarasi A, Rathna GS, Kalaiselvam M. 2012. Taxol producing mangrove endophytic fungi Fusarium oxysporum from Rhizophora annamalayana. Asian Pac J Trop Biomed. 2(2):S1081–S1085.
- Fauci A, Morens D. 2012. The Perpetual Challenge of Infectious Diseases. New Engl J Med. 366:454–461.
- Fleming A. 1929. On the antibacterial action of cultures of a penicillium, with special reference to their use in the isolation of B. influenzae. Br J Exp Pathol. 10:226–236.
- Gai Y, Zhao LL, Hu CQ, Zhang HP. 2007. Fusarielin E, a new antifungal antibiotic from Fusarium sp. Chin Chem Lett. 18:954–956.
- Garantonakis N, Pappas ML, Varikou K, Skiada V, Broufas G, Kavroulakis N. 2018. Tomato inoculation with the endophytic strain Fusarium solani K results in reduced feeding damage by the zoophytophagous predator Nesidiocoris tenuis. Front Ecol Evol. 6:126.
- Garyali S, Kumar A, Sudhakara RM. 2013. Taxol production by an endophytic fungus Fusarium redolens, isolated from Himalayan Yew. J Microbiol Biotechnol. 23(10):1372–1380.
- Gitler AD, Dhillon P, Shorter J. 2017. Neurodegenerative disease: models, mechanisms, and a new hope. Dis Model Mech. 10(5):499–502.
- Gurudatt PS, Priti V, Shweta S, Ramesha BT, Ravikanth G, Vasudeva R, Amna T, Deepika S, Ganeshaiah KN, Uma Shaanker R, et al. 2010. Attenuation of camptothecin production and negative relation between hyphal biomass and camptothecin content in endophytic fungal strains isolated from Nothapodytes nimmoniana Grahm (Icacinaceae). Curr Sci. 98:1006–1009.
- Hallman J, Sikora RA. 1994. Influence of Fusarium oxysporum, a mutualistic fungal endophyte on Meloidogyne incognita infection of tomato. J Plant Dis Prot. 101:475–481.
- Hamzah T, Lee S, Hidayat A, Terhem R, Faridah-Hanum I, Mohamed R. 2018. Diversity and characterization of endophytic fungi isolated from the tropical mangrove species, Rhizophora mucronata, and identification of potential antagonists against the soil-borne fungus, Fusarium solani. Front Microbiol. 9:1707. doi:10.3389/fmicb.2018.01707.
- Han SM, Weaver FA, Comerota AJ, Perler BA, Joing M. 2010. Efficacy and safety of alfimeprase in patients with acute peripheral arterial occlusion (PAO). J Vasc Surg. 51(3):600–609.
- Hawas WU, Al-Farawati R, El-Kassem LTA, Adnan J, Turk AJ. 2016. Different Culture Metabolites of the Red Sea Fungus Fusarium equiseti optimize the Inhibition of Hepatitis C Virus NS3/4A Protease (HCV PR). Mar Drugs. 14:190.
- Hemphill PFC, Sureechatchaiyan P, Kassack UM, Orfali SR, Lin W, Daletos G, Proksch P. 2017. OSMAC approach leads to new fusarielin metabolites from Fusarium tricinctum. J Antibiot. 70(6): 726–732.
- Hidayat I, Radiastuti N, Rahayu G. 2015. Three Quinine – and Cinchonidine – producing Fusarium species from Indonesia. Curr Res Environ Appl Mycol. 6:20–34.
- Huang YF, Zhao JL, Zhou LG, Wang MA, Wang JG, Li XL, Chen Q. 2009a. Antimicrobial compounds from the endophytic fungus Fusarium sp. Ppf4 isolated from the medicinal plant Paris polyphylla var. yunnanensis. Nat Prod Comm. 4:1455–1458.
- Huang Z, Yang J, She Z, Lin Y. 2012. A new isoflavone from the mangrove endophytic fungus Fusarium sp. (ZZF60). Nat Prod Res. 26(1):11–15.
- Humberto H, Lara V, Ayala-Nunez NV, Carmen LD, Ixtepan T, Cristina RP. 2010. Bactericidal effect of silver nanoparticles against multidrug-resistant bacteria. World J Microbiol Biotechnol. 26:615–621.
- Hung T, Lin S. 2017. Microbial natural products: A promising source for drug discovery. J Appl Microbiol Biochem. 1:2–5.
- Hwang Y, Rowley D, Rhodes D, Gertsch J, Fenical W, Bushman F. 1999. Mechanism of inhibition of poxvirus topoisomerase by the Marine natural product sansalvamide A. Mol Pharmacol. 55:1049–1053.
- Ibrahim SRM, Abdallah HM, Elkhayat ES, Al Musayeib NM, Asfour HZ, Zayed MF, Mohamed GA. 2018a. Fusaripeptide A: new antifungal and anti-malarial cyclodepsipeptide from the endophytic fungus Fusarium sp. J Asian Nat Prod Res. 20(1):75–85.
- Ibrahim SRM, Elkhayat ES, Mohamed GAA, Fat’hi SM, Ross SA. 2016a. Fusarithioamide A, a new antimicrobial and cytotoxic benzamide derivative from the endophytic fungus Fusarium chlamydosporium. Biochem Biophys Res Commun. 479(2):211–216.
- Ibrahim SRM, Mohamed GA, Al Haidari RA, Zayed MF, El-Kholy AA, Elkhayat ES, Ross SA. 2018b. Fusarithioamide B, a new benzamide derivative from the endophytic fungus Fusarium chlamydosporium with potent cytotoxic and antimicrobial activities. Bioorg Med Chem. 26:786–790.
- Ibrahim SRM, Mohamed GA, Rosse SA. 2016b. Integracides F and G: new tetracyclic triterpenoids from the endophytic fungus Fusarium sp. Phytochem Lett. 15:125–130.
- Imazaki I, Kadota I. 2015. Molecular phylogeny and diversity of Fusarium endophytes isolated from tomato stems. FEMS Microbiol Ecol. 91(9):fiv 098.
- Ishii K, Ueno Y. 1981. Isolation and characterization of two new trichothecenes from Fusarium sporotrichioides strain m-1-1. Appl Environ Microb. 42(3):541–543.
- Ji H-F, Li X-J, Zhang H-Y. 2009. Natural products and drug discovery. Can thousands of years of ancient medical knowledge lead us to new and powerful drug combinations in the fight against cancer and dementia? EMBO Rep. 10(3):194–200. doi:10.1038/embor.2009.12.
- Jia M, Chen L, Xin HL, Zheng CJ, Rahman K, Han T. 2016. A friendly relationship between endophytic fungi and medicinal plants: a systematic review. Front Microbiol. 7:906.
- Jiang C-X, Li J, Zhang J-M, Jin X-J, Yu B, Fang J-G, Wu Q-X. 2019. Isolation, identification, and activity evaluation of chemical constituents from soil fungus Fusarium avenaceum SF-1502 and endophytic fungus Fusarium proliferatum AF-04. J Agric Food Chem. 67:1839–1846.
- Jin Z, Gao L, Zhang L, Liu T, Yu F, Zhang Z, Guo Q, Wang B. 2017. Antimicrobial activity of saponins produced by two novel endophytic fungi from Panax notoginseng. Nat Prod Res. 31(22):2700–2703.
- Kaul S, Sharma T, Dhar MK. 2016. “Omics” Tools for Better Understanding the Plant-Endophyte Interactions. Front Plant Sci. 7:955. doi:10.3389/fpls.2016.00955.
- Kaushik NK, Murali TS, Dinkar S, Suryanarayanan TS. 2014. A search for antiplasmodial metabolites among fungal endophytes of terrestrial and marine plants of southern India. Acta Parasitol. 59(4):745–757.
- Kavroulakis N, Ntougias S, Zervakis GI, Ehalioti C, Haralampidis K, Papadopoulou KK. 2007. Role of ethylene in the protection of tomato plants against soil-borne fungal pathogens conferred by an endophytic Fusarium solani strain. J Exp Bot. 58(14):3853–3864.
- Khan N, Afroz F, Begum M, Rony SR, Sharmin S, Moni F, Hasan CM, Shaha K, Sohra M. 2018. Endophytic Fusarium solani: A rich source of cytotoxic and antimicrobial napthaquinone and aza-anthraquinone derivatives. Toxicol Rep. 5:970–976.
- Kharwar RN, Mishra A, Gond SK, Stierle A, Stierle D. 2011. Anticancer compounds derived from fungal endophytes: their importance and future challenges. Nat Prod Rep. 28(7):1208. doi:10.1039/c1np00008j.
- Kildgaard S, Subko K, Phillips E, Goidts V, de la Cruz M, Díaz C, Gotfredsen CH, Andersen B, Frisvad JC, Nielsen KF, et al. 2017. A dereplication and bioguided discovery approach to reveal new compounds from a marine-derived fungus Stilbella fimetaria. Mar Drugs. 15(8):253. doi:10.3390/md15080253.
- Kloepper JW, Ryu C-M. 2006. Bacterial endophytes as elicitors of induced systemic resistance. In: Schulz BJE, Boyle CJC, Sieber TN, editors. Microbial Root Endophytes. Berlin:Springer; p. 33–52 doi: 10.1007/3-540-33526-9.
- Kornsakulkarn J, Dolsophon K, Boonyuen N, Boonruangprapa T, Rachtawee P, Prabpai S. 2011. Dihydronaphthalenones from endophytic fungus Fusarium sp. BCC14842. Tetrahedron. 67(39):7540–7547.
- Kour A, Shawl SA, Rehman S, Sultan P, Qazi PH, Suden P, Khajuria KR, Verma V. 2008. Isolation and identification of an endophytic strain of Fusarium oxysporum producing podophyllotoxin from Juniperus recurva. World J Microbiol Biotechnol. 24:1115–1121.
- Kumar A, Patil D, Rajamohanan PR, Ahmad A. 2013. Isolation, purification and characterization of vinblastine and vincristine from endophytic fungus Fusarium oxysporum isolated from Catharanthus roseus. PLoS One. 8(9):e71805.
- Kumara PM, Soujanya KN, Ravikanth G, Vasudeva R, Ganeshaiah KN, Uma Shaanker R. 2014. Rohitukine, a chromone alkaloid and a precursor of flavopiridol, isproduced by endophytic fungi isolated from Dysoxylum binectariferum Hook.f and Amoora rohituka (Roxb).Wight & Arn. Phytomedicine. 21:541–546.
- Kundu A, Saha S, Walia S, Dutta TK. 2016. Anti-nemic secondary metabolites produced by Fusarium oxysporum f. sp. ciceris. J Asia-Pac Entomol. 19:631–636.
- Kusari S, Zühlke S, Spiteller M. 2009. An endophytic fungus from Camptotheca acuminata that produces camptothecin and analogues. J Nat Prod. 72:2–7.
- Kyekyeku JO, Kusari S, Adosraku RK, Bullach A, Golz C, Strohmann C, Spiteller M. 2017. Antibacterial secondary metabolites from an endophytic fungus, Fusarium solani JK10. Fitoterapia. 119:108–114.
- Lata R, Chowdhury S, Gond SK, White JF Jr. 2018. Induction of abiotic stress tolerance in plants by endophytic microbes. Lett Appl Microbiol. 66(4):268–276.
- Le HTT, Padgham JL, Hagemann MH, Sikora RA, Schouten A. 2016. Developmental and behavioural effects of the endophytic Fusarium moniliforme Fe14 towards Meloidogyne graminicola in rice. Ann Appl Biol. 169:134–143.
- Lee J, Lobkovsky E, Pliam NB, Strobel G, Clardy J. 1995. Subglutinols A & B: immunosuppressive compounds from the endophytic fungus-Fusarium subglutinans. J Org Chem. 60:7076–7077.
- Li C-T, Li Y, Wang Q-J, Sung C-K. 2008. Taxol production by Fusarium arthrosporioides isolated from yew, Taxus cuspidata. J Med Biochem. 27:454–458.
- Li P, Mou Y, Shan T, Xu J, Li Y, Lu S, Zhou L. 2011. Effects of polysaccharide elicitors from endophytic Fusarium oxysporium Dzf17 on growth and diosgenin production in cell suspension culture of Dioscorea zingiberensis. Molecules. 16(11):9003–9016.
- Li YC, Tao WY, Cheng L. 2009. Paclitaxel production using co-culture of Taxus suspension cells and paclitaxel-producing endophytic fungi in a co-bioreactor. Appl Microbiol Biotechnol. 83:233–239.
- Looke FMD, Gottlieb T, Jones CA. 2015. The global challenges of infectious diseases. Med J Aust. 202(5):225–226.
- Mander P, Cho SS, Simkhada JR. 2011. A low molecular weight chymotrypsin-like novel fibrinolytic enzyme from Streptomyces sp. CS624. Process Biochem. 46:1449–1455.
- Marmann A, Aly A, Lin W, Wang B, Proksch P. 2014. Co-cultivation—a powerful emerging tool for enhancing the chemical diversity of microorganisms. Mar Drugs. 12:1043–1065. doi:10.3390/md12021043.
- Martinuz A, Schouten A, Sikora RA. 2012. Post‐infection development of Meloidogyne incognitaon tomato treated with the endophytes Fusarium oxysporum strain Fo162 and Rhizobium etli strain G12. BioControl. 58:95–104.
- Mennan S, Aksoy HM, Ecevit O. 2005. Antagonistic effect of Fusarium oxysporum on Heterodera cruciferae. J Phytopathol. 153:221–225.
- Michelakis ED, Webster L, Mackey JR. 2008. Dichloroacetate (DCA) as a potential metabolic-targeting therapy for cancer. Br J Cancer. 99(7):989–994.
- Mine Y, Wong AHK, Jiang B. 2005. Fibrinolytic enzymes in Asian traditional fermented foods. Food Res Int. 38:243–250. doi:10.1016/j.foodres.2004.04.008.
- Moron LS, Lim YW, Dela Cruz TEE. 2018. Antimicrobial activities of crude culture extracts from mangrove fungal endophytes collected in Luzon Island, Philippines. Philipp Sci Lett. 11:28–36.
- Moussa M, Ebrahim W, Bonus M, Gohlke H, Mándi A, Kurtán T, Proksch P. 2019. Co-culture of the fungus Fusarium tricinctum with Streptomyces lividans induces production of cryptic naphthoquinone dimers. RSC Adv. 9(3):1491–1500.
- Musavi SF, Balakrishnan RJ. 2013. Biodiversity, antimicrobial potential, and phylogenetic placement of an endophytic Fusarium oxysporum NFX 06 isolated from Nothapodytes foetida. J Mycol. doi:10.1155/2013/172056.
- Mwaura P, Dubois T, Losenge T, Coyne D, Kahangi EM. 2010. Effect of endophytic Fusarium oxysporum on paralysis and mortality of Pratylenchus goodeyi. Afr J Biotechnol. 9:1130–1134.
- Mwaura P, Kahangi EM, Losenge T, Dubois T, Coyne D. 2009. In vitro screening of endophytic Fusarium oxysporum against banana nematode (Helicotylenchus multicinctus). Afr J Hortic Sci. 2:103–110.
- Nadeem M, Ram M, Alam P, Ahmad MM, Mohammad A, Al-Qurainy F, Khan S, Abdin MZ. 2012. Fusarium solani, P1, a new endophytic podophyllotoxin producing fungus from roots of Podophyllum hexandrum. Afr J Microbiol Res. 6:2493–2499.
- Nascimento AM, Conti R, Turatti ICC, Cavalcanti BC, Costa-Lotufo LV, Pessoa C, de Moraes MO, Manfrim V, Toledo JS, Cruz AK, et al. 2012. Bioactive extracts and chemical constituents of two endophytic strains of Fusarium oxysporum. Rev Bras Farmacogn. 22(6):1276–1281.
- Nenkep V, Yun K, Zhang D, Choi DH, Kang JS, Son BW. 2010. Induced Production of Bromomethylchlamydosporols A and B from the marine-derived fungus Fusarium tricinctum. J Nat Prod. 73:2061–2063.
- Netzker T, Fischer J, Weber J, Mattern DJ, König CC, Valiante V, Schroeckh V, Brakhage AA. 2015. Microbial communication leading to the activation of silent fungal secondary metabolite gene clusters. Front Microbiol. 6:299.
- Newman DJ, Cragg GM. 2016. Natural products as sources of new drugs from 1981 to 2014. J Nat Prod. 79:629–661.
- Ola RBA, Thomy D, Lai D, Brotz-Oesterhelt H, Proksch P. 2013. Inducing secondary metabolite production by the endophytic fungus Fusarium tricinctum through coculture with Bacillus subtilis. J Nat Prod. 76:2094−2099.
- Pappas ML, Liapoura M, Papantoniou D, Avramidou M, Kavroulakis N, Weinhold A, Broufas GD, Papadopoulou KK. 2018. The beneficial endophytic fungus Fusarium solani strain K alters tomato responses against spider mites to the benefit of the plant. Front Plant Sci. 9:1603. doi:10.3389/fpls.2018.01603.
- Peng Y, Yang X, Zhang Y. 2005. Microbial fibrinolytic enzymes: an overview of source, production, properties, and thrombolytic activity in vivo. Appl Microbiol Biotechnol. 69:126–132.
- Peters RJB, Rijk JCW, Bovee TFH, Nijrolder AWJM, Lommen A, Nielen MWF. 2010. Identification of anabolic steroids and derivatives using bioassay-guided fractionation, UHPLC/TOFMS analysis and accurate mass database searching. Anal Chim Acta. 664:77–88.
- Plummer M, de Martel C, Vignat J, Ferlay J, Bray F, Franceschi S. 2016. Global burden of cancers attributable to infections in 2012: a synthetic analysis. Lancet Glob Health. 4(9):e609–16.
- Qin C, Lin X, Lu X, Wan J, Zhou X, Liao S, Tu Z, Xu S, Liu Y. 2014. Sesquiterpenoids and xanthones derivatives produced by sponge-derived fungus Stachybotry sp. HH1 ZSDS1F1-2. J Antibiot. 68:121–125. doi:10.1038/ja.2014.97.
- Ran X, Zhang G, Li S, Wang J. 2017. Characterization and antitumor activity of camptothecin from endophytic fungus Fusarium solani isolated from Camptotheca acuminate. Afri Health Sci. 17(2):566–574.
- Rather RA, Srinivasan V, Anwar M. 2018. Seasonal deviation effects foliar endophyte assemblage and diversity in Asparagus racemosus and Hemidesmus indicus. BMC Ecol. 18(1):52. doi:10.1186/s12898-018-0211-y.
- Ratnaweera PB, Dilip de Silva E, Williams DE, Andersen RJ. 2015. Antimicrobial activities of endophytic fungi obtained from the arid zone invasive plant Opuntia dillenii and the isolation of equisetin, from endophytic Fusarium sp. BMC Compl Alternat Med. 15:220.
- Rodriguez RJ, Henson J, Van Volkenburgh E, Hoy M, Wright L, Beckwith F, Kim Y, Redman RS. 2008. Stress tolerance in plants via habitat‐adapted symbiosis. Inter Soc Microb Ecol. 2:404–416.
- Rovati JI, Delgado OD, Figueroa LIC, Farina JI. 2010. A novel source of fibrinolytic activity: bionectria sp., an unconventional enzyme-producing fungus isolated from Las Yungas rainforest (Tucumán, Argentina). World J Microb Biot. 26:55–62.
- Schulz B, Rommert AK, Dammann U, Aust HJ, Strack D. 1999. The endophyte‐host interaction: a balanced antagonism? Mycol Res. 10:1275–1283.
- Schulze-Makuch D, Wagner D, Kounaves SP, Mangelsdorf K, Devine KG, de Vera JP, Schmitt-Kopplin P, Grossart HP, Parro V, Kaupenjohann M, et al. 2018. Transitory microbial habitat in the hyperarid Atacama Desert. Proc Natl Acad Sci USA. 115(11):2670–2675. doi:10.1073/pnas.1714341115.
- Selosse M-A, Bessis A, Pozo M-J. 2014. Microbial priming of plant and animal immunity: symbionts as developmental signals. Trends Microbiol. 22:607–613.
- Shah A, Rather MA, Hassan QP, Aga MA, Mushtaq S, Shah AM, Hussain A, Baba SA, Ahmad Z. 2017. Discovery of anti-microbial and anti-tubercular molecules from Fusarium solani: an endophyte of Glycyrrhiza glabra. J Appl Microbiol. 122:1168–1176.
- Shah S, Shrestha R, Maharjan S, Selosse M-A, Pant B. 2019. Isolation and characterization of plant growth-promoting endophytic fungi from the roots of Dendrobium moniliforme. Plants. 8:5. doi:10.3390/plants8010005.
- Shahasi A, Dubois Y, Viljoen A, Nico L, Ragama P, Niere B. 2006. vitro antagonism of endophytic Fusarium oxysporum isolates against the burrowing nematode Radopholus similis. Nematology. 8(4):627–636.
- Shao C, Wang C, Zheng C, She Z, Gu Y, Lin Y. 2010. A new anthraquinone derivative from the marine endophytic fungus Fusarium sp. (No. b77). Nat Prod Res. 24(1):81–85.
- Shao RJ. 2012. Screening of efficient thrombolytic strains and comparative analysis of methods for detecting thrombolytic activity. J Chongqing Normal Univ. 1–1. (in Chinese).
- Shiono Y, Shibuya F, Koseki T, Harizon Supratman U, Uesugi S, Kimura K-I. 2014. A new α-pyrone metabolite from a mangrove plant endophytic fungus, Fusarium sp. J Asian Nat Prod Res. doi:10.1080/10286020.2014.961919.
- Shu RG, Wang FW, Yang YM, Liu YX, Tan RX. 2004. Antibacterial and xanthine oxidase inhibitory cerebrosides from Fusarium sp. IFB-121, an endophytic fungus in Quercus variabilis. Lipids. 39:667–673.
- Shweta S, Zuehlke S, Ramesha BT, Priti V, Mohana Kumar P, Ravikanth G, Spiteller M, Vasudeva R, Uma Shaanker R. 2010. Endophytic fungal strains of Fusarium solani, from Apodytes dimidiata E. Mey. ex Arn (Icacinaceae) produce camptothecin, 10-hydroxycamptothecin and 9-methoxycamptothecin. Phytochemistry. 71:117–122.
- Sim J-H, Chai-Hoon K, Learn-Han L, Yoke-Kqueen C. 2010. Molecular diversity of fungal endophytes isolated from Garcinia mangostana and Garcinia parvifolia. J Microbiol Biotechnol. 20(4):651–658. doi:10.4014/jmb.0909.09030.
- Stępień Ł, Lalak-Kańczugowska J, Witaszak N, Urbaniak M. 2018. Fusarium secondary metabolism biosynthetic pathways: so close but so far away. Co-Evolution of Secondary Metabolites. 1–37. doi:10.1007/978-3-319-76887-8_28-1.
- Stierle A, Strobel GA, Stierle D. 1993. Taxol and taxanes production by Taxomyces andreanae, an entophytic fungus of pacific yew. Science. 260:214.
- Strobel G. 2018. The emergence of endophytic microbes and their biological promise. J Fungi. 4:57. doi:10.3390/jof4020057.
- Strobel G, Daisy B. 2003. Bioprospecting for microbial endophytes and their natural products. Microbiology and Molecular Biology Reviews. 67(4):491–502.
- Strobel GA, Hess WM, Ford E, Sidhu RS, Yang X. 1996. Taxol from fungal endophytes and issue of biodiversity. J Ind Microbiol. 17:417–423.
- Summerell BA, Leslie JF. 2011. Fifty years of Fusarium: how could nine species have ever been enough? Fungal Divers. 50:135–144.
- Sun WJ, Zhu HT, Zhang TY, Yang C-R, Zhang Y-X. 2018. Two new alkaloids from Fusarium tricinctum SYPF 7082, an endophyte from the root of Panax notoginseng. Nat Prod Bioprospect. 8:391.
- Tacconelli E, Carrara E, Savoldi A, Harbarth S, Mendelson M, Monnet D, Pulcini C, Kahlmeter G, Kluytmans J, Carmeli Y, et al., WHO Pathogens Priority List Working Group. 2018. Discovery, research, and development of new antibiotics: the WHO priority list of antibiotic-resistant bacteria and tuberculosis. Lancet Infect Dis. 18(3): 318–327.
- Tan QW, Ouyang MA, Shen S, Li W. 2012. Bioactive metabolites from a marine-derived strain of the fungus Neosartorya fischeri. Nat Prod Res. 26:1402–1407.
- Tan RX, Zou WX. 2001. Endophytes: as rich source of fuctional metabolite. Nat Prod Rep. 18:448–459.
- Tayung K, Barik BP, Jha DK, Deka DC. 2011. Identification and characterization of antimicrobial metabolite from an endophytic fungus, Fusarium solani isolated from bark of Himalayan yew. Mycosphere. 2(3):203–213.
- Thrane U. 2001. Developments in the taxonomy of Fusarium species based on secondary metabolites. In: Summerbell BA, Leslie JF, Backhouse D, Bryden WL, Burgess LW, editors. Fusarium. St. Paul (Minnesota): Paul E Nelson Memorial Symposium. APS Press; p. 29–49.
- Tiwari S, Singh S, Pandey P, Saikia SK, Negi AS, Gupta SK, Pandey R, Banerjee S. 2014. Isolation, structure determination, and antiaging effects of 2,3-pentanediol from endophytic fungus of Curcuma amada and docking studies. Protoplasma. 251:1089–1098.
- Toghueo KRM, Dinkar S, Boyom FF. 2016b. Stimulation of the production of new volatile and nonvolatile metabolites by endophytic Aspergillus niger using small organic chemicals. Curr Res Environ Appl Mycol. 6(4):256–267. doi:10.5943/cream/6/4/3.
- Toghueo KRM, Dinkar S, Inigo Z, Baker B, Boyom FF. 2018. Conditioned media and organic elicitors underpin the production of potent antiplasmodial metabolites by endophytic fungi from Cameroonian medicinal plants. Parasitol Res. 117:2473–2485.
- Toghueo RMK, Kemgne EAM, Eke P, Kanko MIM, Dize D, Sahal D, Boyom FF. 2019. Antiplasmodial potential and GC-MS fingerprint of endophytic fungal extracts derived from Cameroonian Annona muricata. J Ethnopharmacol. 235:111–121.
- Toghueo RMK, Zeuko’o Menkem E, Mbekou Kanko MI, Jesus Marie A-E-C, Ngo Mback N, Eke P, Vázquez de Aldana BR, Íñigo Z, Fekam Boyom F. 2016a. Antimicrobial and antiradical activities of ethyl acetate extracts from endophytic fungi isolated from Cameroonian medicinal plants. J Med Plant Stud. 4(4):290–295.
- Tung CY, Yang DB, Gou M. 2002. A preliminary study on the condition of the culture and isolate of endophytic fungus producing Vincristine. J Chuxiong Normal Univ. 6:39–41.
- Ueda M, Kubo T, Miyatake K, Nakamura T. 2007. Purification and characterization of fibrinolytic alkaline protease from Fusarium sp. BLB. Appl Microbiol Biotechnol. 74(2):331–338.
- Van Bael SA, Seid MA, Wcislo WT. 2012. Endophytic fungi increase the processing rate of leaves by leaf-cutting ants (Atta). Ecol Entomo. 37:318–321.
- Van Dessel P, Coyne D, Dubois T, De Waele D, Franco J. 2011. In vitro nematicidal effect of endophytic Fusarium oxysporum against radopholus similis, Pratylenchus goodeyi and Helicotylenchus multicinctus. Nematropica. 41:154–160.
- Vandenkoornhuyse P, Quaiser A, Duhamel M, Van L, Dufresne A. 2015. The importance of the microbiome of the plant holobiont. New Phytol. 206:1196–1206.
- Vasundhara M, Baranwal M, Kumar A. 2016. Isolation and Analysis of Salt Response of Lactobacillusplantarum FS5-5 from Dajiang. Indian J Microbiol. 56:433.
- Venugopalan A, Potunuru UR, Dixit M, Srivastava S. 2016. Reprint of: effect of fermentation parameters, elicitors and precursors on camptothecin production from the endophyte Fusarium solani. Bioresour Technol. 213:311–318.
- Vu TT, Sikora RA, Hauschild R. 2004. Effects of endophytic Fusarium oxysporum towards Radopholus similis activity in the absence of banana. Commun Agricul Appl Biol Sci. 69:381–385.
- Vujanovic V, Vujanovic J. 2007. Mycovitality and mycoheterotrophy: where lies dormancy in terrestrial orchid and plants with minute seeds. Symbiosis. 44:93–99.
- Wang H, Liu T, Xin Z. 2014. A new glucitol from an endophytic fungus Fusarium equiseti Salicorn 8. Eur Food Res Technol. 239:365–376.
- Wang QX, Li SF, Zhao F, Dai HQ, Bao L, Ding R. 2011. Chemical constituents from endophytic fungus Fusarium oxysporum. Fitoterapia. 82:777–781.
- Wang Z-F, Zhang W, Xiao L, Zhou Y-M, Du F-Y. 2018. Characterization and bioactive potentials of secondary metabolites from Fusarium chlamydosporum. Nat Prod Res. doi:10.1080/14786419.2018.1508142.
- Wani ZA, Ashraf N, Mohiuddin T, Riyaz-Ui-Hassan S. 2015. Plant-endophyte symbiosis, an ecological perspective. Appl Microbiol Biotechnol. 99:2955–2965.
- Wätjen W, Debbab A, Hohlfeld A, Chovolou Y, Kampkötter A, Edrada RA, Ebel R, Hakiki A, Mosaddak M, Totzke F, et al. 2009. Enniatins A1, B and B1 from an endophytic strain of Fusarium tricinctum induce apoptotic cell death in H4IIE hepatoma cells accompanied by inhibition of ERK phosphorylation. Mol Nutr Food Res. 53:431–440.
- Waweru B, Turoop L, Kahangi E, Coyne D, Dubois T. 2014. Non-pathogenic Fusarium oxysporum endophytes provide field control of nematodes, improving yield of banana (Musa sp.). Biol Control. 74:82–88.
- Weller MG. 2012. A unifying review of bioassay-guided fractionation, effect-directed analysis and related techniques. Sensors. 12(7):9181–9209. doi:10.3390/s120709181.
- WHO. 2018. World malaria report. World Health Organization: Geneva (Switzerland)
- Woolhouse M, Gowtage-Sequeria S. 2005. Host range and emerging and reemerging pathogens. Emerging Infect Dis. 11(12):1842–1847.
- Woolhouse ME, Dye C. 2001. Population biology of emerging and re-emerging pathogens—preface. Philos Trans R Soc Lond B Biol Sci. 356:981–982.
- Wu B, Wu L, Ruan L, Ge M, Chen D. 2009. Screening of endophytic fungi with antithrombotic activity and identification of a bioactive metabolite from the endophytic fungal strain CPCC 480097. Curr Microbiol. 58(5):522–527.
- Xiao W-J, Chen H-Q, Wang H, Cai C-H, Mei W-L, Dai H-F. 2018. New secondary metabolites from the endophytic fungus Fusarium sp. HP-2 isolated from “Qi-Nan” agarwood. Fitoterapia. 130:180–183.
- Xiong Z-Q, Yang -Y-Y, Na Zhao N, Wang Y. 2013. Diversity of endophytic fungi and screening of fungal paclitaxel producer from Anglojap yew, Taxus x media. BMC Microbiol. 13:71.
- Xu F, Tao WY, Cheng L, Guo LJ. 2006. Strain improvement and optimization of the media of taxol-producing fungus Fusarium mairei. Biochem Eng J. 31:67–73.
- Xu L, Wang J, Zhao J, Li P, Shan T, Wang J, Li X, Zhou L. 2010. Beauvericin from the endophytic fungus, Fusarium redolens, isolated from Dioscorea zingiberensis and its antibacterial activity. Nat Prod Commun. 5:811–814.
- Yang S-X, Xiao J, Laatsch H, Holstein JJ, Dittrich B, Zhang Q, Gao J-M. 2012. Fusarimine, a novel polyketide isoquinoline alkaloid, from the endophytic fungus Fusarium sp. LN12, isolated from Melia azedarach. Tetrahedron Lett. 53(47):6372–6375.
- Yong YH, Dai CC, Gao FK, Yang QY, Zhao M. 2009. Effects of endophytic fungi on growth and two kinds of terpenoids for Euphorbia pekinensis. Chin Trad Herb Drugs. 40:18–22.
- Zaher AM, Makboul MA, Moharram AM, Tekwani BL, Calderón AI. 2014. A new enniatin antibiotic from the endophyte Fusarium tricinctum Corda. J Antibiot. 1:4.
- Zhan J, Burns AM, Liu MX, Faeth SH, Gunatilaka AAL. 2007. Search for cell motility and angiogenesis inhibitors with potential anticancer activity: beauvericin and other constituents of two endophytic strains of Fusarium oxysporum. J Nat Prod. 70:227–232.
- Zhang J, Liu D, Wang H, Liu T, Xin Z. 2015a. Fusartricin, a sesquiterpenoid ether produced by an endophytic fungus Fusarium tricinctum Salicorn 19. Eur Food Res Technol. 240:805–814.
- Zhang LB, Gou LH, Zeng SV. 2000. Preliminary study on the isolation of endophytic fungus of Catharanthus roseus and its fermentation to produce product of therapeutic value. Chin Trad Herb Drugs. 11:805–807.
- Zhang S, Wang Y, Zhang N. 2015b. Purification and characterisation of a fibrinolytic enzyme from Rhizopus microsporus var. tuberosus. Food Technol Biotechnol. 53(2):243–248.
- Zhao JT, Fu YJ, Luo M, Zu YG, Wang W, Zhao CJ, Gu CB. 2012. Endophytic fungi from pigeon pea [Cajanus cajan(L.) Mill sp.] produce antioxidant Cajaninstilbene Acid. J Agric Food Chem. 60:4314–4319.
- Zhong L, Niu B, Tang L, Chen F, Zhao G, Zhao J. 2016. Effects of polysaccharide elicitors from endophytic Fusarium oxysporum Fat9 on the growth, flavonoid accumulation and antioxidant property of Fagopyrum tataricum sprout cultures. Molecules. 21:1590. doi:10.3390/molecules21121590.