ABSTRACT
Aspergillus flavus
exploits diverse mechanisms to survive during exposure to antifungal agents including morphogenesis. Germination of dormant conidia involves cascades of reactions integrated into the signalling pathway. This study documents the effect of phytochemical-quercetin on A. flavus during germination of conidia using scanning electron microscopy (SEM). Significant inhibition of conidial swelling of A. flavus in comparison to control was observed at 4 and 7 h Quantitative real-time PCR for genes from calcium signalling pathway and heat-shock proteins family showed up-regulation of heat shock (Hsp70 and Hsp90) and calcium signalling pathway genes (calcium-transporting ATPase and calmodulin) in response to quercetin at initial 4 h in comparison to control sample whereas up-regulation of Hsp70, calcineurin and transcription factor Crz1, were observed in both the treated samples. Gene encoding for calcium-kinase, cAMP, Rho-gdp, Plc and Pkc showed a constitutively higher level of expression in quercetin-treated sample in comparison to control at both time points. These data showed a clear response from genes encoding calcineurin-Crz1 signalling pathways and may find its application in the screening of antifungal agents.
Abbreviations
Hsp: Hear shock protein; MIC: Minimum Inhibitory Concentration; SEM: Scanning Electron Microscopy; qRT-PCR: Quantitative Real-Time Polymerase Chain Reaction
Introduction
The morphological transition of dormant conidia of Aspergillus flavus to the mycelial stage is critical for it to be a successful pathogen. A. flavus conidia colonise on crops under the favourable condition and on successful germination produce carcinogenic aflatoxin. It exists in three forms viz. conidia, mycelia, and hyphae and metabolic adaptation are required for the isotropic growth of the conidia to germinate into the hyphae (Shankar et al. Citation2018). The anti-fungal agents available (echinocandins, azoles and amphotericin B) majorly target the fungal cell wall and cell membrane (McCarthy et al. Citation2017). The poor outcome or failure of antifungal agents are generally attributed to the development of drug-resistant clinical or environmental isolates (Shishodia et al. Citation2019). In fungi, complex signal transduction cascades regulating morphogenesis are important to adapt and survive under influence of antifungal drugs (Lengeler et al. Citation2000). Calcium signalling pathway has been widely studied in C. albicans and S. cerevisiae in response to various stress and found to play a major role in cell survival, morphogenesis and virulent activity (Cowen and Steinbach Citation2008; Liu et al. Citation2015a). It is noted that the cellular calcium signalling machinery interacts with many other signalling pathway and is conserved throughout the fungal system (Barrige et al. Citation2003). Also, calcium signalling is directly involved in lateral branching from subapical hyphal compartments separated from the tip by a septum, hence playing a vital role in fungal morphogenesis. Previous researches have shown that binding of Ca2+ with calmodulin activates calmodulin-dependent kinases and the calcineurin phosphatase. Ca2+ binding to the regulatory subunit of calcineurin (CnaB) allowing activation of the catalytic subunit (CnaA) (Joseph and Means Citation2002). CnaA dephosphorylates transcription factor, crzA, to activate nuclear genes controlling processes such as cell-wall remodelling, polarity and conidiophore development and to respond against various stress signals (Yoshimoto et al. Citation2002; Boyce et al. Citation2015). Numerous studies have emphasised the role of Ca2+ in polarised tip growth in filamentous fungi. Tip-high gradients of total and free cytoplasmic Ca2+ have been measured in the pollen tube and growing fungal hyphae (Torralba and Heath Citation2001). Along with calcium signalling, heat shock proteins (Hsps) also impart their role in fungal morphogenesis. Heat shock proteins, being conserved in eukaryotes and its important role in morphogenesis and virulence in response to stress have been found attractive for antifungal studies (Gong et al. Citation2017). In addition, Hsps interacts with other cellular signalling pathways in order to control various physiological activities and virulence in fungi in response to traditional antifungal drugs (Singh et al. Citation2009; Gong et al. Citation2017). Previously it has been shown that Hsp90 and Hsp70 are the two major heat-shock proteins which can alone or together play a major role in morphogenesis and filamentation in Aspergilli (Tiwari et al. Citation2015). It has also been reported that Hsp90 is linked with calcium signalling as it regulates calcineurin (Mkc1) in the maintenance of the integrity of cell wall with the help of Hsp70 (LaFayette et al. Citation2010). Previously it has been shown that Hsp90-calcineurin pathway control conidiation in A. fumigatus and A. nidulans. However, Hsp90-calcineurin pathway inhibition resulted in hyphal growth impairment, cell-wall inhibition and defect in sporulation in A. fumigatus (Juvvadi et al. Citation2014). In addition, calcineurin helps Hsp90 in maintaining environmental changes by regulating dimorphism but not proliferation (Tiwari et al. Citation2015; Lamoth et al. Citation2016).
Anti-fungal studies have been approached towards some novel, environmental friendly compounds, which are basically plant derivatives such as alkaloids, flavanoids, tannins etc. (Arif et al. Citation2009). Phytochemicals against A. flavus showed that quercetin is a potential plant extract having both anti-Aspergillus and antiaflatoxigenic properties using MTT assay analysis (Zhou et al. Citation2015; Tiwari et al. Citation2017). Quercetin, a polyphenolic flavanoid have shown to exhibit oxidative stress in various Aspergillus species as well anti-fungal activities against A. flavus and C. abicans (Tempesti et al. Citation2012; Tiwari et al. Citation2017). Also, a proteomic approach using nLC-Q-TOF analysis of A. flavus in response to quercetin treatment showed activation of oxidative stress response proteins and transmembrane transport proteins, which suggests the efficiency of quercetin as a anti-aspergillus phytochemical (Tiwari and Shankar Citation2018). Therefore, the role of quercetin mediated inhibition of A. flavus morphogenesis via calcium signalling and heat-shock protein need further investigations. Recently, SEM and qRT-PCR have been used as efficient tools to determine the phytochemical activity against morphogenetic transformations and expression on various pathways at genomic levels, respectively (Liu et al. Citation2016; Nishiyama et al. Citation2017). Thus, the objectives of present study were (i) to determine the effect of quercetin on A. flavus conidia at two different time points (4 and 7 h) using scanning electron microscopy; and (ii) to investigate the mechanism of quercetin-mediated inhibition of A. flavus germination via genes encoding for calcium signalling pathway and heat-shock protein using qRT-PCR.
Material and method
Growth conditions for Aspergillus flavus
Toxigenic Aspergillus flavus (MTCC 9367), strain which was previously used by Patel et al. (Citation2014) was selected for this study (Patel et al. Citation2014) and the culture was maintained on potato dextrose agar (potato infusion-20%, dextrose-2%, agar-2%, pH 5.6) slants at 37°C. Spores were harvested after 72 h in phosphate-buffered saline (PBS) with 0.05% tween 20 (PBST) followed by centrifugation at 10,000 rpm (10 min at 4°C). Further, spores were washed with PBS two times and viability check (numbers of CFU/ml) was performed on PDA plates using haemocytometer according to the procedures followed by Tiwari et al. (Citation2016) (Tiwari et al. Citation2016) and 1 × 106 cells/ml were used as a working conidial culture for this study.
SEM analysis of quercetin treated Aspergillus flavus conidia at 4 and 7 h
For SEM analysis, 1 × 106 conidia of A. flavus treated with quercetin at MIC50; 113µg/ml and inoculated in sabouraud dextrose broth (dextrose-4%, peptone-1%, pH 5.6) medium against control (A. flavus without quercetin) for 4 and 7 h. After that conidia were harvested by centrifugation at 2700 rpm and washed with PBS thrice. Conidia were then fixed in 4% glutaraldehyde in PBS under vacuum for 2–4 h. The cells were washed with distilled water and then the cells were post-fixed with 1% osmium tetroxide for 1 h and dehydrated by passage through increasing concentration of (50–100%) ethanol solutions. The sample was then mounted on an aluminium sheet and coated with gold-palladium alloy. The observations were made on a Zeiss SEM (MA EVO −18 Special Edition).
Differential gene expression analysis using qRT-PCR
Total RNA from A. flavus treated with and without quercetin at 4 and 7 h time points was extracted in two independent biological replicates using the TRIzol method (Invitrogen, USA). Nanodrop spectrophotometer (Thermo Scientific, USA) was used at A260/A280 nm to estimate the quality and quantity of extracted RNA (Shankar et al. Citation2011). Further, 1.2% agarose gel electrophoresis was performed to check the integrity of extracted RNA. One micro gram (1 µg) of RNA was used for cDNA synthesis as per manual instructions (Thermo Scientific, USA. Primers for selected A. flavus genes were designed using Primer-Blast tool (NCBI) for expression study (Ye et al. Citation2012) enlisted in . Further, Bio-Rad machine CFX96 used for qRT-PCR to study the expression of selected genes in tested samples. 100ng of cDNA in12.5 µl reaction was used as a template for RT-PCR using SYBR-Green master-mix (BioRad). From two independent biological replicates, three technical replicates from each were carried for qRT-PCR (Thakur and Shankar Citation2017). PCR conditions were; initial denaturation at 95°C for 3 min, and 39 cycles of 95°C for 10 s, Tm (52–60) °C for 30 s, 72°C for 45 s. Reference gene tubulin was considered for data analysis and melting curve analysis was performed for the replicates for the each gene to check the specificity of primers. In order to calculate the relative expression of genes in samples, ‘2-∆∆CT” method was used to quantify the expression of selected genes in this study (Livak and Schmittgen Citation2001)
Table 1. List of primers for genes from calcium signalling pathway, tubulin and genes encoding for heat shock proteins.
Statistical analysis
From each independent time point (treated vs control), the significance of the mean values of the data at 4 and 7 h time-point in qRT-PCR was calculated using two-way ANOVA with Bonferronipost test. GraphPad software (GraphPad Prism v 5) was used for the analysis (*** represents P-value<0.001).
Results and discussion
Germination is the key step in the morphogenesis of conidia, which involves the activation of signalling pathways depending on environmental factors and stress response (Wang et al. Citation2012). To depict the effect of quercetin on the conidial cell wall of A. flavus, SEM analysis was performed at 4 and 7 h to determine the swelling of conidia and isotropic growth. Our study revealed that untreated A. flavus conidia were 4 µm in diameter, was found to be more swollen than the quercetin-treated A. flavus at 4 h that is 3.6 µm in diameter. Also, at 7 h it has been observed that the size of untreated A. flavus conidia was 4.4 µm in comparison to quercetin treated conidia, 2.3 µm. Significant swelling in control A. flavus conidia in comparison to quercetin treated conidia were observed (). Furthermore, a consistant increase in the size of control conidia from 4 to 7 h was observed in comparison to the treated conidia. Protuberance was observed more in control A. flavus conidia during the early stages when compared with the quercetin-treated. Morphogenesis of conidia of A. flavus with and without quercetin at 4 and 7 h using SEM have been shown in .
Figure 1. SEM images at different magnifications showing morphological changes in response to quercetin treatment in A. flavus.
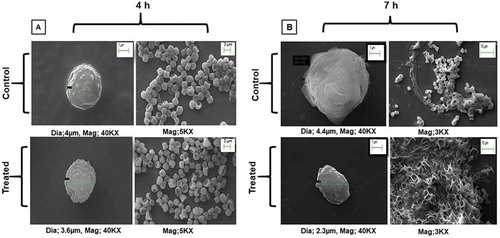
Using SEM analysis, we demonstrated that quercetin effectively delayed the morphogenesis of A. flavus at two different time points (4 and 7 h). The findings showed that the morphological inhibition starts at the early germination stage of A. flavus which increases significantly with time, in response to quercetin. Also, at 4 h, the swelling of conidia and flat-ball shape was not observed in quercetin treated conidia in comparison to control. Whereas at 7 h, less protuberance from the cell surface along with the shrunken and collapsed conidia incapable of polarised hyphal growth were observed in quercetin treated conidia. Further, the SEM studies conducted by Singh et al. (Citation2015) showed apoptotic effects in C. albicans in response to quercetin treatment (Singh et al. Citation2015). Furthermore, in a study by Rauha et al. (Citation2000), quercetin mediated growth inhibition in A. niger, B. subtilis and S. cerevisiae were observed (Rauha et al. Citation2000). Overall results showed that quercetin mediated in delaying of swelling as well as isotropic growth of conidia in A. flavus.
In order to quantify the expression profile of selected genes from calcium signalling pathway in response to quercetin, listed genes in were considered. A. flavus conidia (with and without quercetin) at 4 and 7 h time points were studied. Up-regulation of genes encoding for Calcium-transporting ATPase (Ca-ATPase) and Calmodulin (Cmd) at 4 h time point in quercetin treated sample and also in comparison to 7 h were observed. Whereas, up-regulation of genes encoding for calcineurin and transcription factor (Crz1) were observed at 7 h in treated sample as well as in comparison to 4 h. Other genes such as Ca-kinase, cAMP, Rho-gdp and Pkc were not significantly modulated with their expression in quercetin treatment in A. flavus at 4 and 7 h, however, showed a constitutively higher level of expression in quercetin-treated sample in comparison to control A. flavus ().
Figure 2. Differential gene expression patterns of heat shock proteins and calcium signalling pathway genes in response to quercetin in A. flavus. Panel A illustrated the fold change in expression of genes encoding for heat shock proteins in quercetin treated A. flavus in comparison to control at 4 and 7 h time points. Panel B illustrated the fold change in expression of genes related to calcium pathway in quercetin treated A. flavus in comparison to control at 4 and 7 h time points. The expression value of each gene was normalised with reference gene “tubulin” with their respective time points from treated and control sample. The data represented from the mean of triplicate for each gene from qRT-PCR using two independent biological replicates.
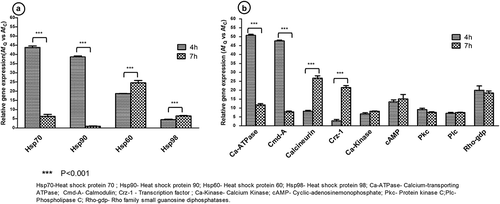
We further report a potential mechanism leading to modulation of Hsp dependent calcium signalling pathway in A. flavus in response to quercetin. Calcineurin-Crz1 signalling pathway is the well-established pathway in morphogenesis/dimorphism, fungi virulence and drug resistance (Juvvadi et al. Citation2017). Activation of calcineurin is mediated by various regulatory pathways which rely on the stress factors which further activates downstream transcriptional factor Crz1 (Juvvadi et al. Citation2014; Liu et al. Citation2015b).
Several studies showed that Hsp90 functions synergistically with calcineurin (key mediator) in response to azoles and echinocandins by activating calcium kinases and provided resistance in C. albicans (Singh et al. Citation2009; Gong et al. Citation2017). Also, Hsp90-calcineurin interaction is important for fungal dimorphism and virulence in fungi such as, C. albicans, S. cerevisiae and P. brasiliensis (Lee et al. Citation2013; Tiwari et al. Citation2015). In response to oxidative stress Hsp90 is also known to activate calmodulin kinases which in turn activates calcineurin-Crz1 pathway (Rodriguez-Caban et al. Citation2011). In our gene expression study in A. flavus under influence of quercetin, we found up-regulation of calcineurin and Crz1 genes at both 4 and 7 h time points, which suggested the activation of calcineurin-Crz1 signalling pathway in response to quercetin-mediated stress. Interestingly, Cramer et al. (Citation2008) using calcineurin and CrzA gene mutant of A. fumigatus showed lack of polarised hyphal growth in SEM images (Cramer et al. Citation2008).
Genes encoding for heat-shock proteins like Hsp60, Hsp70, Hsp90 and Hsp98 were selected for expression analysis with and without quercetin treated (control) in A. flavus at two different time points (4 and 7 h). qRT-PCR results showed significant up-regulation of Hsp70, Hsp90, at 4 h time point in treated samples in comparison to control and also in comparison to 7 h whereas Hsp60 and Hsp98 were up-regulated at 7 h in the treated sample and also in comparison to 4 h (). Quercetin-mediated response showed up-regulation of both Hsp70 and Hsp90 at the early time point (4 h) under phytochemical stress. Up-regulation of Hsp70 and Hsp90 at 4 h (in comparison to 7 h and control) suggests that quercetin modulated the expression of Hsp90 potentially to overcome the inhibited swelling of conidia. Other signalling pathway involves the co-operative function of Hsp70 and 90 which activates mitogen-activated protein kinase/protein kinase C (MAPK/PKC) pathway involved in the cell proliferation. Also, Hsp70 and 90 showed a positive interaction with calcineurin in response to drugs (Rodriguez-Caban et al. Citation2011; Nagao et al. Citation2012). These findings suggested that under quercetin stress, expression of genes encoding for Hsp70 and Hsp90, and calcineurin-Crz1 signalling pathway are up-regulated to overcome the inhibitory effect of phytochemical. To initiate swelling of conidia, influx of Ca+2 in the cytoplasm by calcium-transporting ATPase activates calmodulin, a calcium sensor protein. Calmodulin by activating calcium/calmodulin kinase signals calcineurin pathway (Odom Citation2014).
On the note, free cytoplasmic Ca2+ during polarised growth of fungal hyphae have been observed (Torralba and Heath Citation2001). In addition, it has been observed that abundant expression of calcium transporter protein, i.e. calcium-transporting ATPase under quercetin treatment in A. flavus (Tiwari and Shankar Citation2018). The effect of quercetin on Ca+2 concentration has also been studied using HepG2 cells (Cui et al. Citation2015) that showed an increase in calcium ion concentration inside the cells and the flow of calcium ions among cell organelles. Whereas in case of breast cell cancer quercetin suggested to induce apoptosis in MDA-MB-231 cells by a reduction in calcium dependent urokinase which leads to decrease in intracellular calcium uptake in cells (Devipriya et al. Citation2006). Gene transcripts data encoding for calcium transporting ATPase and calmodulin was significantly up-regulated at 4 h (in comparison to 7 h and control) in quercetin treated conidia. These data suggested that quercetin may modulate Ca+2 concentrations, and intracellular Ca+2 homoeostasis may play an important role during morphogenesis of A. flavus conidia.
The next pathway is G-protein coupled receptor pathway which stimulates phospholipase C (Plc) involved in the activation of the calcineurin pathway via IP3 (Schumacher et al. Citation2008). We have observed constitutive higher expression of genes encoding for cAMP, Pkc, Plc and Rho-gdp at 4 and 7 h in response to quercetin to sustain the swelling and polarised growth of the conidia in both the conditions (Tsai et al. Citation2013; Tsai and Chung Citation2014). Overall analysis showed that quercetin modulated the expression of key genes and other elements from signalling pathway.
Conclusion
Quercetin is a potential flavonoid capable of inhibiting morphogenesis of conidia of A. flavus, an essential step in the germination, by modulating the gene expression from calcium signalling pathway, Hsp90 and 70. These findings highlight the potential of calcineurin-Crz signalling pathway for antifungal targets. It also elucidated the role of conserved elements of signal transduction cascades under the phytochemical stress.
Compliance with ethical standards
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Acknowledgements
Authors are thankful to Department of Biotechnology and Bioinformatics, Jaypee University of Information Technology, Solan, Himachal Pradesh, India, for providing facilities and financial support to PhD student SKS and ST. We also acknowledge Amity University for SEM facilities, Noida Uttar Pradesh, India.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Arif T, Bhosale J, Kumar N, Mandal T, Bendre R, Lavekar G, Dabur R. 2009. Natural products–antifungal agents derived from plants. J Asian Nat Prod Res. 11(7):621–638.
- Barrige M, Bootman M, Roderick H. 2003. Calcium signaling: dynamics, homeostasis, and remodeling. Nature. 4:517–529.
- Boyce KJ, Andrianopoulos A. 2015. Fungal dimorphism: the switch from hyphae to yeast is a specialized morphogenetic adaptation allowing colonization of a host. FEMS Microbiol Rev. 39(6):797–811.
- Cowen LE, Steinbach WJ. 2008. Stress, drugs, and evolution: the role of cellular signaling in fungal drug resistance. Eukaryot Cell. 7(5):747–764.
- Cramer RA Jr., Perfect BZ, Pinchai N, Park S, Perlin DS, Asfaw YG, Heitman J, Perfect JR, Steinbach WJ. 2008. Calcineurin target CrzA regulates conidial germination, hyphal growth, and pathogenesis of Aspergillus fumigatus. Eukaryot Cell. 7(7):1085–1097. eng.
- Cui X, Luo Y, Li C, Li Y, Wang Z. 2015. Changes of intracellular Ca2+ in quercetin-induced autophagy progression. Acta Biochim Biophys Sin (Shanghai). 47(11):908–914. eng.
- Devipriya S, Vani G, Ramamurthy N, Shyamaladevi CS. 2006. Regulation of intracellular calcium levels and urokinase activity in MDA MB 231 cells by quercetin. Chemotherapy. 52(2):60–65.
- Gong Y, Li T, Yu C, Sun S. 2017. Candida albicans heat shock proteins and Hsps-associated signaling pathways as potential antifungal targets. Front Cell Infect Microbiol. 7:520.
- Joseph JD, Means AR. 2002. Calcium binding is required for calmodulin function in Aspergillus nidulans. Eukaryot Cell. 1(1):119–125.
- Juvvadi PR, Lamoth F, Steinbach WJ. 2014. Calcineurin as a multifunctional regulator: unraveling novel functions in fungal stress responses, hyphal growth, drug resistance, and pathogenesis. Fungal Biol Rev. 28(2–3):56–69.
- Juvvadi PR, Lee SC, Heitman J, Steinbach WJ. 2017. Calcineurin in fungal virulence and drug resistance: prospects for harnessing targeted inhibition of calcineurin for an antifungal therapeutic approach. Virulence. 8(2):186–197.
- LaFayette SL, Collins C, Zaas AK, Schell WA, Betancourt-Quiroz M, Gunatilaka AL, Perfect JR, Cowen LE. 2010. PKC signaling regulates drug resistance of the fungal pathogen Candida albicans via circuitry comprised of Mkc1, calcineurin, and Hsp90. PLoS Pathog. 6(8):e1001069.
- Lamoth F, Juvvadi PR, Steinbach WJ. 2016. Heat shock protein 90 (Hsp90): a novel antifungal target against Aspergillus fumigatus. Crit Rev Microbiol. 42(2):310–321.
- Lee SC, Li A, Calo S, Heitman J. 2013. Calcineurin plays key roles in the dimorphic transition and virulence of the human pathogenic zygomycete Mucor circinelloides. PLoS Pathog. 9(9):e1003625.
- Lengeler KB, Davidson RC, D’souza C, Harashima T, Shen W-C, Wang P, Pan X, Waugh M, Heitman J. 2000. Signal transduction cascades regulating fungal development and virulence. Microbiol Mol Biol Rev. 64(4):746–785.
- Liu F-F, Pu L, Zheng Q-Q, Zhang Y-W, Gao R-S, Xu X-S, Zhang S-Z, Lu L. 2015a. Calcium signaling mediates antifungal activity of triazole drugs in the Aspergilli. Fungal Genet Biol. 81:182–190.
- Liu S, Hou Y, Liu W, Lu C, Wang W, Sun S. 2015b. Components of the calcium-calcineurin signaling pathway in fungal cells and their potential as antifungal targets. Eukaryot Cell. 14(4):324–334.
- Liu S, Yue L, Gu W, Li X, Zhang L, Sun S. 2016. Synergistic effect of fluconazole and calcium channel blockers against resistant Candida albicans. PLoS One. 11(3):e0150859.
- Livak KJ, Schmittgen TD. 2001. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods. 25(4):402–408.
- McCarthy MW, Kontoyiannis DP, Cornely OA, Perfect JR, Walsh TJ. 2017. Novel agents and drug targets to meet the challenges of resistant fungi. J Infect Dis. 216(suppl_3):S474–S483.
- Nagao J-I, Cho T, Uno J, Ueno K, Imayoshi R, Nakayama H, Chibana H, Kaminishi H. 2012. Candida albicans Msi3p, a homolog of the Saccharomyces cerevisiae Sse1p of the Hsp70 family, is involved in cell growth and fluconazole tolerance. FEMS Yeast Res. 12(6):728–737.
- Nishiyama Y, Takahata S, Abe S. 2017. Morphological effect of the new antifungal agent ME1111 on hyphal growth of trichophyton mentagrophytes, determined by scanning and transmission electron microscopy. Antimicrob Agents Chemother. 61(1):e01195–01116.
- Odom AR. 2014. The triphenylethylenes, a novel class of antifungals. MBio. 5(3):e01126–01114.
- Patel TK, Anand R, Singh AP, Shankar J, Tiwary BN. 2014. Evaluation of aflatoxin B1 biosynthesis in A. flavus isolates from central india and identification of atoxigenic isolates. Biotechnol Bioprocess Eng. 19(6):1105–1113.
- Rauha J-P, Remes S, Heinonen M, Hopia A, Kähkönen M, Kujala T, Pihlaja K, Vuorela H, Vuorela P. 2000. Antimicrobial effects of Finnish plant extracts containing flavonoids and other phenolic compounds. Int J Food Microbiol. 56(1):3–12.
- Rodriguez-Caban J, Gonzalez-Velazquez W, Perez-Sanchez L, Gonzalez-Mendez R, Rodriguez-del Valle N. 2011. Calcium/calmodulin kinase1 and its relation to thermotolerance and HSP90 in Sporothrix schenckii: an RNAi and yeast two-hybrid study. BMC Microbiol. 11(1):162.
- Schumacher J, Viaud M, Simon A, Tudzynski B. 2008. The Gα subunit BCG1, the phospholipase C (BcPLC1) and the calcineurin phosphatase co‐ordinately regulate gene expression in the grey mould fungus Botrytis cinerea. Mol Microbiol. 67(5):1027–1050.
- Shankar J, Tiwari S, Shishodia SK, Gangwar M, Hoda S, Thakur R, Vijayaraghavan P. 2018. Molecular insights into development and virulence determinants of Aspergilli: A proteomic perspective. Front Cell Infect Microbiol. 8:180.
- Shankar J, Wu TD, Clemons KV, Monteiro JP, Mirels LF, Stevens DA. 2011. Influence of 17β-estradiol on gene expression of Paracoccidioides during mycelia-to-yeast transition. PLoS One. 6(12):e28402.
- Shishodia SK, Tiwari S, Shankar J. 2019. Resistance mechanism and proteins in Aspergillus species against antifungal agents. Mycology. 10(3):151–165.
- Singh B, Upreti D, Singh B, Pandey G, Verma S, Roy S, Naqvi A, Rawat A. 2015. Quercetin sensitizes fluconazole-resistant Candida albicans to induce apoptotic cell death by modulating quorum sensing. Antimicrob Agents Chemother. 59(4):2153–2168.
- Singh SD, Robbins N, Zaas AK, Schell WA, Perfect JR, Cowen LE. 2009. Hsp90 governs echinocandin resistance in the pathogenic yeast Candida albicans via calcineurin. PLoS Pathog. 5(7):e1000532.
- Tempesti TC, Alvarez MG, de Araújo MF, Júnior FEAC, de Carvalho MG, Durantini EN. 2012. Antifungal activity of a novel quercetin derivative bearing a trifluoromethyl group on Candida albicans. Med Chem Res. 21(9):2217–2222.
- Thakur R, Shankar J. 2017. Proteome profile of Aspergillus terreus conidia at germinating stage: identification of probable virulent factors and enzymes from mycotoxin pathways. Mycopathologia. 182(9–10):771–784.
- Tiwari S, Gupta N, Malairaman U, Shankar J. 2017. Anti-aspergillus properties of phytochemicals against aflatoxin producing Aspergillus flavus and Aspergillus parasiticus. National Acad Sci Lett. 40(4):267–271.
- Tiwari S, Shankar J. 2018. Integrated proteome and HPLC analysis revealed quercetin-mediated inhibition of aflatoxin B1 biosynthesis in Aspergillus flavus. 3 Biotech. 8(1):47.
- Tiwari S, Thakur R, Goel G, Shankar J. 2016. Nano-LC-Q-TOF analysis of proteome revealed germination of Aspergillus flavus conidia is accompanied by MAPK signalling and cell wall modulation. Mycopathologia. 181(11–12):769–786.
- Tiwari S, Thakur R, Shankar J. 2015. Role of heat-shock proteins in cellular function and in the biology of fungi. Biotechnol Res Int. 2015:132635.
- Torralba S, Heath IB. 2001. Cytoskeletal and Ca2+ regulation of hyphal tip growth and initiation. Curr Top Dev Biol. 51:135–187.
- Tsai H-C, Chung K-R. 2014. Calcineurin phosphatase and phospholipase C are required for developmental and pathological functions in the citrus fungal pathogen Alternaria alternata. Microbiology. 160(7):1453–1465.
- Tsai H-C, Yang SL, Chung K-R. 2013. Cyclic AMP-dependent protein kinase A negatively regulates conidia formation by the tangerine pathotype of Alternaria alternata. World J Microbiol Biotechnol. 29(2):289–300.
- Wang L, Lin X. 2012. Morphogenesis in fungal pathogenicity: shape, size, and surface. PLoS Pathog. 8(12):e1003027.
- Ye J, Coulouris G, Zaretskaya I, Cutcutache I, Rozen S, Madden TL. 2012. Primer-blast: a tool to design target-specific primers for polymerase chain reaction. BMC Bioinformatics. 13(1):134.
- Yoshimoto H, Saltsman K, Gasch AP, Li HX, Ogawa N, Botstein D, Brown PO, Cyert MS. 2002. Genome-wide analysis of gene expression regulated by the calcineurin/Crz1p signaling pathway in Saccharomyces cerevisiae. J Bio Chem. 277(34):31079–31088.
- Zhou W, Hu L-B, Zhao Y, Wang M-Y, Zhang H, Mo H-Z. 2015. Inhibition of fungal aflatoxin B1 biosynthesis by diverse botanically-derived polyphenols. Trop J Pharm Res. 14(4):605–609.
