?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
Polysaccharides in boletes (Boletales) are economically significant to both function food and medicinal industries. The polysaccharides were extracted from the fruit bodies of eight boletes, namely, Aureoboletus longicollis, Butyriboletus hainanensis, Crocinoboletus rufoaureus, Hemioporus japonicus, Neoboletus infuscatus, Neoboletus obscureumbrinus, Tylopilus otsuensis, Xanthoconium fusciceps, which were collected from tropical China; their physicochemical properties and antioxidant activities were characterised and evaluated, respectively. The results revealed that the polysaccharides among the eight boletes were mainly composed of glucose, mannose, and galactose, with a broad molecular weight range, and contained a pyranose ring revealed by FT-IR and NMR spectral analyses. Many factors such as different species of boletes, geographic conditions, molecular weight, configuration, and monosaccharide content may affect the antioxidant power of polysaccharides, simultaneously, instead of one single factor. The antioxidant activities of the polysaccharides were measured according to in vitro assays of DPPH scavenging, superoxide anion scavenging, and ferrous ion reducing tests. The polysaccharide of C. rufoaureus has greatly superior antioxidant activity and it could serve as potential functional food or medicine.
1. Introduction
Polysaccharides, one of the most abundant constituents in mushrooms, have attracted much attention for their special physicochemical properties and potential activities (Zhang et al. Citation2018). For example, polysaccharides derived from mushrooms, like ganoderan, lentinan, and grifolan have been investigated as the focus of research (Wu Citation2018; López-Legarda et al. Citation2020; Yang et al. Citation2021). Boletes (Boletales), one important group of mushrooms, have been reported recently, showing high species diversity (Yang et al. Citation2008; Zhang et al. Citation2011; Zeng et al. Citation2015, Citation2018b; Liang et al. Citation2016; Wu et al. Citation2019; Jiang et al. Citation2021). Polysaccharides extracted from boletes also display a wide range of bioactivities, especially strong antioxidant activities (Sun et al. Citation2016a; Yang et al. Citation2008; Zhang et al. Citation2011, Citation2018). However, only a few polysaccharides from boletes were investigated (Sun et al. Citation2016; Wu et al. Citation2016; Su et al. Citation2018; Xiao et al. Citation2019; Meng et al. Citation2021), polysaccharides from more boletes should be noted.
Recently, eight boletes were collected from tropical China, crude polysaccharides were extracted from the fruit bodies of eight boletes. The structures of the crude polysaccharides were characterised according to high-performance liquid chromatography (HPLC) and Fourier-transform infrared spectroscopy (FT-IR), respectively; their antioxidant properties were studied using in vitro assays of DPPH scavenging, superoxide anion scavenging, and ferrous ion reducing tests. The purposes of this study are: (i) to elucidate the structures of polysaccharides and explore their antioxidant activities from eight boletes; and (ii) to uncover more medicinal boletes from China.
2. Materials and methods
2.1. Materials and chemicals
Fruit bodies of eight boletes collected from Hainan Island, a tropical area of China, are demonstrated in . The voucher specimens were deposited in the Fungal Herbarium of Hainan Medical University, Haikou City, Hainan Province, China (FHMU). Techniques for species identification using morphological and molecular phylogenetic analyses followed those in Zeng et al. (Citation2015, Citation2018), Liang et al. (Citation2016), Chai et al. (Citation2019), Wu et al. (Citation2019), Jiang et al. (Citation2021), and references therein. Five sequences [2 of nuc 28S rDNA D1-D2 domains (28S) (MW826956, OK316964), 2 of nuc rDNA region encompassing the internal transcribed spacers 1 and 2, along with the 5.8S rDNA (ITS) (MW830220, OK298390), and 1 of the translation elongation factor 1-α gene (TEF1) (MW925783)] were newly generated and deposited in GenBank. The results of species identification indicated that the eight boletes were Aureoboletus longicollis (Ces.) N.K. Zeng & Ming Zhang, Butyriboletus hainanensis N.K. Zeng, Zhi Q. Liang & S. Jiang, Crocinoboletus rufoaureus (Massee) N.K. Zeng, Zhu L. Yang & G. Wu, Hemioporus japonicas (Hongo) E. Horak, Neoboletus infuscatus N.K. Zeng, S. Jiang & Zhi Q. Liang, N. obscureumbrinus (Hongo) N.K. Zeng, H. Chai & Zhi Q. Liang, Tylopilus otsuensis Hongo, and Xanthoconium fusciceps N.K. Zeng, Zhi Q. Liang & S.Jiang, respectively. The abbreviations of A. longicollis, But. hainanensis, C. rufoaureus, H. japonicas, N. infuscatus, N. obscureumbrinus, T. otsuensis, and X. fusciceps in this work were Al., Bh., Cr., Hj., Ni., No., To., Xf., respectively.
Figure 1. Basidiomata of eight boletes. (a). Aureoboletus longicollis (FHMU398). (b). Butyriboletus hainanensis (FHMU2410). (c). Crocinoboletus rufoaureus (FHMU1975). (d). Hemioporus japonicus (FHMU887). e. Neoboletus infuscatus (FHMU3372). (f). N. obscureumbrinus (FHMU2052). g. Tylopilus otsuensis (FHMU914). (h–i). Xanthoconium fusciceps (FHMU4759). Photos by N.K. Zeng.

1,1-Dipheneyl-2-picrylhydrazyl (DPPH) was purchased from Tokyo Kasei Kogyo Co. Ltd. (Shanghai, China); 2,6-ditert-butyl-4-methylphenol (BHT) and 1-phenyl-3-methyl-5-pyrazolone (PMP) were bought from Shanghai Macklin Biochemical Technology Co. Ltd (Shanghai, China); ascorbic acid (ASC) was obtained from Beijing Solarbio Science & Technology Co., Ltd. (Beijing, China); standard monosaccharide samples were all from Sigma-Aldrich (Germany); other chemicals used were of analytical grade, and mainly produced by Xilong Scientific Co., Ltd. (Guangdong, China).
2.2. Preparation of water-soluble crude polysaccharides
The dried samples were ground into fine powders (40 meshes). The crude polysaccharides were prepared as the previously reported method (Liu et al. Citation2016), with some minor modifications. The powders (10 g) were subjected to macerating with 95% ethanol (v/v) overnight. The residues were dried and then extracted with hot water at 85°C for 3 h and then repeated twice. The filtrates were combined and evaporated to 20 mL in vacuum. The proteins in the solution were eliminated by Sevag reagent. Then, the deproteinized liquid was dialysed against tap water for 24 h and distilled water for 12 h. The dialysate was concentrated and then precipitated with 4 volumes of ethanol-water solution and centrifuged, and finally lyophilised to obtain the crude polysaccharides. The crude polysaccharide content was determined using the phenol-sulphuric acid spectrophotometric method according to the established method (Kaewnarin et al. Citation2020).
2.3. Molecular weight determination
Molecular weight (MW) distributions of the crude polysaccharides were measured by referring to the reported method (Zhang et al. Citation2018). Each sample (10 mg) was dissolved in 5 mL of deionised water to obtain a sample solution at the concentration of 2 mg/mL. The solution was stirred with a magnetic stirrer for 30 min before analysis. Then the MW distributions were determined using high-performance gel permeation chromatography (HPGPC) matching a TSK-GEL G5000 PW column (7.8 × 300 mm, Tosoh Corp, Japan) on an Agilent 1100 HPLC system equipment equipped with a Waters 2410 refractive index detector, and eluted with ultrapure water at a flow rate of 0.8 mL/min. The column temperature was maintained at 30°C. A 20 μL of polysaccharide solution was injected in each run. Dextran standards in the range of 3.5 to 670 kDa were used to establish the standard curve. The results were processed and analysed using Breeze Software.
2.4. Monosaccharide composition
The monosaccharide compositions of the polysaccharides from eight boletes were analysed using PMP-HPLC according to a previously reported method (Ni et al. Citation2014), with minor modifications. Each dried crude polysaccharide sample (10 mg) was hydrolysed using 2 mL of 2 mol/L trifluoroacetic acid (TFA) at 110°C for 2 h. After being cooled down to room temperature, 1 mL of solution was mixed with 1 mL of methanol, then the mixture was dried by nitrogen at 70°C in a water bath. The procedure was repeated twice to eliminate the excessive acid. One millilitre of sodium hydroxide solution (0.3 M) was subsequently added to dissolve it completely.
Prepare a mixed monosaccharide solution containing mannose, rhamnose, glucosamine, glucose, galactose, xylose, arabinose, and fucose at a concentration of 0.4 mg/mL, then mix it with sodium hydroxide solution (0.6 M) at an equivalent volume. A 400 μL of PMP methanol solution was reacted with 400 μL of the mixture solution at 70°C for 2 h. After the reaction solution was cooled down to room temperature, 400 μL dilute hydrochloric acid (0.3 M) was then added to neutralise the solution, and its pH value was kept between 6 and 7. Then, 1.2 mL water was added and partitioned with an equivalent volume of chloroform. The procedure was repeated twice. The water phases were combined and filtered with microporous membrane (0.45 μm). The polysaccharide hydrolysates were derivatised under the same conditions as PMP.
The monosaccharide composition analysis was performed through a C18 column (250 mm × 4.6 mm, 5 μm, Agilent Corp., USA) on an Agilent 1100 HPLC with a DAD detector. The mobile phase was sodium phosphate buffer (100 mM, pH 6.7) (A) – acetonitrile (B). The gradient elution mode was as follows: 0–9 min, 86%A→83%A; 9–28 min, 83%A→78%A; 28–29 min, 78%A→50%A; 29–31 min, 50%A; 31–32 min, 50%A→86%A; 32–36 min, 86%A. The detection wavelength was set at 250 nm, the column temperature was maintained at 30°C, and the injection volume was 10 μL aliquot for each run at a flow rate of 1 mL/min.
2.5. FT-IR spectral analysis
FT-IR spectra of crude polysaccharides were recorded on a Fourier-transform infrared spectrometer (Nicolet iS5 FT-IR, Thermo Fisher Corporation, USA) in the range of 400–4000 cm−1 at room temperature. Each polysaccharide was incorporated with KBr powder at a ratio of 1: 100, pressed into a pellet, and then scanned for FT-IR measurement.
2.6. NMR spectral analysis
The polysaccharides were dried for 72 h in a vacuum dryer filled with silica gel and then dissolved in 0.6 mL deuterium oxide (D2O). The 1H NMR spectra of eight samples were performed on a JEOL 400 MHz NMR spectrometer. All NMR data were analysed using MestReNova software.
2.7. In vitro antioxidant activities
2.7.1. DPPH radical scavenging activity
The DPPH radical scavenging assay of the eight samples was investigated according to the report by (Kaewnarin et al. Citation2020), with minor modifications. Polysaccharide samples (10 mg) were dissolved in distilled water and then diluted to 10 mL to obtain the sample solution at a concentration of 1 mg/mL. The antioxidant activity at various doses of polysaccharides was evaluated using DPPH in a 96-well plate. And the absorbance at 517 nm was measured with a pan-wavelength automatic microplate reader (Spectra MAX1900, Meigu Molecular Devices Co. Lit., Shanghai, China). Lower absorbance indicates high free radical scavenging activity. The positive controls were BHT and ASC, the negative and blank control was the reaction solution without polysaccharide and distilled water. Each test was repeated three times in parallel. The DPPH radical scavenging rate was determined as follows:
Where Ai was the absorbance of the solution containing a sample; A0 was the absorbance of the mixture of DPPH solution and distilled water. The EC50 value, which was measured by establishing DPPH radical scavenging ability versus polysaccharide concentration, denoted the concentration of polysaccharide producing 50% inhibition.
2.7.2. Superoxide anion scavenging ability
The scavenging ability of superoxide radical was determined in accordance with the previously described method (Vamanu Citation2012), with some minor modifications. In brief, each polysaccharide was dissolved in water, and a series of sample solutions were diluted to various concentrations. The ability of the investigated fraction to scavenge superoxide radical was determined with various doses of samples (0.1–0.5 mg/mL), which were mixed in the 96-well plate and OD values were measured at 325 nm. The positive controls were BHT and ASC, the blank control was the reaction mixture without polysaccharides. Each test was repeated three times in parallel. The scavenging rate was also calculated by the following equation:
Where A0 was the absorbance of blank control, Ai represented the absorbance of the solution including a sample, and Aj stood for the absorbance of the pyrogallol solution substituted by pyrogallol-HCl solution.
2.7.3. Ferrous ion reducing power
The power of the polysaccharide to reduce ferrous ions was detected using the method of Vamanu E (Vamanu Citation2012), with minor modifications. The test solution consisted of the mixture of 1 mL of sodium phosphate buffer (pH 6.6), 1% potassium hexacyanoferrate solution (w/v), and test sample solution. The polysaccharide samples with different concentrations (0.2–1.0 mg/mL) were mixed in the 96-well plate and absorbance was calculated at 700 nm with a pan-wavelength automatic microplate reader (Spectra MAX1900, Meigu Molecular Devices Co. Lit., Shanghai, China). Higher absorbance was indicative of greater reducing ability. BHT and ASC were used as the positive controls and the reaction solution without polysaccharides was served as the blank control.
2.8. Statistical analysis
All experiments were carried out in triplicate and the results were shown as means ± standard deviation. Statistical significance was assessed using one-way analysis of variance (ANOVA) followed by Tukey´s tests. A value of P < 0.05 was judged statistically significant. Pearson correlation and multiple linear regression analysis were applied to investigate the relationships between polysaccharide characteristics and antioxidant activities. All analyses of data from the experiment were processed by SPSS and Origin software.
3. Results
3.1. Assay of crude polysaccharides
The glucose standard curve was plotted with the concentration values of the standard solution as the horizontal coordinates and the OD values at 490 nm as the vertical coordinates. The regression equation of the standard curve was obtained as follows: y = 7.3714x + 0.0037, R2 = 0.9994. The result indicated that the standard curve showed good linearity in the concentration ranging from 0 to 0.07 mg/mL.
Eight crude polysaccharides were obtained through defatting, aqueous extraction, deproteinization, and ethanol precipitation, consecutively. The yield of the polysaccharides was calculated according to the standard curve. The yield of crude polysaccharides of the eight samples followed the order of Cr. < Xf. < Bh. < Hj. < Al. < Ni. < To. < No., which was 29.38 ± 1.72%, 32.46 ± 1.1%, 35.62 ± 0.55%, 37.25 ± 1.51%, 37.70 ± 1.72%, 48.83 ± 2.21%, 54.53 ± 2.72%, and 60.50 ± 0.95%, respectively. The highest yield of crude polysaccharides, which was 60.50%, was obtained from N. obscureumbrinus (sample No.), followed by the T. otsuensis (sample To.), which was 54.53%. However, the crude polysaccharide yield of C. rufoaureus (sample Cr.) was the lowest among the eight boletes, which was only 29.38%. Among these samples, there was no significant difference in the crude polysaccharide yield of A. longicollis (sample Al.), B. hainanensis (sample Bh.), and H. japonicus (sample Hj.), all of which ranged from 35% to 37%.
3.2. Molecular weight determination
Weight-average molecular weight (Mw), number-average molecular weight (Mn), and the Mw/Mn of polysaccharide fractions from eight boletes are shown in , and the average Mw of crude polysaccharides isolated from these boletes was different. The polysaccharide of H. japonicus (sample Hj.) exhibited the highest Mw among the eight boletes (Mw = 31,665 Da), while C. rufoaureus (sample Cr.) had the lowest Mw (Mw = 5286 Da). The crude polysaccharides from eight boletes showed an Mw range from 5.29 × 103 Da to 3.17 × 104 Da, and the average Mw of the polysaccharides from these boletes were similar, ranging from 103 Da to 104 Da.
Table 1. The molecular weight of crude polysaccharides from eight boletes.
3.3. Monosaccharide composition
The monosaccharide composition and HPLC chromatograms of the crude polysaccharides from the eight boletes are presented in and , respectively. The crude polysaccharides from the investigated boletes mainly consisted of mannose, glucosamine, glucose, galactose, xylose, and fucose; mannose, glucose, and galactose were the most abundant monosaccharides of the crude polysaccharides, while the content of glucose was the highest among them.
Figure 2. Fingerprint chromatograms of the monosaccharide compositions of crude polysaccharides from eight boletes and fourteen kinds of reference monosaccharides. 1. GulUA. 2. ManUA. 3. Man. 4. Rib. 5. Rham. 6. GlcN. 7. GlcUA. 8. GalUA. 9. Glc. 10. GalN. 11. Gal. 12. Xyl. 13. Ara. 14. Fuc.
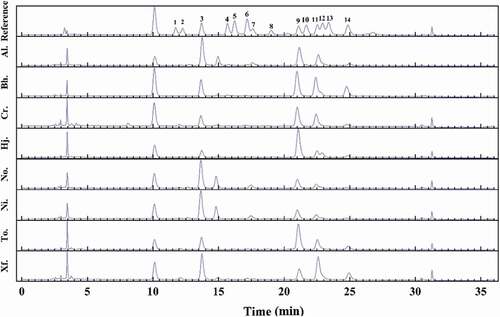
Table 2. The monosaccharide composition of crude polysaccharides from eight boletes.
The monosaccharide compositions of the crude polysaccharides from eight boletes were different, for example, the arabinose in the crude polysaccharide of H. japonicus was not detected, with glucose content being the most abundant (201.87 mg/g); polysaccharide in N. obscureumbrinus had the highest content of mannose, with rhamnose and arabinose not detected; additionally, arabinose was not detected in But. hainanensis, C. rufoaureus, and H. japonicus, rhamnose was not found in X. fusciceps, and neither glucose nor rhamnose was detected in N. obscureumbrinus.
3.4. FT-IR spectral analysis
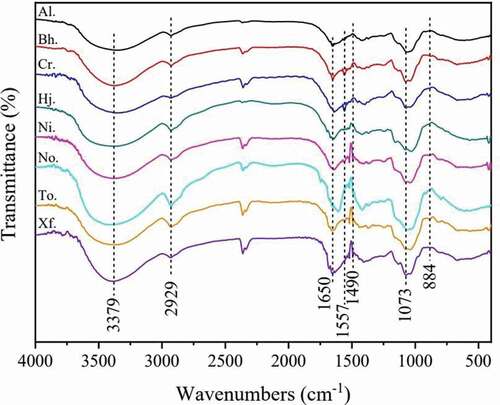
The experimental result of the FT-IR of crude polysaccharides from eight boletes exhibited in . As seen in , the spectra of eight crude polysaccharides were almost similar to each other, but there were some differences in the absorption peaks and their positions, which could be attributed to different compositions and content of monosaccharides from the eight boletes. All samples suggested a broad stretching peak at 3370 cm−1 typical of hydroxyl groups as well as a C-H absorption peak at 2930 cm−1, which was thought to be the characteristic of polysaccharides (Liu et al. Citation2012). Besides, the absorption band 1800–1500 cm−1 was accounted for the stretching vibration of the bound water. The peaks near 1600 cm−1 could be due to symmetrical deformation vibration of the carbonyl group of the polysaccharides, while the centre of the peaks at 1500 cm−1 may be correlated to the symmetrical formation vibration of carbonyl group. Moreover, the absorption peak in the range of 1200–1000 cm−1 suggested the existence of the pyranose ring among all samples. In previous studies, the peak near 798 cm−1 exhibited the existence of an alpha configuration, and the peak around 888 cm−1 showed the presence of a β-configuration (Liu et al. Citation2012; Fan et al. Citation2018). From what has been analysed above, we could conclude that all samples from the eight boletes mainly contain the β-configuration, which was in line with those of two other boletes revealed by previous studies, viz. L. rugosiceps and Boletes sp. (Yang et al. Citation2008; Li et al. Citation2020).
3.5. NMR analysis
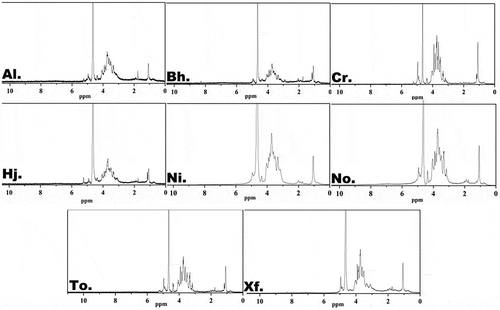
The 1H NMR spectra of polysaccharides from eight boletes are displayed in . The crowded and narrow regions of eight samples ranged from 3.0 to 5.5 ppm were typical signals of polysaccharides, which was consistent with our studies (Wan et al. Citation2022). In general, the anomeric hydrogen signals of polysaccharides were generated at δ 4.3–5.9, and the anomeric proton signals at δ 4.47–5.0 were responsible for the β-pyranose unit (Corsaro et al. Citation2005). Besides, some of the absorption peaks above 5 ppm could relate to the purity of crude polysaccharides and the existence of α-pyranose. And the 1H NMR spectra suggested that β-pyranose existed in eight polysaccharides, which was in line with the analysis of the FT-IR spectra. Then, the results with other polysaccharides in NMR spectra were very similar to these (Cheng et al. Citation2020; Li et al. Citation2020; Wan et al. Citation2022). Li et al. (Citation2020) suggested the 1H NMR spectrum of LRP-1 was crowded in narrow regions ranging from 3.1 to 4.5 ppm. Furthermore, some absorption peaks around 1 ppm could be associated with the existence of the methylated polysaccharides.
3.6. Antioxidant activities in vitro
3.6.1. DPPH radical scavenging activity
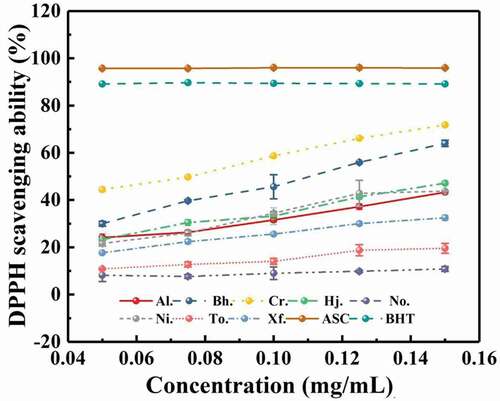
The scavenging ability of all samples from eight boletes on hydroxyl-free radical is summarised in . In the concentration range of 0.05–0.15 mg/mL, DPPH scavenging ability was improved with increasing concentration of these crude polysaccharides, although the DPPH scavenging abilities of BHT and ASC were significantly higher than those of eight boletes. In this study, DPPH scavenging abilities from different polysaccharides samples were different. The polysaccharide from C. rufoaureus presented the strongest DPPH scavenging ability, followed by that of But. hainanensis, and followed by T. otsuensis, and followed by A. longicollis, H. japonicus, and N. infuscatus, which had similar scavenging DPPH ability. In addition, N. obscureumbrinus had the lowest scavenging ability. The scavenging ability of the crude polysaccharides from eight boletes ranked as Cr. (71.75%) > Bh. (64%) > Hj. (47.16%) > Ni. (43.75%) > Al. (43.31%) > Xf. (32.49%) > To. (19.56%) > No. (10.81%), when the concentration of the crude polysaccharides came to 0.15 mg/mL.
3.6.2. Superoxide anion scavenging ability
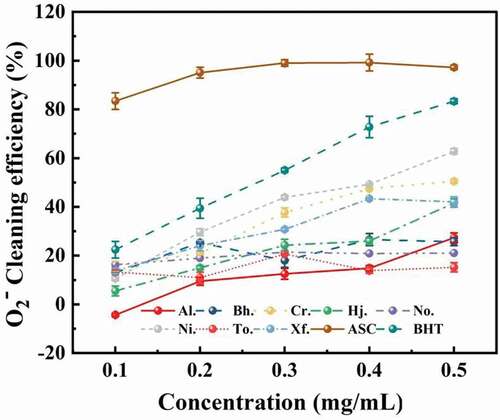
Previous studies have revealed that antioxidant activities of boletes have a relationship with scavenging ability on superoxide radicals (Zhang et al. Citation2011). The results of the superoxide anion scavenging ability of the samples is exhibited in . In the concentration range of 0.1–0.5 mg/mL, the scavenging ability of polysaccharides from most boletes improved with the concentration increased, except for But. hainanensis and T. otsuensis. When the concentration of polysaccharide came to 0.5 mg/mL, N. infuscatus and C. rufoaureus had greater scavenging ability on superoxide radical than other samples, but weaker than that on the positive controls (BHT and ASC), with 62.78% and 50.52%, respectively. When the concentration of crude polysaccharide from eight boletes reached 0.5 mg/mL, the scavenging ability on superoxide scavenging decreased in the order of Ni. (62.78%) > Cr. (50.52%) > Xf. (42.04%) > Hj. (41.56%) > Al. (27.31%) > Bh. (25.72%) > No. (21.05%) > To. (15.19%), indicating some differences compared with those in the DPPH scavenging activity assay.
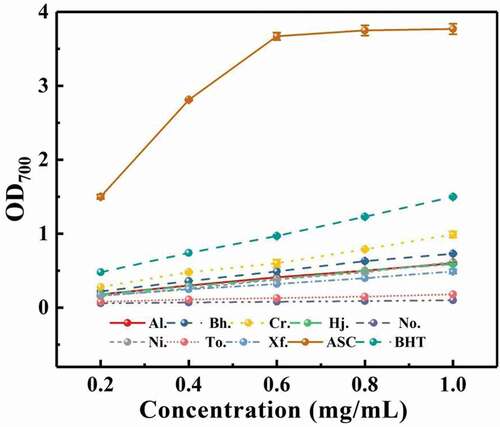
3.6.3. Ferrous ion reducing power
The result of the ferrous ion reducing power of polysaccharides can be seen in . The result clearly presented that the reducing ability of eight samples was in concentration-dependent manner in the range of 0.2–1 mg/mL, higher OD700 value meant stronger reducing ability of samples from boletes. Among these samples, C. rufoaureus presented the greater reducing power than the other crude polysaccharide samples, with the OD700 values being 0.99 at the concentration of 1 mg/mL, although the OD700 value of C. rufoaureus was still weaker than that of positive control; while ferrous ion reducing tests indicated that the polysaccharide extracted from N. obscureumbrinus had the least significant effect compared with other samples. When the concentration of polysaccharide came to 1 mg/mL, the reducing power (OD700 value) of eight boletes ranked as Cr. (0.99) > Bh. (0.73)> Ni. (0.62) > Al. (0.60) > Hj. (0.59) > Xf. (0.49) > To. (0.18) > No. (0.10). Experimental results were similar to those of previous DPPH radical scavenging assays, the extracts from C. rufoaureus showed stronger reducing activities than those of the other seven polysaccharide extracts.
4. Discussion
Although the antioxidant activity of polysaccharides produced by boletes including B. aereus Bull., B. edulis Bull., B. speciosus Frost, B. violaceo-fuscus W.F. Chiu, Suillus bovinus (L.) Roussel, and S. luridus (Schaeff.) Richon & Roze was investigated by Ding et al. (Citation2012), Luo et al. (Citation2012), and Zhang et al. (Citation2018), more boletes used for antioxidant should be investigated for the structural characterisations and activities of polysaccharides have been proved to be different for different species (Jiao et al. Citation2010; Ren et al. Citation2014; Zheng et al. Citation2014; Meng et al. Citation2015; Sun et al. Citation2016b; Su and Li Citation2020; Li et al. Citation2020). In the present study, the structural characterisations and activities of polysaccharides of eight boletes from Hainan, tropical area of China were never investigated before.
The previous study showed that average molecular weight of some polysaccharide fractions isolated from B. edulis and B. speciosus were ranging from 103 Da to 104 Da (Luo et al. Citation2012), which was consistent with the results of eight boletes in our study. However, Sun et al. (Citation2016), Zhang et al. (Citation2018); Li et al. (Citation2020) reported that the average molecular weights of crude polysaccharides extracted from boletes including B. aereus, B. edulis, B. speciosus, B. violaceo-fuscus, Leccinellum rugosiceps (Peck) C. Hahn [basionym: Leccinum rugosiceps (Peck) Singer], Rubroboletus sinicus (W.F. Chiu) Kuan Zhao & Zhu L. Yang (basionym: B. sinicus W.F. Chiu), S. bovinus, and S. luridus were 105 to 107 Da. Recently, Ren et al. (Citation2014) demonstrated that polysaccharides with a low MW or a β-configuration in the pyranose form displayed a higher antioxidant activity. Xie et al. (Citation2016) demonstrated that polysaccharides with a larger molecular weight had a weaker antioxidant activity than lower MW polysaccharides due to the steric hinderance. However, Zheng et al. (Citation2014) presented that the extracts isolated from the submerged culture of B. aereus, with the higher molecular weight, showed remarkably great antioxidant power among the three polysaccharide fractions. Simultaneously, in the present study, C. rufoaureus with a lower molecular weight (5.29 × 103), also exhibited a stronger DPPH scavenging power and ferrous ion reducing ability than other samples with a higher molecular weight while N. infuscatus with a higher molecular weight (9.46 × 103) among the samples, also possessed best superoxide anion scavenging ability. Our result was agreed well with Zhang et al. (Citation2018) that effect of polysaccharide Mw on antioxidant activity is maintained unclear.
Then, our resulting data coincided with those of previous studies, which exhibited that mannose, glucose, and galactose were also the most abundant monosaccharides in B. edulis and R. sinicus (Jiao et al. Citation2010; Sun et al. Citation2016b); B. aereus, B. edulis, B. violaceo-fuscus, S. bovinus, and S. luridus in which glucose was the most abundant monosaccharide constituents of the polysaccharides (Zhang et al. Citation2018), while A. longicollis, X. fusciceps, and N. obscureumbrinus with mannose being the most abundant were exhibited in our study. According to Sun et al. (Citation2016), BSP-2b, a novel polysaccharide from R. sinicus, mainly consisted of mannose, glucuronic acid, glucosamine glucose, and galactose, however, glucuronic acid and glucosamine glucose were not found in our study. The monosaccharide compositions of crude polysaccharides from eight boletes in our study were different from those revealed in previous studies. Moreover, Zhang et al. (Citation2018) reported that the scavenging abilities for the DPPH radical exhibited an obvious correlation with the arabinose and the galactose content of the polysaccharides. According to the linear regression analysis, there were no obvious correlations between the monosaccharide content and the DPPH scavenging percentages of the polysaccharides in our study (P > 0.05). Su and Li (Citation2020) also demonstrated that there were no significant correlations between the monosaccharide content and DPPH scavenging ability of polysaccharides, but significant correlations between monosaccharide content and molecular weight. In addition, Meng et al. (Citation2015) put forward a different view and reported that the antioxidant activity of polysaccharides showed correlation with mannose content (P < 0.01) and glucose content (P < 0.05), instead of galactose content (P > 0.05). Thus, the ability of monosaccharide components to affect antioxidant activity may vary from species to species.
Additionally, previous studies indicated that other factors except for MW, including molecular configuration, sulphation, and so on, also played a significant role in the antioxidant activities of polysaccharides (Wang et al. Citation2017). For example, β-configuration of polysaccharides tended to form a stable triple-helical structure, which had biological activities such as antitumor, antiviral, and antioxidant activities, whereas α-configuration usually has no biological activities (Li and Wang Citation2002). And thus, the β-configuration of the glucose molecule in a helix structure was considered to be significant for the biological and pharmacological activity (Mizuno Citation1999). In the present study, the results revealed that the polysaccharides among the eight boletes contained a pyranose ring revealed by FT-IR and NMR spectral analyses. The polysaccharides extracted from C. rufoaureus, with a β-configuration in the pyranose form, suggested the greater scavenging power on DPPH and reducing ability. Thus, we can speculate that the antioxidant activity of boletes may be due to the presence of β-configuration.
In previous investigations, the antioxidant properties of polysaccharides could be associated with their structural characteristics such as monosaccharide composition, molecular weight distribution and degree of branching or sulphation (Lo et al. Citation2007; Sun et al. Citation2009; Lo et al. Citation2012). According to the all the above, we speculated that many factors such as species, molecular weight, configuration and monosaccharide content, may affect antioxidant power of the crude polysaccharides, simultaneously, instead of one single factor.
5. Conclusions
All crude polysaccharides were extracted from the fruit bodies of eight boletes after defatting, aqueous extraction, deproteinization, and ethanol precipitation, subsequently. Eight polysaccharides from boletes were characterised by PMP-HPLC, HPGPC, FT-IR spectroscopy, and NMR spectra analysis, and their antioxidant abilities were consecutively evaluated. The results demonstrated that the crude polysaccharides from eight boletes in tropical China were mainly composed of mannose, glucose, and galactose, and possessed the beta configuration in the pyranose form; yield, Mw, and the monosaccharide composition of the crude polysaccharides from different boletes were greatly different in the present study. Moreover, the antioxidant assay indicated that C. rufoaureus presented significantly great antioxidant activities among those of eight boletes, and exhibited superior potential in the application of the natural antioxidant activity.
CRediT authorship contribution statement
RT: Methodology, Formal analysis, Writing—original draft preparation, Performing. HC: Methodology, Formal analysis, Performing. JQQ: Writing—original draft preparation. ZQL: Resources. HJX: Performing. YW: Conceptualization, Writing—review, and editing. NKZ: Conceptualization, Writing—review, and editing, Resources, Funding Acquisitions.
Acknowledgements
We are grateful to Mrs. S. Jiang and the forest rangers (Yinggeling Substation, Hainan Tropical Rainforest National Park, China) for their kind help during the sample collections.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Chai H, Liang ZQ, Xue R, Jiang S, Luo SH, Wang Y, Wu LL, Tang LP, Chen Y, Hong D, et al. 2019. New and noteworthy boletes from subtropical and tropical China. MycoKeys. 46:55–96. doi:10.3897/mycokeys.46.31470.
- Cheng J, Song J, Wei H, Wang Y, Huang X, Liu Y, Lu N, He L, Lv G, Ding H, et al. 2020. Structural characterization and hypoglycemic activity of an intracellular polysaccharide from Sanghuangporus sanghuang mycelia. Int J Bio Macromol. 164:3305–3314. doi:10.1016/j.ijbiomac.2020.08.202.
- Corsaro MM, Castro CD, Naldi T, Parrilli M, Tomás JM, Regué M. 2005. 1H and 13C NMR characterization and secondary structure of the K2 polysaccharide of Klebsiella pneumoniae strain 52145131. Carbohydr Res. 340:2212–2217. doi:10.1016/j.carres.2005.07.006.
- Ding X, Hou Y, Hou W. 2012. Structure elucidation and antioxidant activity of a novel polysaccharide isolated from Boletus speciosus Forst. Int J Bio Macromol. 50:613–618. doi:10.1016/j.ijbiomac.2012.01.021.
- Fan HY, Liu JY, Chen GL. 2018. Application of IR and effective ingredients analysis on extraction and isolation of Cynomorium songaricum polysaccharides. Spectrosc Spectral Anal. 38:75–76. doi:10.26914/c.cnkihy.2018.017551.
- Jiang S, Mi HX, Xie HJ, Zhang X, Chen Y, Liang ZQ, Zeng NK. 2021. Neoboletus infuscatus, a new tropical bolete from Hainan, southern China. Mycoscience. 62:205–211. doi:10.47371/mycosci.2021.03.001.
- Jiao LM, Ji CY, Kang JS, Chen HM, Yang YH. 2010. Separation, purification and component analysis of extracellular polysaccharide of Boletus edulis Bull. Sci Technol Food Ind. 31:164–166. doi:10.13386/j.1002-0306.2010.05.086.
- Kaewnarin K, Suwannarach N, Kumla J, Choonpicharn S, Tanreuan K, Lumyong S. 2020. Characterization of polysaccharides from wild edible mushrooms from Thailand and their antioxidant, antidiabetic, and antihypertensive activities. Int J Med Mushrooms. 22:221–223. doi:10.1615/IntJMedMushrooms.2020034092.
- Li Y, Wang Q. 2002. A review of the studies on the relationship between activity and structure of polysaccharides from fungi. J Jilin Agric Univ. 24(2):70–74. doi:10.3969/j.1000-5684.2002.02.022.
- Li YM, You LJ, Dong F, Yao WZ, Chen J. 2020. Structural characterization, antiproliferative and immunoregulatory activities of a polysaccharides from Boletus Leccinum rugosiceps. Int J Bio Macromol. 157:106–118. doi:10.1016/j.ijbiomac.2020.03.250.
- Liang ZQ, An DY, Jiang S, Su MZ, Zeng NK. 2016. Butyriboletus hainanensis (Boletaceae, Boletales), a new species from tropical China. Phytotaxa. 267:256–262. doi:10.11646/phytotaxa.267.4.2.
- Liu Y, Chen D, You Y, Zeng S, Li Y, Tang Q, Han G, Liu A, Feng C, Li C, et al. 2016b. Nutritional composition of boletus mushrooms from Southwest China and their antihyperglycemic and antioxidant activities. Food Chem. 211:83–91. doi:10.1016/j.foodchem.2016.05.032.
- Liu H, Fan Y, Wang W, Liu N, Zhang H, Zhu Z, Liu A. 2012. Polysaccharides from Lycium barbarum leaves: isolation, characterization and splenocyte proliferation activity. Int J Bio Macromol. 54:417–422. doi:10.1016/j.ijbiomac.2016.12.010.
- Liu XC, Zhu ZY, Tang YL, Wang MF, Wang Z, Liu AJ, Zhang YM. 2016a. Structural properties of polysaccharides from cultivated fruit bodies and mycelium of Cordyceps militaris. Carbohydr Polym. 142:63–72. doi:10.1016/j.carbpol.2016.01.040.
- Lo TCT, Chang CA, Chiu KH, Tsay PK, Jen JF. 2012. Correlation evaluation of antioxidant properties on the monosaccharide components and glycosyl linkages of polysaccharide with different measuring methods. Carbohydr Polym. 86:320–327. doi:10.1016/j.carbpol.2011.04.056.
- Lo TCT, Kang MW, Wang BC, Chang CA. 2007. Glycosyl linkage characteristics and classifications of exo-polysaccharides of some regionally different strains of Lentinula edodes by amplified fragment length polymorphism assay and cluster analysis. Anal Chim Acta. 592:146–153. doi:10.1016/j.aca.2007.04.021.
- López-Legarda X, Arboleda-Echavarría C, Parra-Saldivar R, Rostro-Alanis M, Alzate JF, Villa-Pulgarín JA, Segura-Sánchez F. 2020. Biotechnological production, characterization and in vitro antitumor activity of polysaccharides from a native strain of Lentinus crinitus. Int J Bio Macromol. 164:3133–3144. doi:10.1016/j.ijbiomac.2020.08.191.
- Luo AX, Luo AS, Huang JD, Fan YJ. 2012. Purification, characterization and antioxidant activities in Vitro and in Vivo of the polysaccharides from Boletus edulis Bull. Molecules. 17:8079–8090. doi:10.3390/molecules17078079.
- Meng L, Sun S, Li R, Sheng Z, Wang P, Jiang X. 2015. Antioxidant activity of polysaccharides produced by Hirsutella sp. and relation with their chemical characteristics. Carbohydr Polym. 117:452–457. doi:10.1016/j.carbpol.2014.09.076.
- Meng T, Yu SS, Ji HY, Xu XM, Liu AJ. 2021. A novel acid polysaccharide from Boletus edulis: extraction, characteristics and antitumor activities in vitro. Glycoconjugate J. 84:13–24. doi:10.1007/s10719-021-09972-0.
- Mizuno T. 1999. The extraction and development of antitumour-active polysaccharides from medicinal mushrooms in Japan. Int J Med Mushr. 1:9–29. doi:10.1615/IntJMedMushrooms.v1.i1.20.
- Ni L, Wang Y, He W, Zhang L. 2014. Monosaccharide composition, activity and their correlation analysis in eight polysaccharides. J Tianjin Univ. 47:326–330. doi:10.11784/tdxbz201207047.
- Ren L, Hemar Y, Perera CO, Lewis G, Krissansen GW, Buchanan PK. 2014. Antibacterial and antioxidant activities of aqueous extracts of eight edible mushrooms. Bioact Carbohydr Diet Fibre. 3:41–51. doi:10.1016/j.bcdf.2014.01.003.
- Su Y, Li L. 2020. Structural characterization and antioxidant activity of polysaccharide from four auriculariales. Carbohydr Polym. 229:115407. doi:10.1016/j.carbpol.2019.115407.
- Su S, Wang M, Ding X, Hou X, Tang J, Liu L, Dong M, Jing L. 2018. Protein chip of Boletus speciosus Frost polysaccharide revealed the molecular mechanism of antitumor and immunostimulatory activities on macrophages. Indian J Pharm Sci. 80:1029–1038. doi:10.4172/pharmaceutical-sciences.1000453.
- Sun L, Li X, Su X. 2016a. Preparation and antioxidant activities of the polysaccharides from Boletus snicus. Sci Technol Food Ind. 37:173–175. doi:10.13386/j.1002-0306.2016.24.025.
- Sun LP, Su XJ, Zhuang YL. 2016b. Preparation, characterization and antiglycation activities of the novel polysaccharides from Boletus snicus. Int J Bio Macromol. 92:42–47. doi:10.1016/j.ijbiomac.2016.07.014.
- Sun L, Wang C, Shi Q, Ma C. 2009. Preparation of different molecular weight polysaccharides from Porphyridium cruentum and their antioxidant activities. Int J Bio Macromol. 45:42–47. doi:10.1016/j.ijbiomac.2009.03.013.
- Vamanu E. 2012. Biological activities of the polysaccharides produced in submerged culture of two edible Pleurotus ostreatus mushrooms. J Biomed Biotechnol. 2012:565974. doi:10.1155/2012/565974.
- Wan X, Jin X, Wu X, Yang X, Lin D, Chang L, Hu Y, Liu Y, Liu X, Lv J, et al. 2022. Structural characterization and antitumor activity against non-small cell lung cancer of polysaccharides from Sanghuangporus vaninii. Carbohydr Polym. 276:118798. doi:10.1016/j.carbpol.2021.118798.
- Wang M, Zhu P, Zhao S, Nie C, Wang N, Du X, Zhou Y. 2017. Characterization, antioxidant activity and immunomodulatory activity of polysaccharides from the swollen culms of Zizania latifolia. Int J Bio Macromol. 95:809–817. doi:10.1016/j.ijbiomac.2016.12.010.
- Wu S. 2018. Hypolipidaemic and anti-lipidperoxidant activities of Ganoderma lucidum polysaccharide. Int J Bio Macromol. 118:2001–2005. doi:10.1016/j.ijbiomac.2018.07.082.
- Wu LL, Liang ZQ, Xue R, Fan YG, Jiang S, Fu YQ, Zeng NK. 2019. The genus Crocinoboletus (Boletaceae, Boletales): a new species and updated information for previously described species. Phytotaxa. 419:91–99. doi:10.11646/phytotaxa.419.1.6.
- Wu S, Wang G, Yang R, Cui Y. 2016. Anti-inflammatory effects of Boletus edulis polysaccharide on asthma pathology. Am J Transl Res. 8:4478–4489.
- Xiao Y, Chen L, Fan Y, Yan P, Li S, Zhou X. 2019. The effect of boletus polysaccharides on diabetic hepatopathy in rats. Chem Biol Interact. 308:61–69. doi:10.1016/j.cbi.2019.05.013.
- Xie JH, Wang ZJ, Shen MY, Nie SP, Gong B, Li HS, Zhao Q, Li WJ, Xie MY. 2016. Sulfated modification, characterization and antioxidant activities of polysaccharide from Cyclocarya paliurus. Food Hydrocolloid. 53:7–15. doi:10.1016/j.foodhyd.2015.02.018.
- Yang L, Liu L, Zhong X, Feng P, Cai D, Ma C. 2008. Seperation and identification of polysaccharides from natural Boletus and their antioxidant activities. Food Sci. 29:335–338. doi:10.3321/j.1002-6630.2008.08.074.
- Yang W, Wu J, Liu W, Ai Z, Cheng Y, Wei Z, Zhang H, Ma H, Cui F, Zhou C, et al. 2021. Structural characterization, antioxidant and hypolipidemic activity of Grifola frondosa polysaccharides in novel submerged cultivation. Food Biosci. 42:101187. doi:10.1016/j.fbio.2021.101187.
- Zeng NK, Chai H, Jiang S, Xue R, Wang Y, Hong D, Liang ZQ. 2018a. Retiboletus nigrogriseus and Tengioboletus fujianensis, two new boletes from the south of China. Phytotaxa. 367:45–54. doi:10.11646/phytotaxa.367.1.5.
- Zeng NK, Chai H, Liang ZQ, Tang LP, Yang ZL. 2018b. The genus Heimioporus in China. Mycologia. 110:1110–1126. doi:10.1080/00275514.2018.1512303.
- Zeng NK, Zhang M, Liang ZQ. 2015. A new species and a new combination in the genus Aureoboletus (Boletales, Boletaceae) from southern China. Phytotaxa. 222:129–137. doi:10.11646/phytotaxa.222.2.5.
- Zhang L, Hu Y, Duan XY, Tang TT, Shen YB, Hu B, Liu AP, Chen H, Li C, Liu YT. 2018. Characterization and antioxidant activities of polysaccharides from thirteen boletus mushrooms. Int J Bio Macromol. 113:1–7. doi:10.1016/j.ijbiomac.2018.02.084.
- Zhang A, Xiao N, He P, Sun P. 2011. Chemical analysis and antioxidant activity in vitro of polysaccharides extracted from Boletus edulis. Int J Bio Macromol. 49:1092–1095. doi:10.1016/j.ijbiomac.2011.09.005.
- Zheng JQ, Wang JZ, Shi CW, Mao DB, He PX. 2014. Characterization and antioxidant activity for exopolysaccharide from submerged culture of Boletus aereus. Process Biochem. 49:1047–1053. doi:10.1016/j.procbio.2014.03.009.