?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
The search for a novel microbial producer of cellulases including a glucose tolerant β-glucosidase is a challenge as most are inhibited by their product glucose. This study aims to screen for cellulolytic fungi using qualitative and quantitative screening methods. Primary screening revealed 34 of 46 fungal isolates with β-glucosidase activity. Eleven and 13 of these also displayed endoglucanase and exoglucanase activities, respectively. During secondary screening, this number was reduced to 26 β-glucosidase producers with 13 also having endoglucanase and exoglucanase activities. Isolate C1 displayed enhanced production of β-glucosidases in the presence of 0.05 M glucose (69% higher activity). Optimisation of growth conditions for β-glucosidase production by one variable at a time experiments improved production for (isolates) PS1 (64%), MB5 (84%), and C2 (69%). Isolate PS1 identified as Chaetomella sp. BBA70074 displayed the highest tolerance to glucose, retaining 10% of β-glucosidase activity in the presence of 0.8 M glucose. Tolerance to glucose increased to 14% when produced under optimal conditions. β-Glucosidase had a molecular weight of 170 kDa with a pH and temperature optima of 6 and 70 °C, respectively. Future studies will include optimisation of the production of the glucose tolerant enzyme by Chaetomella sp. BBA70074.
1. Introduction
The depletion of fossil fuels, the negative environmental impact resulting from their combustion, and the increasing energy demand have paved the way for sustainable biofuels and bioproducts from renewable resources by biorefineries (Sørensen et al. Citation2013). The transportation, chemical, and plastic production sectors are dependent on oil as their primary energy source. Soon biorefineries are expected to supplement, if not completely replace oil refineries by maximising the use of the tonnes of renewable biomass currently being disposed off in landfills, to produce biofuels and basic molecules as building blocks for the synthesis of chemicals and polymeric materials (Cherubini Citation2010; Kanakaraju et al. Citation2020). Not only are landfills reaching their maximum capacity, but they pose environmental and financial concerns, therefore, biomass valorisation will solve both issues of concern (Srivastava et al. Citation2019).
During the pulp and paper manufacturing process, paper mills generate large amounts of solid waste products referred to as pulp and paper mill sludge (PPMS). In South Africa, four to five hundred thousand wet tonnes of PPMS are produced, which makes the pulp and paper mill industry one of the highest polluting industries (Darwesh et al. Citation2020). Pulp and paper mill sludge contain organic and inorganic materials; however, the majority is organic material that includes lignocellulosic residuals originating from wood, the raw material used in the pulp and paper making process (Naicker et al. Citation2020).
Biorefineries rely upon this lignocellulosic biomass as an abundant and renewable energy source (Sørensen et al. Citation2013). Most biorefineries focus on hydrolysing the biomass into simple sugars that serve as feedstocks for chemical and microbial conversion to biofuels (ethanol, butanol, and hydrocarbons), different organic acids, and other higher value products (Naicker et al. Citation2020). Enzymatic hydrolysis is the preferred method of hydrolysis compared to chemical methods that eventually release harmful chemicals, thus increasing environmental pollution (Srivastava et al. Citation2019).
Enzymatic hydrolysis of lignocellulosic biomass is carried out by cellulases, a group of three different enzymes, namely, endoglucanases (1, 4-β-D-glucanohydrolase) which hydrolyse the β 1–4 glycosidic bonds within the cellulose chain exposing reducing and non-reducing ends, which are then acted upon by exoglucanases (1, 4-β-D-glucan cellobiohydrolase) releasing cellobiose and cello-oligosaccharides. In the final step β-glucosidases (β-D-glucopyranoside glucohydrolases) convert these products into free glucose units (Singh et al. Citation2016). The majority of cellulase cocktails from native fungal producers are unable to completely hydrolyse cellulose due to the low titres of β-glucosidases (Kumar and Wyman Citation2009; Rani et al. Citation2014). Darwesh et al. (Citation2020) reported that cellulases from Aspergillus Niger MK543209 in a 2:1:1 (endoglucanases: exoglucanases: β-glucosidases) ratio resulted in the complete hydrolysis of cellulose to glucose.
The activity of β-glucosidase is the rate-limiting step that determines the overall rate of conversion of cellulose into glucose molecules (Srivastava et al. Citation2019). The main obstacles to commercialising an efficient microbial β-glucosidase include product inhibition and thermal inactivation, which reduce process/production yields, as well as the high cost of enzyme production (Yao et al. Citation2016). During lignocellulose biomass conversion, cellobiose is an inhibitor of cellobiohydrolases and endoglucanases; therefore, β-glucosidases are incorporated to mitigate this by hydrolysing the cellobiose to glucose; however, β-glucosidases in turn are often inhibited by their own product, which is glucose, thus making them the rate-limiting step during the hydrolysis of cellulosic biomass (Ahmed et al. Citation2017). It has been postulated that a glucose tolerant β-glucosidases would ensure high hydrolysis rates as they will perform efficiently even when glucose levels are high (Yao et al. Citation2016). Hydrolysis may also be inhibited by transglycosylation events due to the reaction being a reversible process; this occurs when the active site of the β-glucosidase is used to catalyse the formation of a glycosidic bond rather than its hydrolysis (Sørensen et al. Citation2013). β-Glucosidases are classified into three classes based on substrate specificity, (i) aryl-β-D-glucosidases, which have a strong affinity for aryl-β-D-glucosides; (ii) cellobiases that hydrolyse only disaccharides; and (iii) broad specificity glucosidases that exhibit activity on many substrate types and are most common (Singh et al. Citation2016).
Glucose tolerant β-glucosidase enzymes can be divided into three groups: (i) those that are strongly inhibited by low glucose concentrations, which are most β-glucosidases; (ii) β-glucosidases that are tolerant to low glucose concentrations but strongly inhibited by high glucose concentrations, such as glucosidases from Aspergillus oryzae and Candida peltata; and (iii) β-glucosidases that are stimulated by low but inhibited by high concentrations of glucose (Cao et al. Citation2015).
Microbial cellulases have become key players as biocatalysts due to their complex nature and widespread industrial applications. Both fungi and bacteria produce cellulases, however, fungi have the potential to secrete large amounts of enzymes, utilise a variety of substrates through the extracellular secretion of hydrolytic enzymes, display simpler structures, and have the ability to penetrate complex substrates (Kuhad et al. Citation2011). Trichoderma reesei, a commercial producer of cellulases, utilises sophorose as an inducer of cellulases that increases production by 2,500 times as compared to cellobiose; however, production costs would be high as sopharose is expensive (Zhang et al. Citation2022). Challenges lie in searching for a cheaper alternative with similar effects that will reduce production costs. A recent study by de Souza et al. (Citation2021) reported efficient hydrolysis of cellulosic material by a cellulase enzyme cocktail produced by T. reesei utilising wheat bran as a carbon source. These researchers also discovered that the pH during the fermentation process played a key role in reducing feedback inhibition of the enzyme, thus achieving efficient cellulose hydrolysis.
Several studies have reported on species of Aspergillus that express glucose tolerant β-glucosidases that are insensitive to product inhibition (Sørensen et al. Citation2013; Zhang et al. Citation2017; Srivastava et al. Citation2019). One of the largest sources of commercial β-glucosidases is Aspergillus niger (A. niger) sold under the name Novazym 188 (Ahmed et al. Citation2017).
Crude cellulase cocktails are preferred for the hydrolysis of lignocellulosic biomass in industry as they may contain the full lignocellulolytic set of enzymes avoiding the use of pure analytical-grade enzymes from different sources that are expensive and may not work as well in a cocktail compared to a native producer (Zang et al. Citation2018, Monteiro et al. Citation2020, Naicker et al. Citation2020; de Souza et al. Citation2021). Current research lies in searching for a thermophilic glucose tolerant β-glucosidase producer that prefers a slightly acidic to basic pH as well as a native cellulase producer that produces thermophilic enzymes in a cocktail with optimal and proportional activities required for the complete hydrolysis of lignocellulose to free glucose units.
Therefore, the aim of this study was to search for a cellulolytic fungal isolate with the potential to produce a crude cocktail of endoglucanases, exoglucanases, and glucose tolerant β-glucosidases by isolating fungi from the environment using the serial dilution method, screening for endoglucanase, exoglucanase, and β-glucosidase activity using quantitative and qualitative methods, and further screening of the highest β-glucosidase producers for glucose tolerance.
2. Materials and methods
2.1. Sample collection and isolation of fungal isolates
Tree bark and soil samples were collected from three locations. Tree bark samples were collected from three different trees; tree one: Acer negrundo (Site A: 29°49ʹ01” S 30°56ʹ41” E), tree two: Juglans regia (Site B: 29°49ʹ03” S 30°56ʹ29” E), and tree three: Citrus limone (Site C: 29°16ʹ13” S 31°22ʹ06” E). Microorganisms living in this environment are expected to produce cellulases capable of cellulose degradation (Steffen et al. Citation2017). Tenfold serial dilutions were performed; 0.1 ml of each dilution was plated onto potato dextrose agar (PDA) plates and incubated at 30 °C for five days until fungal growth was observed. Two types of long-term stocks were prepared for all isolated fungi; 25% glycerol stocks were prepared and stored at −20 °C, and mineral oil slants using PDA were stored at 4 °C (Adesina and Onilude Citation2013).
2.2. Primary and secondary screening of fungi for cellulase and β-glucosidase activities
The fungal isolates were grown on PDA supplemented with 1.0 g of aesculin and 0.3 g of ferric citrate per litre and incubated at 30 °C for seven days to determine β-glucosidase activity. Positive β-glucosidase activity was indicated by a black precipitate around the fungal mycelium (Kao et al. Citation2019). The potential of their activity was determined by measuring the diameter of the black precipitate (Pérez et al. Citation2011). Endoglucanase and exoglucanase (cellulases) activities were determined on a minimal agar medium (0.5 g NaCl, 1.0 g KH2PO4, 0.5 g MgSO4.7H2O, 0.01 g MnSO4.H2O, 0.3 g NH4NO3, 0.01 g FeSO4.7H2O 12 g bacteriological agar) supplemented with 1% carboxymethyl cellulose (CMC) or avicel, respectively. These plates were inoculated with a standardised inoculum and incubated at 30 °C for seven days. Thereafter, the plates were flooded with 0.1% Congo red solution for 15 minutes, followed by destaining with 1 mol/L sodium chloride (NaCl) for 10 min, and were examined for zones of clearing around the point of inoculation. The enzyme activity was determined by measuring the cellulolytic index (CI) by measuring the diameter millimetre (mm) of clearing divided by the diameter (mm) of colony growth (Saini et al. Citation2017).
Extracellular enzyme extracts were produced using a submerged fermentation process. Crude extracellular β-glucosidases and glucanases were produced in a minimal media using the methods of Kao et al. (Citation2019) and Adesina and Onilude (Citation2013), respectively. Each 250 mL flask contained 25 mL of medium and three 5 mm mycelial plugs of actively growing hyphae and was incubated at 30 °C for seven days at 125 rpm (New Brunswick Scientific, Incubator Shaker series, Innova 44, Germany). After incubation, the extracellular supernatant was obtained by centrifugation at 16837 ×g (Eppendorf Centrifuge 5418, Germany) for 10 minutes and used as the crude enzyme for quantification of enzyme activity (Kao et al. Citation2019).
2.3. Enzyme assays
The crude enzyme extracts were assayed to quantify β-glucosidase, endoglucanase, and exoglucanase activities. β-Glucosidase activity was quantified using the method of Kao et al. (Citation2019). The reaction mixture contained 100 µL of enzyme added to 100 µL of 4 (millimolar) mmol/L 4-nitrophenyl-β-D-glucopyranoside (4-NPG) in 0.05 mol/L sodium acetate buffer (pH 5.0) and incubated at 55 °C for 5 min of shaking. After 5 min 600 µL of 1 mol/L Na2CO3 was added to terminate the reaction. The absorbance was read at 410 nm using a spectrophotometer (Shimadzu UV1800, Japan). One unit of activity was defined as the amount of enzyme needed to release one µmol of phenol equivalents per minute at 55 °C. All the experiments and assays were duplicated and a standard curve using 4-nitrophenol (4-NP) in sodium acetate buffer (50 mmol/L, pH 5.0) was established (Kao et al. Citation2019). The Beer-Lambert equation was used to calculate enzyme activity:
Where A = Change in absorbance, V = Total volume of the reaction (mL) divided by ε = Molar extinction co-efficient of 4-nitrophenol (µmol/L), t = reaction time (minutes), and V = Volume of enzyme (mL).
Endoglucanase and exoglucanase activities were quantified using the dinitrosalicylic acid (DNS) method with 1% avicel or carboxymethyl cellulose (CMC) as the substrate in 0.05 mol/L citrate buffer (pH 5.0), respectively. The reaction mixture contained 66.6 µL of the enzyme, 600 µL of 1% avicel or CMC, incubated in a water bath (Labcon 5032 U, South Africa) for 15 min at 55 °C. The reaction was terminated by adding 1 mL of DNS to the reaction mixture, boiled at 100 °C for 5 min and the absorbance was read at 540 nm using a spectrophotometer (Shimadzu UV1800, Japan). One unit of activity was defined as the amount of enzyme needed to release one µmol of glucose equivalent per minute at 55 °C (Adesina and Onilude Citation2013). All assays were carried out in duplicate. A standard curve was established at 595 nm using a spectrophotometer (Shimadzu UV1800, Japan) and used to calculate enzyme activity (Adesina and Onilude Citation2013).
2.4. Production of β-glucosidases in the presence of glucose for the seven highest β-glucosidase producers
The ability of isolates C1, C2, C4, MS8, MB5, PB8, and PS1 to produce β-glucosidases in the presence of glucose was determined by supplementing the minimal salt medium with different concentrations of glucose (0.05 mol/L, 0.25 mol/L, 0.5 mol/L, 0.75 mol/L, and 1 mol/L) and incubating at 30 °C, 125 rpm for five days and assaying the crude extract for enzyme activity as per section 2.3.
2.5. Determination of glucose tolerance of the β-glucosidases produced by the seven isolates displaying the highest β-glucosidase activities
The ability of β-glucosidases produced by isolates C1, C2, C4, MS8, MB5, PB8, and PS1 to withstand the presence of glucose was determined by the method of Ariaeenejad et al. (Citation2020). The reaction mixture that included 100 µL of the enzyme, 100 µL of 4-NPG, and 100 µL of glucose (0.05 mol/L, 0.25 mol/L, 0.5 mol/L, 0.75 mol/L, and 1 mol/L) was incubated at 55 °C for 5 min. The reaction was terminated by the addition of 600 µL of 1 mol/L Na2CO3 and the absorbance was read at 410 nm using a spectrophotometer (Shimadzu UV1800, Japan).
2.6. Identification of fungal isolates C1, C2, C4, MS8, MB8, PS1, and MB5 and phylogenetic analysis
The fungal isolates were identified based on the sequences of the ITS2 region of 18S rRNA gene. Genomic DNA was isolated according to the manufacturer’s instructions of the ZR soil Microbe DNA Miniprep™ kit (Zymo Research, USA). The polymerase-chain reaction (PCR)
mixture consisted of 1 µL 10 mmol/L each of forward ITS5F (5’-GGAAGTAAAAGTCGTAACAAGG-3’) and reverse ITS4R (5’- CTCCTCCGCTTATTGATATGC-3’) primer, 12.5 µL of PCR master mix (Thermo Scientific, USA), DNA template 1 µL, and 9.5 µL of nuclease free water (White et al. Citation1990). Amplification was conducted in a thermal cycler (Biorad, USA) as follows: initial denaturation 95 °C (2 min), denaturation 95 °C (30 s), annealing 53 °C (45 s), and extension 72 °C (1 min), and the last three steps were repeated for 25 cycles (Ramnath et al. Citation2014). The PCR products were then subjected to electrophoresis in a 1% agarose gel and stained with ethidium bromide (0.5 µg/mL). The PCR amplicons were sequenced by the Inqaba Biotechnology DNA Sequencing Unit and the consensus sequences submitted to the National Centre for Biotechnology Information (NCBI) blast database to determine their identities.
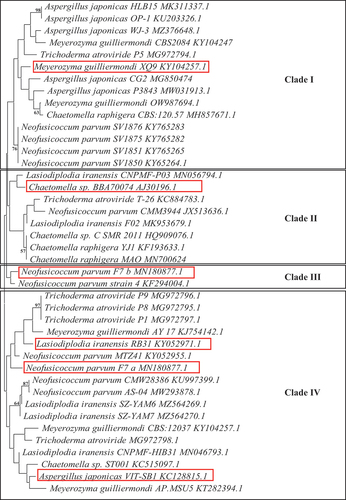
The 18S ITS2 rRNA sequences of the seven fungal isolates were compared to other closely related strains using BLAST and the NCBI GenBank database. Alignment and phylogenetic tree construction were done using the MEGA 11 software. The phylogenetic tree was constructed using the neighbour-joining method, and the tree was evaluated using 100 bootstrap replications (Tamura et al. Citation2011) ().
2.8. Optimisation of enzyme production by the three isolates displaying the highest tolerance to glucose C2, MB5, and PS1
Optimisation of enzyme production for isolates C2, MB5, and PS1 was conducted by evaluating the effect of a single variable at a time and thereafter, manifesting it as a standard condition before the optimisation of the next parameter. Enzyme activity was assayed after each step to determine the optimal yield. The experiments were conducted in two independent experiments with duplicate assays from each experiment (Kao et al. Citation2019). The variables tested included incubation time, pH, temperature, agitation, carbon, and nitrogen sources.
To determine the optimum incubation time for enzyme production, shake flasks consisting of 25 mL of minimal salt medium were inoculated and incubated as per section 2.3. Samples were obtained every 48 hours for 12 days, and enzyme activity was assayed.
Optimum pH was determined by inoculation of a minimal salt medium with different pH buffers 0.05 mol/L sodium acetate buffer (pH 3.0 to 5.0), 0.05 mol/L sodium phosphate buffer (pH 6.0 to 8.0), and 0.05 mol/L glycine-NaOH buffer (pH 9.0–10.0) incubated for their optimal time for production and thereafter assayed for enzyme activity.
The optimum incubation temperature was determined by setting up shake flask cultures with media at the optimal pH and incubating them at different temperatures (25 °C, 30 °C, 40 °C, 50 °C, 60 °C, 70 °C, and 80 °C) at 125 rpm for the optimal time duration. Thereafter, the extracts were assayed for enzyme activity.
To determine the optimum agitation rate for fermentation, broth media with optimal pH were inoculated and incubated at the optimal temperature at different agitation rates (100, 125, 150, 175, and 200 r/min), and thereafter assayed for enzyme activity.
To maximise enzyme production, various carbon and nitrogen sources were supplemented into the standard minimal medium at optimal pH in section 2.3. The following carbon (1%) lactose, maltose, glucose, sucrose, and glycerol and nitrogen sources (1%) casein, glycine, yeast extract, ammonium sulphate, and ammonium acetate were (Mahapatra et al. Citation2016) inoculated with standardised culture and incubated under optimal conditions as described above.
2.9. Effect of pH on β-glucosidase activity and stability of isolate PS1
Optimum pH was determined at 55 °C for 5 min in various buffers: sodium acetate (0.05 mol/L, pH 3–5), sodium phosphate (50 mmol/L, pH 6–8), and Glycine-NaOH (50 mmol/L, pH 9–10) containing 4 mol/L 4-nitrophenyl-β-D-glucopyranoside. The pH stability was carried out by pre-incubating the enzyme in sodium phosphate buffer (50 mmol/L, pH 6) for 4 h with aliquots sampled every 30 min. Residual activity was determined using the assay as per section 2.3. The enzyme in the optimum pH buffer with no incubation served as the control (100% activity).
2.10. Effect of temperature on isolate PS1 β-glucosidase activity and stability
The optimum temperature of the enzyme was determined in sodium phosphate buffer (0.05 mol/L, pH 6.0) containing 4 mmol/L 4-nitrophenyl-β-D-glucopyranoside. The stability of the enzyme was determined by pre-incubating the enzyme at 55 °C and 70 °C in the absence of 4-nitrophenyl-β-D-glucopyranoside for 4 h with aliquots sampled every 30 min. The residual activity was determined at 55 °C for 5 min as per section 2.3. Residual activity was determined by using the enzyme in the optimum pH buffer with no incubation as the control (100% active).
2.11. SDS polyacrylamide gels
SDS-PAGE was carried out according to the procedure by Laemmli (Citation1970). A 12% polyacrylamide was prepared. Electrophoresis was carried out at 50 V for 3 h and the gel was stained with Coomassie Brilliant Blue (More et al. Citation2011). The approximate molecular weight of the protein was determined from the bands that developed on the gel from the spectra multicolour broad range molecular weight markers (ThermoScientific, USA).
2.12. Native polyacrylamide gels
A native PAGE gel was used to detect the β-glucosidase enzyme. After electrophoresis, the gel was soaked in 0.05 mol/L sodium acetate buffer (pH 5.0) for 10 min at room temperature, thereafter, incubated in 0.05 mol/L sodium acetate buffer supplemented with 0.1% aesculin and 0.03% ferric chloride for 5 min at 55 °C. After incubation, a black band should form corresponding to the protein band, thus confirming β-glucosidase activity (Kwon et al. Citation1994).
3. Results
3.1. Isolation, primary screening, and secondary screening of fungal isolates
A total of 46 isolates were obtained from three different sampling sites. Primary screening for β-glucosidase activity using aesculin revealed that 12 isolates had no precipitate indicating an absence of β-glucosidase activity, 34 displayed precipitation indicative of positive β-glucosidase activity as follows: 7 isolates displayed a slight precipitate indicating the presence of activity, 13 displayed a medium precipitate indicative of good activity, and 14 isolates displayed large precipitates indicating excellent β-glucosidase activity (Supplementary Figure 1 and ). All 14 excellent producers (C1; CB9; CB11, MS2; MS3; MS5; MS7; MB2; MB5; PS1; PB2; PB4; PB6; PB8) displayed large precipitate zones with the largest precipitate diameter of 75 mm for 10 isolates (C1; CB11; MS2; MS3; MS5; MB2; PB2; PB4; PB6; PB8) which displayed complete hydrolysis indicated by complete blackening of the entire surface of the substrate agar plates. The black precipitate and the zones of hydrolysis around the fungal growth are indicative of extracellular β-glucosidase and cellulase production, respectively (Bonciani et al. Citation2018).
Table 1. Identity of the seven fungal isolates after NCBI BLAST analysis.
Fungal isolates MB2, MB5, PB4, and PB8 displayed a black pigment when grown on PDA. This made it difficult to distinguish between the black fungal pigment and the precipitate due to hydrolysis of the aesculin substrate. Therefore, secondary screening was performed to quantify β-glucosidase activity. The β-glucosidase activity of all 27 isolates displaying medium-to-large zones of precipitation indicative of enzyme activity from primary screening were quantified using 4-nitrophenyl-β-D-glucopyranoside as a substrate (). Of these, 13 isolates displayed (C1, C2, C4, CB1, MS2, MS8, MB2, MB4, MB5, PS1, PB2, PB4, PB8) enzyme activities between 0.4 U/mL and 0.9 U/mL, with isolate PS1 displaying the highest activity. All 13 isolates were then screened for endoglucanase and exoglucanase activity. Isolates C2, C4, MS8, PB2, and PB8 completely hydrolysed the CMC substrate, indicating good endoglucanase production (Supplementary Figure 1 and ). Isolates C1, C2, MS8, MB2, MB4, PS1, PB2, and PB8 completely hydrolysed the avicel substrate indicated by complete clearing of the plate after qualitative screening methods compared to the control indicating excellent exoglucanase production (Supplementary Figure 1 and ). Cellulase activity was then quantified for all 13 isolates, and the results in revealed that isolate C1 was the highest producer of both endoglucanases (36.3 U/mL) and exoglucanases (61.58 U/mL). The ability to produce β-glucosidases in the presence of glucose and the ability of the β-glucosidase enzyme to tolerate glucose were tested for the seven highest β-glucosidase producers (PS1; MS8; C4; C1; C2, MB5; PB8).
Figure 1. Quantification of β-glucosidase activity for the 27 fungal isolates with the largest precipitate zones from qualitative screening in the crude enzyme extracts produced at 30 °C, 125 r/min and 4-nitrophenyl-β-D-glucopyranoside as a substrate at OD410 nm,(Mean ± SD, N = 4).
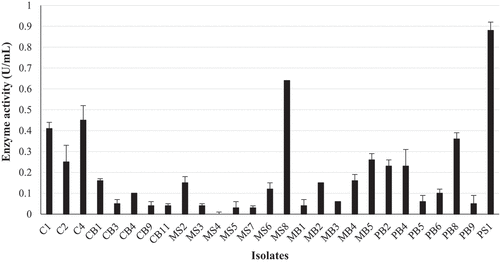
Figure 2. Quantification of endoglucanase and exoglucanase activity of the 13 isolates that displayed the highest β-glucosidase activity using the crude enzyme extract produced at 30 °C, 125 r/min and the DNS assay with avicel and CMC as substrates, respectively at OD 540 nm (Mean ± SD, N = 4).
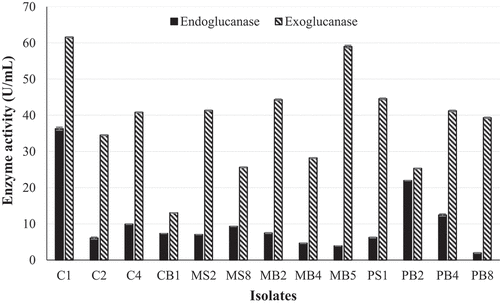
Table 2. Ratio of β-glucosidases to glucanases in the crude extracts of the fungal isolates.
3.2. Identification of fungal isolates and phylogenetic analysis
The above seven fungal isolates were identified by sequencing of the ITS2 region of the 600 bp 18S ITS2 ribosomal RNA amplicons. Genomic DNA was successfully isolated from all seven isolates, and the 18S ribosomal RNA was amplified and the expected 600 bp amplicon was obtained. The BLAST analysis of the consensus sequences revealed the identities of all seven isolates. Isolate (C1) Aspergillus japonicasVIT-SB1, (C2) Neofusicoccum parvuma, (C4) Meyerozyma guilliermondi XQ9, (MS8) Trichoderma atroviride, (PB8) Lasiodiplodia iranensis, (PS1) Chaetomella sp. BBA70074, and (MB5) Neofusicoccum parvumb. Isolates C2 and MB5 displayed the same identities with different accession numbers indicating that they are different strains (). The phylogenetic tree displayed four clades, with nodes representing common ancestry (). Several species were grouped in clade I, including M. guilliermondi (XQ9), T. atroviride, C. raphigera, A. japonicas, and N. parvum. Clade II only displays different Chaetomella sp. The N. parvuma and N. parvumb isolates surprisingly did not group together in one clade but were in clades III and IV, forming sister taxa with N. parvum strain 4 KF294004.1 and N. parvum MTZ41 KY052955.1 respectively. Lasiodiplodia iranensis RB31 and N. parvuma F7 have descended from the same common ancestor into two separate lineages. All the A. japonicas isolates are grouped together in clade I except for A. japonicas VIT-SB1 which formed sister clades with Chaetomella sp. ST001 and M. guilliermondi AP. MSU5.
3.3. Production of β-glucosidases in the presence of glucose
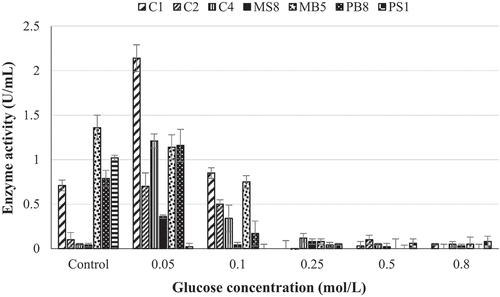
The effect of glucose on β-glucosidase production was determined to ensure that the enzyme produced is able to remain active in the presence of glucose. Production in the presence of 0.05 mol/L glucose was enhanced for five isolates (C1, C2, C4, MS8, and PB8) by greater than 50% and decreased marginally at a higher glucose concentration (0.1 M). From glucose concentrations ≥0.25 mol/L the enzyme production decreased by greater than 70% for all seven isolates ().
Figure 3. Effect of glucose on the production of β-glucosidases by (C1) Aspergillus japonicas, (C2) Neofusicoccum parvuma, (C4) Meyerozyma guilliermondi, (MS8) Trichoderma atroviride, (MB5) Neofusicoccum parvumb, (PB8) Lasiodiplodia iranensis, and (PS1) Chaetomella sp. at 30 °C, 125 r/min using 4-nitrophenyl-β-D-glucopyranoside as a substrate at OD 410 nm (Mean ± SD, N = 4).
3.4. Glucose tolerance assays
The glucose tolerance studies indicated that five of the seven isolates, namely Aspergillus japonicas (A. japonicas), N. parvuma, N. parvumb, Lasiodiplodia iranensis (L. iranensis), and Chaetomella sp. retained greater than 50% of their β-glucosidase activities in the presence of 0.05 mol/L glucose. Meyerozyma guilliermondi (M. guilliermondi) and Trichoderma atroviride (T. atroviride) lost almost all their activity (). The three isolates that retained the highest β-glucosidase activity in the presence of 0.05 mol/L glucose were Chaetomella sp. (92%), N. parvuma (55%), and N. parvumb (51%). Chaetomella sp. displayed the highest tolerance to glucose and retained 11% of activity, N. parvuma displayed no activity, and N. parvumb 1% β-glucosidase activity in the presence of 0.8 mol/L glucose. These isolates were taken forward for optimisation of enzyme production by one variable at a time experiments. The optimised crude enzyme extract of Chaetomella sp. retained 31% β-glucosidase activity in the presence of 0.8 mol/L glucose, this is 20% more enzyme activity retained compared to the initial crude extract whilst the optimised crude extract of isolates N. parvuma and N. parvumb did not show an increase in tolerance to glucose when compared to the initial crude extract.
Figure 4. Glucose tolerance of crude β-glucosidases produced by (C1) Aspergillus japonicas, (C2) Neofusicoccum parvuma, (C4) Meyerozyma guilliermondi, (MS8) Trichoderma atroviride, (MB5) Neofusicoccum parvumb, (PB8) Lasiodiplodia iranensis, (PS1) Chaetomella sp., at 30 °C, 125 r/min using 4-nitrophenyl-β-D-glucopyranoside as a substrate at OD 410 nm (Mean ± SD, N = 4).
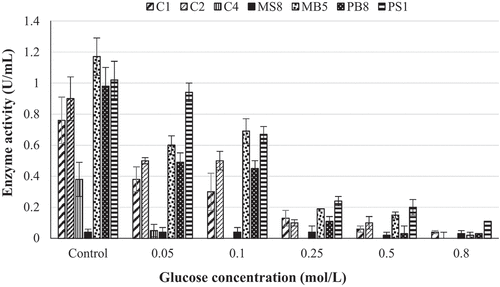
3.5. Optimisation of enzyme production by N. parvuma, N. parvumb and Chaetomella sp
Optimisation of enzyme production was achieved by determining optimal growth parameters so as to achieve maximal enzyme levels from fermentation, thus decreasing costs. The optimal incubation time for the production of β-glucosidase by Chaetomella sp. was 6 days, N. parvuma 9 days, and N. parvumb 10 days (). Maximum production was obtained at pH 6.0 and 30 °C for all three isolates (). The agitation speeds of 150, 125, and 175 rpm yielded optimal enzyme titres for Chaetomella sp., N. parvuma, and N. parvumb, respectively (). Casein and glycerol were the best nitrogen and carbon sources for N. parvumb yielding an enzyme activity of 1.8 U/mL in 1.75% and 0.5% nitrogen and carbon, respectively (). Isolates N. parvuma and Chaetomella sp. displayed optimal β-glucosidase production with supplementation of soy peptone and cellobiose, respectively, displaying enzyme activities of 0.80 U/mL in 1% soy peptone and 1% cellobiose and 2.44 U/mol/L in 0.5% soy peptone and 1.25% cellobiose, respectively (). Chaetomella sp. produced the highest β-glucosidase activity of 2.44 U/mL ().
Figure 6. Optimal time of incubation (a) optimum pH (b) temperature (c) and agitation (d) using one factor at a time experiments for the production of β-glucosidases by isolates (C2) Neofusicoccum parvuma, (MB5) Neofusicoccum parvumb, and (PS1) Chaetomella sp. using the crude enzyme extracts and 4-nitrophenyl-β-D-glucopyranoside as substrate at OD 410 nm (Mean ±SD, N = 4).
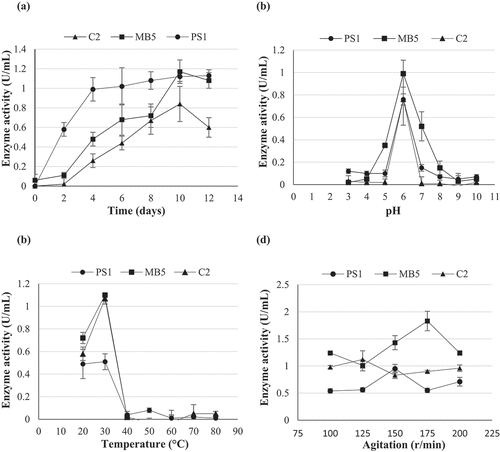
Figure 7. Optimum (a) nitrogen source and (b) nitrogen concentration for the optimal production of β-glucosidase by isolates (C2) Neofusicoccum parvuma, (MB5) Neofusicoccum parvumb, and (PS1) Chaetomella sp. using the crude enzyme extracts and 4-nitrophenyl-β-D-glucopyranoside as substrate at OD410 nm (Mean ±SD, N = 4).
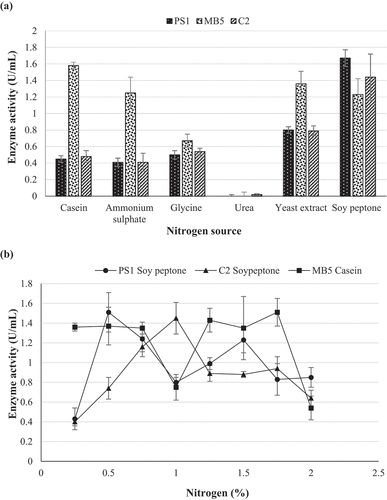
Figure 8. Optimum (a) Carbon source and (b) Carbon concentration for the optimal production of β-glucosidase by isolates (C2) Neofusicoccum parvuma, (MB5) Neofusicoccum parvumb, and (PS1) Chaetomella sp. using the crude enzyme extracts and 4-nitrophenyl-β-D-glucopyranoside as substrate at OD410 nm (Mean ±SD, N = 4).
3.6. Polyacrylamide gel electrophoresis
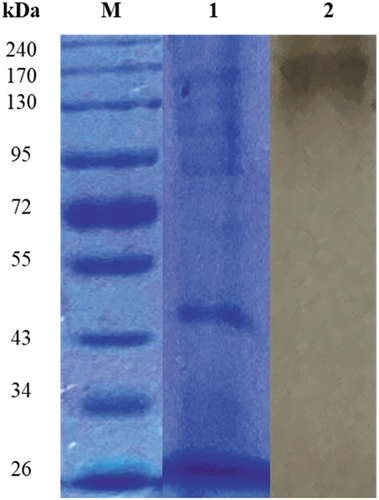
The SDS-PAGE gel of the crude β-glucosidase revealed multiple-protein bands in the crude extract. Zymography using non denaturing gel electrophoresis revealed a 170 kDa protein subunit displaying β-glucosidase activity ().
Figure 9. SDS and Native PAGE of (PS1) Chaetomella sp. crude β-glucosidase extract produced under optimal conditions (Day 12, 3 °֯C, 150 r/min, 0.5% soy peptone, 1.25% cellobiose). Lanes M: spectra multicolour broad range molecular weight marker (ThermoScientific, USA), 1: crude enzyme, and 2: crude enzyme displaying β-glucosidase activity.
3.6.1. Effect of pH and temperature on the activity of crude β-glucosidase enzymes produced by Chaetomella sp. BBA70074
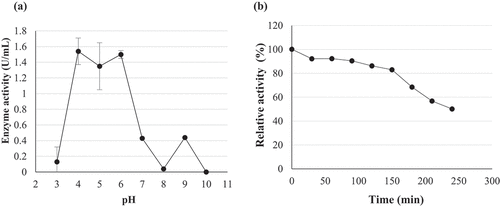
The pH optima for β-glucosidase activity of Chaetomella sp. BBA70074 was pH 4.0 and 6.0 (). β-Glucosidase displayed maximal activity at 70 °C in pH 6.0 buffer () indicating a thermophilic enzyme. pH and temperature stability studies were conducted focusing on the parameters and levels that were similar to those required for industrial enzymes.
Figure 10. (a) pH optima and (b) stability at pH 6 of (PS1) Chaetomella sp. β-glucosidase crude extract produced under optimal conditions (Day 12; 30 °C; 150 r/min; 0.5% soy peptone; 1.25% cellobiose) and assayed using 4-nitrophenyl-β-D-glucopyranoside as substrate at 55 °C and OD410 nm (Mean ±SD, N = 2).
3.6.2. pH and temperature stability of crude Chaetomella sp. β-glucosidase enzymes
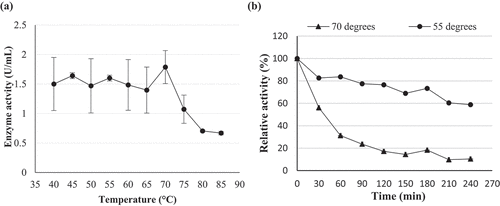
The enzyme was fairly stable for 150 min, retaining 80% activity at pH 6.0. At 180 min the β-glucosidase activity started to decline with the enzyme retaining only 50% activity at 240 min (). At 70 °C, the enzyme was unstable and lost approximately 40% activity in the first 30 min with a further 30% activity lost after 60 min and only 10% activity remaining at 240 min (). Since the enzyme was not stable at its optimal temperature, thermal stability was tested at the next optimal temperature point (55 °C). Better stability was observed at 55 °C compared to 70 °C. The enzyme was fairly stable retaining 80% activity within the first 90 min, from 120 to 180 min a decline in enzyme activity and at 240 min the enzyme retained 60% activity ().
Figure 11. (a) Temperature optima and (b) stability at 55 °C and 70 °C of (PS1) Chaetomella sp. crude β-glucosidase extract produced under optimal conditions (Day 12, 3 °֯C, 150 r/min, 0.5% soy peptone, 1.25% cellobiose) and assayed using 4-nitrophenyl-β-D-glucopyranoside as substrate at 55 °C and OD410 nm (Mean ±SD, N = 2).
4. Discussion
In this study, a total of 46 isolates were obtained from soil, tree, and bark samples from three different sites and screened for β-glucosidase activity and 13 of these were screened for glucanase activity by primary screening methods. The colour intensity of precipitation and the zones of hydrolysis in primary screening display no correlation with the enzyme activities obtained in secondary screening. It is, therefore, important to utilise both primary and secondary screening methods as qualitative screening methods are less sensitive compared to quantitative methods (Bonciani et al. Citation2018).
A. japonicas was the highest cellulase producer with β-glucosidases with promise as a native producer of cellulase cocktails for the degradation of cellulosic biomass in industrial applications. It produced all three cellulase enzymes with glucose tolerant β-glucosidases (). Rani et al. (Citation2014) reported that the commercial T. reesei cellulase cocktail with ratios of β-glucosidase: endoglucanase: exoglucanase of <1%: 18%: 72% resulted in hydrolysis of cellulosic material; however, this cocktail had to be supplemented with β-glucosidases from A.niger as the β-glucosidase in the T. reesei cocktail was not at an appropriate concentration and was inhibited by high levels of glucose.
Based on the different effects of glucose on β-glucosidase activity in this study those from isolates M. guilliermondi and T. atroviride can be grouped into group one whilst all of the others belong to group two (Cao et al. Citation2015). Chaetomella sp. displayed the highest tolerance to glucose retaining 92% and 11% of its activity at low 0.05 mol/L and high 0.8 mol/L glucose concentrations, respectively. Cao et al. (Citation2015) reported 10% retention of activity in 0.6 mol/L glucose, which indicates that isolate Chaetomella sp. has great promise as its β-glucosidase retained 11% activity in 0.8 mol/L glucose, which is a higher concentration of glucose.
Studies have reported the isolation of β-glucosidases and cellulases from Chaetomella sp. and M. guilliermondi; however, very low activities were observed (Sari et al. Citation2017; Kao et al. Citation2019; Zhang et al. Citation2021). Species of Aspergillus and Trichoderma display great potential for biotechnological processes. A study by Decker et al. (Citation2000) reported β-glucosidases from A. japonicus with enzyme activity of 29.5 U/mL; however, the tolerance to glucose was not tested. In another study, Valappil et al. (Citation2019) reported glucose tolerant β-glucosidases from A. unguis that retained less than 10% β-glucosidase activity in 0.5 mol/L of glucose. Cellulases including β-glucosidases from A. niger and species of Trichoderma are generally regarded as yardsticks for commercial production; however, the proportion of β-glucosidases in these cellulase cocktails is meagre, and they require supplementation with β-glucosidases. Industrial-level scale-up of the process is inefficient as naturally-occurring cocktails do not contain optimal ratios and specific activities of all three components. T. reesei has already been commercialised and utlised in biotechnological applications (Ahmed et al. Citation2017). Two other Trichoderma strains, namely, T. atroviridae and T. virens display potential for commercialisation.
The whole-genome sequence of T. atroviride and T. virens is incomplete, but that of T. reesei has been completed. The sequence data shows that the genome consists of few genes encoding hemicellulolytic and cellulolytic enzymes (Schmoll et al. Citation2016). The whole-genome sequence will enable scientists to obtain the β-glucosidase gene sequences, which may be utilised to produce recombinant enzymes with potentially enhanced β-glucosidase activity. Makraki et al. (Citation2020) produced a recombinant plasmid by cloning the T. reesei (Trbgl2, AB003110) gene into a pET vector. The appropriate codon in the recombinant plasmid was then substituted with the E367Q gene fragment by site directed mutagenesis. The resulting mutant displayed a 188% increase in activity. Cao et al. (Citation2015) isolated a Bgl6 clone from a 260 Mb metagenomic library with an open reading frame of 1371 bp encoding 456 amino acids displaying homology similar to glucose tolerant β-glucosidases from the GH1 family.
The isolates under study were identified by NCBI nucleotide blast and the identities of the respective unknown isolates are represented in . All seven isolates belong to the phylum Ascomycota, which represents the most diverse group of fungi. The principal characteristic of this group includes an ascus, which is a sac-like structure that contains ascopores (Naranjo-Ortiz and Gabaldón Citation2019). Phylogenetic analysis plays an important role in understanding the evolution of species, populations, and genes (). Evolutionary relationships between species are represented in phylogenetic trees (Chen and Zhuang Citation2017). Neofusicoccum parvuma and N. parvumb isolated from two different areas descended from the same common ancestor into clades IV and III, respectively. These two isolates were morphologically identical except for a different pigmentation pattern. The fungal species A. japonicas and N. parvuma isolated from the same area belong to the same clade indicating that they share a common ancestor and similar characteristics but are from different genera. These two scenarios are an indication that the nucleotide sequences encoding genes in different species may be influenced by environmental factors (Taylor et al. Citation2015). N. parvuma, N. parvumb, and L. iranensis are known endophytic fungi; however, this is the first report of the production of β-glucosidases and cellulases from these isolates. N. parvum is also a plant pathogen on different trees in various areas worldwide (Chan et al. Citation2021) while L. iranensis inhabits eucalyptus, citrus, and many other plants as an endophyte (Du et al. Citation2020). In this study, two isolates were identified as N. parvum, however, they were isolated from two different areas and although both isolates displayed similar morphologies, their black pigmentation on PDA differed (Supplementary Figure 1). N. parvuma displayed a central black circular pigmentation pattern on the bottom of the plate whilst N. parvumb displayed a patchy black pigmentation on both sides of the plates. In addition, the biochemical character of the isolates differed in one variable at a time experiments except for temperature and pH. They displayed different preferences for nutrients (carbon and nitrogen). N. parvumb displayed higher tolerance to glucose compared to N. parvuma with 0.2% and 0.1% activity retained in 0.5 mol/L glucose, respectively. This change may be the result of different selective pressures caused by environmental differences to which they adapted, thus culminating in different growth parameters for optimal β-glucosidase production. This phenomenon is not surprising and can be attributed to Darwin’s law of natural selection, which states that “A population in equilibrium with its environment under natural selection will have a phenotype which maximises the fitness locally” (Haufe Citation2012).
Chaetomella sp. displayed the highest enzyme production on day 12; however, day 6 was selected as the optimal day of production as a t-test displayed a p-value > 0.5 indicating that difference in enzyme levels between days 6 and 12 was not significant; however, the reduced production time will translate to production cost savings. N. parvuma and N. parvumb also required long incubation periods for optimal enzyme production and an optimal pH and temperature of six and 30 °C, respectively. Optimal β-glucosidase activity was also reported to occur after incubation periods longer than 5 days by Gao et al. (Citation2012, Citation2016, Ahmed et al. (Citation2017) and Kao et al. (Citation2019). The optimal pH of 6 and temperature of 30 °C are expected as a study by Agarwal et al. (Citation2014) reported optimal β-glucosidase production at the same pH and temperature. Chaetomella sp. and N. parvuma displayed optimal production of β-glucosidases when supplemented with soy peptone and cellobiose as carbon and nitrogen sources, respectively. This is not remarkable as many studies report variations in carbon and nitrogen source preferences by different fungi (Gao et al. Citation2012; Sørensen et al. Citation2014; Ahmed et al. Citation2017). A study by Mahapatra et al. (Citation2016) reported the optimal production of β-glucosidase enzymes from A. niger using glycerol as a carbon source. Soy peptone has also been reported for optimal β-glucosidase production by Chaetomium thermophilum (Mahapatra et al. Citation2016). Other carbon sources preferred by microorganisms that were not tested in this study include avicel, pectin, and quercetin and nitrogen sources include beef extract, L- asparagine, and tryptone (Ahmed et al. Citation2017).
Hydrolysis of the lignocellulosic biomass requires a basic pH of 5–9 and thermophilic temperatures between 50 °C and 85 °C (Kao et al. Citation2019). Chaetomella sp. displayed an optimal pH and temperature at pH 6 and 70 °C, respectively. Two other studies reported pH and temperature optima within the ranges (pH 5.0–9.0) and (50–70 °C) indicating their thermophilic nature (Karnchanatat et al. Citation2007; Karami et al. Citation2020). Karami et al. (Citation2020) reported a fairly good stability of β-glucosidase after 1 hour at 70 °C, whereas the enzyme in the current study was not stable at 70 °C; however, thermal stability was tested at 55 °C and revealed that the enzyme was stable for 120 min indicating that the β-glucosidase enzyme is still suitable for application in cellulose hydrolysis processes.
The present study focused on searching for a novel cellulase producer with glucose tolerant β-glucosidase with optimal specific activities, which would be more cost-effective in scaling up. The strains isolated displayed promising activity for both cellulases and glucose tolerant β-glucosidases. A. japonicas displayed promising activity as a prospective producer of a cocktail of all three enzymes for application in industry for the hydrolysis of cellulose. In addition to high glucanase activity, it also has a promising ratio of β-glucosidase:exoglucanase:endoglucanase. The β-glucosidase from this isolate also showed tolerance to glucose; however, the Chaetomella sp. and N. parvumb isolates displayed higher tolerance to glucose compared to that of A. japonicas. These isolates may also be suitable for application in industry as producers of glucose tolerant β-glucosidases for supplementation of existing cellulase cocktails for the hydrolysis of cellulose (Karami et al. Citation2020).
Future studies include optimisation of enzyme production using one variable at a time experiments, Plackett Burman, Response Surface Methodology, purification, and characterisation of the enzymes of interest.
Supplemental Material
Download MS Word (18.4 KB)Acknowledgements
The authors would like to express their gratitude to the University of KwaZulu-Natal Westville campus for their support.
Disclosure statement
No potential conflict of interest was reported by the authors.
Supplementary material
Supplemental data for this article can be accessed online at https://doi.org/10.1080/21501203.2022.2155261
Additional information
Funding
References
- Adesina F, Onilude A. 2013. Isolation, identification, and screening of xylanase and glucanase-producing microfungi from degrading wood in Nigeria. Afr J of Agric Res. 8(34):4414–4421. doi:10.5897/AJAR2013.6993.
- Agarwal T, Saxena MK, Chandrawat MK 2014. Production and optimization of cellulase enzyme by Pseudomonas aeruginosa MTCC 4643 using sawdust as a substrate. IJSRP. 4(1):1–3.
- Ahmed A, Nasim FH, Batool K, Bibi A. 2017. Microbial β-glucosidase: sources, production, and applications. Appl & Environ Microbiol. 5(1):31–46.
- Ariaeenejad S, Noshi-Nedamani S, Rahban M, Kavousi K, Pirbalooti AG, Mirghaderi SS, Mahammadi M, Mirzael M, Salekdeh GH. 2020. A novel high glucose-tolerant β-glucosidase: a targeted computational approach for metagenomic screening. Front Bioeng and Biotechnol. 8(2296–4185):1–14. doi:10.3389/fbioe.2020.00813.
- Bonciani T, Vero D, Giannuzzi E, Verspohl A, Giudici P. 2018. Qualitative and quantitative screening of the β-glucosidase activity in Saccharomyces cerevisiae and Saccharomyces uvarum strains isolated from refrigerated must. Lett Appl Microbiol. 67(1):72–78. doi:10.1111/lam.12891.
- Cao L, Wang Z, Ren G, Kong W, Li L, Xie W, Liu Y. 2015. Engineering a novel glucose-tolerant β-glucosidase as supplementation to enhance the hydrolysis of sugarcane bagasse at high glucose concentration. Biotechnol Biofuels. 8(202):1–12. doi:10.1186/s13068-015-0383-z.
- Chan DJ, Yang D, Ye Y, Zhang ZB, Pan LF, Zhang F, Gang F. 2021. First report of Neofusicoccum parvum causing leaf spot on Geodorum eulophioides in China. Plant Dis. 5(2):486.
- Chen K, Zhuang WY. 2017. Discovery from a large-scaled survey of Trichoderma in soil of China. Sci. 7:9090.
- Cherubini F. 2010. The biorefinery concept : using biomass instead of oil for producing energy and chemicals. Energy Convers. 51(7):1412–1421. doi:10.1016/j.enconman.2010.01.015.
- Darwesh OM, El-Maraghy SH, Abdel-Rahman HM, Zaghloul RA. 2020. Improvement of paper wastes conversion to bioethanol using novel cellulose degrading fungal isolate. J Fuel. 262(16–2361):116518. doi:10.1016/j.fuel.2019.116518.
- Decker CH, Visser J, Schreier P. 2000. β-Glucosidases from five black Aspergillus species: a study of their physicochemical and biocatalytic properties. J Agric Food Chem. 48(10):4929–4936. doi:10.1021/jf000434d.
- de Souza MF, da Silva BEP, da Silva AS. 2021. Production of cellulases and β-glucosidases by Trichoderma reesei Rut C30 using steam-pretreated sugarcane bagasse: an integrated approach for onsite enzyme production. Braz J Chem Eng. 38(2021):435–442. doi:10.1007/s43153-021-00114-5.
- Du H, Parit M, Xinpeng MW, Wang CY, Zhang M, Wang R, Zhang X, Jiang Z, Li B 2020. Sustainable valorization of paper mill sludge into cellulose nanofibrils and cellulose nanopaper. J Hazard. 400:1–5.
- Gao Z, Hop DV, Yen LTH, Ando K, Hiyamuta S, Kondo R. 2012. The production of β-glucosidases by Fusarium proliferatum NBRC109045 isolated from Vietnamese forest. AMB Express. 2(1):49–62.
- Haufe C. 2012. Darwin’s laws. Stud Hist Philos Biol Biomed Sci. 43(1):269–280.
- Kanakaraju Y, Uma A, Palety K. 2020. Aspergillus Niger based production of cellulase-A study on submerged and solid state fermentation. Int J Sci Res. 64(3):60–65.
- Kao M, Kuo H, Lee C, Huang K, Huang T, Li C, Chen W, Andrew H, Wang J, Yu S, et al. 2019. Chaetomella raphigera β-glucosidase D2-BGL has intriguing structural features and a high substrate affinity that renders it an efficient cellulase supplement for lignocellulosic biomass hydrolysis. Biotechnol for Biofuels. 12(1):258–276. doi:10.1186/s13068-019-1599-0.
- Karami F, Ghorbani M, Mahoonak AS, Khodarahmi R 2020. Fast, inexpensive purification of β-glucosidase from Aspergillus niger and improved catalytic/physicochemical properties upon the enzyme immobilization: Possible broad prospects for industrial applications. Food Sci Technol. 118:1–9.
- Karnchanatat A, Petsom A, Sangvanich P, Piaphukiew J, Whalley AJS, Reynolds CD, Sihanonth P 2007. Purification and biochemical characterization of an extracellular β-glucosidase from the wood-decaying fungus Daldinia eschscholzii. FEMS Microbiol Lett. 270:162–170.
- Kuhad RC, Gupta R, Singh A. 2011. Microbial cellulases and their industrial applications. Enzyme Res. 2011(1):1–10. doi:10.4061/2011/280696.
- Kumar R, Wyman CE. 2009. Effects of cellulase and xylanase enzymes on the deconstruction of solids from pretreatment of poplar by leading technologies. Biotechnol Prog. 25(2):302–314. doi:10.1002/btpr.102.
- Kwon K, Lee J, Kang HG, Hah YC. 1994. Detection of β-Glucosidase activity in polyacrylamide gels with esculin as substrate. Appl Environ Microbiol. 60(12):4584–4586. doi:10.1128/aem.60.12.4584-4586.1994.
- Laemmli UK. 1970. Cleavage of structural proteins during the assembly of the head of bacteriophages T4. Nature. 270(1970):680–685. doi:10.1038/227680a0.
- Mahapatra S, Vickram AS, Sridharan TB, Parameswari M, Pathy R. 2016. Screening, production, optimisation, and characterisation of β-glucosidase using microbes from shellfish waste. Biotechnology. 6(2):213–223.
- Makraki E, Darby JF, Carneiro MG, Firth JD, Heyam A, E AB, O’Brien P, Siegal G, Hubbard RE. 2020. Fragment derived modulators of an industrial β-glucosidase. Biochem J. 477(22):4383–4395. doi:10.1042/BCJ20200507.
- Monteiro LMO, Vici AC, Pinheiro PM, Heinin PR, de Oliveira AHC, Ward RJ, Prade RA, Buckeridge MS, de Moraes Polizeli MDT 2020. A highly glucose tolerant ß-glucosidase from Malbranchea pulchella (MpBg3) enables cellulose saccharification. Sci Rep. 10(6998):1–12.
- More SS, Renuka PS, Sweta M, Malini S, Veena SM. 2011. Isolation, purification, and characterisation of fungal laccase from Pleurotus species. Enzyme Res. 10(2011):248735.
- Naicker JE, Govinden R, Lekha P, Sithole B. 2020. Transformation of pulp and paper mill sludge (PPMS) into a glucose-rich hydrolysate using green chemistry: assessing pretreatment methods for enhanced hydrolysis. J Environ Manage. 270(301–4797):110914. doi:10.1016/j.jenvman.2020.110914.
- Naranjo-Ortiz MA, Gabaldón T. 2019. Fungal evolution: diversity, taxonomy, and phylogeny of the fungi. Biol Rev. 94(6):2101–2137. doi:10.1111/brv.12550.
- Pérez G, Fariña L, Barquet M, Boido E, Gaggero C, Dellazassa E, Carrau F. 2011. A quick screening method to identify β-glucosidase activity in native wine yeast strains: application of Esculin Glycerol Agar (EGA) medium. World J Microbiol Biotechnol. 27(1):47–55. doi:10.1007/s11274-010-0425-4.
- Ramnath L, Bush T, Roshini, Govinden G. 2014. Method optimization for denaturing gradient gel electrophoresis (DGGE) analysis of microflora from Eucalyptus sp. wood chips intended for pulping. Afr J Biotechnol. 13(3):356–365. doi:10.5897/AJB2013.12899.
- Rani V, Mohanram S, Tiwari R, Nain L, Arora A. 2014. β-Glucosidase: key enzyme in determining efficiency of cellulases and biomass hydrolysis. J Bioproces Biotechnol. 5(1):1–8.
- Saini A, Aggarwal NK, Yadav A. 2017. Isolation and screening of cellulose hydrolysing bacteria from different ecological niches. Bioeng and Biosci. 5(1):7–13.
- Sari SLA, Setyaningsih R, Wibowo NFA. 2017. Isolation and screening of cellulolytic fungi from Salacca edulis leaf litter. Biodiversity. 18(3):1282–1288. doi:10.13057/biodiv/d180355.
- Schmoll MC, Dattenböck N, Mendoza-Mendoza C, Tisch MI, Aleman SE, Baker C, Brown MG, Cervantes-Badillo J, Cetz-Chel GR, Cristobal-Mondragon L 2016. The genomes of three uneven siblings: footprints of the lifestyles of three Trichoderma species. Microbiol Mol Biol Rev. 80(1):205–327.
- Singh G, Verma AK, Kumar V. 2016. Catalytic properties, functional attributes and industrial applications of β-glucosidases. Biotechnology. 6(1):57–73.
- Sørensen A, Andersen JJ, Ahring BK, Phillip JT, Lübeck M. 2014. Screening of carbon sources for β-glucosidase production by Aspergillus saccharolyticus. Int Biodeterior Biodegrad. 93(2014):78–83. doi:10.1016/j.ibiod.2014.05.011.
- Sørensen A, Lübeck M, Lübeck PS, Birgitte KA. 2013. Fungal β-glucosidases: a bottleneck in industrial use of lignocellulosic materials. Biomolecules. 3(3):612–631. doi:10.3390/biom3030612.
- Srivastava N, Rathour R, Jha S, Pandey K, Srivastava M, Thakur VK, Sengar RS, Gupta VK, Mazumder PB, Khan AF, et al. 2019. Microbial β-glucosidase enzymes: recent advances in biomass conversation for biofuels application. Biomolecules. 9(6):220–243. doi:10.3390/biom9060220
- Steffen F, Janzon R, Wenig R, Saake B. 2017. Valorisation of waste streams from deinked pulp mills through anaerobic digestion of deinking sludge. Bioresearch. 12(3):4547–4566. doi:10.15376/biores.12.3.4547-4566.
- Tamura K, Peterson D, Peterson N, Stecher G, Nei M, Kumar S. 2011. MEGA5: molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance and maximum parsimony methods. Mol Biol Evol. 28(10):9–2731. doi:10.1093/molbev/msr121.
- Taylor NT, Krings M, Taylor EL. 2015. Ascomycota. Fossil Fuels Chapter. 8:171.
- Valappil PK, Rajasree KP, Abraham A, Christopher M, Sukumaran RK. 2019. Characterisation of a glucose tolerant β-glucosidase from Aspergillus unguis with high potential as a blend in for biomass hydrolysing enzyme cocktails. Biotechnology. 41(10):1201–1211.
- White TJ, Bruns T, Lee SB, Taylor J 1990. Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. PCR protocols: a guide to methods and application. 315–322.
- Yao G, Wu R, Kan Q, Gao L, Liu M, Yang P, Du J, Li Z, Qu Y. 2016. Production of a high-efficiency cellulase complex via β-glucosidase engineering in Penicillium oxalicum. Biotechnol Biofuels. 9(2016):78. doi:10.1186/s13068-016-0491-4.
- Zang X, Liu M, Fan Y, Xu J, Xu X, Li H 2018. The structural and functional contributions of β-glucosidase producing-microbial communities to cellulose degradation in composting. Biotechnol Biofuels. 11(51):1–13.
- Zhang L, Fu Q, Li W, Wang B, Yin X, Liu S, Xu Z, Niu Q, Nagini S. 2017. Identification and characterisation of a novel β-glucosidase via metagenomic analysis of Bursaphelenchus xylophilus and its microbial flora. Sci Rep. 7(1):2045–2322. doi:10.1038/s41598-017-01960-5.
- Zhang P, Li Q, Chen Y, Peng N, Liu W, Wang X, Li Y. 2022. Induction of cellulase production in Trichoderma reesei by a glucose-sophorose mixture as an inducer prepared using stevioside. RSC Adv. 12(2022):17392. doi:10.1039/D2RA01192A.
- Zhang P, Zhang R, Sirisena S, Gan R, Fang Z. 2021. The β-glucosidase activity of wine yeasts and its impacts on wine volatiles and phenolics: a mini-review. Food Microbiol. 100(740–0020):103859. doi:10.1016/j.fm.2021.103859.