ABSTRACT
Generally, Cryptococcus initially infects the respiratory tract, but can spread, eventually crossing the blood-brain barrier (BBB) and causing meningitis or meningoencephalitis. Specifically, Cryptococcus invades the vascular endothelial cells of the BBB, from which it enters the brain. The main mechanisms through which Cryptococcus crosses the BBB are transcellular traversal, the paracellular pathway, and via Trojan horse. In this paper, the mechanisms by which Cryptococcus crosses the BBB were explained in detail. In addition to pathways of entry to the brain, this paper presents a discussion on some rare cryptococcal infections and provides some insights for future research directions.
1. Introduction
Cryptococcus is a genus of basidiomycetous yeast, a pathogenic fungus that can cause cryptococcal meningitis and pneumonia. Cryptococcus neoformans (Cn) and Cryptococcus gattii (Cg) are the main pathogenic types (Francisco et al. Citation2021), both of which can cause nervous system disease. Cg commonly infects healthy hosts whereas Cn typically affects HIV-infected or other immunocompromised hosts (Sorrell et al. Citation2016; Yu et al. Citation2021). Some people infected with Cn may present with nervous system disease (Zaragoza Citation2019), especially immunocompromised individuals, who present with poorly controlled diabetes, malignant tumours, acute granulocytopenia, or severe burns; have a history of long-term antibiotic use; are receiving chemotherapy or glucocorticoids; or have undergone organ transplantation or have AIDS and therefore taking immunosuppressants long term (Thompson and Patterson Citation2012; Craig Citation2019). Globally, there are almost 250,000 cases of cryptococcal meningitis per year, and 181,000 deaths are attributed to cryptococcal meningitis annually, the mortality rate is 100% if the infection remains untreated (Iyer et al. Citation2021). Cryptococcal meningitis which is more common in adults with HIV (Ratemo and Denning Citation2023), accounts for 15% of AIDS-related deaths (Li et al. Citation2021).
Inhalation of cryptococcal basidiospores or yeast in the environment can result in pulmonary infection and meningitis if the infections spread to the central nervous system (CNS) (Leongson et al. Citation2013). Cryptococcal meningitis is most commonly caused by haematogenous dissemination, in which Cryptococcus spreads through the bloodstream from the lungs and crosses the blood-brain barrier (BBB), causing neurological disease. It also infects other parts of the body, resulting in cryptococcal laryngitis (Nadrous et al. Citation2004); however, occurrences of infection at alternative sites are uncommon. The CNS includes three important barriers: BBB, the blood-cerebrospinal fluid (CSF) barrier (BCSFB), and the CSF-brain barrier. These barriers share several functional similarities that protect the brain from toxins, maintain brain cell metabolism, and prevent pathogen entry (Dunn and Isaacs Citation2021), and among these barriers, the BBB has been the most extensively studied. Cryptococci crossing of the BBB is key to cryptococcal meningitis; therefore, it is important to study the interaction between cryptococci and BBB. Moreover, the sinuses are connected to the cranial cavity, making it easy for Cryptococcus to enter the brain. This review focuses on the different pathways of Cryptococcus entry into the brain and the mechanism by which Cryptococcus crosses the BBB.
2. The pathways by which Cryptococcus enters the brain
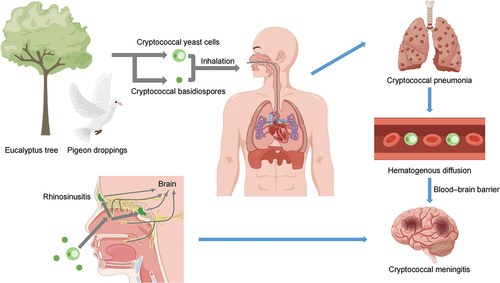
Cryptococci are inhaled and typically infect the lungs or CNS, after entering the respiratory tract, but can cause disease after accumulating in any part of the body (). This means that cryptococci enter the CNS from different sites via multiple pathways possibly. For example, in the rare case of cryptococcal laryngitis, cryptococci can accumulate in abundance in the throat, where they can enter the blood circulatory system and ultimately cross the BBB into the brain. The mechanism is discussed in detail in the following part.
Figure 1. The pathways by which Cryptococcus result in infection. Cryptococcus were found in eucalyptus trees and pigeon droppings. Inhalation of cryptococcal basidiomycetes or yeast cells from the environment can cause pulmonary infection and meningitis. The intracranial infections originate from direct spread through bone defects and nerves, or indirect haematogenous diffusion, ultimately resulting in the pathogen crossing the blood-brain barrier (BBB) (By Figdraw).
Moreover, the infectious particles in human cryptococcosis are yeast and spores (Velagapudi et al. Citation2009). Much attention has been paid to yeast cells, while how spores cause infections and disseminate to the brain remain poorly understood. Some studies have shown that spore-infected mice exhibit a higher brain fungal burden compared to yeast-infected mice (Walsh et al. Citation2019; Frerichs et al. Citation2021). It was found that such differences in outcome were the results of events occurring in the lungs before spreading to the bloodstream (Walsh et al. Citation2019). The current study suggests no difference between yeast cells and spores in the mechanism by which they cross the BBB from the blood into the brain. In addition, some studies have demonstrated that spores germinated into yeast inside alveolar macrophages and disseminate into the brain (Giles et al. Citation2009; Frerichs et al. Citation2021). We speculate that spores can also cause sinusitis infections, then germinate into yeast inside macrophages and enter the brain through direct spread.
In addition to the BBB, the barriers of the CNS include the BCSFB and the CSF-brain barrier. Is there a mechanism by which cryptococci enter the CSF after crossing brain capillaries? The choroid plexus is responsible for the production of CSF, suggesting that there is a mechanism by which cryptococci cross the choroid plexus of the brain and enter the CSF. Despite the discovery of cryptococcal choroid plexitis cases (Dubbioso et al. Citation2013), which suggests a possible mechanism for the entry of cryptococci into the cerebrospinal fluid through the choroid plexus, no yeast cells have been observed in the choroid plexus of mouse models (Chang et al. Citation2004), and no evidence of disruption to the epithelial layer or the basal lamina in choroid plexus sections has been observed (Charlier et al. Citation2005).
In addition, other pathways of entry into the brain have rarely been reported, for example, cryptococcal sphenoid sinusitis with meningitis, skull base osteomyelitis (Prendiville et al. Citation2016), and cryptococcal endophthalmitis (Crump et al. Citation1992; Amphornphruet et al. Citation2018). The sinuses are connected to the nasal cavity through an ostium and are separated from the brain by only a thin bony structure (Goldman-Yassen et al. Citation2021), which is an important site of intracranial complications of sinusitis. Inflammation of the paranasal sinuses may lead to complications, such as meningitis or endophthalmitis (Younis et al. Citation2002). Various fungi have been reported to be pathogenic and cause acute invasive fungal rhinosinusitis (AIFR), these fungi include Mucor/Rhizopus, Aspergillus, Alternaria, Scedosporium, Candida, and Fusarium (Luo et al. Citation2023). Accelerated reproduction of fungi may cause sinus bone destruction accompanied by intraorbital, pterygopalatine fossa, and even intracranial dissemination of the pathogen (Aribandi et al. Citation2007). However, AIFR is a rare disease and occurs mostly in immunosuppressed patients, and the incidence of intracranial infection is low (Thompson and Patterson Citation2012; Craig Citation2019). Intracranial infection usually originates from indirect haematogenous diffusion (De Vita et al. Citation2021), with the pathogen ultimately crossing the BBB (). Direct pathogen spreading is rare (Carr Citation2016), and usually involves bone defects, the space between sinuses (), the intracranial space (Jones et al. Citation2002; Younis et al. Citation2002), or the nerves in the brain (Sekaran et al. Citation2022), possibly via the sheath of the optic nerve (Ghobrial et al. Citation2016), olfactory nerve or trigeminal nerve (Hanson and Frey Citation2007, Citation2008).
These are some of the rare pathways through which cryptococcus enters the brain. Although these cases are rare, attention should be paid to these possibilities in the clinical, particularly when examining patients who are immunocompromised.
3. The blood-brain barrier
3.1. Anatomy and physiology of the BBB
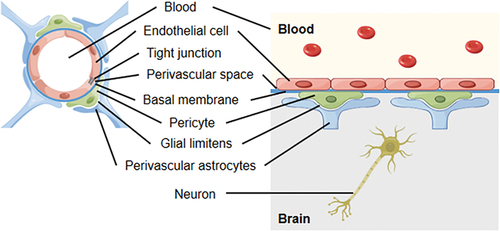
The level of difficulty by which solutes in the blood move from brain capillaries into brain tissue varies: pass quickly, some pass slowly, and some cannot pass at all. The highly selective permeability of the brain suggests that a structure restricts the passage of solutes; this structure is the BBB (Verkhratsky and Pivoriunas Citation2023). The BBB separates blood plasma from brain cells and can prevent certain substances (mostly harmful substances or drugs) in the bloodstream from entering the brain (Zhang et al. Citation2022). This structure exhibits selective permeability, preventing or minimising brain tissue damage caused by harmful substances in the circulatory system blood, ensuring the dynamic balance of the environment within the brain, and helping maintain the physiological homoeostasis of the CNS (Zhang et al. Citation2022).
The BBB is composed of a continuous layer of brain endothelial cells connected with tight junctions (TJs) between cells, as well as a complete basement membrane with pericytes, glial limiting membrane surrounded by astrocyte endfeet (Zhang et al. Citation2022). Together, these components constitute the brain endothelium and the main structure of the BBB (). Compared with capillaries in other tissues and organs, brain capillaries differ in structure and function: (a) Brain capillaries lack pores (or have a few small pores) that non-brain capillaries have; (b) Endothelial cells overlap each other and are tightly connected through TJs (Abbott et al. Citation2006; Pivoriunas and Verkhratsky Citation2021), effectively preventing large molecules from crossing between endothelial cells; (c) Human brain microvascular endothelial cells (HBMECs) exhibit a low endocytosis rate (Daneman and Prat Citation2015; Pivoriunas and Verkhratsky Citation2021), and (d) Endothelial cells are surrounded by a continuous basement membrane, and approximately 85% of the surface of BMECs outside the basement membrane is surrounded by the end feet of perivascular astrocytes (Daneman and Prat Citation2015). At present, it is generally believed that the permeability of the BBB depends mainly on BMECs. However, some studies have shown that astrocytes secrete bioactive molecules and extracellular vesicles that can be taken up by endothelial cells to regulate the permeability of the BBB (Verkhratsky and Pivoriunas Citation2023), possibly controlling the integrity of the BBB by regulating the expression of TJ proteins (Delpino et al. Citation1995; Wey et al. Citation2008; Pivoriunas and Verkhratsky Citation2021; Woo and Martinez Citation2021). In conclusion, the function of astrocytes and their interactions with cryptococci need to be further explored. Other studies have shown that the loss of pericytes may increase the permeability of the BBB, and pericytes exhibit different recovery rates after the BBB is damaged (Geranmayeh et al. Citation2019). However, there are only a few related studies, with most studies having been focused on HBMECs.
CNS diseases can cause marked changes in the structure and function of the BBB, such as disruption of TJs, enlargement of the intercellular space, and a large increase in the permeability of the barrier, allowing large molecules such as plasma albumin to pass through the barrier (Zhang et al. Citation2022). Damage to the BBB leads to the entry of pathogens into the brain, causing CNS diseases (Shi et al. Citation2012). In addition, pathogen infections abrogate the integrity and permeability of the BBB and lead to increased entry rates of viruses, immune cells, or cytokines into the brain, leading to increased inflammatory responses in the CNS and further damage to the brain (Liu et al. Citation2019). A study revealed that cryptococcal meningitis in patients with AIDS can lead to reduced activation of astrocytes (Tripathi et al. Citation2014), which can further damage the BBB.
3.2. The in vitro model of BBB
In various studies on the mechanism by which microorganisms cross the BBB, in vitro models are often used (Kim Citation2006; Sorrell et al. Citation2016). The establishment of an in vitro model takes advantage of the speciality of BMECs, that is, TJ formation, as well as the ability to form a polar monolayer in culture (Weksler et al. Citation2005). The simplest model is a single-cell culture model in which a culture medium is added to the upper chamber and a lower chamber of a permeable insert, with BMECs inoculated onto the membrane that separates the chambers (Kim et al. Citation2021). In this model, the upper chamber represents blood and the lower chamber represents the brain, therefore, the system simulates the process of microorganisms penetrating BMECs into the brain. Coculture models, including double-layer coculture models (BMECs and astrocytes) and triple-layer coculture models (BMECs, astrocytes, and pericytes), can also be established. Notably, in vitro models represent static conditions, not dynamic conditions simulating blood flow in capillaries (Kim et al. Citation2021).
4. The mechanisms by which Cryptococcus crosses the BBB
In most cases, cryptococcal meningitis is believed to result from haematopoietic dissemination, with cryptococci colonising the lungs and then travelling through the bloodstream to the BBB, which they penetrate to enter the brain directly (via transcellular traversal or a paracellular pathway) or indirectly (via a Trojan horse mechanism) by transversing BMECs, causing CNS diseases. A study on Cn-infected mouse models showed that paravascular microabscesses formed after cryptococci entered the brain and moved through cortical microvessels and blood vessels (Perfect Citation2004), with the development of meningitis after secondarily spreading to the meninges. Regarding the mechanisms underlying cryptococci crossing the BBB, debates have focused on whether cryptococci are transported through the blood and across the BBB as free cells or encased in phagocytes (the so-called Trojan horse mechanism). In cellular and animal models of cryptococcosis, it has been shown that free cryptococci can either engage in specific ligand-receptor interactions and then cross the BBB via transcytosis (Jong et al. Citation2007a, Citation2008a, Citation2008b, Citation2012) or cross the BBB after mechanical blockage mediated by cryptococci in capillary branches followed by mechanical or biochemical disruption of the capillary walls, which consist of endothelial cells and their TJs (Olszewski et al. Citation2004; Shi et al. Citation2010). An increasing number of recent studies have shown that cryptococci are transported through the bloodstream within phagocytes and then cross the BBB via the Trojan horse mechanism.
Sorrell et al. (Citation2016) suggested that Cn typically causes meningitis in HIV-infected hosts. In addition, of 86 Cg-infected patients in Australia between 2000 and 2007, most patients (85%) presented with meningoencephalitis (Chen et al. Citation2012; Miyazato Citation2016). However, no studies have revealed whether Cn and Cg cause meningitis differently or whether Cn or Cg is more likely to cause meningitis via a preferential route through the BBB. Many studies have shown that HIV promotes cryptococci crossing the BBB, leading to cryptococcal meningitis in patients with AIDS. Moreover, the HIV gp41 protein has been found to stimulate the binding of Cn to BMECs, thereby increasing the risk of brain infection (Jong et al. Citation2007b; Huang et al. Citation2011b). In addition, corresponding studies have shown that HIV infection increases the ability of peripheral monocytes to enter the brain (Wang et al. Citation2008). Relevant mechanistic studies have revealed that the high-affinity FC-γ receptor 3A on phagocytes promotes the phagocytosis of cryptococci, thereby causing cryptococcal meningoencephalitis (Rohatgi et al. Citation2013). MYOC, a cytoskeleton-related gene identified as an important factor in the Trojan horse mechanism, is one of the central regulators of the miRNA-mRNA regulatory network and is significantly downregulated during Cn infection. In addition, MYOC is overexpressed in THP-1-derived macrophages in HIV-infected patients infected with cryptococci, which increases the phagocytosis activity and migration ability of macrophages, thereby inducing more cryptococci to be taken up macrophages and enhance the Trojan horse mechanism mediated by macrophages (Li et al. Citation2022).
4.1. Transcellular traversal
Transcellular traversal refers to the penetration of barrier cells by cryptococci through cellular endocytosis (Charlier et al. Citation2005; Chang et al. Citation2011). By this mechanism, cryptococci must adhere to and be internalised from the surface of the brain microvascular lumen (blood side) by BMECs, after which cryptococci migrate through the endothelial cytoplasm to the parietal lumen side of the BMECs (the brain side) to cross BBB. Chang et al. (Citation2004) directly observed the whole process of cryptococci passing through BMECs in vitro via electron microscopy. After cryptococci adhered to the surface of endothelial cells, the formation of microvillus-like membrane protrusions was observed on the surface of BMECs, and then, the yeast cells were gradually enveloped. However, this process was only directly observed in vitro, not in vivo. The histopathology results obtained by Chang et al. (Citation2004) showed that cryptococci initially accumulated near brain capillaries and then appeared in the brain parenchyma far away from these capillaries, and ten days later, cryptococci were observed near the meninges. The adherence of cryptococci to HBMECs is the first step in pathogen crossing the BBB via transcellular traversal. Cryptococci promote their transcellular traversal across the BBB via different mechanisms described in detail below.
4.1.1. Inositol
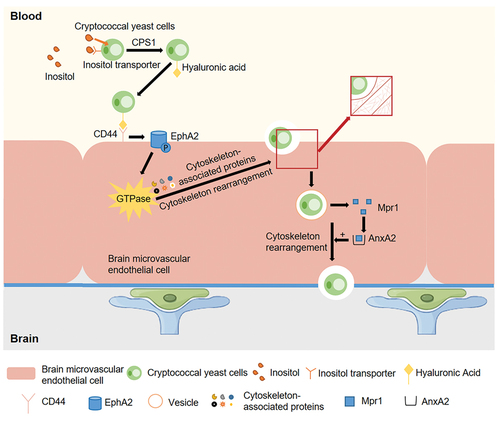
Inositol is one of the most abundant metabolites in the human brain. A high concentration of inositol has been detected in astrocytes directly associated with the BBB, and inositol can be released from cells rapidly under hypertonic conditions (Isaacks et al. Citation1994; Fisher et al. Citation2002). It has been suggested that the high abundance of inositol in the host brain is a factor contributing to the high incidence of cryptococcal meningitis (Wang et al. Citation2011a). Liu et al. (Citation2013) found that cryptococci leverage a complex system to obtain inositol from the environment and that they use host inositol, which binds to their inositol transporter, thereby promoting their adhesion to HBMECs (). It has also been reported that inositol is absorbed from the inositol transporter on the cryptococcal cell membrane, followed by high CPS1 gene expression, which stimulates the expression of hyaluronic acid on the cryptococcal cell membrane (). Hyaluronic acid binds CD44 receptors on BMECs of the CNS, further increasing the adherence of cryptococci to HBMECs (Liu et al. Citation2013; Miyazato Citation2016).
Figure 3. Model of transcellular traversal by which Cryptococcus crosses the blood-brain barrier (BBB). Cryptococci use host inositol which binds to their inositol transporter. Inositol is absorbed from the inositol transport tube on the cryptococcal cell membrane, followed by activating genes such as CPS1, which induces the expression of hyaluronic acid on the cryptococcal cell membrane. Hyaluronic acid binds to the CD44 receptor on the surface of HBMECs. Cryptococci induce EPH-EphrinA1 (EphA2) phosphorylation through transactivation of CD44, which promotes GTPase-dependent signalling. It induces the up-regulation of cytoskeleton-related proteins, reorganises the actin cytoskeleton, and internalises cryptococci via endocytosis. Cryptococci secrete metalloproteinase Mpr1, which binds to the Mpr1 target protein AnnexinA2 (AnxA2) inside the HBMECs. This also induces the up-regulation of cytoskeleton-related proteins, which triggers the reorganisation of actin cytoskeleton, thus cryptococci transcytosis and exit through HBMECs to cross BBB (By Figdraw).
4.1.2. CD44 receptor
Hyaluronic acid binds to CD44 receptors (Vu et al. Citation2013) (). CD44, a cluster of differentiation glycoprotein, is located in lipid rafts/vesicles on the surface of BMECs (Huang et al. Citation2011a; Long et al. Citation2012). It has been shown that CD44 knockout mice infected with Cn survived longer than infected wild-type mice and presented with a lower brain fungal burden (Jong et al. Citation2012), suggesting that CD44 may play a critical role as the host receptor during cryptococci crossing the BBB. The expression of hyaluronic acid on the surface of the cryptococcal capsule is closely related to the expression of the CPS1 gene, and when the hyaluronic acid on the capsule is abrogated, the ability of cryptococci to adhere to HBMECs is significantly reduced (Jong et al. Citation2007a). After successful adhesion of cryptococci to the surface of BMECs, protein kinase Cα/dual-specificity tyrosine phosphorylation-regulated kinase 3-mediated downstream signalling pathways [the CPS1-CD44-PKCα/DYRK3-actin filament signalling pathway (Jong et al. Citation2008a)] and the CD44-EPH-EphrinA1 (EphA2) tyrosine kinase receptor signalling pathway (Aaron et al. Citation2018) are activated, triggering actin cytoskeleton reorganisation and phagocytosis (Jong et al. Citation2008b, Citation2012) ().
4.1.3. Metalloprotease Mpr1
A secreted metalloprotease Mpr1 was recently discovered in the extracellular proteome of Cn (CnMpr1), and some studies have indicated that it is required for fungal infection of the CNS (Vu et al. Citation2014; Pombejra et al. Citation2017; Aaron et al. Citation2020). Vu et al. (Citation2014) found that Mpr1 facilitates the passage of Cn through BMECs and into the CNS by promoting the attachment of cryptococci to the endothelial cell surface, suggesting a key role for M36 protease in fungal pathogenesis, as it plays a key role in transcellular traversal. In addition, Pombejra et al. (Citation2017) identified 62 protein targets of Mpr1 on the surface of the BBB, and among these proteins, transcytosis of cells lacking AnnexinA2 (AnxA2) and exit through HBMECs are prevented (). Moreover, they found that Mpr1 promoted cytoskeletal remodelling of HBMECs and then facilitated BBB permeation mediated by the AnxA2-MPR1 interaction.
4.1.4. Molecules related to the cytoskeleton
Host actin cytoskeleton rearrangement was needed for Cryptococcus binding, invading, and migrating into HBMECs. Vu et al. (Citation2013) found upregulated expression of cytoskeleton-associated proteins, such as myosin, transgelin, annexin A2, and S2A100 (proteins involved in the recruitment of factors to the cytoskeleton), which may have been a result of the rearrangement of the cytoskeleton in BMECs after the attachment and internalisation of Cn. These cytoskeleton-associated proteins have also been identified in other studies (Chen et al. Citation2003, Citation2011; Wang et al. Citation2011b). Kim et al. (Citation2012) found that the three major Rho-GTPases, namely RhoA, Rac1, and Cdc42, were closely associated with the rearrangement of the actin cytoskeleton and that cryptococci induced the phosphorylation of focal adhesion kinase (FAK), ezrin, and protein kinase C alpha (PKCα), all of which are involved in the rearrangement of the host actin cytoskeleton. Their study showed that downregulation of FAK, ezrin, or PKCα significantly reduced the ability of cryptococci to cross a HBMEC monolayer, meaning that Cn-activated Rho-GTPases, FAK, ezrin, and PKCα to facilitate their subsequent crossing of the HBMEC monolayer, which is a critical step in the development of cryptococcal brain infection and meningitis (Kim et al. Citation2012). In addition, Li et al. (Citation2021) found that Pdlim2 was associated with cytoskeletal factors in the cytoplasm, while myosin phosphatase Rho-interacting protein (Mprip) targeted myosin phosphatase to the actin cytoskeleton. Pdlim2 and Mprip were shown to cooperatively regulate intracellular traversal and exocytosis of Cryptococcus in HBMECs.
4.1.5. Others
Meredith et al. (Citation2014) found that, in association with increased mitochondrial metabolism induced by glucose, the binding of advanced glycation end products (AGEs) to receptors for advanced glycation end products (RAGEs) led to increased BMEC permeability. This finding may explain the greater susceptibility of diabetic patients to cryptococcal meningitis. In addition, some studies have shown that inhibition of TLR-4/NF-κB/MMP-9 signalling pathways attenuates neuroinflammation reactions and reduces damage to the BBB (Nighot et al. Citation2019; Fu et al. Citation2021; Geng et al. Citation2022; Liu et al. Citation2022), but it is unclear whether Cryptococcus infection causes alterations in these signalling pathways. Given these findings, transcellular traversal causing Cryptococcus crossing the BBB is a hot topic, but there are still more mechanisms to be explored.
4.2. Paracellular pathway
The paracellular pathway refers to cryptococci crossing the BBB into the brain via spaces between damaged or weakened endothelial cell TJs (Kim Citation2008; Stie et al. Citation2009; Zhang et al. Citation2022). Notably, some studies have reported the temporary relaxation of TJs caused by osmotic or bioactive agents (Barar et al. Citation2016), such as bradykinin, cereport, and histamine. However, it is not clear whether Cryptococcus infection activates these alterations and thus promotes pathogen crossing BBB via the paracellular pathway. There are several studies on Cryptococcus crossing the BBB via the paracellular pathway.
4.2.1. The urokinase-plasminogen-plasmin pathway
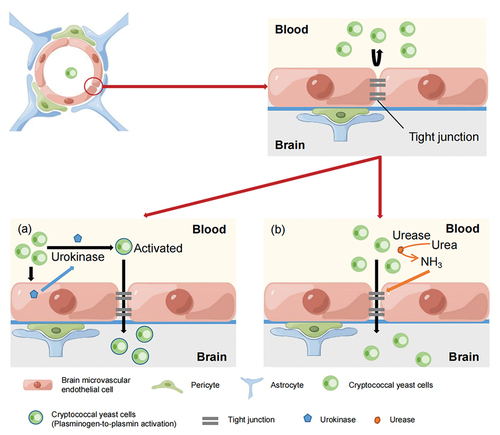
Urokinase is a plasminogen activator that activates the conversion of plasminogen to plasmin in the body. Plasmin degrades the fibrin-enriched extracellular matrix and basement membranes and activates other zymogen-protease systems that affect vascular permeability, thereby enhancing pathogen invasiveness. Stie et al. (Citation2009) found that cryptococcal cell wall surface receptors bound to host plasminogen, and in the presence of tissue-derived plasminogen activator (tPA), cryptococci that bind plasminogen induced its conversion into plasmin, which binds and breaks down the extracellular matrix and cell membrane, thereby enhancing the ability of cryptococci to invade the extracellular matrix. Moreover, coculturing plasminogen-bound Cryptococcus with BMECs promoted the conversion of plasminogen to plasmin without the addition of tPA and enhanced the ability of cryptococci to invade the extracellular matrix (Stie and Fox Citation2012), suggesting that HBMECs may have the ability to activate plasminogen. This group also found that HBMECs in coculture with live Cryptococcus were induced to secrete urokinase and that urokinase activated plasminogen on the cryptococcal surface into plasmin, enhancing the ability of cryptococci to cross the BBB, while siRNA-mediated silencing of urokinase gene expression or the use of specific inhibitors of urokinase activity eliminated plasminogen-to-plasmin conversion and reduced the capacity of cryptococci to cross the BBB (Stie et al. Citation2012). These findings suggest that Cryptococcus can use the host urokinase-plasminogen-plasmin pathway to promote its traversal of the BBB ().
Figure 4. Model of the paracellular pathway by which Cryptococcus crosses the blood-brain barrier (BBB). (a) HBMEC was induced to secrete urokinase by Cryptococcus stimulation. Urokinase activates the conversion of plasminogen on the surface of cryptococci to plasmin, which degrades the fibrin-enriched extracellular matrix and basement membranes. This facilitates Cryptococcus to cross the BBB. (b) Cryptococcus secretes urease, which converts urea into ammonia. The local production of ammonia may damage endothelial cells, impair tight junctions, and widen endothelial cell gaps, thereby causing the migration of cryptococci into the brain (By Figdraw).
4.2.2. Urease
Olszewski et al. (Citation2004) utilised C. neoformans H99, the urease knockout strain H99 (ure1), and urease recovery strain ure1+URE1-1 to explore the role of cryptococcal urease in lung-to-CNS cryptococci transmission and invasion. They speculated that urease potentiates yeast adhesion to BMECs during haematogenous transmission, thereby facilitating blood-to-brain invasion. In addition, Charlier et al. (Citation2005) employed horseradish peroxidase (HRP) to determine whether the entry of cryptococcal yeast cells into the brain parenchyma was associated with functional lesions in the BBB, and they found that damage causing lesions in the BBB and BMECs were evident as early as 6 hours in mice inoculated with Cn and observed leakage of HRP as well as alterations in the basal lamina and endothelium cells. They thus speculated that functional and morphological changes in the BBB appear shortly after cryptococcal fungemia and may lead to the disruption of TJs between HBMECs, causing yeast cells to invade the brain through the intercellular spaces formed. Shi et al. (Citation2010) injected fluorescently labelled urease-mutant strain C. neoformans intravenously into mice and performed cerebrovascular imaging of the mice. The results showed that the ability of the Cn urease mutant strain to enter the brain was significantly reduced. This result suggested that urease may cause biochemical disruptions to brain endothelial connections (Vu et al. Citation2013). Another study found that accessory proteins Ure4, Ure6, and Ure7 were all required for the function of urease, while mutants of the core urease protein Ure1, accessory protein Ure7, and transporter protein Nic1 all attenuated the invasion of pathogen into the CNS, suggesting that urease activity may directly affect the integrity of TJs between the endothelial cells of the BBB (Morrow and Fraser Citation2013). Urease, which can convert urea to ammonia, may act be one of the enzymes that dissolves tight junction proteins. Specifically, the local production of ammonia may damage endothelial cells, impair tight junctions, and widen endothelial cell gaps, thereby increasing the permeability of HBMECs and leading to the migration of cryptococci (Yang et al. Citation2017) ().
Notably, these experiments provide only indirect evidence of the paracellular pathway, and there is no direct evidence showing that cryptococci enter the brain through this pathway. As a result, finding direct evidence or even observing cryptococci invading the brain via the paracellular pathway via image-assisted technology is a future research suggestion.
4.3. Trojan horse mechanism
The Trojan horse mechanism refers to pathogens, after being internalised by phagocytes, that are not digested but survive within the phagocytes (Alanio et al. Citation2015) and enter the brain as the phagocytes cross the BBB (Charlier et al. Citation2009; Vu et al. Citation2013). The Trojan horse mechanism has been a hot research topic in recent years.
The Trojan horse mechanism has been shown to exist in bacterial pathogen infections, such as beta-haemolytic Streptococcus (Nizet et al. Citation1997) and Listeria monocytogenes infection (Drevets et al. Citation2001, Citation2004; Drevets and Bronze Citation2008; Disson and Lecuit Citation2012), and viral pathogen infection, such as human immunodeficiency virus infection (Fischer-Smith and Rappaport Citation2005). In addition, different research teams have provided strong evidence for the migration of cryptococci via the Trojan horse mechanism. Charlier et al. (Citation2005, Citation2009). found that depletion of circulating monocytes induced by chlorophosphate reduced the severity of cryptococcal meningitis and the brain fungal burden. This means that a decrease in the phagocytosis rate leads to a decrease in brain fungal burden. In mice injected with monocytes infected with Cn in vitro showed increased brain fungal burden. In this regard, Santangelo et al. (Citation2004) performed a similar experiment in which lung macrophages and peripheral blood mononuclear cells from mice infected with Cn via inhalation were injected into healthy mice, which developed cerebral cryptococcosis. Sorrell et al. (Citation2016) performed experiments with FITC-labelled cryptococci, and after phagocytes engulfed the FITC-labelled cryptococci, the unengulfed cryptococci were stained with Uvitex to distinguish them from the engulfed cryptococci. Then, fluorescence microscopy was used to observe the process of phagocytosis and BBB crossing in vitro. The result suggested that the cryptococci engulfed by phagocytes crossed the BBB via phagocyte migration (Sorrell et al. Citation2016) (). In addition, they found that compared with Cg, more Cn crossed the BBB via the Trojan horse mechanism (Sorrell et al. Citation2016), which may support the idea that Cn is more likely to cause neurological disorders. However, this experiment illustrated only the difference between the two fungal isolates that cross the BBB via the Trojan horse mechanism; the exact reason for this difference is still unknown. Moreover, this study illustrated only the difference between Cn and Cg penetration rates of the BBB through the Trojan horse mechanism, the exact reason for which is still unknown, and the differences in the infectivity through the other two pathways remain to be verified.
Figure 5. Model of Trojan horse mechanism by which Cryptococcus crosses the blood-brain barrier (BBB). The phagocytosis of Cn inhibited the chemotaxis of macrophages stimulated by CX3CL1 and CSF-1. Phagocytes infected with Cryptococcus adhere to HBMEC and utilise their nuclear lobes to create gaps between (a) or within (b) endothelial cells. Nuclear lobes insert into the HBMEC gap, and then phagocytes enter the brain. Then cryptococci in phagocytes are carried to the side of the brain by this behaviour of phagocytes, and cryptococci continue to be wrapped in phagocytes or be released.
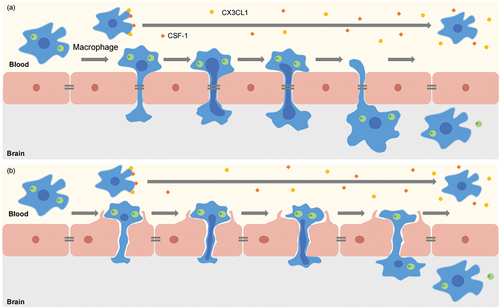
In addition, the interpretation of the Trojan horse mechanism is difficult and complex because cryptococci can grow both inside and outside phagocytes and can move to both sides of the phagocyte membrane while remaining viable in vivo. In other words, cryptococci may be transported through the bloodstream within phagocytes but are expelled after phagocytes reach the BBB, or after they are expelled before phagocytes attach the BMECs. That is, free cryptococci cross the BBB. The first possibility seems to have been verified by Santiago-Tirado et al. (Citation2017), who directly observed phagocytes containing cryptococci crossing the BBB via live-cell microscopy, providing evidence of the Trojan horse mechanism. In addition, they observed that phagocytes moved from the blood to the brain through a “donut hole” formed in endothelial cells without entering the cytoplasm and moving between HBMECs (). However, this finding has not been verified by others. Whether phagocytes pass the BBB through a “donut hole” formed in the endothelium, through intercellular spaces, or both remains to be explored.
A recent study found that the phagocytosis of Cn inhibited the chemotaxis of macrophages stimulated by CX3CL1 and CSF-1 (Luo et al. Citation2009), and Zhang et al. (Citation2015) suggested that this effect may slow the crawling of Cn-containing macrophages along the cerebral vasculature, thereby facilitating the transport of Cn (). To induce an appropriate immune response to infection, macrophage metabolism is increasingly switched to aerobic glycolysis with the simultaneous decrease in the oxidative phosphorylation rate, so that the end product of glycolysis, lactic acid, accumulates in the extracellular environment; this process is similar to the Warburg effect (Cruz-Gregorio et al. Citation2023), which refers to the fact that pyruvate produced by massive glucose uptake by tumour cells does not undergo oxidative phosphorylation, but is lysed in the cytoplasm to form a large amount of lactate in the normoxic environment. Moreover, a study revealed that lactate significantly increased bacterial clearance from human macrophages infected with Mycobacterium tuberculosis (Maoldomhnaigh et al. Citation2021). The accumulation of lactic acid helps macrophages clear cryptococci and thus reduce the brain fungal burden is a possibility worthy of deeper investigation. In studies on the mechanisms by which leukocytes cross the endothelial barrier, it has been shown that the nuclear lobes in leukocytes are the sites of gap and pore generation between and inside endothelial cells, enabling reversible bending of endothelial actin stress fibres and leukocyte movement through the endothelial barrier via rapid turnover of endothelial actin filaments, not endothelial cell contraction (Barzilai et al. Citation2017) (). Whether a similar mechanism enables phagocytes to cross the cerebral vasculature and BBB should be further investigated.
The studies on the Trojan horse mechanism have produced mostly indirect evidence, and the direct evidence that has been presented to date is not sufficient. Moreover, the experiments related to the Trojan horse mechanism have been mostly based on in vitro models because the results of experiments performed in vivo are more difficult to visualise. Recent research on this mechanism has largely fallen short of confirming that cryptococci cross the BBB via this mechanism, and therefore, the true mechanism underlying entry into the brain remains to be identified.
5. Conclusions
This paper focuses on the interaction between cryptococci and the BBB and describes the three mechanisms related to cryptococci crossing the BBB that have been reported thus far, but the human body is a complex system with a multilayered structure, and there are still more mechanisms to be explored.
In addition, among numerous case reports of cryptococcal infections, both Cn and Cg cause cryptococcal meningitis. Do these passages follow different routes of entry into the brain? Most studies have focused on Cn and not Cg, so we know little about the mechanisms by which Cg crosses the BBB and the reasons for the differences between Cn and Cg infection rates. We speculate that there are differences in the mechanisms by which Cn and Cg cross the BBB. It may be manifested in the speed of crossing the BBB, and it may be reflected in the difficulty crossing the BBB. In another, Cn is more likely to infect immunocompromised patients, the deficiency of immunocytes that interfere with the clearance of Cn, and mechanisms by which HIV promotes Cn to cross the BBB are also related. In subsequent studies, attention should be paid to whether both Cg and Cn penetrate the BBB by all three mechanisms or whether only one or two mechanisms are involved, differences in the amount or rate of Cg and Cn entering the brain via the same mechanism need to be resolved and whether Cg or Cn is more likely to cause meningitis needs to be determined. Furthermore, it is inconclusive which of the three mechanisms for crossing the BBB predominates, although transcellular traversal was the first to be identified. In addition, due to the high mortality rate of cryptococcal meningitis, the development of therapeutic drugs is urgently needed. There are currently a few drugs for the treatment of fungal infections caused by Cryptococcus, such as Amphotericin B, which is a commonly used antifungal drug (Mahendrarajan and Bari Citation2022). The elucidation of the mechanism by which cryptococcal cells cross the BBB will facilitate the development of relevant drugs that impede them into the brain. This type of prevention will greatly reduce the incidence and mortality of cryptococcal central nervous system diseases. In conclusion, blocking cryptococcal brain entry may facilitate the development of a new therapeutic intervention that will benefit patients suffering from cryptococcal meningitis.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Aaron PA, Jamklang M, Uhrig JP, Gelli A. 2018. The blood-brain barrier internalises Cryptococcus neoformans via the EphA2-tyrosine kinase receptor. Cell Microbiol. 20(3):e12811. doi: 10.1111/cmi.12811.
- Aaron PA, Vu K, Gelli A. 2020. An antivirulence approach for preventing Cryptococcus neoformans from crossing the blood-brain barrier via novel natural product inhibitors of a fungal metalloprotease. mBio. 11(4):e01249–20. doi: 10.1128/mBio.01249-20.
- Abbott NJ, Ronnback L, Hansson E. 2006. Astrocyte-endothelial interactions at the blood-brain barrier. Nat Rev Neurosci. 7(1):41–53. doi: 10.1038/nrn1824.
- Alanio A, Vernel-Pauillac F, Sturny-Leclere A, Dromer F. 2015. Cryptococcus neoformans host adaptation: Toward biological evidence of dormancy. mBio. 6(2):e02580–14. doi: 10.1128/mBio.02580-14.
- Amphornphruet A, Silpa-Archa S, Preble JM, Foster CS. 2018. Endogenous cryptococcal endophthalmitis in immunocompetent host: Case report and review of multimodal imaging findings and treatment. Ocul Immunol Inflamm. 26(4):518–522. doi: 10.1080/09273948.2017.1298820.
- Aribandi M, McCoy VA, Bazan C III. 2007. Imaging features of invasive and noninvasive fungal sinusitis: A review. Radiographics. 27(5):1283–1296. doi: 10.1148/rg.275065189.
- Barar J, Rafi MA, Pourseif MM, Omidi Y. 2016. Blood-brain barrier transport machineries and targeted therapy of brain diseases. BioImpacts. 6(4):225–248. doi: 10.15171/bi.2016.30.
- Barzilai S, Yadav SK, Morrell S, Roncato F, Klein E, Stoler-Barak L, Golani O, Feigelson SW, Zemel A, Nourshargh S, et al. 2017. Leukocytes breach endothelial barriers by insertion of nuclear lobes and disassembly of endothelial actin filaments. Cell Rep. 18(3):685–699. doi:10.1016/j.celrep.2016.12.076.
- Carr TF. 2016. Complications of sinusitis. Am J Rhinol Allergy. 30(4):241–245. doi: 10.2500/ajra.2016.30.4322.
- Chang YC, Stins MF, McCaffery MJ, Miller GF, Pare DR, Dam T, Paul-Satyasee M, Kim KS, Kwon-Chung KJ. 2004. Cryptococcal yeast cells invade the central nervous system via transcellular penetration of the blood-brain barrier. Infect Immun. 72(9):4985–4995. doi: 10.1128/IAI.72.9.4985-4995.2004.
- Chang YC, Wang Z, Flax LA, Xu D, Esko JD, Nizet V, Baron MJ. 2011. Glycosaminoglycan binding facilitates entry of a bacterial pathogen into central nervous systems. PLoS Pathol. 7(6):e1002082. doi: 10.1371/journal.ppat.1002082.
- Charlier C, Chretien F, Baudrimont M, Mordelet E, Lortholary O, Dromer F. 2005. Capsule structure changes associated with Cryptococcus neoformans crossing of the blood-brain barrier. Am J Pathol. 166(2):421–432. doi: 10.1016/S0002-9440(10)62265-1.
- Charlier C, Nielsen K, Daou S, Brigitte M, Chretien F, Dromer F. 2009. Evidence of a role for monocytes in dissemination and brain invasion by Cryptococcus neoformans. Infect Immun. 77(1):120–127. doi: 10.1128/IAI.01065-08.
- Chen Y, Chen J, Wen H, Gao P, Wang J, Zheng Z, Gu J. 2011. S100A10 downregulation inhibits the phagocytosis of Cryptococcus neoformans by murine brain microvascular endothelial cells. Microb Pathog. 51(3):96–100. doi: 10.1016/j.micpath.2011.05.003.
- Chen SC, Slavin MA, Heath CH, Playford EG, Byth K, Marriott D, Kidd SE, Bak N, Currie B, Hajkowicz K, et al. 2012. Clinical manifestations of Cryptococcus gattii infection: Determinants of neurological sequelae and death. Clin Infect Dis. 55(6):789–798. doi:10.1093/cid/cis529.
- Chen SHM, Stins MF, Huang SH, Chen YH, Kwon-Chung KJ, Chang Y, Kim KS, Suzuki K, Jong AY. 2003. Cryptococcus neoformans induces alterations in the cytoskeleton of human brain microvascular endothelial cells. J Med Microbiol. 52(11):961–970. doi: 10.1099/jmm.0.05230-0.
- Craig JR. 2019. Updates in management of acute invasive fungal rhinosinusitis. Curr Opin Otolaryngol Head Neck Surg. 27(1):29–36. doi: 10.1097/MOO.0000000000000507.
- Crump JR, Elner SG, Elner VM, Kauffman CA. 1992. Cryptococcal endophthalmitis: Case report and review. Clin Infect Dis. 14(5):1069–1073. doi: 10.1093/clinids/14.5.1069.
- Cruz-Gregorio A, Aranda-Rivera AK, Amador-Martinez I, Maycotte P. 2023. Mitochondrial transplantation strategies in multifaceted induction of cancer cell death. Life Sci. 332:122098. doi: 10.1016/j.lfs.2023.122098.
- Daneman R, Prat A. 2015. The blood-brain barrier. Cold Spring Harb Perspect Biol. 7(1):a020412. doi: 10.1101/cshperspect.a020412.
- Delpino MMS, Peterson DR, Hawkins RA. 1995. Neutral amino-acid-transport characterization of isolated luminal and abluminal membranes of the blood-brain-barrier. J Biol Chem. 270(25):14913–14918. doi: 10.1074/jbc.270.25.14913.
- De Vita C, Sollini G, Zoli M, Mazzatenta D, Pasquini E. 2021. When is a multidisciplinary approach required in management of intracranial complications of sinonasal inflammatory disorders? Acta Otorhinolaryngol Ital. 41(Suppl. 1):S67–S75. doi: 10.14639/0392-100X-suppl.1-41-2021-07.
- Disson O, Lecuit M. 2012. Targeting of the central nervous system by Listeria monocytogenes. Virulence. 3(2):213–221. doi: 10.4161/viru.19586.
- Drevets DA, Bronze MS. 2008. Listeria monocytogenes: Epidemiology, human disease, and mechanisms of brain invasion. FEMS Immun Med Microbiol. 53(2):151–165. doi: 10.1111/j.1574-695X.2008.00404.x.
- Drevets DA, Jelinek TA, Freitag NE, Tuomanen EI. 2001. Listeria monocytogenes-infected phagocytes can initiate central nervous system infection in mice. Infect Immun. 69(3):1344–1350. doi: 10.1128/IAI.69.3.1344-1350.2001.
- Drevets DA, Leenen PJM, Greenfield RA. 2004. Invasion of the central nervous system by intracellular bacteria. Clin Microbiol Rev. 17(2):323–347. doi: 10.1128/CMR.17.2.323-347.2004.
- Dubbioso R, Pappata S, Quarantelli M, D’Arco F, Manganelli F, Esposito M, Santoro L. 2013. Atypical clinical and radiological presentation of cryptococcal choroid plexitis in an immunocompetent woman. J Neurol Sci. 334(1–2):180–182. doi: 10.1016/j.jns.2013.08.010.
- Dunn JF, Isaacs AM. 2021. The impact of hypoxia on blood-brain, blood-CSF, and CSF-brain barriers. J Appl Physiol. 131(3):977–985. doi: 10.1152/japplphysiol.00108.2020.
- Fischer-Smith T, Rappaport J. 2005. Evolving paradigms in the pathogenesis of HIV-1-associated dementia. Expert Rev Mol Med. 7(27):1–26. doi: 10.1017/S1462399405010239.
- Fisher SK, Novak JE, Agranoff BW. 2002. Inositol and higher inositol phosphates in neural tissues: Homeostasis, metabolism and functional significance. J Neurochem. 82(4):736–754. doi: 10.1046/j.1471-4159.2002.01041.x.
- Francisco EC, de Jong AW, Hagen F. 2021. Cryptococcosis and Cryptococcus. Mycopathologia. 186(5):729–731. doi: 10.1007/s11046-021-00577-7.
- Frerichs AB, Huang M, Ortiz SC, Hull CM. 2021. Methods for manipulating Cryptococcus spores. J Fungi (Basel). 8(1):4. doi: 10.3390/jof8010004.
- Fu X, Yang C, Chen B, Zeng K, Chen S, Fu Y. 2021. Qi-Long-tian formula extract alleviates symptoms of acute high-altitude diseases via suppressing the inflammation responses in rat. Respir Res. 22(1):52. doi: 10.1186/s12931-021-01645-8.
- Geng Y, Yang J, Cheng X, Han Y, Yan F, Wang C, Jiang X, Meng X, Fan M, Zhao M, et al. 2022. A bioactive gypenoside (GP-14) alleviates neuroinflammation and blood brain barrier (BBB) disruption by inhibiting the NF-kappa B signaling pathway in a mouse high-altitude cerebral edema (HACE) model. Int Immunopharmacol. 107:108675. doi: 10.1016/j.intimp.2022.108675.
- Geranmayeh MH, Rahbarghazi R, Farhoudi M. 2019. Targeting pericytes for neurovascular regeneration. Cell Commun Signal. 17(1):26. doi: 10.1186/s12964-019-0340-8.
- Ghobrial GM, Pisculli ML, Evans JJ, Bilyk JR, Farrell CJ. 2016. Odontogenic sinusitis resulting in abscess formation within the optic chiasm and tract: Case report and review. J Neuroophthalmol. 36(4):393–398. doi: 10.1097/WNO.0000000000000430.
- Giles SS, Dagenais TR, Botts MR, Keller NP, Hull CM. 2009. Elucidating the pathogenesis of spores from the human fungal pathogen Cryptococcus neoformans. Infect Immun. 77(8):3491–3500. doi: 10.1128/IAI.00334-09.
- Goldman-Yassen AE, Meda K, Kadom N. 2021. Paranasal sinus development and implications for imaging. Pediatr Radiol. 51(7):1134–1148. doi: 10.1007/s00247-020-04859-y.
- Hanson LR, Frey WH II. 2007. Strategies for intranasal delivery of therapeutics for the prevention and treatment of neuroAIDS. J Neuroimmune Pharmacol. 2(1):81–86. doi: 10.1007/s11481-006-9039-x.
- Hanson LR, Frey WH. 2008. Intranasal delivery bypasses the blood-brain barrier to target therapeutic agents to the central nervous system and treat neurodegenerative disease. BMC Neurosci. 9(Suppl 3):S5. doi: 10.1186/1471-2202-9-S3-S5.
- Huang SH, Long M, Wu CH, Kwon-Chung KJ, Chang YC, Chi F, Lee S, Jong A. 2011a. Invasion of Cryptococcus neoformans into human brain microvascular endothelial cells is mediated through the lipid Rafts-Endocytic pathway via the dual specificity tyrosine phosphorylation-regulated kinase 3 (DYRK3). J Biol Chem. 286(40):34761–34769. doi: 10.1074/jbc.M111.219378.
- Huang SH, Wu CH, Jiang S, Bahner I, Lossinsky AS, Jong AY. 2011b. HIV-1 gp41 ectodomain enhances Cryptococcus neoformans binding to human brain microvascular endothelial cells via gp41 core-induced membrane activities. Biochem J. 438:457–466. doi: 10.1042/BJ20110218.
- Isaacks RE, Bender AS, Kim CY, Prieto NM, Norenberg MD. 1994. Osmotic regulation of myoinositol uptake in primary astrocyte cultures. Neurochem Res. 19(3):331–338. doi: 10.1007/BF00971582.
- Iyer KR, Revie NM, Fu C, Robbins N, Cowen LE. 2021. Treatment strategies for cryptococcal infection: challenges, advances and future outlook. Nat Rev Microbiol. 19(7):454–466. doi: 10.1038/s41579-021-00511-0.
- Jones NS, Walker JL, Bassi S, Jones T, Punt J. 2002. The intracranial complications of rhinosinusitis: Can they be prevented? Laryngoscope. 112(1):59–63. doi: 10.1097/00005537-200201000-00011.
- Jong A, Wu CH, Chen HM, Luo F, Kwon-Chung KJ, Chang YC, LaMunyon CW, Plaas A, Huang SH. 2007a. Identification and characterization of CPS1 as a hyaluronic acid synthase contributing to the pathogenesis of Cryptococcus neoformans infection. Eukaryot Cell. 6(8):1486–1496. doi: 10.1128/EC.00120-07.
- Jong A, Wu CH, Gonzales-Gomez I, Kwon-Chung KJ, Chang YC, Tseng HK, Cho WL, Huang SH. 2012. Hyaluronic acid receptor CD44 deficiency is associated with decreased Cryptococcus neoformans brain infection. J Biol Chem. 287(19):15298–15306. doi: 10.1074/jbc.M112.353375.
- Jong AY, Wu CH, Jiang S, Feng L, Chen HM, Huang SH. 2007b. HIV-1 gp41 ectodomain enhances Cryptococcus neoformans binding to HBMEC. Biochem Biophys Res Commun. 356(4):899–905. doi: 10.1016/j.bbrc.2007.03.100.
- Jong A, Wu CH, Prasadarao NV, Kwon-Chung KJ, Chang YC, Ouyang Y, Shackleford GM, Huang SH. 2008a. Invasion of Cryptococcus neoformans into human brain microvascular endothelial cells requires protein kinase C-alpha activation. Cell Microbiol. 10(9):1854–1865. doi: 10.1111/j.1462-5822.2008.01172.x.
- Jong A, Wu CH, Shackleford GM, Kwon-Chung KJ, Chang YC, Chen HM, Ouyang Y, Huang SH. 2008b. Involvement of human CD44 during Cryptococcus neoformans infection of brain microvascular endothelial cells. Cell Microbiol. 10(6):1313–1326. doi: 10.1111/j.1462-5822.2008.01128.x.
- Kim KS. 2006. Microbial translocation of the blood-brain barrier. International J Parasitol. 36(5):607–614. doi: 10.1016/j.ijpara.2006.01.013.
- Kim KS. 2008. Mechanisms of microbial traversal of the blood-brain barrier. Nat Rev Microbiol. 6(8):625–634. doi: 10.1038/nrmicro1952.
- Kim JC, Crary B, Chang YC, Kwon-Chung KJ, Kim KJ. 2012. Cryptococcus neoformans activates RhoGTPase proteins followed by protein kinase C, focal adhesion kinase, and ezrin to promote traversal across the blood-brain barrier. J Biol Chem. 287(43):36147–36157. doi: 10.1074/jbc.M112.389676.
- Kim J, Lee KT, Lee JS, Shin J, Cui B, Yang K, Choi YS, Choi N, Lee SH, Lee JH, et al. 2021. Fungal brain infection modelled in a human-neurovascular-unit-on-a-chip with a functional blood-brain barrier. Nat Biomed Eng. 5(8):830–846. doi:10.1038/s41551-021-00743-8.
- Leongson K, Cousineau-Cote V, Goupil M, Aumont F, Senechal S, Gaboury L, Jolicoeur P, Kronstad JW, de Repentigny L, Deepe GS. 2013. Altered immune response differentially enhances susceptibility to Cryptococcus neoformans and Cryptococcus gattii infection in mice expressing the HIV-1 transgene. Infect Immun. 81(4):1100–1113. doi: 10.1128/IAI.01339-12.
- Li Z, Bruno VM, Kim KS, Monack D. 2021. Central nervous system-infecting pathogens Escherichia coli and Cryptococcus neoformans exploit the host Pdlim2 for intracellular traversal and exocytosis in the blood-brain barrier. Infect Immun. 89(10):e0012821. doi: 10.1128/IAI.00128-21.
- Li H, Han X, Du W, Meng Y, Li Y, Sun T, Liang Q, Li C, Suo C, Gao X, et al. 2022. Comparative miRNA transcriptomics of macaques and mice reveals MYOC is an inhibitor for Cryptococcus neoformans invasion into the brain. Emerg Microbes Infect. 11(1):1572–1585. doi:10.1080/22221751.2022.2081619.
- Liu TB, Kim JC, Wang Y, Toffaletti DL, Eugenin E, Perfect JR, Kim KJ, Xue C. 2013. Brain inositol is a novel stimulator for promoting Cryptococcus penetration of the blood-brain barrier. PLoS Pathog. 9(4):e1003247. doi: 10.1371/journal.ppat.1003247.
- Liu P, Pan L, Cui L, Li T, Zhao S, Hu Y, Tao X, Deng H, Jiang J, Zhao B, et al. 2022. Cordycepin ameliorates acute hypobaric hypoxia induced blood-brain barrier disruption, and cognitive impairment partly by suppressing the TLR4/NF-kappaB/MMP-9 pathway in the adult rats. Eur J Pharmacol. 924:174952. doi: 10.1016/j.ejphar.2022.174952.
- Liu H, Qiu K, He Q, Lei Q, Lu W. 2019. Mechanisms of blood-brain barrier disruption in herpes simplex encephalitis. J Neuroimmune Pharmacol. 14(2):157–172. doi: 10.1007/s11481-018-9821-6.
- Long M, Huang SH, Wu CH, Shackleford GM, Jong A. 2012. Lipid raft/caveolae signaling is required for Cryptococcus neoformans invasion into human brain microvascular endothelial cells. J Biomed Sci. 19(1):19. doi: 10.1186/1423-0127-19-19.
- Luo Y, Isaac BM, Casadevall A, Cox D. 2009. Phagocytosis inhibits f-actin-enriched membrane protrusions stimulated by fractalkine (CX3CL1) and colony-stimulating factor 1. Infect Immun. 77(10):4487–4495. doi: 10.1128/IAI.00530-09.
- Luo Y, Zhu C, He B, Yan A, Wei H. 2023. Diagnostic and therapeutic strategies of acute invasive fungal rhinosinusitis. Asian J Surg. 46(1):58–65. doi: 10.1016/j.asjsur.2022.05.006.
- Mahendrarajan V, Bari VK. 2022. A critical role of farnesol in the modulation of amphotericin B and aureobasidin a antifungal drug susceptibility. Mycology. 13(4):305–317. doi: 10.1080/21501203.2022.2138599.
- Maoldomhnaigh CO, Cox DJ, Phelan JJ, Mitermite M, Murphy DM, Leisching G, Thong L, O’Leary SM, Gogan KM, McQuaid K, et al. 2021. Lactate alters metabolism in human macrophages and improves their ability to kill Mycobacterium tuberculosis. Front Immunol. 12:663695. doi: 10.3389/fimmu.2021.663695.
- Meredith ME, Qu ZC, May JM. 2014. Ascorbate reverses high glucose- and RAGE-induced leak of the endothelial permeability barrier. Biochem Biophys Res Commun. 445(1):30–35. doi: 10.1016/j.bbrc.2014.01.078.
- Miyazato A. 2016. Mechanism of Cryptococcus meningoencephalitis. Med Mycol J. 57(1):J27–32. doi: 10.3314/mmj.57.J27.
- Morrow CA, Fraser JA. 2013. Is the Nickel-dependent urease complex of Cryptococcus the pathogen’s achilles’ heel? mBio. 4(4):e00408–13. doi: 10.1128/mBio.00408-13.
- Nadrous HF, Ryu JH, Lewis JE, Sabri AN. 2004. Cryptococcal laryngitis: Case report and review of the literature. Ann Otol Rhinol Laryngol. 113(2):121–123. doi: 10.1177/000348940411300207.
- Nighot M, Rawat M, Al-Sadi R, Castillo EF, Nighot P, Ma TY. 2019. Lipopolysaccharide-induced increase in intestinal permeability is mediated by TAK-1 activation of IKK and MLCK/MYLK gene. Am J Pathol. 189(4):797–812. doi: 10.1016/j.ajpath.2018.12.016.
- Nizet V, Kim KS, Stins M, Jonas M, Chi EY, Nguyen D, Rubens CE. 1997. Invasion of brain microvascular endothelial cells by group B streptococci. Infect Immun. 65(12):5074–5081. doi: 10.1128/iai.65.12.5074-5081.1997.
- Olszewski MA, Noverr MC, Chen GH, Toews GB, Cox GM, Perfect JR, Huffnagle GB. 2004. Urease expression by Cryptococcus neoformans promotes microvascular sequestration, thereby enhancing central nervous system invasion. Am J Pathol. 164(5):1761–1771. doi: 10.1016/S0002-9440(10)63734-0.
- Perfect JR. 2004. Management of invasive mycoses in hematology patients: current approaches. Oncology (Williston Park). 18(13 Suppl 7):5–14.
- Pivoriunas A, Verkhratsky A. 2021. Astrocyte-endotheliocyte axis in the regulation of the blood-brain barrier. Neurochem Res. 46(10):2538–2550. doi: 10.1007/s11064-021-03338-6.
- Pombejra SN, Salemi M, Phinney BS, Gelli A. 2017. The metalloprotease, Mpr1, engages AnnexinA2 to promote the transcytosis of fungal cells across the blood-brain barrier. Front Cell Infect Microbiol. 7:296. doi: 10.3389/fcimb.2017.00296.
- Prendiville S, Bielamowicz SA, Hawrych A, Deeb ZE. 2016. Isolated cryptococcal sphenoid sinusitis with septicemia, meningitis, and subsequent skull base osteomyelitis in an immunocompetent patient. Otolaryngol Head Neck Surg. 123(3):277–279. doi: 10.1067/mhn.2000.104777.
- Ratemo SN, Denning DW. 2023. Burden of fungal infections in Kenya. Mycology. 14(2):142–154. doi: 10.1080/21501203.2023.2204112.
- Rohatgi S, Gohil S, Kuniholm MH, Schultz H, Dufaud C, Armour KL, Badri S, Mailliard RB, Pirofski LA. 2013. Fc gamma receptor 3A polymorphism and risk for HIV-associated cryptococcal disease. mBio. 4(5):e00573–13. doi: 10.1128/mBio.00573-13.
- Santangelo R, Zoellner H, Sorrell T, Wilson C, Donald C, Djordjevic J, Shounan Y, Wright L. 2004. Role of extracellular phospholipases and mononuclear phagocytes in dissemination of cryptococcosis in a murine model. Infect Immun. 72(4):2229–2239. doi: 10.1128/IAI.72.4.2229-2239.2004.
- Santiago-Tirado FH, Onken MD, Cooper JA, Klein RS, Doering TL. 2017. Trojan horse transit contributes to blood-brain barrier crossing of a eukaryotic pathogen. mBio. 8(1):e02183–16. doi: 10.1128/mBio.02183-16.
- Sekaran A, Patil N, Sabhapandit S, Sistla SK, Reddy DN. 2022. Rhino-orbito-cerebral mucormycosis: An epidemic in a pandemic. IJID Reg. 2:99–106. doi: 10.1016/j.ijregi.2021.12.009.
- Shi M, Calaruso P, Mody CH. 2012. Real-time in vivo imaging of fungal migration to the central nervous system. Cell Microbiol. 14(12):1819–1827. doi: 10.1111/cmi.12027.
- Shi M, Li SS, Zheng C, Jones GJ, Kim KS, Zhou H, Kubes P, Mody CH. 2010. Real-time imaging of trapping and urease-dependent transmigration of Cryptococcus neoformans in mouse brain. J Clin Invest. 120(5):1683–1693. doi: 10.1172/JCI41963.
- Sorrell TC, Juillard PG, Djordjevic JT, Kaufman-Francis K, Dietmann A, Milonig A, Combes V, Grau GER. 2016. Cryptococcal transmigration across a model brain blood-barrier: evidence of the Trojan horse mechanism and differences between Cryptococcus neoformans var. grubii strain H99 and Cryptococcus gattii strain R265. Microbes Infect. 18(1):57–67. doi: 10.1016/j.micinf.2015.08.017.
- Stie J, Bruni G, Fox D, Lin X. 2009. Surface-associated plasminogen binding of Cryptococcus neoformans promotes extracellular matrix invasion. PLoS One. 4(6):e5780. doi: 10.1371/journal.pone.0005780.
- Stie J, Fox D. 2012. Blood–brain barrier invasion by Cryptococcus neoformans is enhanced by functional interactions with plasmin. Microbiol. 158(1):240–258. doi: 10.1099/mic.0.051524-0.
- Stie J, Fox D, Jacobsen ID. 2012. Induction of brain microvascular endothelial cell urokinase expression by Cryptococcus neoformans facilitates blood-brain barrier invasion. PLoS One. 7(11):e49402. doi: 10.1371/journal.pone.0049402.
- Thompson GR III, Patterson TF. 2012. Fungal disease of the nose and paranasal sinuses. J Allergy Clin Immunol. 129(2):321–326. doi: 10.1016/j.jaci.2011.11.039.
- Tripathi S, Patro I, Mahadevan A, Patro N, Phillip M, Shankar SK. 2014. Glial alterations in tuberculous and cryptococcal meningitis and their relation to HIV co-infection - A study on human brains. J Infect Dev Ctries. 8(11):1421–1443. doi: 10.3855/jidc.3894.
- Velagapudi R, Hsueh YP, Geunes-Boyer S, Wright JR, Heitman J. 2009. Spores as infectious propagules of Cryptococcus neoformans. Infect Immun. 77(10):4345–4355. doi: 10.1128/IAI.00542-09.
- Verkhratsky A, Pivoriunas A. 2023. Astroglia support, regulate and reinforce brain barriers. Neurobiol Dis. 179:106054. doi: 10.1016/j.nbd.2023.106054.
- Vu K, Eigenheer RA, Phinney BS, Gelli A, Deepe GS. 2013. Cryptococcus neoformans promotes its transmigration into the central nervous system by inducing molecular and cellular changes in brain endothelial cells. Infect Immun. 81(9):3139–3147. doi: 10.1128/IAI.00554-13.
- Vu K, Tham R, Uhrig JP, Thompson GR III, Pombejra SN, Jamklang M, Bautos JM, Gelli A. 2014. Invasion of the central nervous system by Cryptococcus neoformans requires a secreted fungal metalloprotease. mBio. 5(3):e01101–14. doi: 10.1128/mBio.01101-14.
- Walsh NM, Botts MR, McDermott AJ, Ortiz SC, Wüthrich M, Klein B, Hull CM, Rivera A. 2019. Infectious particle identity determines dissemination and disease outcome for the inhaled human fungal pathogen Cryptococcus. PLoS Pathog. 15(6):e1007777. doi: 10.1371/journal.ppat.1007777.
- Wang Y, Liu T, Delmas G, Park S, Perlin D, Xue C. 2011a. Two major inositol transporters and their role in cryptococcal virulence. Eukaryot Cell. 10(5):618–628. doi: 10.1128/EC.00327-10.
- Wang H, Sun J, Goldstein H. 2008. Human immunodeficiency virus type 1 infection increases the in vivo capacity of peripheral monocytes to cross the blood-brain barrier into the brain and the in vivo sensitivity of the blood-brain barrier to disruption by lipopolysaccharide. J Virol. 82(15):7591–7600. doi: 10.1128/JVI.00768-08.
- Wang XJ, Zhu YJ, Cui JG, Huang X, Gu J, Xu H, Wen H. 2011b. Proteomic analysis of human umbilical vein endothelial cells incubated with Cryptococcus neoformans var. neoformans. Mycoses. 54(5):E336–E343. doi: 10.1111/j.1439-0507.2010.01920.x.
- Weksler BB, Subileau EA, Perriere N, Charneau P, Holloway K, Leveque M, Tricoire-Leignel H, Nicotra A, Bourdoulous S, Turowski P, et al. 2005. Blood-brain barrier-specific properties of a human adult brain endothelial cell line. FASEB J. 19(11):1872–1874. doi:10.1096/fj.04-3458fje.
- Wey SP, Wu HY, Chang FC, Jan TR. 2008. Methamphetamine and diazepam suppress antigen-specific cytokine expression and antibody production in ovalbumin-sensitized BALB/c mice. Toxicol Lett. 181(3):157–162. doi: 10.1016/j.toxlet.2008.07.015.
- Woo YH, Martinez LR. 2021. Cryptococcus neoformans-astrocyte interactions: Effect on fungal blood brain barrier disruption, brain invasion, and meningitis progression. Crit Rev Microbiol. 47(2):206–223. doi: 10.1080/1040841X.2020.1869178.
- Yang CL, Wang J, Zou LL. 2017. Innate immune evasion strategies against cryptococcal meningitis caused by Cryptococcus neoformans. Exp Ther Med. 14(6):5243–5250. doi: 10.3892/etm.2017.5220.
- Younis RT, Lazar RH, Anand VK. 2002. Intracranial complications of sinusitis: A 15-year review of 39 cases. Ear Nose Throat J. 81(9):636–638, 640–632, 644. doi: 10.1177/014556130208100911.
- Yu CH, Sephton-Clark P, Tenor JL, Toffaletti DL, Giamberardino C, Haverkamp M, Cuomo CA, Perfect JR. 2021. Gene expression of diverse Cryptococcus isolates during infection of the human central nervous system. mBio. 12(6):e0231321. doi: 10.1128/mBio.02313-21.
- Zaragoza O. 2019. Basic principles of the virulence of Cryptococcus. Virulence. 10(1):490–501. doi: 10.1080/21505594.2019.1614383.
- Zhang S, Gan L, Cao F, Wang H, Gong P, Ma C, Ren L, Lin Y, Lin X. 2022. The barrier and interface mechanisms of the brain barrier, and brain drug delivery. Brain Res Bull. 190:69–83. doi: 10.1016/j.brainresbull.2022.09.017.
- Zhang M, Sun D, Shi M. 2015. Dancing cheek to cheek: Cryptococcus neoformans and phagocytes. Springerplus. 4(1):410. doi: 10.1186/s40064-015-1192-3.