ABSTRACT
Ganoderma, a traditional medicine in Asian countries, has been used to prevent and treat various ailments for centuries. Ganoderma neo-japonicum (synonym Ganoderma bambusicola), also known as purple Lingzhi, is a species that is currently underutilised when compared to Ganoderma lucidum (Lingzhi). However, in recent decades, this mushroom has garnered significant attention due to its ethnomedicinal uses, especially in Southeast Asia regions like Malaysia. The taxonomy and nomenclature of this mushroom have been extensively studied. Numerous publications have reported that G. neo-japonicum displays a variety of medicinal properties, including antioxidation, anticancer, anti-hyperglycaemic, genoprotective, hepatoprotective, neuritogenic, and antidiabetic effects, both in vitro and in vivo. With the surge of research findings on this mushroom, this review aims to provide a systematic bibliometric analysis of G. neo-japonicum, published between 1991 to 2021. Additionally, the taxonomic description of this mushroom is discussed in detail. Our review reveals that G. neo-japonicum contains polysaccharides (α/β-D-glucans), triterpenoids, and sterols/ergosterol. However, the existing literature suggests that these active compounds have not yet been explored to their full potential as drug candidates. Moreover, most of the studies are preclinical and have several drawbacks. In conclusion, G. neo-japonicum possesses valuable pharmacological activities that merit further exploration.
1. Introduction
Malaysia remains a natural reservoir for macrofungi with myriad health benefits (Samsudin and Abdullah Citation2019). Nevertheless, many wild edible species have been neglected due to the gradual loss of ancestral knowledge within the indigenous communities. The traditional use of macrofungi (mushrooms) is often undocumented. Preserving this eroding knowledge thus becomes of utmost importance as it holds great potential for drug discovery.
Oriental medicine has long used the genus Ganoderma, which contains more than 400 species worldwide (Richter et al. Citation2015). Numerous pharmacological activities of Ganoderma, including anticancer, anti-hyperglycaemia, anti-inflammation, antioxidant, and antivirus, have been extensively evaluated (Wang et al. Citation2020). Despite its profound medicinal values, the traditional use of Ganoderma is relatively sparse in Malaysia, as indigenous communities commonly prefer other genera like Amauroderma, Lignosus, Microporus, and Xylaria (Lee et al. Citation2009; Foo et al. Citation2018). Malaysia hosts a variety of Ganoderma species (Lee et al. Citation2012). However, only G. applanatum (Pers.) Pat., G. neo-japonicum Imazeki, and G. lucidum (Curt.: Fr.) Karst are consumed among indigenous tribes in Malaysia (Ayu et al. Citation2019), implying that some related species with ethnomycological potential may have been overlooked.
In recent years, Ganoderma neo-japonicum Imazeki [Bull. Tokyo Sci. Mus., 1:37 (1939)] is drawing considerable attention from researchers (Tan et al. Citation2015). This endemic polypore is found in several Asian nations, including China, Japan, and Korea (Tan et al. Citation2015). It is a saprophytic species that feeds on dead coniferous trees (Hapuarachchi et al. Citation2019). In Malaysia, G. neo-japonicum (“cendawan senduk”) is well-recognised by ethnic tribes including “Bateq”, “Jahai”, “Jakun”, “Kensiu”, “Kintak”, “Lanoh”, “Semai”, “Temiar”, and “Temuan” (Tan et al. Citation2015). It is prepared as a tonic for different ailments, such as joint discomfort, cancer, fever, and asthma. It also serves as a topical preparation to aid in wound healing. Such therapeutic claims are encouraging enough to merit scientific validation. Unfortunately, although it is non-poisonous, consumption of G. neo-japonicum was linked to cases of reversible pancytopenia in Korea (Yoon et al. Citation2011).
The fruiting season of G. neo-japonicum occurs between May and October every year (Tan et al. Citation2015). The abundance of basidiocarps is highly affected by regional climate and habitat availability. Given its unique growth cycle, commercial foraging alone is far insufficient to support the surge in market demand. Hence, domestication has become crucial to ensure a sustainable harvest and thus mitigate supply shortages. Successful cultivation of G. neo-japonicum on bag logs was reported by Tan et al. (Citation2015). However, the cultivated strain possessed a different antioxidant power as compared to the wild strain. This finding aligns with the outcomes demonstrated by Lignosus rhinocerotis (Cooke) Ryvarden (Jamil et al. Citation2017), suggesting that an improved cultural process is warranted to retain the bioactivity of G. neo-japonicum.
There are scattered publications about G. neo-japonicum research. On this basis, through bibliometric analysis, the present review aims to uncover the historical exploration and identify the emerging research trend on G. neo-japonicum. This review also discusses the domestication and medicinal properties of G. neo-japonicum.
2. Bibliometric analysis
Bibliometric analysis is widely accepted as an essential tool for monitoring the state of a research domain (Chan et al. Citation2020; Moral-Muñoz et al. Citation2020; Tang et al. Citation2022). It involves a quantitative evaluation of published works in a specific discipline or subject area. In this study, the bibliometric analysis was performed by retrieving publications from several data sources such as Scopus, Web of Science, Dimension, and PubMed, in April 2022. The search string included terms [title-abstract-keywords] of “Ganoderma neo-japonicum” or “Ganoderma neojaponicum”; but was not limited to article type, country, date, and language. After removing duplicates, a total of 36 articles were found between 1991 and 2021 (); Scopus represented the most comprehensive database (n = 32), followed by Dimension (n = 28), Web of Science (n = 27), and PubMed (n = 20). These articles consisted of original papers (n = 34), reviews (n = 1), and conference abstracts (n = 1); published in English, Korean, and Russian languages ().
Figure 1. A venn diagram illustrating the number of articles (n) deposited in four reputed databases, namely Scopus, Web of Science, Dimension, and PubMed, with coverage of Ganoderma neo-japonicum study.
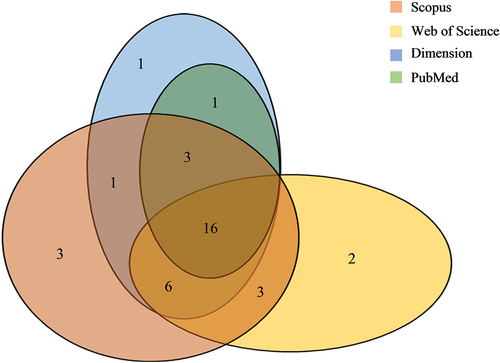
Table 1. List of journals for ganoderma neo-japonicum research output between 1991 and 2021.
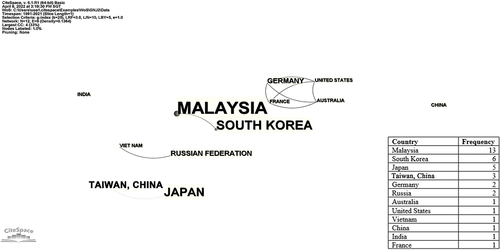
The publication counts (n) over a recent 31-year period can be preliminarily divided into two phases (): A lag phase (from 1991 to 2008, where n ≤ 1) and a log phase (from 2009 to 2021, where n ≥ 2). Despite the low productivity in the early years, the upward trend depicted in suggested that G. neo-japonicum has gained increasing attention since 2009. Malaysia emerged as the most prolific country in G. neo-japonicum research, contributing about 35% of total publication counts (). The research output of Western countries was relatively poorer compared to that of South Korea, Japan, and Taiwan, China. Based on the co-authorship mapping, the mutual writing relationship among countries remained very sparse (), underscoring a need for international collaborations to boost current research productivity. Notable crossover collaborations have been evident between Malaysia and South Korea, Vietnam, and Russia, as well as Germany, France, the United States, and Australia. Meanwhile, all the articles (n = 36) were dispersed across 29 different journals (), according to Bradford’s law of scattering (Tang et al. Citation2022). Core journals encompassed “International Journal of Medicinal Mushrooms” (n = 4), “Mycobiology” (n = 3), and “Phytochemistry” (n = 3). “Phytochemistry” with 216 citation counts (representing nearly 24% of the total) stood out as the most influential journal for G. neo-japonicum study.
Figure 2. Publication counts of Ganoderma neo-japonicum study reported from 1991 to 2021 (a 31-year time span). The line curve indicates annual cumulative frequency (right axis).
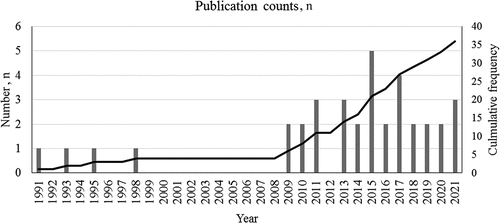
Figure 3. Contributions by country to Ganoderma neo-japonicum research in Scopus database. The country co-authorship network was visualised via citespace software (version 6.1.R1). The connection among nodes (countries) represented a mutual writing relationship, and the strength was indicated by line shade.
3. Taxonomy
The genus Ganoderma Karst. is widely distributed in both temperate and tropical areas (Richter et al. Citation2015). There are 419 epithets in Species Fungorum (http://www.speciesfungorum.org/Names/Names.asp; retrieval date: April 2022) and 511 records in Mycobank (http://www.mycobank.org/; retrieval date: April 2022).
Principally, Ganoderma comprises species with both “laccate” (subgen. Ganoderma) and “dull” [subgen. Elfvingia (Karst.) Imazeki] pileal surfaces. However, its species delimitation based on morphological traits can be challenging (Richter et al. Citation2015). Besides having overlapping appearances, Ganoderma shows high morphological variability due to geographic and climatic influences, causing its taxonomical status to be controversial. Moreover, the lack of standardised taxonomic criteria has resulted in a confusing nomenclature within the genus (Richter et al. Citation2015). Under such circumstances, DNA fingerprinting has emerged as an essential tool for discriminating between Ganoderma species. The useful gene markers include internal transcribed spacer region (ITS), large subunit ribosomal RNA (LSU), second-largest subunit of RNA-polymerase II (RPB2), translation elongation factor 1-α (TEF1α), partial ß-tubulin II (TUB2), and (Stielow et al. Citation2015).
Ganoderma neo-japonicum is well recognised by its laccate basidiocarp and long slender stipe (Lee Citation1981; Hattori and Ryvarden Citation1994). Based on the phylogenetic analysis (TUB2 sequences), it was shown to form a distinct cluster along with related species such as Ganoderma resinaceum Boud. and G. valesiacum Boud (Park et al. Citation2012a, Citation2012b). Likewise, concordant phylogenies inferred from ITS sequences have been generated by using the same strain in Korea (Park et al. Citation1999, Citation2012a, Citation2012b) or other representative strains originating in Japan (Wu et al. Citation2020), Laos (Hapuarachchi et al. Citation2019), Myanmar (Hapuarachchi et al. Citation2019), and Taiwan, China (Wang and Yao Citation2005). Taxonomic assessments on global Ganoderma have further demonstrated that G. neo-japonicum is segregated from G. boninense Pat., G. sichuanense Zhao & Zhang, and G. sinense Zhao et al. (Jargalmaa et al. Citation2017; Thawthong et al. Citation2017; Fryssouli et al. Citation2020; He et al. Citation2021).
In truth, G. neo-japonicum has been misidentified due to morphological similarities with Ganoderma bambusicola Wu et al., which shares a shiny reddish-black pileus and a long concolorous stipe (Wu et al. Citation2020). Both species have similar-sized basidiospores with smooth walls and narrow inter-wall pillars as described by Tsivileva et al. (Citation2016), nonetheless, G. neo-japonicum differs in having a heterogeneous pileal context. Upon revisiting the genomic dataset (), several native “G. neo-japonicum” strains from Laos (Hapuarachchi et al. Citation2019), Myanmar (Hapuarachchi et al. Citation2019), and Taiwan, China (Hsieh and Yeh Citation2004; Wang and Yao Citation2005) were found to be conspecific with G. bambusicola (Wu et al. Citation2020). These misidentified strains share a host preference behaviour analogous to the Malaysian G. neo-japonicum found on dead bamboo clumps (Schizostachyum brachycladium) (Tan et al. Citation2015). However, the phylogenetic relationship between G. neo-japonicum (Malaysia) and G. bambusicola (Taiwan, China) remains inconclusive, necessitating further validation.
Table 2. Taxon identities of Ganoderma neo-japonicum.
4. Domestication
The domestication of Ganoderma has been introduced to fulfill the expanding worldwide market demand (Hapuarachchi et al. Citation2018). Species like G. lucidum, G. resinaceum, G. sichuanense, and G. tropicum (Jungh.) Bres. are now commercially cultivated as reliable sources of medicinal materials.
Collecting wild G. neo-japonicum presents challenges due to its unique growth cycle and host preference. In this context, Jo et al. (Citation2010) were the first to attempt artificial cultivation of G. neo-japonicum using the bag method. The yield (dried fruiting bodies) obtained was 52–61 g per 2.4 kg substrate (90% oak sawdust and 10% rice bran). Subsequently, some patented refinements were made using a culture bottle containing larch sawdust, corn cob meal, and rice bran (3:1:1 ratio) under specific conditions: Illuminance, 500 lux; temperature, 23 °C; and humidity, 90% (Inose and Yamamoto Citation2013, Citation2015). The authors reported a yield of dried fruiting bodies at 35–60 g dried per 470 g substrate. A revisited study even found that the use of lignocellulosic substrates, particularly rubberwood sawdust, shortened mycelial colonisation (≈40 days) and primordial formation (≈60 days) in a 500 g substrate bag (Tan et al. Citation2015).
Apart from fruiting bodies, cultural factors for biomass production of G. neo-japonicum have been elucidated over the past decades. Typically, the pure colony manifests as a white mycelial mat with brownish-yellow pigmentation on solid agar media (Hsieh and Yeh Citation2004). Its hyphal anatomy is characterised by generative hyphae bearing (with or without) clam connections, nonbranched or moderately branched skeletal hyphae, and relatively thin binding hyphae (Tsivileva et al. Citation2016). Several physiological assays have found that G. neo-japonicum mycelia exhibit optimal growth on malt extract agar (MEA, enriched with 40 g/L glucose) at pH 6 and 24–28 °C (Hsieh and Yeh Citation2004). Growth could be further improved upon supplementation of brown sugar and spent yeast at a carbon/nitrogen (C/N) ratio of 1.74 (Ubaidillah et al. Citation2015), corresponding to the impact of media composition on G. neo-japonicum’s colony development (Tsivileva et al. Citation2016).
Submerged fermentation provides an alternative way of acquiring biomass and bioactive metabolites from Ganoderma (Zhou et al. Citation2012). To produce G. neo-japonicum ergothioneine (an antioxidant), optimal yields have been achieved in a formulated fungal growth medium (FGM, pH 4.5) containing methionine (Lee et al. Citation2009, Citation2010). Likewise, its phenolic content has been significantly enhanced by adding tryptophan and yeast to FGM under similar cultural conditions (Park and Lee Citation2010). Immunomodulatory polysaccharides have been produced in a stirred tank bioreactor with the following settings: aeration rate, 1.3 vessel volumes per minute (vvm); agitation speed, 160 r/min; and thermal point, 27 °C. Although various extracellular enzymatic activities, including amylase, avicelase, ß-glucosidase, cellulose, laccase, ligninase, pectinase, protease, and xylanase, were detected in G. neo-japonicum (Hsieh and Yeh Citation2004; Jo et al. Citation2011), the production of such enzymes remains inadequate due to elusive cultural conditions.
5. Medicinal properties
Despite a longstanding history in Oriental medicine, numerous Ganoderma species remain underexplored for their medicinal and pharmaceutical benefits. These unexplored species, including G. neo-japonicum, offer a promising avenue for further research and discovery in the field of medicine.
5.1. Functional molecules in G. neo-japonicum
Ganoderma neo-japonicum is one of the underappreciated species with significant ethnomedicinal potential. Its nutritional constituents include carbohydrates, dietary fibre, protein, and trace elements (Subramaniam et al. Citation2020). This polypore also produces bioactive substances, such as ergothioneine (Lee et al. Citation2009) and phenolic compounds (Park and Lee Citation2010), which contribute to its compelling antioxidant capacity (Subramaniam et al. Citation2014, Citation2017). G. neo-japonicum possesses phenolic compounds like catechin, chlorogenic acid, gallic acid, p-coumaric acid, protocatechuic acid, quercetin, and vanillin (Park and Lee Citation2010). In line with this, both the wild and cultivated strains have been shown to have potent free radical scavenging activity (Tan et al. Citation2015), comparable to that of G. lucidum (Veljović et al. Citation2017; Kolniak-Ostek et al. Citation2022) and G. Lingzhi (Dong et al. Citation2019).
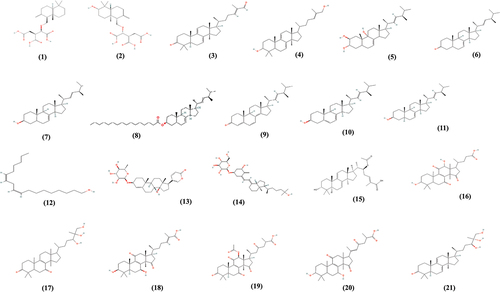
Like other Ganoderma species (Baby et al. Citation2015; Galappaththi et al. Citation2023), sporadic studies have unveiled the diverse array of secondary metabolites (1–21, ) produced by G. neo-japonicum (). Hirotani et al. (Citation1991) initially reported the isolation of two drimane sesquiterpenoids (crytoporic acids H and I) from this polypore. Thereafter, the isolation of lanostanoids (ganoderal A and ganodermadiol) and steroids (2β,3α,9α-trihydroxyergosta-7,22-diene, ergosta-7,22-dien-3-one, ergosta-7,22-dien-3β-ol, ergosta-7,22-dien-3β-yl palmitate, and ergosta-4,6,8(14),22-tetraen-3-one) was conducted by Gan et al. (Citation1998). Bui et al. (Citation2014) isolated another steroid called ergosterol (ergosta-5,7,22-trien-3-ol). A recent phytochemical screening further identified the presence of 47 lanostane-type triterpenoids in G. neo-japonicum (Zhang et al. Citation2023). This versatile chemical profile has been found in G. applanatum, G. lucidum, and G. tsugae Murr (Hapuarachchi et al. Citation2017), which are significantly associated with anticancer properties (Hsu et al. Citation2008; Li et al. Citation2017; Elkhateeb et al. Citation2018). Likewise, G. neo-japonicum has imposed a strong cytotoxic effect on human hepatomas (Bui et al. Citation2014) and adenocarcinomas (Lau et al. Citation2021).
Table 3. Myco-nutrient screening of Ganoderma neo-japonicum extracts with potent bioactivities.
5.2. Apoptotic and anti-cancer properties
B-cell lymphoma-2 (BCL-2) protein is implicated in various malignancies via its regulation of apoptosis (Warren et al. Citation2019). Consistent with previous findings on G. lucidum (Li et al. Citation2017) and G. tsugae (Elkhateeb et al. Citation2018), Lau et al. (Citation2021) demonstrated that G. neo-japonicum serves as a competitive inhibitor of BCL-2 and thus induces apoptosis in colonic carcinoma cells. The authors identified four potential compounds with inhibitory effects against BCL-2, including stellasterol, proscillaridin A, 1,25-dihydroxyvitamin D3, and linoleyl alcohol.
In their follow-up study, apart from cell death, G. neo-japonicum was shown to trigger cell cycle arrest in colonic carcinoma cells, under both normal and hyperglycaemic conditions (Lau et al. Citation2022). Furthermore, this polypore was concomitantly found to diminish high-glucose-induced glutathione, thereby enforcing lethal oxidative stress on the carcinoma cells (Lau et al. Citation2022). G. neo-japonicum, as a fungal material, holds the feasibility for synthesising silver nanoparticles to combat breast cancer through DNA damage (Gurunathan et al. Citation2013).
5.3. Hepato- and genoprotective effects
Ganoderma neo-japonicum can be a protective agent against liver illness. Lin et al. (Citation1995) reported its hepatoprotective effects by reducing serum levels of glutamic oxaloacetic transaminase (GOT) and lactic dehydrogenase (LDH) in a carbon tetrachloride (CCl4)-injured rat model. The CCl4-mediated lipid peroxidation was impaired partly due to its potent free radical scavenging activity. In addition, Tan et al. (Citation2018) highlighted the genoprotective effects of G. neo-japonicum on hydrogen peroxide (H2O2)-damaged macrophage cells. According to the study, ethanol extracts of wild basidiocarps imposed superior protection against H2O2-induced DNA damage compared to aqueous extracts of wild basidiocarps, as well as domesticated basidiocarps. Nonetheless, both the aqueous and ethanol extracts of mycelia from the submerged culture showed no appreciable DNA repair ability (Tan et al. Citation2018). It is postulated that variances in their protective effects and cellular DNA repair capacity may be attributed to factors such as growing conditions and substrate types.
5.4. Anti-viral, anti-inflammatory and immunomodulating effects
Enterovirus A71 (EVA71) and coxsackievirus A16 (CV-A16) are the culprits behind hand, foot, and mouth disease (HFMD). Despite ongoing HFMD outbreaks, there are currently no vaccines or antiviral drugs tailored to combat the enteroviruses responsible for HFMD. However, in a recent study, G. neo-japonicum was found to impede enterovirus infection and replication in human primary oral fibroblast cells (Ang et al. Citation2021). The most potent extract, S2, demonstrated virucidal activities with the presence of active polysaccharides.
Ubaidillah et al. (Citation2015) took steps to isolate and characterise intracellular polysaccharides (IPSs) and extracellular polysaccharides (EPSs) from G. neo-japonicum. Both IPSs and EPSs were observed to increase the proliferation and phagocytosis activities of RAW264.7 macrophages. An oral toxicity test unveiled no significant adverse effects in Sprague-Dawley rats that were fed on dried G. neo-japonicum mycelium (Ubaidillah et al. Citation2015), suggesting the potential use of its polysaccharides as immunomodulating agents to activate the innate immune system in the fight against infectious diseases.
Moreover, G. neo-japonicum was corroborated to possess anti-inflammatory effects that support cellular longevity (Zhang et al. Citation2023). It effectively mitigated lipopolysaccharide (LPS)-induced inflammation by downregulating the mRNA levels of pro-inflammatory cytokines, such as TNF-α, IL-1β, and IL-6, resulting in a concurrent decrease in the expression of nitric oxide synthase (iNOS) and cyclooxygenase-2 (COX-2). It also suppressed the LPS-mediated oxidative stress through the inhibition of reactive oxygen species. Upon further investigation, these anti-inflammatory activities were found to be significantly associated with the deactivation of the nuclear factor-kappa B (NF-κB) and the activation of the nuclear factor erythroid 2-related factor 2 (NRF2)/haem oxygenase-1 (HO-1) signalling pathways.
5.5. Neuritogenic effect
Age-related neurodegenerative illnesses are believed to be strongly influenced by neuronal senescence, associated with reduced levels of nerve growth factor (NGF). There is a growing focus on the search for neuroactive compounds that can mimic the activity of NGF (Sabaratnam et al. Citation2013).
Ganoderma neo-japonicum has undergone testing to assess its ability to promote neurite outgrowth in developing mouse neuroblastoma (N2a) cells (Phan et al. Citation2013). For possible embryo- and neuro-toxic effects, in vitro cytotoxicity was studied using mouse embryonic fibroblast (BALB/3T3) and N2a cells, respectively. The aqueous extracts of G. neo-japonicum stimulated neurite outgrowth in N2a cells, with average neurite-bearing cells ranging from 26.4% to 29.6%. The neurite outgrowth activities, at a dosage of 20 µg/mL, even showed no significant difference from other medicinal mushrooms with superior neuritogenic characteristics, particularly, Hericium erinaceus (Bull.) Persoon (Phan et al. Citation2013). It was also been confirmed absence of embryotoxic and neurotoxic effects in BALB/3T3 and N2a cells.
Seow et al. (Citation2013) investigated the neuritogenic effects of aqueous extracts of G. neo-japonicum on pheochromocytoma cells (PC12). The study showed that G. neo-japonicum promotes neuritogenesis via mitogen-activated protein kinase/extracellular signal-regulated kinase 1/2 (MEK/ERK1/2) and phosphatidylinositol 3-kinase/protein kinase B (PI3K/Akt) signalling pathways.
5.6. Anti-hyperglycaemic effects
The ethanol extract of wheat grains fermented with G. neo-japonicum mycelia was found to exhibit an insulin-like effect in 3T3-L1 adipocytes, where it increased adipogenesis and exerted modest anti-epinephrine-induced lipolytic activities (Subramaniam et al. Citation2015). The ethanol extract also upregulated the expression of target genes such as adiponectin, peroxisome proliferator-activated receptor gamma (PPAR), glucose transporter 4 (GLUT4), and hormone-sensitive lipase (HSL).
Meanwhile, G. neo-japonicum hot aqueous extract (AE-3) was demonstrated to have anti-glycaemic properties, as evidenced by α-amylase and α-glucosidase enzyme inhibition assays (Subramaniam et al. Citation2017). A purified polysaccharide fraction (PF) was separated from AE-3 by column chromatography. Fourier transform infrared spectroscopic assessment of the purified polysaccharide fraction (PF) confirmed the presence of typical polysaccharide bands with an estimated β-glucan content of 39.26%.
Subsequently, Subramaniam et al. (Citation2020) isolated β-D-glucan polysaccharide from G. neo-japonicum and reported its ability to induce insulin-independent adipogenesis in 3T3-L1 adipocytes. This β-D-glucan, designated as “GNJP”, stimulated glucose uptake and adiponectin release while inhibiting lipid formation. Most recently, GNJP has been tested for its potential in treating obesity-induced type 2 diabetes mellitus (T2DM) in mice. The supplementation of GNJP at a dosage of 50 mg/kg body weight was found to inhibit weight gain and liver steatosis (Subramaniam et al. Citation2023). Moreover, it improved serum lipid profile and glucose tolerance, leading to the successful attenuation of hyperglycaemia and hyperinsulineamia. The increased HSL and decreased Akt-1 and PPAR gene expressions may have contributed to the prevention of obesity and lipid dysregulation. Therefore, supplementing with an appropriate amount of GNJP holds promise for preventing metabolic abnormalities and obesity-induced T2DM.
6. Future prospectives
One of the challenges that are persistent in the development of G. neo-japonicum study is its domestication process, even though a pilot-scale study was reported (Tan et al. Citation2015). Cultivating a new mushroom species is a complicated procedure that depends on temperature, moisture, soil type, habitat, and spore management. Other potential hurdles might include the non-availability of raw materials (particularly spawn and compost), irregular fluctuating production, perishable nature, lack of knowledge about improved cultivation technology, lengthy and cumbersome methods of compost preparation, and limited post-harvest processing options. Thus, G. neo-japonicum research in the future should focus on improving and refining solid-state fermentation (cultivation) of the basidiocarps.
Besides that, the discovery of new chemical compositions (e.g. novel terpenoids) from this mushroom, whether from mycelial extract or basidiocarps, should be closely followed up. Pre-clinical research should involve carefully planned animal studies. To establish pharmacological effects for human use, there is an urgent need for high-quality clinical data. We lack clinical investigations into the safety and effectiveness of G. neo-japonicum, its interactions with foods and beverages, its actions with chronic usage, teratogenicity, mutagenicity, and genotoxicity. Supporting clinical trials for drug formulations of G. neo-japonicum is essential to broaden its acceptance and understanding in the medical community. This will help in harnessing the potential benefits of this mushroom and making it more accessible for therapeutic applications.
7. Limitations
Data collection for our bibliometric study was carried out in April 2022 and upon re-searching the publication in 2023, we found an additional study done in this period. This could be due to the inconclusive nomenclature of this mushroom. However, to mitigate any potential bias, we included the discussion of both Ganoderma neo-japonicum Imazeki and Ganoderma bambusicola sp. nov., so that scholars would benefit from this comprehensive review.
8. Conclusions
The nomenclature of G. neo-japonicum and G. bambusicola remains a subject of debate due to their similar lustrous “dark reddish brown to purplish black” pileus surface and a long blackish stipe, although G. neo-japonicum differs from the latter in having a uniform pileal context. Notably, G. bambusicola is exclusively known to grow on bamboo roots (Wu et al. Citation2020). Since its widespread use in tribal and local communities in various Asian countries, particularly Malaysia, research on G. neo-japonicum has made substantial progress. Various investigations from several perspectives have elucidated the mushroom’s biological properties, including anticancer, antidiabetic, antiviral, and immunomodulating actions (). Research on chemical composition has shed light on the mushroom’s active compounds, including phenolics that contribute to its compelling antioxidative effect, and polysaccharides which are important immunomodulatory agents. Despite successful domestication, G. neo-japonicum has not made much headway in the industrial sector. It is believed that this medicinal mushroom, like G. lucidum, can be effectively cultivated, although this would necessitate additional research and refining, such as substrate formulation and quality spawn production. There are opportunities to expand the cultivation of G. neo-japonicum, leveraging cutting-edge technology for mass production.
Table 4. A summary of medicinal properties of Ganoderma neo-japonicum in the literature.
Acknowledgments
The authors would like to thank Mr. Shamsudin Fatani Mohamad, Health and Safety Environment Manager (retired) who introduced this indigenous medicinal mushroom, “cendawan senduk”.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Ang WX, Sarasvathy S, Kuppusamy UR, Sabaratnam V, Tan SH, Wong KT, Perera D, Ong KC. 2021. In vitro antiviral activity of medicinal mushroom Ganoderma neo-japonicum Imazeki against enteroviruses that caused hand, foot and mouth disease. Trop Biomed. 38(3):239–247. doi: 10.47665/tb.38.3.063.
- Ayu K, Linatoc A, Ahmad A, Mansor P, Sanusi MS, Mohamed M, Lili T. 2019. Documentation of macrofungi traditionally used by Jakun people in Johor, Malaysia in treatment of various illnesses. IOP Conf Ser: Earth Environ Sci. 269(1):12013. doi: 10.1088/1755-1315/269/1/012013.
- Baby S, Johnson AJ, Govindan B. 2015. Secondary metabolites from Ganoderma. Phytochemistry. 114:66–101. doi: 10.1016/j.phytochem.2015.03.010.
- Bui TTH, Nguyen TT, Nguyen QT, Le XT, Dang NQ. 2014. Cytotoxic steroids in Vietnamese Lingzhi Ganoderma neo-japonicum. J Sci HNUE. 59(9):25–29.
- Chan XH, Sabaratnam V, Abdullah N, Phan CW. 2020. A 53-year bibliometric and scientometric analysis of research in culinary and medicinal mushrooms. Int J Med Mushrooms. 22(6):521–534. doi: 10.1615/IntJMedMushrooms.2020035031.
- Dong Q, Li Y, Liu G, Zhang Z, Zhou H, Yang H. 2019. High oxygen treatments enhance the contents of phenolic compound and ganoderic acid, and the antioxidant and DNA damage protective activities of Ganoderma lingzhi fruiting body. Front Microbiol. 10:2363. doi: 10.3389/fmicb.2019.02363.
- Elkhateeb WA, Zaghlol GM, El-Garawani IM, Ahmed EF, Rateb ME, Abdel Moneim AE. 2018. Ganoderma applanatum secondary metabolites induced apoptosis through different pathways: In vivo and in vitro anticancer studies. Biomed Pharmacother. 101:264–277. doi: 10.1016/j.biopha.2018.02.058.
- Foo S, Saikim F, Kulip J, Sathiya Seelan J, Seelan S. 2018. Distribution and ethnomycological knowledge of wild edible mushrooms in Sabah (Northern Borneo), Malaysia. Biotropica. 15:203–222. doi: 10.51200/jtbc.v15i0.1494.
- Fryssouli V, Zervakis GI, Polemis E, Typas MA. 2020. A global meta-analysis of ITS rDNA sequences from material belonging to the genus Ganoderma (Basidiomycota, Polyporales) including new data from selected taxa. MycoKeys. 75:71–143. doi: 10.3897/mycokeys.75.59872.
- Galappaththi MCA, Patabendige NM, Premarathne BM, Hapuarachchi KK, Tibpromma S, Dai DQ, Suwannarach N, Rapior S, Karunarathna SC. 2023. A review of Ganoderma triterpenoids and their bioactivities. Biomolecules. 13(1):24. doi: 10.3390/biom13010024.
- Gan KH, Kuo SH, Lin CN. 1998. Steroidal constituents of Ganoderma applanatum and Ganoderma neo-japonicum. J Nat Prod. 61(11):1421–1422. doi: 10.1021/np980184j.
- Gurunathan S, Raman J, Abd Malek SN, John PA, Vikineswary S. 2013. Green synthesis of silver nanoparticles using Ganoderma neo-japonicum Imazeki: A potential cytotoxic agent against breast cancer cells. Int J Nanomed. 8:4399–4413. doi: 10.2147/IJN.S51881.
- Hapuarachchi KK, Cheng CR, Wen TC, Jeewon R, Kakumyan P. 2017. Mycosphere essays 20: Therapeutic potential of Ganoderma species: Insights into its use as traditional medicine. Mycosphere. 8(10):1653–1694. doi: 10.5943/mycosphere/8/10/5.
- Hapuarachchi KK, Elkhateeb WA, Karunarathna SC, Cheng CR, Bandara AR, Kakumyan P, Hyde KD, Daba GM, Wen TC. 2018. Current status of global Ganoderma cultivation, products, industry and market. Mycosphere. 9(5):1025–1052. doi: 10.5943/mycosphere/9/5/6.
- Hapuarachchi KK, Karunarathna SC, Phengsintham P, Yang HD, Kakumyan P, Hyde KD, Wen TC. 2019. Ganodermataceae (polyporales): Diversity in greater Mekong subregion countries (China, Laos, Myanmar, Thailand and Vietnam). Mycosphere. 10(1):221–309. doi: 10.5943/mycosphere/10/1/6.
- Hattori T, Ryvarden L. 1994. Type studies in the polyporaceae 25 species described from Japan by RImazeki and A. Yasuda. Mycotaxon. 50:27–46.
- He J, Luo ZL, Tang SM, Li YJ, Li SH, Su HY. 2021. Phylogenetic analyses and morphological characters reveal two new species of Ganoderma from Yunnan province, China. MycoKeys. 84:141–162. doi: 10.3897/mycokeys.84.69449.
- Hirotani M, Furuya T, Shiro M. 1991. Cryptoporic acids H and I, drimane sesquiterpenes from Ganoderma neo-japonicum and Cryptoporus volvatus. Phytochemistry. 30(5):1555–1559. doi: 10.1016/0031-9422(91)84208-A.
- Hsieh FG, Yeh ZY. 2004. Cultural and physiological studies of Ganoderma neo-japonicum and G. zonatum. Bio Formosa. 39(1):23–32. Chinese.
- Hsu SC, Ou CC, Li JW, Chang TC, Kuo HP, Liu JY, Chen CS, Lin SC, Su CH, Kao MC. 2008. Ganoderma tsugae extracts inhibit colorectal cancer cell growth via G(2)/M cell cycle arrest. J Ethnopharmacol. 120(3):394–401. doi: 10.1016/j.jep.2008.09.025.
- Inose T, Yamamoto H 2013. New Ganoderma neo-japonicum strain and method of artificial cultivation of the same. Japan Patent JP2013226130A.
- Inose T, Yamamoto H 2015. New Ganoderma neo-japonicum strain and method of artificial cultivation of the same. Japan Patent JP2015162380A.
- Jamil NAM, Rashid NMN, Hamid MHA, Rahmad N, Al-Obaidi JR. 2017. Comparative nutritional and mycochemical contents, biological activities and LC/MS screening of tuber from new recipe cultivation technique with wild type tuber of tiger’s milk mushroom of species Lignosus rhinocerus. World J Microb Biot. 34(1):1. doi: 10.1007/s11274-017-2385-4.
- Jargalmaa S, Eimes JA, Park MS, Park JY, Oh SY, Lim YW. 2017. Taxonomic evaluation of selected Ganoderma species and database sequence validation. PeerJ. 5:e3596. doi: 10.7717/peerj.3596.
- Jo WS, Park HN, Cho DH, Yoo YB, Park SC. 2011. Detection of extracellular enzyme activities in Ganoderma neo-japonicum. Mycobiology. 39(2):118–120. doi: 10.4489/MYCO.2011.39.2.118.
- Jo WS, Park HN, Park SH, Jung HY, Yoo YB. 2010. Fruit-body production of Ganoderma neo-japonicum by sawdust cultivation. Korean J Mycol. 38(2):199–201. Korean. doi:10.4489/KJM.2010.38.2.199.
- Kolniak-Ostek J, Oszmiański J, Szyjka A, Moreira H, Barg E. 2022. Anticancer and antioxidant activities in Ganoderma lucidum wild mushrooms in Poland, as well as their phenolic and triterpenoid compounds. IJMS. 23(16):9359. doi: 10.3390/ijms23169359.
- Lau MF, Chua KH, Sabaratnam V, Kuppusamy UR. 2021. In vitro and in silico anticancer evaluation of a medicinal mushroom, Ganoderma neo-japonicum Imazeki, against human colonic carcinoma cells. Biotechnol Appl Bioc. 68(4):902–917. doi: 10.1002/bab.2013.
- Lau MF, Chua KH, Sabaratnam V, Kuppusamy UR. 2022. In vitro anti-colorectal cancer potential of the medicinal mushroom Ganoderma neo-japonicum imazeki in hyperglycemic condition: Impact on oxidative stress, cell cycle and apoptosis. Nutr Cancer. 74(3):978–995. doi: 10.1080/01635581.2021.1931701.
- Lee JY. 1981. Taxonomical studies on Korean higher fungi for the publication of colored illutrations. Korean J Mycol. 9:77–91. Korean.
- Lee SS, Alias S, Jones E, Zainuddin N, Chan H. 2012. Checklist of fungi of Malaysia. Selangor, Malaysia: Institut Penyelidikan PerhutanaInstitut Penyelidikan Perhutanan.
- Lee SS, Chang YS, Noraswati MNR. 2009. Utilization of macrofungi by some indigenous communities for food and medicine in Peninsular Malaysia. For Ecol Manag. 257(10):2062–2065. doi: 10.1016/j.foreco.2008.09.044.
- Lee WY, Park EJ, Ahn JK. 2009. Supplementation of methionine enhanced the ergothioneine accumulation in the Ganoderma neo-japonicum mycelia. Appl Biochem Biotechnol. 158(1):213–221. doi: 10.1007/s12010-008-8322-0.
- Lee WY, Park EJ, Ahn JK, inventors; 2010. Culture medium of Ganoderma neo-japonicum for increasing ergothioneine and culture method of Ganoderma neo-japonicum. Korean Patent KR20100025825A.
- Li K, Na K, Sang T, Wu K, Wang Y, Wang X. 2017. The ethanol extracts of sporoderm-broken spores of Ganoderma lucidum inhibit colorectal cancer in vitro and in vivo. Oncol Rep. 38(5):2803–2813. doi: 10.3892/or.2017.6010.
- Lin JM, Lin CC, Chen MF, Ujiie T, Takada A. 1995. Radical scavenger and antihepatotoxic activity of Ganoderma formosanum, Ganoderma lucidum and Ganoderma neo-japonicum. J Ethnopharmacol. 47(1):33–41. doi: 10.1016/0378-8741(95)01251-8.
- Moral-Muñoz J, Herrera-Viedma E, Espejo A, Cobo M. 2020. Software tools for conducting bibliometric analysis in science: An up-to-date review. Prof Inf. 29(1):e290103. doi: 10.3145/epi.2020.ene.03.
- Park DS, Go SJ, Ryu JC, Sung JM. 1999. Phylogenetic study of Ganoderma spp. based on the DNA sequences in ITS II region. Korean J Mycol. 27(1):39–43. Korean.
- Park YJ, Kwon OC, Son ES, Yoon DE, Han W, Nam JY, Yoo YB, Lee CS. 2012a. Genetic diversity analysis of Ganoderma species and development of a specific marker for identification of medicinal mushroom Ganoderma lucidum. Afr J Microbiol Res. 6(25):5417–5425. doi: 10.5897/AJMR12.846.
- Park YJ, Kwon OC, Son ES, Yoon DE, Han W, Yoo YB, Lee CS. 2012b. Taxonomy of Ganoderma lucidum from Korea based on rDNA and partial ß-tubulin gene sequence analysis. Mycobiology. 40(1):71–75. doi: 10.5941/MYCO.2012.40.1.071.
- Park EJ, Lee WY. 2010. Tryptophan enhanced accumulation of phenolic compounds via chorismate mutase activation in the Ganoderma neo-japonicum mycelia. J Korean Soc Appl Biol Chem. 53(3):364–370. doi: 10.3839/jksabc.2010.056.
- Phan CW, David P, Naidu M, Wong KH, Sabaratnam V. 2013. Neurite outgrowth stimulatory effects of culinary-medicinal mushrooms and their toxicity assessment using differentiating neuro-2a and embryonic fibroblast BALB/3T3. BMC Complement Altern Med. 13(1):261. doi: 10.1186/1472-6882-13-261.
- Richter C, Wittstein K, Kirk PM, Stadler M. 2015. An assessment of the taxonomy and chemotaxonomy of Ganoderma. Fung Divers. 71(1):1–15. doi: 10.1007/s13225-014-0313-6.
- Sabaratnam V, Kah-Hui W, Naidu M, Rosie David P. 2013. Neuronal health - Can culinary and medicinal mushrooms help? J Tradit Complement Med. 3(1):62–68. doi: 10.4103/2225-4110.106549.
- Samsudin N, Abdullah N. 2019. Edible mushrooms from Malaysia: A literature review on their nutritional and medicinal properties. Int Food Res J. 26(1):11–31.
- Seow SLS, Naidu M, David P, Wong KH, Sabaratnam V. 2013. Potentiation of neuritogenic activity of medicinal mushrooms in rat pheochromocytoma cells. BMC Complem Altern M. 13(1):157. doi: 10.1186/1472-6882-13-157.
- Stielow JB, Lévesque CA, Seifert KA, Meyer W, Iriny L, Smits D, Renfurm R, Verkley GJM, Groenewald M, Chaduli D, et al. 2015. One fungus, which genes? Development and assessment of universal primers for potential secondary fungal DNA barcodes. Persoonia. 35(1):242–263. doi:10.3767/003158515X689135.
- Subramaniam S, Ong KC, Sabaratnam V, Chua KH, Kuppusamy UR. 2023. The medicinal mushroom Ganoderma neo-japonicum (Imazeki) polysaccharide extract prevents obesity-induced diabetes in C57BL/6J mice. Int J Med Mushrooms. 25(4):27–42. doi: 10.1615/IntJMedMushrooms.2023047595.
- Subramaniam S, Raman J, Sabaratnam V, Heng CK, Kuppusamy UR. 2017. Functional properties of partially characterized polysaccharide from the medicinal mushroom Ganoderma neo-japonicum (agaricomycetes). Int J Med Mushrooms. 19(10):849–859. doi: 10.1615/IntJMedMushrooms.2017024355.
- Subramaniam S, Sabaratnam V, Heng CK, Kuppusamy UR. 2020. The medicinal mushroom ganoderma neo-japonicum (agaricomycetes) from Malaysia: Nutritional composition and potentiation of insulin-like activity in 3T3-L1 cells. Int J Med Mushrooms. 22(1):65–67. doi: 10.1615/IntJMedMushrooms.2020033250.
- Subramaniam S, Sabaratnam V, Kuppusamy UR. 2015. Solid-substrate fermentation of wheat grains by mycelia of indigenous Ganoderma spp. enhanced adipogenesis and modulated PPARγ expression in 3T3-L1 cells. Chiang Mai J Sci. 41(2):269–281.
- Subramaniam S, Sabaratnam V, Kuppusamy UR, Tan YS. 2014. Solid substrate fermentation of wheat grains by mycelia of indigenous species of the genus Ganoderma (higher basidiomycetes) to enhance the antioxidant activities. Int J Med Mushrooms. 16(3):259–262. doi: 10.1615/IntJMedMushr.v16.i3.60.
- Tang JKS, Phan CW, Tan YS, Sabaratnam V, Seelan JSS, Bolhassan MH. 2022. Bibliometric analysis of mushroom poisoning: From diversity to clinical management. Int J Med Mushrooms. 24(7):1–19. doi: 10.1615/IntJMedMushrooms.2022044313.
- Tan WC, Kuppusamy UR, Phan CW, Sabaratnam V. 2018. Cell proliferation and DNA repair ability of Ganoderma neo-japonicum (agaricomycetes): An indigenous medicinal mushroom from Malaysia. Int J Med Mushrooms. 20(2):155–163. doi: 10.1615/IntJMedMushrooms.2018025445.
- Tan WC, Kuppusamy UR, Phan CW, Tan YS, Raman J, Anuar AM, Sabaratnam V. 2015. Ganoderma neo-japonicum Imazeki revisited: Domestication study and antioxidant properties of its basidiocarps and mycelia. Sci Rep. 5(1):12515. doi: 10.1038/srep12515.
- Thawthong A, Hapuarachchi KK, Wen TC, Raspé O, Thongklang N, Kang JC, Hyde KD. 2017. Ganoderma sichuanense (Ganodermataceae, Polyporales) new to Thailand. MycoKeys. 22:27–43. doi: 10.3897/mycokeys.22.13083.
- Tsivileva O, Nguyen T, Vu L, Yurasov N, Chernyshova P, Galushka A, Markin V, Koftin A, KOFTIN O. 2016. Vietnamese Ganoderma: growth, peculiarities, and low-molecular composition compared to European and Siberian strains. Tur J Bot. 40:269–286. doi: 10.3906/bot-1410-15.
- Ubaidillah NHN, Abdullah N, Annuar MS, Sabaratnam V. 2015. Statistical optimisation of radial growth rates of Ganoderma neo-japonicum (KLUM61076) in low-cost solid agar plate’s medium using full factorial design and central composite design. J Trop Agri Food Sci. 43:61–72.
- Ubaidillah NHN, Abdullah N, Sabaratnam V. 2015. Isolation of the intracellular and extracellular polysaccharides of Ganoderma neojaponicum (Imazeki) and characterization of their immunomodulatory properties. Electron J Biotechnol. 18(3):188–195. doi: 10.1016/j.ejbt.2015.03.006.
- Veljović S, Veljović M, Nikićević N, Despotović S, Radulović S, Nikšić M, Filipović L. 2017. Chemical composition, antiproliferative and antioxidant activity of differently processed Ganoderma lucidum ethanol extracts. J Food Sci Technol. 54(5):1312–1320. doi: 10.1007/s13197-017-2559-y.
- Wang L, Li J, Zhang J, Li Z, Liu H, Wang Y. 2020. Traditional uses, chemical components and pharmacological activities of the genus Ganoderma P. Karst.: a review. RSC Adv. 10(69):42084–42097. doi: 10.1039/D0RA07219B.
- Wang D, Yao YJ. 2005. Intrastrain internal transcribed spacer heterogeneity in Ganoderma species. Can J Microbiol. 51(2):113–121. doi: 10.1139/w04-118.
- Warren CFA, Wong-Brown MW, Bowden NA. 2019. BCL-2 family isoforms in apoptosis and cancer. CDD. 10(3):177. doi: 10.1038/s41419-019-1407-6.
- Wu SH, Chern CL, Wei CL, Chen YP, Akiba M, Hattori T. 2020. Ganoderma bambusicola sp. nov. (polyporales, Basidiomycota) from southern Asia. Phytotaxa. 456(1):75–85. doi: 10.11646/phytotaxa.456.1.5.
- Yoon YH, Choi SH, Cho HJ, Moon SW, Kim JY, Lee S. 2011. Reversible pancytopenia following the consumption of decoction of Ganoderma neojaponicum Imazeki. Clin Toxicol. 49(2):115–117. doi: 10.3109/15563650.2011.553834.
- Zhang RR, Zhang J, Guo X, Chen YY, Sun JY, Miao JL, Carpena M, Prieto MA, Li NY, Zhou QX, et al. 2023. Molecular mechanisms of the chemical constituents from anti-inflammatory and antioxidant active fractions of Ganoderma neo-japonicum Imazeki. Curr Res Food Sci. 6:100441. doi: 10.1016/j.crfs.2023.100441.
- Zhou XW, Su KQ, Zhang YM. 2012. Applied modern biotechnology for cultivation of Ganoderma and development of their products. Appl Microbiol Biotechnol. 93(3):941–963. doi: 10.1007/s00253-011-3780-7.