?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
China has a huge area of diverse landscapes and is believed to conceive incredibly high fungal diversity. To systematically and promptly report Chinese fungal species, we initiate the series of Catalogue of fungi in China here. In the first paper of this series, we focus on plant-inhabiting fungi. A total of 33 new taxa are described all over China. These taxa include two new genera, viz. Cremeoefibula and Nothopucciniastrum, 18 new species, viz. Annulohypoxylon lancangensis, Ascotaiwania coffeae, Clitocella neofallax, Coleopuccinia yunnanensis, Cremeoefibula hengduanensis, Crepidotus furcaticystidiosus, C. tomentellus, Diachea macroverrucosa, Helicogloea hangzhouensis, Hyalopsora caprearum, Nemania polymorpha, Phanerochaetella austrosinensis, Physalacria tianzhongshanensis, Setophaeosphaeria panlongensis, Subulicystidium boreale, Trechispora subaraneosa, Vikalpa dujuanhuensis, and Xylaria pteridicola, and 13 new combinations, viz. Nothopucciniastrum actinidiae, N. boehmeriae, N. coriariae, N. corni, N. coryli, N. fagi, N. kusanoi, N. hikosanense, N. hydrangeae-petiolaris, N. miyabeanum, N. styracinum, N. tiliae, and N. yoshinagae. The morphological characteristics and phylogenetic evidence are used to support the establishment of these new taxa and the accuracy of their taxonomic placements. We hope that the series of Catalogue of fungi in China will contribute to Chinese fungal diversity and promote the significance of recording new fungal taxa from China.
1. Introduction
It is estimated that 2.2–3.8 million fungal species exist worldwide; however, less than 10% of the estimated species are currently recorded (Hawksworth et al. Citation2017). Therefore, as one important group of strategic biological resources (Bai et al. Citation2023), fungi, generally comprising Fungi, slime moulds, and oomycetes, deserve more attention, and much more efforts need to be made by global mycologists to recognise, utilise, and conserve fungal resources.
China has a vast area of territory and possesses highly diverse landscapes ranging from boreal to tropical zones. It is expected that a huge number of fungi exist in China. Indeed, Chinese mycologists have contributed significantly to fungal diversity in China as well outside China for the last decades (Wang and Cai Citation2023). The number of new fungal taxa described from China accounts for almost 7% of global recorded species, occupying the second highest worldwide. In addition, the taxonomic frameworks of certain important fungal groups have been updated by Chinese mycologists (Wang et al. Citation2021, Citation2023; Liu et al. Citation2022, Citation2023; Bao et al. Citation2023). Moreover, a series of Flora Fungorum Sinicorum (more than 60 volumes), and many other monographs focusing on special fungal groups (e.g. Li and Yang Citation2021) and Chinese localities (e.g. Liu et al. Citation2023) have been published. Nevertheless, although books can systematically summarise fungal diversity, papers normally with a short publication cycle will report new taxa much more rapidly. However, most taxonomic papers reported less than 10 new taxa from China in a single paper. This phenomenon slows the publication rate, and more importantly, lowers the significance of new taxa. Given the above, a new series compiling new fungal taxa all over China, named Catalogue of Fungi in China, is urgently needed to be published in Mycology, the official journal of the Mycological Society of China.
In the first paper of the series Catalogue of Fungi in China, we focus on plant-inhabiting fungi. Almost all types of fungi inhabiting plants are accommodated, including corticioid fungi, agarics, jelly fungi, plant pathogens, aquatic fungi, and slime moulds. We hope that the Catalogue of Fungi in China will attract wide attention to fungal diversity in China. Beyond taxonomy, the accumulated knowledge of species diversity in this series will also benefit evolutionary biology, ecology, conservation, and resource utilisation of fungi (Zhou and May Citation2023).
2. Materials and methods
The specimens investigated in this study were collected from various plant substrata around China. The strains isolated from some of these specimens were cultivated on potato dextrose agar (PDA), malt extract agar (MEA), and synthetic low nutrient agar (SNA). Voucher specimens were deposited in the fungarium of HMAS, ZHKU, HKAS, BJFC, HMJAU, and IFRD. The morphological observation procedure followed the methods of Zhang and Li (Citation2016), Luo et al. (Citation2018), and Liu et al. (Citation2022). The taxa were described in detail along with illustrations and photographs. Total genomic DNA was extracted from the above-mentioned specimens and strains using the CTAB Genomic DNA Rapid Extraction Kit. PCR was performed to amplify various loci with selected primers as outlined in .
Table 1. Selected primers for amplifying various loci.
The newly generated sequences were deposited in the GenBank (https://www.ncbi.nlm.nih.gov/genbank/) and utilised for phylogenetic analyses. Information on all sequences used for phylogenetic analyses is available in the supplementary materials (Supplementary Tables 1–15). Generally, for a given phylogenetic analysis, each locus was separately aligned, and then the resulting alignments were concatenated as a single alignment. The best-fit evolutionary models of the single alignments were estimated using jModelTest 2.0 (Posada Citation2008). Phylogenetic analyses were carried out using RaxML (Stamatakis Citation2014; Edler et al. Citation2021) and MrBayes 3.2 (Ronquist et al. Citation2012), respectively, for maximum likelihood (ML) and Bayesian inference (BI) methods. The bootstrap (BS) values and Bayesian posterior probability (BPP) were used to judge the reliability of phylogenetic topology.
3. Taxonomy
Fungi
Ascomycota
Dothideomycetes
Pleosporales Luttr. ex M.E. Barr, Prodr. Cl. Loculoasc. (Amherst): 67 (1987)
Dictyosporiaceae Boonmee & K.D. Hyde, Fungal Diversity 80: 462 (2016)
Notes: Dictyosporiaceae was introduced to accommodate a holomorphic group of Dothideomycetes that are saprobes on decaying wood and plant debris (Boonmee et al. Citation2016). Up to now, Dictyosporiaceae comprises 20 genera, the species of these genera are distributed worldwide, and most taxa are saprobes on plant litter, especially dead or decaying wood in freshwater and terrestrial habitats (Boonmee et al. Citation2016; Li et al. Citation2017; Shen et al. Citation2022; Tian et al. Citation2022).
Vikalpa D’souza, Boonmee, Bhat & K.D. Hyde, Fungal Diversity 80: 479 (2016)
Notes: Vikalpa was introduced by Boonmee et al. (Citation2016) based on morphological characteristics and multi-gene phylogenetic analysis, with Vikalpa australiensis as the type species (Boonmee et al. Citation2016). Vikalpa is a special group of cheirosporous hyphomycete, mainly characterised by euseptate conidia, with three rows of cells in different planes (Boonmee et al. Citation2016). Based on specific morphological and phylogenetic evidence, Vikalpa australiensis (Dictyosporium australiense), V. freycinetiae (D. freycinetiae), and V. micronesiaca (D. micronesiacum) were transferred from Dictyosporium to Vikalpa by Boonmee et al. (Citation2016). Currently, the genus includes six species that are saprophytic on wood and decaying leaves from freshwater and terrestrial habitats (Sutton Citation1985; McKenzie Citation2008; Boonmee et al. Citation2016).
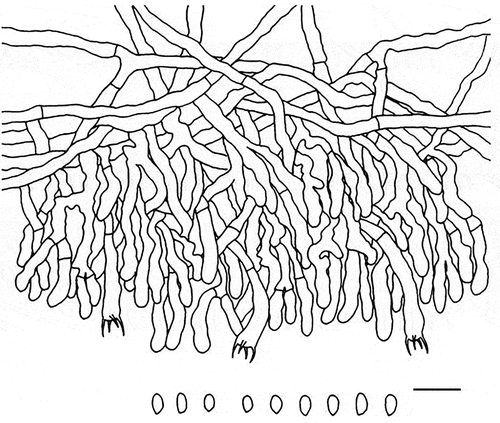
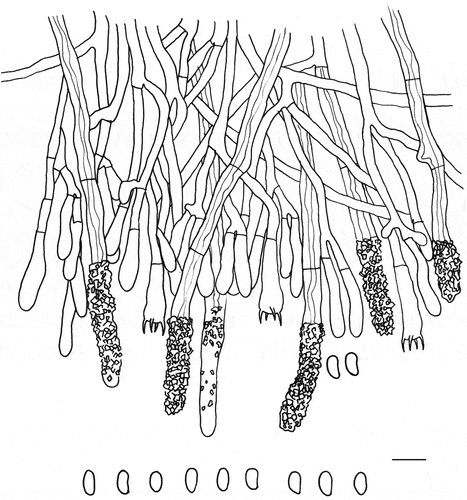
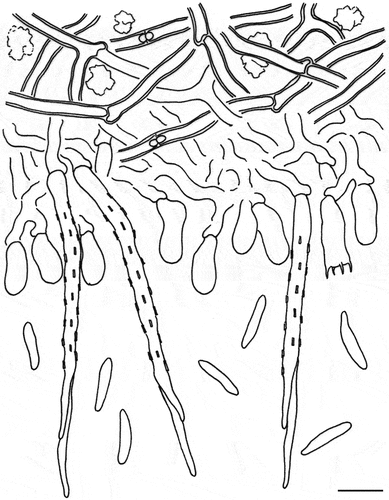
Vikalpa dujuanhuensis H.W. Shen & Z.L. Luo, sp. nov.
Figure 1. Vikalpa dujuanhuenesis (holotype). (a,b) Colonies on the substratum. (c,d) Conidiophores, conidiogenous cells, and conidia. (e) Conidiogenous cells. (f) Conidiogenous cells with conidia. (g–j) Conidia. (k) Germinating conidium. (l) Culture on PDA (from above and from below). Scale bars: a = 1,000 μm; b = 500 μm; c – d = 20 μm; g = 30 μm; e – f, h – k = 10 μm; l = 2 cm.

Fungal Names: FN 571593.
Etymology: dujuanhuensis (Latin), refers to Dujuanhu Lake, in Yunnan Province, where this fungus was collected.
Diagnosis: Differing from Vikalpa freycinetiae by having a lower number of cells per row (8–10 cells vs. 9–13 cells) (McKenzie Citation2008).
Description: Saprobic on submerged decaying wood in a plateau freshwater lake. Asexual morph: Hyphomycetous. Colonies on natural substrate, punctiform, sporodochial, scattered, dark brown. Mycelium composed of immersed or partly superficial, branched, septate, hyaline to pale brown hyphae. Conidiophores mononematous, micronematous, cylindrical, unbranched, septate, hyaline to pale brown, smooth-walled, sometimes reduced to conidiogenous cells. Conidiogenous cells holoblastic, cylindrical, subglobose, sometimes flat at the base, septate, hyaline to pale brown, smooth-walled. Conidia (30–)34–41(−43) × (11–)12–16(−20) μm ( = 38 × 14 μm, n = 50), solitary, cheiroid, pale brown to brown, consisting of three rows of cells in different planes, 8–10 cells in each row, euseptate, irregular, constricted at the septa, guttulate, each row 6–8(−9) μm (
= 7 μm, n = 50) wide, with or without transparent thin gelatinous appendages. Sexual morph: Undetermined.
Culture characteristics: Conidia germinating on PDA within 12 h and germ tubes produced at ends. Colony growth on PDA; reaching about 2.5–3 cm in 45 days at room temperature. Mycelium papillary in the middle part, dense, creamy yellow, brown to reddish-brown, sparse, white at edge; pale cream yellow on the edge and dark reddish brown in the middle on the reverse side.
Materials examined: China. Yunnan Province, Puer City, Jingdong Yi Autonomous County, Dujuanhu Lake, 24°32’32ʺ N, 101°01’38ʺ E (2,500 m), on submerged decaying wood, 25 February 2022, H.W. Shen, YJ 26-28-1 (holotype in KUN-HKAS 125,817), ex-type living cultures (CGMCC 3.24268 = KUNCC 22–12670).
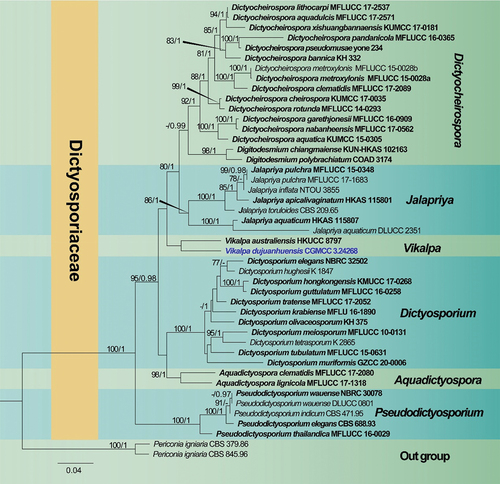
Notes: Phylogenetic analysis showed that Vikalpa dujuanhuensis clusters with V. australiensis with low support (). Morphologically, V. dujuanhuensis fits with Vikalpa species in having euseptate conidia, with three rows of cells in different planes. However, V. dujuanhuensis has longer conidia than V. lignicola (34–41 μm vs. 25–40.5 μm) and V. micronesiaca (34–41 μm vs. 20–30 μm) (Matsushima Citation1981; Boonmee et al. Citation2016). Vikalpa dujuanhuensis can be distinguished from V. freycinetiae by having a lower number of cells per row (8–10 cells vs. 9–13 cells) (McKenzie Citation2008). Therefore, V. dujuanhuensis is introduced as a new species from a freshwater habitat, based on morphological characters and phylogenetic analysis.
Figure 2. Phylogenetic tree of combined ITS, LSU, nSSU, and tef1α sequence data. Bootstrap support values for maximum likelihood ≥ 75% and Bayesian posterior probabilities ≥ 0.97 are indicated above the nodes as BS/BPP. The new species are represented in blue and the type species are in bold.
Phaeosphaeriaceae M.E. Barr, Mycologia 71(5): 948 (1979)
Notes: Barr (Citation1979) first described the family Phaeosphaeriaceae, which is represented by Phaeosphaeria, with P. oryzae serving as the type species. The ascomata of Phaeosphaeriaceae immersed to superficial, globose to subglobose, papilla short, asci bitunicate, hyaline to pigmented, ascospores fusiform to ellipsoidal, filiform, or muriform (Maharachchikumbura et al. Citation2019; Yang et al. Citation2019). Species of Phaeosphaeriaceae, especially the asexual taxa, are important plant pathogens infecting major crops (Yang et al. Citation2019).
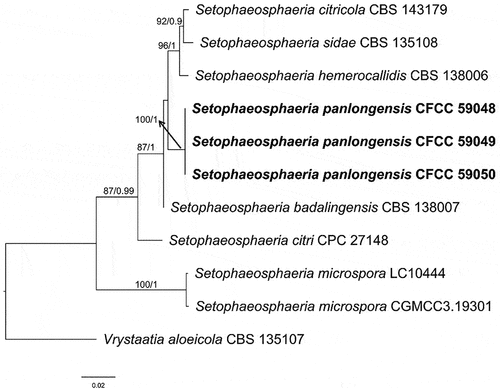
Setophaeosphaeria Crous & Y. Zhang ter, in Crous et al., Persoonia 32: 271 (2014)
Notes: Setophaeosphaeria was established by Crous et al. (Citation2014) to accommodate ascomycetes that differ from Phaeosphaeria by lacking ascomatal setae and possessing phoma-like anamorphs. Setophaeosphaeria currently comprises seven species associated with leaf spots and branch dieback, including six verifiable species with living cultures and DNA data, and doubtful Setophaeosphaeria setosa previously known as Phaeosphaeria setosa (Crous et al. Citation2014, Citation2017). Setophaeosphaeria hemerocallidis was designed as the type species (Crous et al. Citation2014).
Setophaeosphaeria panlongensis L. Lin & X.L. Fan, sp. nov.
Figure 3. Setophaeosphaeria panlongensis (ex-holotype). (a) Culture on PDA. (b) Culture on MEA. (c) Culture on SNA. (d,e) Constricting ring. Scale bars: d – e = 10 μm.
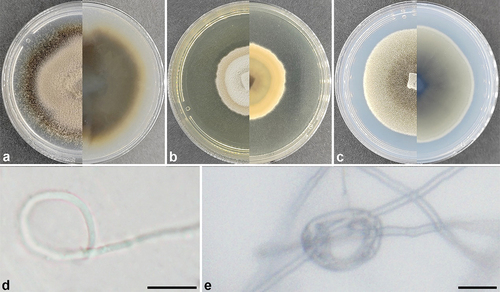
Fungal name number: FN 571655.
Etymology: panlongensis (Latin), refers to the collection site of the type specimen, Panlong District in Kunming City, Yunnan Province, China.
Diagnosis: Differing from other species by the DNA sequence data.
Description: Culture sterile. On MEA, PDA, and SNA, hyphae septate, occasionally forming the constricting ring. Setophaeosphaeria panlongensis differs from its closest phylogenetic affinities, S. citricola, S. sidae, and S. hemerocallidis by unique fixed alleles of three loci based on alignments of the separate loci ().
Table 2. Sequence differences of three loci used to delimit Setophaeosphaeria panlongensis from its phylogenetic affinities.
Culture characteristics: Colonies covering the Petri dish after 21 d at 25 °C, without aerial mycelium, dense. On PDA surface salmon to hazel and buff to isabelline on the reverse. On MEA surface white to buff and buff to salmon on the reverse. On SNA surface olivaceous buff to olivaceous and smoke grey to olivaceous on the reverse.
Materials examined: China. Yunnan Province, Kunming City, Panlong District, on branches of Juniperus formosana, 4 August 2022, holotype in BJFC CF20231001, ex-holotype living culture CFCC 59048; ibid. BJFC CF20231002, living culture CFCC 59049; ibid. BJFC CF20231003, living culture CFCC 59050.
Notes: Setophaeosphaeria panlongensis was isolated on symptomatic branches of Juniperus formosana in China. It differs from other known species in this genus based on forming constricting rings on MEA, PDA, and SNA media, but significant conidial sporulation did not occur. Phylogenetically, the isolates CFCC 59048–59050 formed a distinct clade with high support value (BS = 100, BPP = 1; ) and were different with the sequences of S. citricola, S. sidae, and S. hemerocallidis: for ITS 15/466 bp, 18/466 bp, and 15/466 bp, respectively; for nLSU 5/752 bp, 6/752 bp, and 5/752 bp, respectively; and for tub 8/140 bp, 7/140 bp, and unknown (tub unavailable from S. hemerocallidis), respectively. Thus, we introduce it here as a new species.
Figure 4. Phylogenetic relationship among species of Setophaeosphaeria inferred from the combined dataset of ITS, nLSU, and tub regions. The topology is generated by the maximum likelihood algorithm. Bootstrap values and Bayesian posterior probabilities, when simultaneously above 50% and 0.8, respectively, are labelled at the nodes. The newly described species are in boldface.
Sordariomycetes
Savoryellales Boonyuen, Suetrong, Sivichai, Pang & E.B.G. Jones, Mycologia 103 (6): 1368 (2011)
Savoryellaceae Jaklitsch & Réblová, Index Fungorum 209: 1 (2015)
Notes: Savoryellaceae, established by Jaklitsch and Réblová (Citation2015), was typified by the genus Savoryella. The taxonomic placement of Savoryella has been changed several times based on morphology and phylogeny (Kohlmeyer and Kohlmeyer Citation1979; Jones and Hyde Citation1992; Dhanasekaran et al. Citation2006), and later a multi-gene phylogenetic analysis showed that Savoryella formed a monophyletic clade close to the genera Ascotaiwania and Canalisporium (Boonyuen et al. Citation2011). Hence, a new order Savoryellales was proposed to accommodate Ascotaiwania and Canalisporium (Jaklitsch and Réblová Citation2015). The members of species in Savoryellaceae are abundant in submerged wood in aquatic habitats, and some species have been reported from terrestrial woody plants (Linder Citation1929; Jones et al. Citation2015; Du et al. Citation2022). Savoryellaceae always forms a monophyletic group in phylogenetic analyses, with the species belonging to Ascotaiwania (=Neoascotaiwania), Bactrodesmium, Canalisporium, and Savoryella (Boonyuen et al. Citation2011; Dayarathne et al. Citation2019). Currently, six genera are included in Savoryellaceae (Réblová et al. Citation2020; Yang et al. Citation2022).
Ascotaiwania Sivan. & H.S. Chang, Mycological Research 96: 481 (1992)
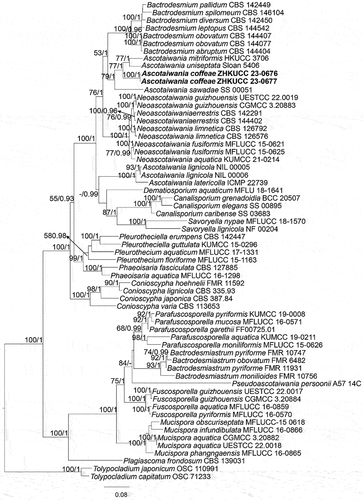
Notes: Ascotaiwania, introduced by Sivanesan and Chang (Citation1992), was typified by A. lignicola. Fourteen species of Ascotaiwania are listed in Index Fungorum (2023), which have been isolated from submerged and decomposing wood in freshwater or terrestrial habitats, and they are widely distributed worldwide (Sivanesan and Chang Citation1992; Ranghoo et al. Citation1999; Dayarathne et al. Citation2019). The members of species in Ascotaiwania are saprobic lignicolous fungi with sexual and asexual morphs (Réblová et al. Citation2020). The sexual morph of Ascotaiwania is characterised by dark-brown to black with lateral neck ascomata, 8-spore asci, cylindrical and unitunicate with a prominent J-refractive and apical ring, containing versicolorous ascospore, 3–7 septate, middle cells brown, end cells hyaline to subhyaline, yellow or pale brown (Tsui and Hyde Citation2003; Boonyuen et al. Citation2011; Dayarathne et al. Citation2019). Asexual morphs of Ascotaiwania are bactrodesmium-like, monotosporella-like, monodictys-like, or trichocladium-like (Ranghoo and Hyde Citation1998; Chang Citation2001; Tsui and Hyde Citation2003; Réblová et al. Citation2016). In this study, a new species of Ascotaiwania with trichocladium-like characteristics is introduced from coffee in Yunnan Province, China.
Ascotaiwania coffeae L. Lu & Karun., sp. nov.
Figure 5. Microscopic structures of ascotaiwania coffeae (holotype). (a,b) Colonies on host substrate. (c–g) Conidiogenous cells with conidia. (i) Germinated conidium. (j,k) Colony on PDA (j from above, k from below). (h,l–s) Conidia. (t) Clusters of conidia formed on submerged hyphae in the agar. (u–w) Developing conidia with attached conidiogenous cells. (x) Mycelia. (y1–y4) Conidia. Scale bars: c, u – x = 20 μm, d – i, l – s, y1 – y4 = 10 μm.

Fungal Names: FN 571656.
Etymology: coffeae (Latin), refers to the host genus “Coffea” from which the holotype of the new species was isolated.
Diagnosis: Differing from Ascotaiwania uniseptata by pyriform conidia and basal cell with a guttulate (Hughes and Pirozynski Citation1972).
Description: Saprobic on decaying branch of Coffea arabica. Sexual morph: Undernimmed. Asexual morph: Trichocladium-like. Colonies effuse, black, glistening, punctiform distributed on substrates. Mycelium 1.5–2.5 μm, subhyaline, septate, branched. Conidiophores inconspicuous or micronematous, mononematous, smooth. Conidiogenous cells 3–4.5 × 2.5–4 µm ( = 3.2 × 3 µm, n = 20), holoblastic, determinate, integrated, terminal becoming intercalary, subglobose or ampulliform. Conidia 9–14 × 6−10 µm (
= 11 × 7 μm, n = 30), solitary, pyriform to obovoid, broadly rounded at the apex, straight or slightly curved, guttulate, 1-septate, septa thick and band-like, dividing the conidium into unequal cells, the upper cell being largest, 6–10 μm long (
= 7.5 μm, n = 30), subhyaline or dark brown, and the basal cell subhyaline or pale brown.
Culture characteristics: Conidia germinating on PDA within 24 h. Colonies on PDA reaching 2 cm diam. After three months at 25 °C, irregular, smooth, with an entire edge, flat to slightly raised, from above cream, from below brown. After three months, vegetative hyphae produced on the reverse side, immersed, subhyaline to light brown, 2.5–3.5 μm wide ( = 3 µm, n = 30), septate and branched, often monilioid. Conidiophores macronematous, semi-macronematous, arising from aerial or submerged hyphae, or micronematous often reduced to conidiogenous cells. Conidiogenous cells holoblastic, 4–8 × 3–6 µm (
= 6 × 4.5 µm, n = 30), hyaline to pale brown, thin-walled, sometimes thickened and pigmented near the base, sub-globose, or irregularly cylindrical, bearing a single terminal conidium. Conidia 8–15 × 6–10 µm (
= 10 × 7 µm, n = 30), sub-globose or obovoid, guttulate, normally 1-septate, sometimes constricted at the septa when mature, hyaline to pale brown.
Materials examined: China. Yunnan Province, Dali City, on a decaying branch of Coffea arabica, (101°91’E, 26°09’ N, 1,416.46 m), 25 July 2022, DL-C19 (holotype in ZHKU 23-0083), living culture ZHKUCC 23-0676 (ex-type), ZHKUCC 23-0677.
Notes: In our phylogenetic analyses (), Ascotaiwania coffeae grouped in a clade comprising A. uniseptata and A. mitriformis. Ascotaiwania coffeae morphologically resembles A. uniseptata, as both have trichocladium-like asexual morphs (Hughes and Pirozynski Citation1972; Ranghoo and Hyde Citation1998). In morphology, A. coffeae is easily distinguishable from A. uniseptata by the pyriform conidia and basal cell with a guttulate (Hughes and Pirozynski Citation1972). Based on nucleotide comparisons, A. coffeae (ZHKUCC 23-0676) is different from A. uniseptata (Sloan 5406) by 12/365 bp (3.3%) of the nLSU, and from A. uniseptata only nLSU region is available (Réblová et al. Citation2016). Therefore, we introduce A. coffeae as a distinct new species based on morphology. In the future, fresh collections of A. uniseptata are needed to obtain single spore isolates and check their phylogenetic affinities.
Figure 6. Phylogenetic relationship selected members of Savoryellomycetidae (Sordariomycetes) inferred from the combined dataset of LSU, nSSU, ITS, rpb2, and tef1α regions. The topology is generated by the maximum likelihood algorithm. Bootstrap values and Bayesian posterior probabilities, when simultaneously equal to or above 50% and 0.8, respectively, are labelled at the nodes. The newly described species are in boldface.
Xylariales Nannf., Nova Acta R. Soc. Scient. upsal., Ser. 4 8(no. 2): 66 (1932)
Hypoxylaceae DC., in Lamarck & de Candolle, Fl. franç., Edn 3 (Paris) 2: 280 (1805)
Notes: The Hypoxylaceae in the Xylariales was introduced by de Candolle in 1805. This family comprises 18 genera, with Hypoxylon as the type genus (Wijayawardene et al. Citation2022). Asexual morph of Hypoxylon is characterised by varying from erect to effused-pulvinate, solitary or confluent stromata, waxy or carbonaceous peridium, with or without KOH-extractable pigments, dark brown to black, persistent or loculate. Some genera present peculiar features, such as alternating zones under the perithecial layer, or hollow and filled with liquid, observed in the Daldinia and Entonaema, respectively. Ostioles can be umbilicate, at the same level or higher than the level of the stroma surface, with or without discs. The asci are typically 8-spored, cylindrical, with apical ring discoid, distinct, highly reduced, or lacking. Ascospores brown, ellipsoid, or short fusoid, with acute, narrowly rounded, or broadly rounded ends, and most species bear a germ slit; perispore dehiscent or indehiscent in 10% KOH (Hsieh et al. Citation2005). The sexual morph is Nodulisporium-like with branching patterns varying from “regular” nodulisporium, periconiella, virgariella, or sporothrix-like (Silva et al. Citation2020).
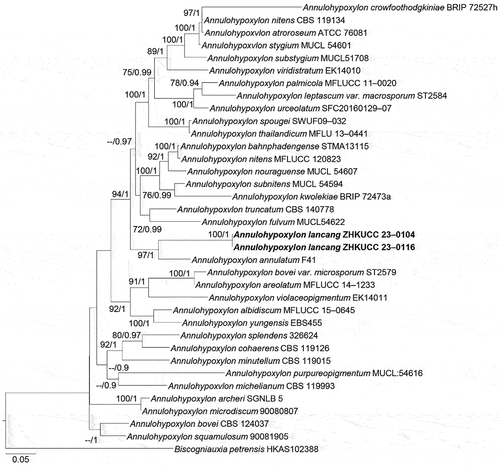
Annulohypoxylon Y.M. Ju, J.D. Rogers & H.M. Hsieh, in Hsieh, Ju & Rogers, Mycologia 97(4): 855 (2005)
Notes: Annulohypoxylon was introduced by Hsieh et al. (Citation2005) with the type species Annulohypoxylon truncatum and the genus has 60 species (Wijayawardene et al. Citation2022). Annulohypoxylon is characterised by effused-pulvinate or pulvinate, glomerate stromata, waxy or carbonaceous tissue immediately beneath the surface and between perithecia, with KOH-extractable pigments in most cases; spherical, obovoid, with carbonaceous stromata layer surrounding individual perithecia; higher than the level of stromata surface ostioles; light- to dark-coloured, 8-spored, cylindrical, stipitate, persistent, with apical ring discoid, amyloid or infrequently inamyloid, distinct asci; light- to dark-coloured, ellipsoid or short fusoid, inequilateral, narrowly rounded, or broadly rounded ends, with a germ slit, perispore dehiscent or indehiscent in 10% KOH (Li et al. Citation2016).
Annulohypoxylon lancangensis R.F. Xu & S. Tibpromma, sp. nov.
Figure 7. Annulohypoxylon lancangensis (holotype). (a–c) Stromata habit on wood. (d) Section of peridium. (e) Section of ostiole. (f) Asci with paraphyses. (g) Paraphyses. (h–k) Asci. (l–n) Ascospore. (o) Ascospore in 10% KOH. (p) Ascus in Melzer’s reagent, showing the J+, subapical ring. (q) Germinating ascospore. (r,s) Colony on PDA medium. Scale bars: d, q = 20 μm, e = 200 μm, f, g, k = 50 μm, h – j = 30 μm, l – o = 5 μm, p = 10 μm.

Fungal Names: FN 571657.
Etymology: lancangensis (Latin), refers to the place Lancang, China where the fungus was first discovered.
Description: Saprobic on unidentified dead wood. Sexual morph Stromata 330–496 × 323–460 μm, ( = 417 × 400 μm, n = 10), glomerate, pulvinate to effused-pulvinate, with conspicuous perithecial mounds, surface shiny black, subglobose to globose, carbonaceous. Ostiole conical, papillate, encircled with a white. Peridium laterally 64–140 μm thick, composed of carbonaceous, thick-walled, dark brown to black cells of textura angularis. Hamathecium 3.5–6 μm wide comprising long, septate paraphyses, hyaline. Asci 91–110 × 4–6 μm (
= 101 × 5.5 μm, n = 10), 8-spored, unitunicate, cylindrical, long pedicellate, J+ apical ring. Ascospores 7.3–9.6 × 3–4.5 μm (
= 8 × 3.8 μm, n = 20), uniseriate, 1-celled, inequilaterally ellipsoidal, with narrowly rounded ends, pare brown to brown, guttules, germ slit straight, running along the entire spore-length on flattened side. Asexual morph Undetermined.
Culture characteristics: Ascospores germinating on PDA within 12 h and germ tubes produced from ends. Colonies growing fast on PDA, whitish colonies, flossy, circular with the entire edge, light yellow in reverse.
Materials examined: China. Yunnan Province, Pu’er City, Lancang, on an unidentified dead wood piece, 11 July 2020, Tian-Ye Du, LCD01 (holotype in ZHKU 23-0053), ex-type living culture, ZHKUCC 23-0104 = ZHKUCC 23-0116.
Notes: The phylogenetic analyses showed Annulohypoxylon lancangensis forms a monophyletic clade with A. annulatum (BS = 97%, BPP = 1.00; ). Moreover, the base pair comparison of Annulohypoxylon lancangensis and A. annulatum shows 131/637 bp differences in ITS (20.56%, gaps 45 bp), 41/261 bp differences in ACT (15.70%, gaps 9 bp) and 74/443 bp differences in tub (16.70%, gaps 20 bp). Based on the recommendation of Jeewon and Hyde (Citation2016), a minimum of > 1.5% nucleotide differences in the ITS region indicate a new species. Morphological comparison of the new taxon (Annulohypoxylon lancangensis) and Annulohypoxylon annulatum revealed that Annulohypoxylon lancangensis has smaller ascospores (7.3–9.6 × 3–4.5 μm vs. 9.3–11.3 × 4.1–4.6 μm; Khodaparast Citation2012). Thus, based on both morphological characteristics and phylogenetic analyses, we introduce Annulohypoxylon lancangensis as a distinct new species.
Figure 8. Phylogenetic relationship among species of Annulohypoxylon inferred from the combined dataset of ITS, LSU, ACT, and tub regions. The topology is generated by the maximum likelihood algorithm. Bootstrap values and Bayesian posterior probabilities, when simultaneously equal to or above 70% and 0.9, respectively, are labelled at the nodes. The newly described species is in boldface.
Xylariaceae Tul. & C. Tul. [as “Xylariei”], Selecta fungorum carpologia. (Paris) 2: 3 (1863)
Notes: The family Xylariaceae (Xylariales), one of the largest and most commonly encountered families of Ascomycota (Maharachchikumbura et al. Citation2015, Citation2016), was erected based on the type genus Xylaria (Tulasne and Tulasne Citation1863). The majority of Xylariaceae species are widely known as saprobes, growing on wood, seeds, fruits, or leaves of angiosperms (Edwards et al. Citation2003; Visser et al. Citation2009). Some Xylariaceae species are endophytes and plant pathogens (Stadler et al. Citation2013). Sexual morphs in this family are characterised by stromata extremely variable in size, shape, and colour, perithecia embedded in more or less well-developed, 8-spored, unitunicate, cylindrical asci with or without an amyloid apical ring, and brown ascospores with germ slits. However, the classification of some genera in this family is controversial. Currently, Xylariaceae resides in the order Xylariales, and has 37 genera in the family (Wendt et al. Citation2018).
Nemania Gray, Nat. Arr. Brit. Pl. (London) 1: 516 (1821)
Notes: The genus Nemania was erected in 1821 with Nemania serpens as the type species. The species of this genus are found throughout the temperate and tropical regions of the world (Pouzar Citation1985; Van Citation1995; Granmo et al. Citation1999; Ju and Rorgers Citation1999; Fournier et al. Citation2018). Nemania is characterised by carbonaceous, dark- or dull-coloured pulvinate stromata, cylindrical asci usually with long-stipitate, bear apical rings that have a height/breadth ratio larger than 1/2, and yellowish to dark brown ascospores with an inconspicuous to conspicuous germ slit (Ju and Rogers Citation1996).
Nemania polymorpha Hai X. Ma & Y. Li, sp. nov.
Figure 9. Nemania polymorpha (holotype). (a,c) Stromata. (b) Close-up view of stromatal surface, showing perithecial mounds and ostiolar disks. (d) Section through stromata, showing perithecia. (e,f) Asci in 5% KOH. (g,h) Asci in Melzer’s reagent. (i) Ascus apical ring in 5% KOH. (j,k,o,p) Ascospores. (l) Ascus apical ring in Melzer’s reagent. (m,n) Ascospore with germ slit. Scale bars: a = 1 mm; b, c = 0.2 mm; d = 0.5 mm; e – h = 10 µm; i – p = 5 µm.
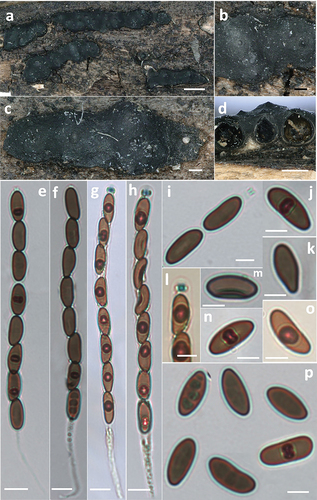
Fungal Names: FN 571663.
Etymology: polymorpha (Latin), refers to various shapes of ascospores.
Diagnosis: Differing from Nemania caries by larger ascospores (Fournier et al. Citation2018), and from N. serpens by apical ring bluing in Melzer’s iodine reagent and larger ascospores (Petrini and Rogers Citation1986).
Description: Stromata superficial, irregularly effused-pulvinate, 2.5–18.6 mm long, 1.3–3.9 mm wide, 0.25–0.58 mm thick, with inconspicuous perithecial contours, roughened by deep cracks and wrinkles, sloping margins; surface greyish-black to black, carbonaceous outer crust immediately surface, 30–58 µm; interior whitish to cream-coloured fibrous soft tissue sometimes between the perithecia in immature stromata, turning black at maturity, carbonaceous; subperithecial tissue absent or inconspicuous. Perithecia subglobose, 0.4–0.7 mm high, 0.4–0.6 mm diam. Ostioles papillate, obtusely conical. Asci with eight ascospores, obliquely or straightly arranged in uniseriate manner, occasionally overlapping, cylindrical, long-stipitate, (140–)150–175(−185) µm total length, the spore-bearing part (80–)88–98(−107) µm long, (6.5–)7–8(−8.6) µm diam., the stipes 55–85 µm long, with apical ring bluing in Melzer’s iodine reagent, short-cylindrical, 2.2–3.0 µm high, 1.9–3.0 µm diam. (Me = 2.5 × 2.3 µm, N = 20). Ascospores brown, unicellular, inequilateral to sometimes slightly equilateral, ellipsoid, suballantoid to nephroid, or pyriform infrequently, with broadly rounded ends, smooth, (12.0–)13.0–14.2(−15.1) × (5.8–)6.0–7.0(−7.5) µm, Q = (1.7–)1.9–2.2(−2.4) (Me = 13.4 × 6.7 µm, Qe = 2.0, N = 60), with a straight, short, inconspicuous germ slit straight, much less than spore length.
Materials examined: China. Heilongjiang Province, Hulin City, Wulingdong Town, on decorticated wood, 21 August 2014, Du ZW, Col. D39 (holotype in FCATAS 410); Jilin Province, Jiaohe City, Ailin Forest Farm, on decorticated wood, 30 August 2013, Hai X. Ma, Col. 085 (FCATAS 408); Liaoning Province, Qingyuan County, Hunheyuan Nature Reserve, on decorticated wood, 16 September 2014, Du ZW, Col. D18 (FCATAS 409).
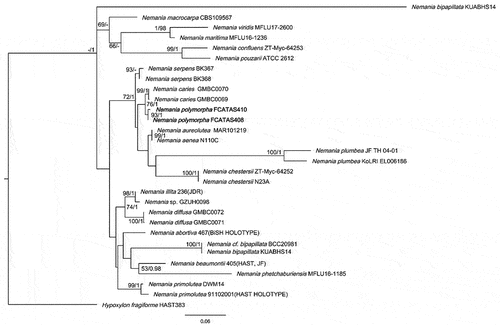
Notes: The phylogenetic analyses showed that the two strains of N. polymorpha clustered together with high support values (BS = 93, PP = 1.00) and formed a sister clade with N. caries (). Nemania caries resembles the new species in stromatal morphology (), but the former has smaller ascospores (9.1–)9.4–11(−11.3) × (3.9–)4–4.5(−4.8) µm (Me = 10.2 × 4.3 µm, Qe = 2.4) (Fournier et al. Citation2018). Nemania serpens is different in having ascus annulus dextrinoid in Lugol’s solution, none or a barely discernible dextrinoid reaction in Melzer’s iodine reagent, and slightly smaller ascospores 10–14.5(−16.5) × 4–6 µm (Me = 12.5 × 4.8 µm) (Petrini and Rogers Citation1986; Granmo et al. Citation1999).
Figure 10. Phylogenetic tree of ITS and nLSU sequences of Nemania. Bootstrap values (≥50%, before the slash markers) and Bayesian posterior probabilities (≥0.95, after the slash markers) are shown.
Xylaria Hill ex Schrank, Baier. Fl. (München) 1: 200 (1789)
Notes: Xylaria is one of the most important genera in the family Xylariaceae (Fournier et al. Citation2011) and more than 300 species have been reported in the world (Kirk et al. Citation2008). The type species, X. hypoxylon, was described by Linnaeus (Citation1745) as Clavaria hypoxylon from Sweden and transferred to the genus Xylaria by Greville (Citation1824). Species of this genus are characterised by having upright, stipitate, woody stromata with perithecia immersed Ju and Rorgers (Citation1999). The genus is widely distributed in tropical, subtropical, and temperate regions.
Xylaria pteridicola Hai X. Ma & A.H. Zhu, sp. nov.
Figure 11. Xylaria pteridicola (holotype). (a–d) Stromata on decomposing petioles of Pteridium. (e–g) Stromatal surface. (h–j) Asci. (k–l) Ascal apical ring. (m–n) Ascospore with germ silt. (o) Ascospores in India ink. (p) Ascospores in Melzer’s iodine. Scale bars: a – d = 1 mm; e, g = 0.5 mm; f = 0.2 mm; h – j = 10 µm; k – p = 5 µm.

Fungal Names: FN 571664.
Etymology: pteridicola (Latin), refers to the host which the fungus inhabits.
Diagnosis: Differing from Xylaria amphithele by smaller ascospores without hyaline appendage (San Martín and Rogers Citation1989), and from Xylaria juruensis by smaller ascospores and inverted hat-shaped to urn-shaped ascal apical ring (San Martín and Rogers Citation1989).
Description: Stromata upright or prostrate, solitary, irregularly fusiform, unbranched, 0.5–5 cm total length, with a mucronate sterile apex up to 1.2 mm; fertile part usually consisting of few scattered exposed perithecial contours, carbonaceous, 2–5 mm long, 0.8–1.3 mm diam.; stipe black, glabrous, the base slightly swollen, 2.5–43 mm long, 0.3–0.5 mm diam.; externally black, interior white. Texture soft. Perithecia immersed, subglobose, 0.4–0.5 mm diam. Ostioles papillate. Asci cylindrical, long-stipitate, 102–135 µm total length, the spore-bearing part 55–70 × 5.5–7.5 µm, the stipes 28–61 µm long, with eight obliquely uniseriate ascospores, with apical ring bluing in Melzer’s iodine reagent, inverted hat-shaped to urn-shaped, 2.5–3.3 µm high, 2.0–2.5 µm broad. Ascospores light brown to brown, unicellular, ellipsoid-inequilateral, with narrowly rounded ends, one end slightly truncate sometimes, smooth, (8.4–)9–10.3(−10.7) × (4.7–)5.0–6.0(−6.5) µm (M = 9.5 × 5.5 µm, n = 30), with straight germ slit spore length, without sheath or appendages visible in India ink.
Materials examined: China. Yunnan Province, Jinghong City, Forest Park, on decomposing petioles of Pteridium sp., 22 January 2015, Hai X. Ma, Cel. 251 (holotype in FCATAS752); Cel. 296 (FCATAS 754); Dadugang Town, on decomposing petioles of Pteridium sp., 21 January 2015, Hai X. Ma, Cel. 288 (FCATAS 753).
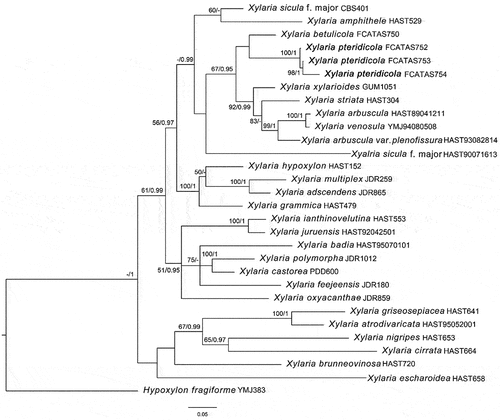
Notes: Xylaria pteridicola somewhat resembles X. amphithele and X. juruensis in stromatal morphology, but X. amphithele differs in having larger ascospores (9–10.3 × 5–6 μm vs. 12–14 × 7–8 μm) with hyaline noncellular appendage 2–5 μm long and 2–5 μm broad, and X. juruensis differs from the new species by its larger ascospores (14.5–17 × 5–5.5 μm) and larger, rectangular ascal apical ring 5–7 μm high and 2–4 μm broad (San Martín and Rogers Citation1989). Moreover, phylogenetic analysis showed that X. pteridicola is distinct from X. amphithele and X. juruensis (). In the phylogenetic tree, X. pteridicola groups together with X. betulicola from China with low support (). Moreover, X. betulicola has longer stromata with a long sterile filiform apex up to 4 cm and larger ascospores 12–14 × 5–6 μm (Ma and Li Citation2018).
Figure 12. Strict consensus tree of ITS and tub sequences of Xylaria and allied genera in Xylariaceae. Bootstrap values (≥50%, before the slash markers) and Bayesian posterior probabilities (≥0.95, after the slash markers) are shown.
Basidiomycota
Agaricomycetes
Agaricales Underw., Moulds, mildews, and mushrooms. A guide to the systematic study of the Fungi and Mycetozoa and their literature (New York): 97 (1899)
Crepidotaceae (S. Imai) Singer, Lilloa 22: 584 (1951) [1949]
Notes: Singer (Citation1949) proposed the tribe Crepidoteae with the type genus Crepidotus (Aime Citation1998; Consiglio and Setti Citation2008). A total of eight genera, namely Tubaria, Melanomphalia, Crepidotus, Simocybe, Pellidiscus, Chromocyphella, Phaeosolenia, and Episphaeria were included in Crepidoteae, which is now named Crepidotaceae s.l (Singer Citation1986). As the number of morphoanatomical and phylogenetic studies increased, some genera were grouped into Cortinariaceae or Inocybaceae (Senn-Irlet Citation1995; Aime Citation1998; Kirk et al. Citation2008; Consiglio and Setti Citation2008; Ge Citation2017; Ge and Bau Citation2020). Vizzini et al. (Citation2012) determined that Neopaxillus and Crepidotus had a sister group relationship based on an ITS and nLSU phylogenetic analysis. Additional studies were subsequently conducted. Watling and Aime (Citation2013) first proposed the framework of Crepidotaceae consisting of the genera Crepidotus, Simocybe, and Neopaxillus to develop the concept of Crepidotaceae s.s.
Crepidotus (Fr.) Staude, Schwämme Mitteldeutschl. 1: xxv, 71 (1857)
Notes: Crepidotus, typed by Crepidotus mollis, originally belonged to a tribe of Agaricus, but Donk was the first to designate it as a genus in 1857 (Consiglio and Setti Citation2008; Ge Citation2017). Crepidotus species are mainly characterised by small to medium-sized basidiomes, mostly sessile or lateral stipe, brown basidiospores, and mostly found on dead branches and deciduous leaves (Senn-Irlet Citation1995; Aime Citation1998; Consiglio and Setti Citation2008; Na et al. Citation2022). There are currently 575 recorded Crepidotus taxa (http://www.indexfungorum.org, accessed on 24 May Citation2023), most of which were described in Europe and North America. After the 20th century, most new Crepidotus taxa have been described in Asia (Hesler and Smith Citation1965; Poder and Ferrari Citation1984; Senn-Irlet Citation1991, Citation1992, Citation1993; Liu Citation1995; Aime et al. Citation2002; Ge Citation2017; Ge et al. Citation2017; Guzman-Davalos et al. Citation2017). Current research efforts in Asia are mainly concentrated in China and India. In the last decade, there have been 10 new taxa in China, 7 new taxa in India, and 1 new taxon in Pakistan (Agretious and Prasanth Citation2017; Guzman-Davalos et al. Citation2017; Kumar et al. Citation2018, Citation2020, Citation2022; Ge and Bau Citation2020; Izhar et al. Citation2021).
Crepidotus furcaticystidiosus Q. Na, M.H. Han, R.X. Wei, H. Zeng & Y.P. Ge, sp. nov.
Figure 13. Basidiomes of Crepidotus. (a–d) Crepidotus tomentellus. (e–h) Crepidotus furcaticystidiosus. Scale bars: a, d, g – h = 10 mm; b – c = 5 mm; e – f = 20 mm.

Figure 14. Microscopic features of Crepidotus furcaticystidiosus (holotype). (a–e) Basidiospore. (f–g) Basidia. (h–o) cheilocystidia. (p) Pileipellis. Scale bars: a – e = 5 μm; f – g = 10 μm; h – p = 20 μm.
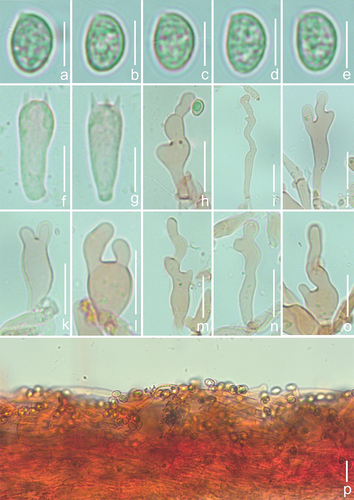
Figure 15. Morphological structures of Crepidotus furcaticystidiosus (holotype). (a) Basidiomes. (b) Basidia. (c) Basidiospores. (d) Cheilocystidia. (e) Pileipellis. Scale bars: a = 10 mm; b, d, e = 10 μm; c = 5 μm.
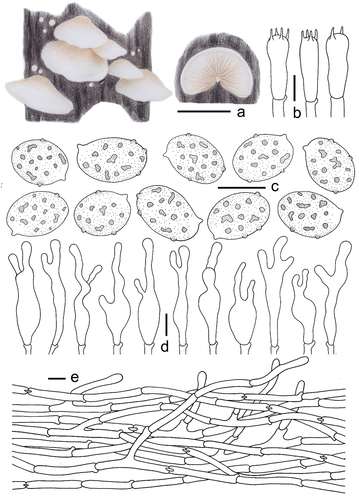
Fungal Names: FN 571591.
Etymology: furcaticystidiosus (Latin), refers to the furcate cheilocystidia.
Diagnosis: Differing from other Crepidotus species by its smooth pileus, white, basidiospores, verrucose to faintly punctate, cheilocystidia branched at the apex, and clamp connections.
Description: Basidiomes small, 5–15 mm in diam., ungulate and campanulate when young, plano-convex to shell-shaped, convex at the near base when mature, white (LIII), but sometimes Cream Colour (XVI19’f), Naples Yellow (XVI19’d), Cartridge Buff (XXX19ʺf), Cream Buff (XXX19ʺd), and Ivory Yellow (XXX21ʺf) because of the basidiospores that dropped from the upper layer of the basidiomes, surface smooth or nearly smooth, edge involuted, non-striated, non-hygrophanous, and covered with sparse and indistinct white (LIII) villose near the attachment. Lamellae 1–2 mm wide, free, L = 9–16, l = 3–9, ventricose, white (LIII) when young, from the base to the edge gradually darkening to Massicot Yellow (XVI21’f) and Straw Yellow (XVI21’d), Primuline Yellow (XVI19’), and Old Gold (XVI19’i) when mature, surface with a few dark spots, Buffy Citrine (XVI19’k), Saccardo’s Olive (XVI19’m), and edge not fimbriate. Stipe cylindrical, 0.5–1.5 mm in length, less than 0.5 mm in diam., white (LIII), nearly transparent, pubescent, and indistinct when mature. Context thin, white (LIII) or nearly transparent. Odour and taste indistinct. Basidiospores (153/6/3) (6.0–)6.3–6.9–7.5(−7.9) × (4.3–)4.5–4.9–5.3(−5.5) μm, Q = (1.20–)1.28–1.51(−1.60), Qm = 1.40 ± 0.07. HOLOTYPE (75/3/1) (6.0–)6.3–6.9–7.4(−7.7) × (4.3–)4.5–4.9–5.3 μm, Q = (1.27–)1.30–1.54(−1.59), Qm = 1.42 ± 0.07, amygdaliform to inaequilateral in the lateral view, ovoid to ellipsoid in the dorsoventral view, light brown, irregularly covered by punctate to faintly verrucous protuberances of varying sizes, sometimes short connections between protuberances. Basidia 17–26 × 6–9 μm, short clavate, obtuse apex, sometimes slightly attenuated in the middle, basal contraction, hyaline, thin-walled, 4-spored, sterigmata 2.8–5.2 μm in length, a few 2-spored. Cheilocystidia 25–64(−73) × 6–16 μm, attenuated at the base, enlarged upwards to globose, subglobose to utriform, apex bifurcate and curved, the bifurcate part is not of equal length, the long bifurcate part can reach up to half the length of cheilocystidia, when immature, cheilocystidia expand indistinctively in the middle, long cylindrical to lageniform, curved, non-bifurcate apex or bifurcate. Pileipellis a cutis, composed of cylindrical hyphae 3.7–5.6 μm wide, bifurcate, hyaline, smooth, some hyphae covered by different coloured punctate ornamentation, some hyphae vertical to the pileus.
Materials examined: China. Jilin Province, Yanbian Korean Autonomous Prefecture, Changbai Mountain Nature Reserve, gregarious on broad-leaved forest twigs, 4 July 2021, GN 1080 (holotype in FFAAS 1026), GN 1074(FFAAS 1027), GN 1093 (FFAAS 1028).
Notes: Based on the faintly punctate to warty ornamented basidiospores, C. furcaticystidiosus should be classified in the series Caspari, sect. Dochmiopus, and subg. Dochmiopus (Consiglio and Setti Citation2008). According to Giovanni’s records, there are four taxa in the series Caspari, viz. Crepidotus caspari var. caspari, Crepidotus caspari var. subglobisporus, Crepidotus fusisporus, and Crepidotus subverrucisporus, of which the basidiospores of C. fusisporus (7.9–9.5 × 4.0–5.2 μm) and C. subverrucisporus (7.7–9.4 × 5.1–6.3 μm) are significantly larger than the basidiospores of the other taxa. The basidiospores of C. caspari var. subglobisporus (5.6–6.6 × 4.5–5.3 μm, Q = 1.15–1.33) are smaller and more globose. The basidiospores of C. furcaticystidiosus are clearly distinguished from those of these three taxa. Cheilocystidia of C. caspari var. caspari are cylindrical to clavate and not branched apically, but the apical bifurcation of the cheilocystidia is the most typical characteristic of C. furcaticystidiosus. Thus, C. furcaticystidiosus can be clearly distinguished from the known European species of the series Caspari (Consiglio and Setti Citation2008).
According to the white basidiomes, absence of pleurocystidia, and morphology of the basidiospores, Crepidotus phaseoliformis, Crepidotus lanuginosus, and Crepidotus villosus in subg. Dochmiopus of sect. Phaseoli and sect. Crepidotellae are closely related to C. furcaticystidiosus. Macroscopically, the tomentose pileus and punctate to spiny ornamented basidiospores of C. villosus can be used to distinguish it from C. furcaticystidiosus. Microscopically, the pileipellis of C. phaseoliformis has a distinct gelatinous layer, whereas the pileipellis of C. lanuginosus comprises brown hyphae. Both taxa are distinct from C. furcaticystidiosus (Hesler and Smith Citation1965). In the current phylogenetic (), the two samples of C. furcaticystidiosus form monophyletic lineages with high statistical support (BS = 100, BPP = 1.00). According to the tree topology, C. furcaticystidiosus belongs to the C. cesatii lineage and is distinct from the previously known taxa of Crepidotus ().
Figure 16. Phylogenetic relationship among species of Crepidotus inferred from ITS region. The topology is generated by the maximum likelihood algorithm. Bootstrap values and Bayesian posterior probabilities, when simultaneously above 75% and 0.95, respectively, are labelled at the nodes. The newly described species are in boldface.
Crepidotus tomentellus Q. Na, M.H. Han, R.X. Wei, H. Zeng & Y.P. Ge, sp. nov.
Figure 17. Morphological structures of Crepidotus tomentellus (holotype). (a) Basidiomes. (b) Basidia. (c) Dasidiospores. (d) Cheilocystidia. (e) Pileipellis. Scale bars: a = 10 mm; b, d, e = 10 μm; c = 5 μm.
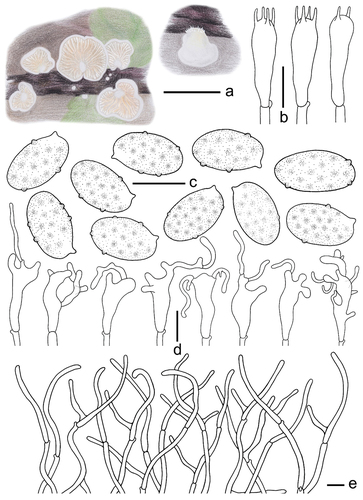
Figure 18. Microscopic features of Crepidotus tomentellus (holotype). (a–e) Basidiospore. (f–g) Basidia. (h–o) Cheilocystidia. (p) Pileipellis. Scale bars: a – e = 5 μm; f – o = 10 μm; p = 20 μm.
Fungal Names: FN 571592.
Etymology: tomentellus (Latin), refers to the tomentose pileus.
Diagnosis: Differing from other Crepidotus species by its tomentose pileus, white, basidiospores ellipsoid to cylindric, cheilocystidia usually with branching upper part, and clamp connections.
Description: Basidiomes small, 5–18 mm in diam., flabelliform to shell-shaped when young, flabelliform to nearly circular, near plano-convex when mature, convex near the attachment, densely pubescent-woolly, white (LIII), margin involuted, non-striated, non-hygrophanous, covered by obvious white (LIII) villose hyphae near the attachment, pileus colour darkens slightly when old, Cream Colour (XVI19’f), Naples Yellow (XVI19’d), and Mustard Yellow (XVI19’b), the hyphae colour darkens slightly near the attachment, Baruta Yellow (IV21f), Martius Yellow (IV23f), some hyphae near the attachment disappear. Lamellae 0.5–1.5 mm wide, free, L = 15–22, l = 3–13, ventricose, Cartridge Buff (XXX19ʺf), Ivory Yellow (XXX21ʺf), Cream Buff (XXX19ʺd) when young, Maize Yellow (IV19f), Buff Yellow (IV19d), Aniline Yellow (IV19i) when mature, the lamellae colour change is consistent, not a gradient, lamellae edge covered by short punctate villose, lamellae colour darkens when old, Orange Citrine (IV19k), Medal Bronze (IV19m), deeply coloured particles or spots at the surface, Russet (XV13’k), Cinnamon Brown (XV15’k), Prout’s Brown (XV15’m). Stipe 0.5–1.5 mm in length, 0.5–1.0 mm in diam., covered with white (LIII) pubescence. Context thin, white (LIII) or nearly transparent. Odour and taste indistinct. Basidiospores (304/6/4) (5.1–)5.5–6.3–7.1(−7.4) × (2.7–)3.0–3.3–3.7(−4.1) μm, Q = (1.60–)1.68–2.12(−2.29), Qm = 1.89 ±0.13. HOLOTYPE (112/2/1) (5.9–)6.2–6.6–7.2(−7.4) × (2.8–)3.0–3.4–3.7(−4.0) μm, Q = (1.66–)1.78–2.13(−2.29), Qm = 1.97 ±0.13, amygdaliform to inaequilateral in the lateral view, narrowly ellipsoid to cylindrical in the dorsoventral view, whitish-yellow to light dark brown, punctate to verrucous protuberance, ornamentation inapparent and difficult to detect (×1,000). Basidia 18–26 × 5–7 μm, cylindrical to short clavate, obtuse apex, sometimes curved in the middle, basal contraction, hyaline, thin-walled, 4-spored, sterigmata 2.9–3.3 μm in length, and a few 2-spored. Cheilocystidia 18–31 × 3–14 μm, unstable shape, most are clavate at the base, irregular upper part, multiple toed bifurcate, bifurcate indistinct, apex with a long cylindrical protuberance, numerous 10–30 μm in length, a few up to 50 μm in length, 2–3 μm in diam., protuberances produce a septum at the connection with the mother cell. Pileipellis a trichoderm, composed of 2.6–3.9 μm wide cylindrical hyphae, bifurcate, but few, hyaline, smooth, the hyphae are spirally or irregularly bent to form a villous structure on the pileipellis.
Materials examined: China. Heilongjiang Province, Greater Khingan Mountains region, Huzhong District, Huzhong National Nature Reserve, gregarious on twigs of Larix, 29 August 2021, GN 1423 (holotype in FFAAS 1024); Heilongjiang Province, Tahe County, Shibazhan Town, gregarious on twigs of Larix, 26 August 2021, GN 1429 (FFAAS 1030), GN 1433 (FFAAS 1029), GN 1434 (FFAAS 1025).
Notes: Based on the elongated ellipsoid to cylindrical basidiospores, Crepidotus tomentellus should be classified in the series Dochmiopus, sect. Dochmiopus, and subg. Dochmiopus (Consiglio and Setti Citation2008). There are seven taxa in the series Dochmiopus, of which Crepidotus variabilis var. variabilis, Crepidotus neotrichocystis, and Crepidotus variabilis var. trichocystis are closely related to C. tomentellus. Microscopically, the basidiospores of C. tomentellus are elongated ellipsoid to cylindrical. The cheilocystidia are much narrower and distinct from those of C. variabilis var. variabilis, C. neotrichocystis, and C. variabilis var. trichocystis (Senn-Irlet Citation1995; Consiglio and Setti Citation2008; Jančovičová et al. Citation2020). Although species in the C. variabilis lineage have no special substrate requirements, they are rarely found on twigs in coniferous forests. In contrast, specimens of C. tomentellus have been found on twigs of Larix trees, which is a distinct feature in Crepidotus (Jančovičová et al. Citation2017). The phylogenetic analysis revealed that C. tomentellus belongs to the C. variabilis lineages, and was resolved as monophyletic with strong support (BS = 100, BPP = 1.00; ).
Entolomataceae Kotl., Pouzar, Česká Mykol. 26(4): 218 (1972)
Notes: The family Entolomataceae was introduced by Kotlába and Pouzar (Citation1972) with the type genus Entoloma. Entolomataceae is a species-rich family that occurs worldwide from arctic to tropical habitats with seven accepted genera viz. Clitocella, Clitopilopsis, Clitopilus, Entocybe, Entoloma, Rhodocybe, and Rhodophana (Co-David et al. Citation2009; He et al. Citation2019; Noordeloos and Hausknecht Citation2007). The family is highly variable in terms of basidiomes and micromorphology (Noordeloos Citation2004). The majority of species live as saprotrophic communities on soil, wood, or moss, while others are parasitic on other mushrooms and plants, or ectomycorrhizal fungi (Antibus et al. Citation1981; Agerer and Waller Citation1993; Noordeloos Citation2004; Montecchio et al. Citation2006). Co-David et al. (Citation2009) first conducted a thorough molecular phylogenetic analysis of the Entolomataceae by multigene analysis (mtSSU, nLSU, and rpb2).
Clitocella Kluting, T.J. Baroni & Bergemann, Mycologia 106(6): 1135 (2014)
Notes: Clitocella (Entolomataceae, Agaricales) was established with C. popinalis as the type species (Kluting et al. Citation2014). The genus Clitocella is characterised by clitocyboid basidiomes; convex to plano-convex or applanate pileus; narrow and crowded, decurrent lamellae; central to eccentric stipe; thin-walled (<0.5 μm) basidiospores with undulate pustules or minute bumps; presence of clamp connections; and usually absence of pleurocystidia and cheilocystidia (Baroni et al. Citation2020; Jian et al. Citation2020; Mao et al. Citation2022).
Clitocella neofallax W.H. Lu, Karun. & S. Tibpromma, sp. nov.
Figure 19. Basidiomes of Clitocella neofallax (holotype). (a) Upper surface. (b) Lower surface. Scale bars: 1 cm.
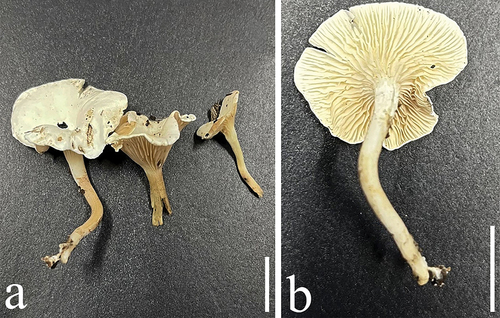
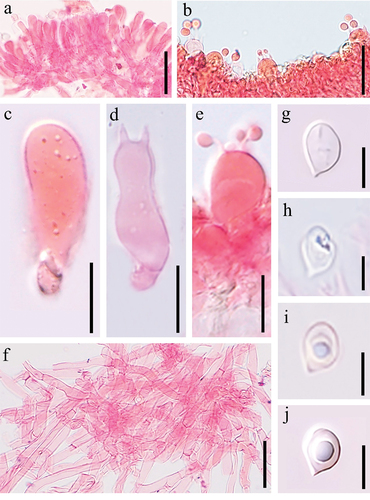
Figure 20. Microscopic structures of Clitocella neofallax (holotype). (a) Hymenium showing basidioles. (b–e) Basidia. (f) Pileipellis. (g–j) Basidiospore. Scale bars: a, b, f = 20 μm; c – e = 10 μm; g – j = 5 μm.
Fungal Name: FN 571612.
Etymology: neofallax (Latin), reflects its morphological similarity to Clitocella fallax.
Diagnosis: Differing from Clitocella fallax by slightly smaller basidiospores and slightly shorter basidia.
Description: Basidiomes clitocyboid, small. Pileus 10–30 mm wide, dry, low convex, sometimes infundibuliform, with a shallow depression at the centre; margin incurved, not striate, often enrolled or flat, sometimes slightly uplifted; surface pale white to greyish white (1B1), context white to greyish white (1B1). Lamellae decurrent, yellowish white (2A2) to pale yellow (2A3), becoming greyish yellow (3B4) or dull yellow (3B5) on drying, crowded, edges entire and concolorous, thin and fragile, lamellulae numerous and concolorous with lamellae. Stipe 10‒25 × 1‒2 mm, central to eccentric, occasionally lateral, cylindrical to subcylindrical, equal or sometimes slightly tapering at base, pale white to greyish yellow (4B6), smooth or tomentose. Odour unrecorded. Taste not recorded. Chemical colour reaction: not reacting with KOH 5% at pileus of dried specimens. Basidiospores ellipsoid to ovoid, occasionally amygdaliform, hyaline, smooth, oil drop at central, obscure pustules (4.0–)4.8–6.3 × (3.5–)4–5 μm, L = 5.3 μm, W = 4.1 μm, Q = 1.2 (n = 20). Basidia clavate, hyaline, with four sterigmata, 15‒23.5 × 6‒9 μm; basidioles similar in shape to basidia, but smaller. Stipitipellis a cutis composed of parallel, compactly arranged, thin-walled, hyaline hyphae, 1‒2 μm wide. Pleurocystidia and cheilocystidia absent.
Materials examined: China. Yunnan Province, Qujing City, Qujing Normal University, on the soil associated with bamboo roots, 25°31’37’’ N, 103°44’40” E, 11 June 2022, QJ 093 (holotype in HKAS 128149); ibid. Wenhua Lu, QJS10 (HKAS 128152).
Notes: In the multilocus phylogenetic tree, Clitocella neofallax formed a distinctly separate sister clade to C. fallax strains with 100% BS and 1.00 of BPP (). Morphologically, Clitocella neofallax closely resembles C. fallax and C. borealichinensis by basidia and basidiospores. However, C. fallax has longer basidia and slightly larger basidiospores 6.5–8 × 4–5 µm vs. (4.0–)4.8–6.3 × (3.5–)4–5 μm (Jian et al. Citation2020), while C. borealichinensis differs by clitocyboid basidiomes in having small basidia and larger pileus (13‒50 μm), and being reported on the ground in a broad-leaved forest (Mao et al. Citation2022). Moreover, C. neofallax is easily confused with C. mundula, but C. mundula differs from C. neofallax by its yellowish grey or brown to dark smoky grey pileus, and somewhat larger basidiospores (4–)4.5–6(−6.5) × 4–5 μm (Jian et al. Citation2020). Based on phylogeny and morphology, our specimens are identified as a new species C. neofallax. In China, four species have been reported from the genus Clitocella, including three new species Clitocella orientalis, C. colorata, C. borealichinensis, and one new record C. mundula (Jian et al. Citation2020; Mao et al. Citation2022), and the diversity of Clitocella will be high based on morphology and phylogeny in the future.
Figure 21. The phylogenetic relationship among species of Clitocella inferred from the combined dataset of nLSU, rpb2, and tef1 regions. The maximum likelihood algorithm has generated the topology. Bootstrap values and Bayesian posterior probabilities, when simultaneously above 70% (left) and 0.90 (right), respectively, are labelled at the nodes. The newly described species is in black bold.
Physalacriaceae Beihefte zur Nova Hedwigia 33: 10 (1970)
Notes: Physalacriaceae belongs to Agaricales (Agaricomycetes) and is typified by Physalacria (Peck Citation1882; Park et al. Citation2017). The family was originally described by Corner (Citation1970) and revised by Berthier (Citation1985). Approximate 21 genera have been reported from Physalacriaceae worldwide (Park et al. Citation2017; Wani et al. Citation2021), and the family was confirmed as a monophyletic group by phylogenetic analyses (Moncalvo et al. Citation2002; Matheny et al. Citation2006; Park et al. Citation2017). This family is characterised by possessing highly variable basidiomes ranging from agaricoid, secotioid, cantharelloid to corticoid, narrowly clavate basidia with two to four basidiospores, and smooth, thin-walled, ellipsoidal, fusiform, cylindrical, or lacrimiform basidiospores (Park et al. Citation2017). Most species in Physalacriaceae are saprobic on decaying leaves and wood, and several species are parasitic (Park et al. Citation2017; Wani et al. Citation2021).
Physalacria Peck, Bull. Torrey bot. Club 9(1): 2 (1882)
Notes: Physalacria typified by P. inflata, includes more than 40 species (Qin and Yang Citation2016; Crous et al. Citation2022). Most species in this genus have shorter than 10 mm, stipitate-capitate (hollow, inflated, head-like caps) basidiomes, a geotropic and smooth hymenium on the surface of the hollow head, sterile stipe, smooth and inamyloid basidiospores, and abundant clamp connections (Berthier Citation1985; He and Xue Citation1996; Desjardin and Hemmes Citation2001; Qin and Yang Citation2016). Physalacria auricularioides has discoid-peltate and cupulate basidiomes with an auriculiform hymenophoral surface and negative geotropism, and is the only one species to be described in Europe (Crous et al. Citation2022). Physalacria has a wide distribution range, being reported in North America, South America, Central America, Oceania, Africa, Europe, and Asia, but mostly in the Southern Hemisphere and the tropics (Kobayasi Citation1951; Berthier Citation1985; He and Xue Citation1996; Inderbitzin and Desjardin Citation1999; Antonín and Mossebo Citation2002; Qin and Yang Citation2016; Crous et al. Citation2022).
Physalacria tianzhongshanensis A. Tohtirjap, L.R. Zhang & F. Wu, sp. nov.
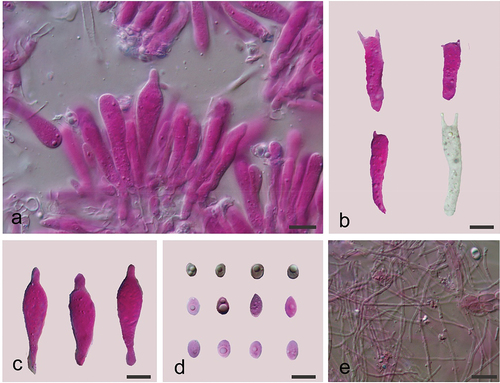
Figure 22. Basidiomes of Physalacria tianzhongshanensis (holotype). (a) A distant view. (b) A close shot. Scale bars = 1 cm.

Figure 23. Microscopic structures of Physalacria tianzhongshanensis (holotype). (a) A vertical section of basidiomes. (b) Basidia. (c) Cystidia. (d) Basidiospores. (e) Hyphae. Scale bars: 10 μm.
Fungal Names: FN 571652.
Etymology: tianzhongshanensis (Latin), referring to the species being found in Tianzhongshan.
Diagnosis: Differing from Physalacria auricularioides by smaller basidia and basidiospores, sessile, whitish to cream, soft coriaceous basidiomes.
Description: Basidiomes soft coriaceous, more or less gelatinous when fresh, whitish to cream, single, scattered, in group, 2–5 mm, discoid-peltate, cupulate, sessile, hymenial surface smooth, negative geotropic, becoming coriaceous and cream to buff after drying. Margin not lobed and rounded. Hyphal system monomitic; usually simple septate, hyaline, thin-walled, and 0.5–2.0 µm in diam. Basidia clavate or cylindrical, thin-walled, with 1–4 sterigmata up to 4 μm long, 17.2–37.2 × 6.2–7.8 μm; basidioles similar in shape to basidia, but smaller. Basidiospores hyaline, thin-walled, smooth, subglobose, ellipsoid to ovoid, subovoid, subcitriform, lacrymoid, usually with one or two oil drops, (5.8–)6.5–10.2(−11.0) × (4.0–)4.5–5.5(−5.8) µm, L = 8.12 µm, W = 5.07 µm, Q = 1.59 (n = 30/1). Cystidia subfusiform, apex narrow, thin-walled, colourless and hyaline, 34.5–42.2 × 9.5–11.4 μm.
Materials examined: China. Zhejiang Province, Hangzhou City, Fuyang District, Tianzhongshan, on fallen angiosperm branch, 26 March 2023, Wu 653 (holotype in BJFC039977), Wu 907 (BJFC040387).
Notes: Physalacria tianzhongshanensis closely resembles P. auricularioides by sharing discoid-peltate and cupulate basidiomes, but the latter species has a stipe or rudimentary pseudo-stipe, hard and leathery basidiomes when fresh, larger basidia (26.6–42.0 × 6.7–12.5 µm) and basidiospores (8.4–12.5 × 5.1–6.8 µm; Crous et al. Citation2022). The new species is also closely related to P. auricularioides in our phylogeny (), but the ITS similarity between P. auricularioides (AMI-SPL676) and P. tianzhongshanensis is only 95.61% (Wu 653) and 95.15% (Wu 907) of 753 bp.
Figure 24. Phylogenetic relationship among species of Physalacria inferred from the combined dataset of ITS and nLSU regions. The topology is generated by the maximum likelihood algorithm. Bootstrap values and Bayesian posterior probabilities, when simultaneously above 50% and 0.8, respectively, are labelled at the nodes. The newly described species are in boldface.
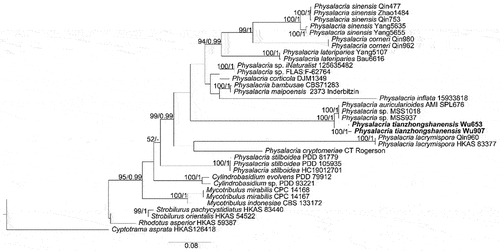
In addition, Physalacria subpeltata also resembles the new species by its discoid-peltate basidiomes, but it has a stipe, larger basidia (30–55 × 9–10 µm) and basidiospores (10–12.5 × 4.5–5 μm), and distribution in Hawaii (Berthier Citation1985). Moreover, P. subpeltata was transferred in Anastrophella by Horak (Horak et al. Citation1994), and its molecular data is not available, so the species is not sure to be one of Physalacria.
Polyporales Gäum., Vergleichende Morphologie der Pilze: 503 (1926)
Phanerochaetaceae Jülich, Bibliotheca Mycologica 85: 384 (1982)
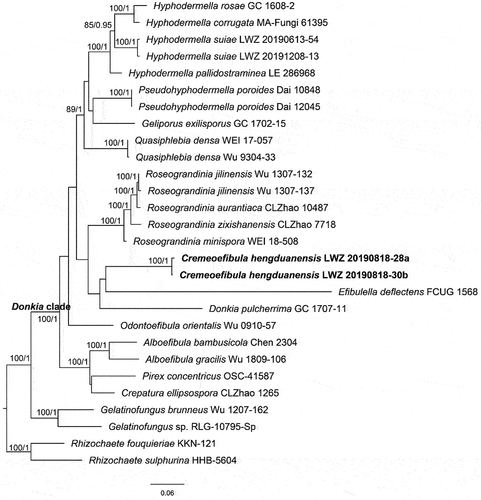
Notes: Phanerochaetaceae is a globally distributed group of wood-inhibiting fungi. This family is typified by Phanerochaete with P. alnea as the type species (Spirin et al. Citation2017). Phanerochaetaceae consists of four well-recognised clades: the Phanerochaete, Donkia, Phlebiopsis, and Bjerkandera clades, encompassing a total of 23 genera (Chen et al. Citation2021). In recent years, the known number of taxa within the Donkia clade has significantly increased (Chen et al. Citation2021; Shen et al. Citation2023). In this study, one more new species occupying an independent position from all known genera was revealed from this clade ().
Figure 25. Phylogenetic relationship among Cremeoefibula and related genera inferred from the combined dataset of ITS, nLSU, and rpb2 regions. The topology is generated by the maximum likelihood algorithm. Bootstrap values and Bayesian posterior probabilities, when simultaneously above 50% and 0.8, respectively, are labelled at the nodes. The newly described species are in boldface.
Cremeoefibula S.L. Liu, Shan Shen & L.W. Zhou, gen. nov.
Fungal Names: FN 571240.
Etymology: Cremeoefibula (Latin), refers to cream basidiomes and simple-septate hyphae.
Type species: Cremeoefibula hengduanensis S.L. Liu, Shan Shen & L.W. Zhou.
Diagnosis: Differing from Efibulella in the absence of cystidia (Li et al. Citation2022).
Basidiomes annual, effused, adnate, membranaceous to subceraceous. Hymenophore cream to straw-yellow, smooth to tuberculate, unchanged in KOH.
Hyphal system monomitic; generative hyphae without clamp connections. Subicular hyphae hyaline, thin to slightly thick-walled. Cystidia absent. Basidia clavate, thin-walled, with four sterigmata. Basidiospores ellipsoid, hyaline, thin-walled, smooth, inamyloid, acyanophilous. On wood.
Notes: Cremeoefibula is characterised by having cream to straw-yellow, membranaceous to subceraceous basidiomes with smooth to tuberculate hymenophores, simple-septate generative hyphae, the absence of cystidia and ellipsoid basidiospores. Morphologically, Efibulella closely resembles Cremeoefibula; however, the presence of leptocystidia in Efibulella is a distinguishing characteristic from the new genus (Zmitrovich Citation2018).
Cremeoefibula hengduanensis S.L. Liu, Shan Shen & L.W. Zhou, sp. nov.
Fungal Names: FN 571261.
Etymology: hengduanensis (Latin), refers to the type locality Hengduan Mountains.
Diagnosis: Characterised by the membranaceous to subceraceous basidiomes with smooth to tuberculate hymenophores, the absence of cystidia, and ellipsoid basidiospores.
Description: Basidiomes annual, resupinate, effused, closely adnate, inseparable from substrate, membranaceous to subceraceous, first as small patches, later confluent up to 10 cm long, 1.5 cm wide, up to 200 µm thick in section. Hymenophore smooth to tuberculate, cream to straw-yellow, unchanged in KOH, not cracked; margin concolorous with hymenophore, slightly arachnoid.
Hyphal system monomitic; generative hyphae without clamp connections; subicular hyphae hyaline, thin-walled to slightly thick-walled, moderately branched, 3–5 µm in diam. Cystidia absent. Basidia clavate, with four sterigmata and a simple septum, 20–26 × 2.5–3 µm. Basidiospores ellipsoid, hyaline, thin-walled, smooth, inamyloid, acyanophilous, 3.8–4.5 × 1.8–2.4 µm, L = 4.1 µm, W = 2.1 µm, Q = 1.9 (n = 60/2).
Materials examined: China. Sichuan Province, Meigu County, Dafengding National Nature Reserve, on fallen angiosperm branch, 18 August 2019, LWZ 20190818-28a (holotype in HMAS 257914); on fallen angiosperm branch, 18 August 2019, LWZ 20190818-30b (paratype in HMAS 257915).
Notes: From the phylogenetic tree, Cremeoefibula hengduanensis formed by two specimens is adjacent to Efibulella deflectens but without reliable statistical support (). Morphologically, the macroscopic appearance of E. deflectens is similar to C. hengduanensis, but differs in the presence of cystidia (Zmitrovich Citation2018).
Irpicaceae Spirin & Zmitr., Mycena 3: 48 (2003)
Notes: Irpicaceae was introduced in Polyporales with Irpex as a genus by Spirin (Citation2003) and currently comprises 13 genera, including a recently described genus Phanerochaetella (Chen et al. Citation2021). The majority of species in Irpicaceae are white rot fungi except for the genus Leptoporus, which is known for causing brown rot (Chen et al. Citation2021).
Phanerochaetella C.C. Chen & Sheng H. Wu, Fungal Diversity 111: 415 (2021)
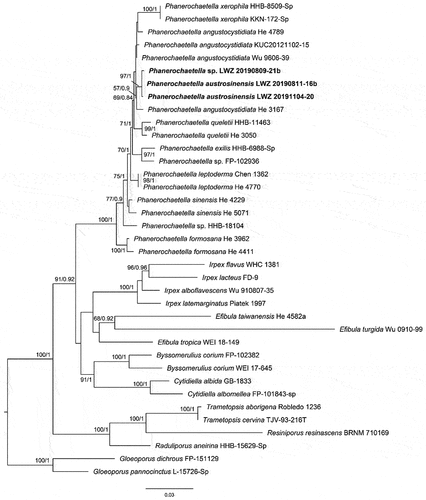
Notes: Phanerochaetella was introduced by Chen et al. (Citation2021), who treated P. angustocystidiata as the type species. This genus is characterised by yellowish cream and membranaceous basidiomes with smooth to tuberculate hymenophores, simple-septate generative hyphae, and ellipsoid to cylindrical basidiospores (Chen et al. Citation2021). Cystidia may be absent or present in the species of Phanerochaetella (Chen et al. Citation2021). Currently, seven species are accepted in this genus worldwide (Li et al. Citation2022). This study uncovered a new distinct lineage from other species within this genus ().
Figure 28. Phylogenetic relationship among species of Phanerochaetella inferred from the combined dataset of ITS and nLSU regions. The topology is generated by the maximum likelihood algorithm. Bootstrap values and Bayesian posterior probabilities, when simultaneously above 50% and 0.8, respectively, are labelled at the nodes. The newly described species are in boldface.
Phanerochaetella austrosinensis S.L. Liu, Shan Shen & L.W. Zhou, sp. nov.
Fungal Names: FN 571658.
Etymology: austrosinensis (Latin), refers to the distribution in southern China.
Diagnosis: Differing from P. sinensis in its wider basidiospores (Li et al. Citation2022).
Description: Basidiomes annual, effused, adnate, inseparable from substrate, membranaceous to coriaceous, up to 10 cm long, 2 cm wide, up to 300 µm thick in section. Hymenophore smooth, white to cream, unchanged in KOH; margin paler or concolorous with hymenophoral surface.
Hyphal system monomitic; generative hyphae without clamp connections. Subicular hyphae indistinct, hyaline, thick-walled, smooth, rarely branched, parallel to substrate, 3–5 µm in diam. Lamprocystidia arising from hymenial layer or medullary layer, cylindrical, hyaline, thick-walled, heavily encrusted, 35–65 × 6–11 µm. Basidia clavate to subcylindrical, hyaline, thin-walled, smooth, with four sterigmata and a basal simple septum, 28–35 × 4–6 µm; basidioles in shape similar to basidia but slightly smaller. Basidiospores cylindrical, hyaline, thin-walled, smooth, inamyloid, acyanophilous, 5–7 × 3–4 µm, L = 5.9 µm, W = 3.3 µm, Q = 1.8–1.9 (n = 60/2).
Materials examined: China. Yunnan Province, Dali, Cangshan-Erhai National Nature Reserve, on fallen trunk of angiosperm, 4 November 2019, LWZ 20191104–20 (holotype in HMAS 257916); Sichuan Province, Xichang, Luoujishan Scenic Spot, on fallen Pinus branch, 11 August 2019, LWZ 20190811-16b (paratype in HMAS 257917).
Notes: Phanerochaetella austrosinensis and P. xerophila are similar in the presence of smooth to tuberculate hymenophores and larger basidiospores than other species in the genus. However, the main difference between them is that P. xerophila lacks cystidia (Burdsall Citation1985).
A specimen, viz. LWZ 20190809-21b collected from Mianning County, the Hengduan Mountains exhibits larger basidiospores (6.9–8.6 × 4.5–5.4 µm) than P. austrosinensis, but cannot be differentiated through the ITS and nLSU regions from this species (). The situation with almost identical ITS and nLSU sequences but differentiated morphological characteristics was also observed between Basidioradulum mayi and B. tasmanicum when Wang et al. (Citation2020) described these two new species. However, due to only one specimen being available in the current case, we would not like to describe one more new species.
Trechisporales K.H. Larss., in Hibbett et al., Mycol. Res. 111(5): 541 (2007)
Hydnodontaceae Jülich, Biblthca Mycol. 85: 372 (1982) [1981]
Notes: Hydnodontaceae was established by Jülich (Citation1981), with Hydnodon as the type genus. However, Hydnodon is now recognised as a later synonym of Trechispora (Ryvarden Citation2002). Currently, 12 genera are accepted within the family Hydnodontaceae (Liu et al. Citation2022). For the latest taxonomic emendation of this family as well as Trechisporales and Sistotremastrales, please refer to Liu et al. (Citation2022).
Subulicystidium Parmasto, Conspectus Systematis Corticiacearum: 120 (1968)
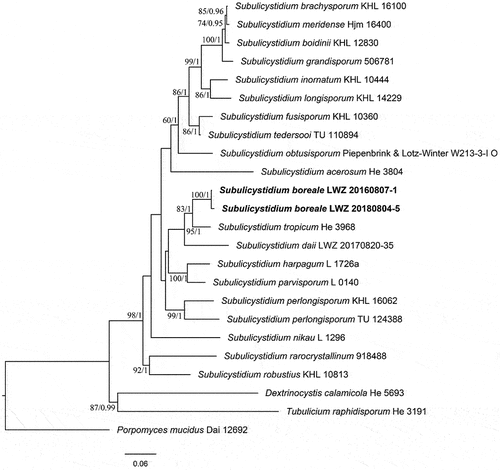
Notes: Subulicystidium, typified by S. longisporum, has unique long subulate or sword-like cystidia in Trechisporales (Ordynets et al. Citation2018; Liu et al. Citation2022). Fifteen new species have been described in the tropical and subtropical areas of the world over the past five years (Ordynets et al. Citation2018; Liu et al. Citation2019, Citation2022). Now, a new species, occupying a distinctive position on the phylogenetic tree (), is described from the temperate forests of China below.
Figure 31. Phylogenetic relationship among species of subulicystidium inferred from the combined dataset of ITS and nLSU regions. The topology is generated by the maximum likelihood algorithm. Bootstrap values and Bayesian posterior probabilities, when simultaneously above 50% and 0.8, respectively, are labelled at the nodes. The newly described species are in boldface.
Subulicystidium boreale S.L. Liu & L.W. Zhou, sp. nov.
Fungal Names: FN 571659.
Etymology: boreale (Latin), refers to the distribution in northern China.
Diagnosis: Differing from S. tropicum in its wider basidiospores and a boreal distribution (Liu et al. Citation2019).
Description: Basidiomes annual, resupinate, effused, thin, up to 10 cm long, 3 cm wide. Hymenophore smooth, white to orange-grey, not cracked; margin undifferentiated.
Hyphal system monomitic; generative hyphae with clamp connections, hyaline, slightly thick-walled, frequently branched and septate, loosely interwoven, 2.5–3.5 µm in diam. Cystidia abundant, subulate, projecting beyond hymenium, hyaline, thick-walled, 60–90 × 4–5 µm. Basidia subclavate to suburniform, hyaline, thin-walled, with four sterigmata and a basal clamp connection, 12–15 × 5–6 µm; basidioles in shape similar to basidia, but slightly smaller. Basidiospores fusiform to slightly vermicular, hyaline, thin-walled, smooth, inamyloid, acyanophilous, 10–13 × (1.8–)2–2.5(−2.8) µm, L = 11.4 µm, W = 2.2 µm, Q = 5.1 (n = 60/2).
Materials examined: China. Jilin Province, Antu County, Changbaishan National Nature Reserve, on fallen angiosperm branch, 7 August 2016, LWZ 20160807-1 (holotype in HMAS 257918); Beijing, Xiaolongmen Forest Park, on fallen angiosperm branch, 5 August 2018, LWZ 20180804-5 (paratype in HMAS 257919).
Notes: Subulicystidium boreale may be confused with S. fusisporum by whitish hymenophores, and similar cystidia and basidiospores. However, S. fusisporum has slightly wider basidiospores (2.5–3.5 µm in width; Ordynets et al. Citation2018).
Trechispora P. Karst.
Notes: Trechispora was introduced by Karsten (Citation1890) with T. onusta as the type species. Species in this genus exhibit a diverse range of hymenophoral configurations, including smooth, grandinioid, odontioid, hydnoid, and poroid forms. A total of 149 names have been recorded in Index Fungorum (http://www.indexfungorum.org) and 93 species have been previously recorded around the world (Liu et al. Citation2022; Luo and Zhao Citation2022; Sommai et al. Citation2023). Based on the latest comprehensive phylogenetic backbone of Trechispora provided by Liu et al. (Citation2022), a new lineage is discovered () and described as a new species in the present study.
Figure 34. Phylogenetic relationship among species of Trechispora inferred from the combined dataset of ITS and nLSU regions. The topology is generated by the maximum likelihood algorithm. Bootstrap values and Bayesian posterior probabilities, when simultaneously above 50% and 0.8, respectively, are labelled at the nodes. The newly described species are in boldface.
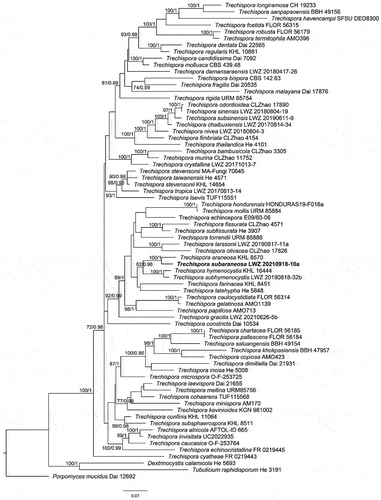
Trechispora subaraneosa S.L. Liu, H.W. Wei & L.W. Zhou, sp. nov.
Figure 36. Microscopic structures of Trechispora subaraneosa (holotype). (a) Vertical section of basidiomes. (b) Hyphae in subiculum. (c) Basidia. (d–f) Basidiaspores. (g) Crystals. Scale bars: a – c = 10 µm; d, g = 5 µm; e = 2 µm; f = 1 µm.
Fungal Names: FN 571660.
Etymology: subaraneosa (Latin) referring to the similarity to T. araneosa.
Diagnosis: Differing from T. araneosa in smaller basidiospores and a distribution in the Northern Hemisphere (Larsson Citation1995).
Description: Basidiomes annual, resupinate, effused, soft and fragile, thin, easily separated from substrates, up to 10 cm long, 3 cm wide. Hymenophore at first smooth, with age grandinioid, white to greyish white when fresh, greyish white when dry; margin thinning out, arachnoid, concolorous, about 2 mm wide. Cords frequent in subiculum, white.
Hyphal system monomitic; generative hyphae with clamp connections. Subicular hyphae distinct, hyaline, thin to slightly thick-walled, frequently branched, smooth, interwoven, 3.5–5.5(−8) μm in diam. Cystidia absent. Crystals butterfly-like, easily broken into irregular shapes. Basidia cylindrical with a slight median constriction, hyaline, thin-walled, with four sterigmata and a basal clamp connection, 9–13 × 4–5.5 µm; basidioles in shape similar to basidia, but slightly smaller. Basidiospores ellipsoid, hyaline, slightly thick-walled, aculeate, inamyloid, acyanophilous, (3.1–)3.2–4 × (2.4–)2.8–3 µm excluding the aculei, L = 3.7 µm, W = 2.9 µm, Q = 1.3 (n = 30/1).
Material examined: China. Hubei Province, Huanggang County, Taohuachong Scenic Spot, on fallen angiosperm branch, 18 September 2021, LWZ 20210918-10a (holotype in HMAS 257920).
Notes: Trechispora subaraneosa is characterised by white to greyish white basidiomes, smooth to grandinioid hymenophores, a monomitic hyphal system, butterfly-like crystals, and aculeate basidiospores. Trechispora subaraneosa has tuberculate ornamentations on the basidiospore aculei (), which reminds us of T. araneosa known only in Australia (Larsson Citation1995). Trechispora minima is morphologically similar to T. subaraneosa, but differs in shorter basidiospores and the absence of butterfly-like crystals (Larsson Citation1996).
Atractiellomycetes
Atractiellales Oberw. & Bandoni, Can. J. Bot. 60(9): 1740 (1982)
Phleogenaceae Weese, Ber. dt. bot. Ges. 37(10): 518 (1920)
Notes: Phleogenaceae belongs to Atractiellales (Atractiellomycetes) and is typified by Phleogena. This family is characterised by stipitate, capitate basidiomes, 4-celled basidia, thick-walled basidiospores, and clamped hyphae (Bandoni and Oberwinkler Citation1982). Currently, some species with effused and gelatinous basidiomes, thin-walled basidiospores, and simple-septate hyphae are included in the family (Schoutteten et al. Citation2018; Malysheva et al. Citation2019).
Helicogloea Pat., in Patouillard & Lagerheim, Bull. Soc. mycol. Fr. 8(3): 121 (1892)
Notes: Helicogloea was described by Patouillard and de (Citation1892) based on a single species H. lagerheimii from Ecuador. The genus is characterised by effused basidiomes varying in texture from soft gelatinous to floccose, probasidia with a lateral probasidial sac, in which karyogamy occurs, and segmented hypobasidia with the segments in linear series (Baker Citation1936, Citation1946; Wells Citation1990; Spirin et al. Citation2018; Malysheva et al. Citation2019). Nowadays, a total of 36 valid names are listed in Index Fungorum (www.indexfungorum.org/Names/Names.asp, accessed on 3 July 2023), but only 17 species were confirmed by molecular data (Malysheva et al. Citation2019). Helicogloea species were widely reported in North America, South America, Europe, and Asia, but mostly in Europe and South America (Spirin et al. Citation2018; Malysheva et al. Citation2019).
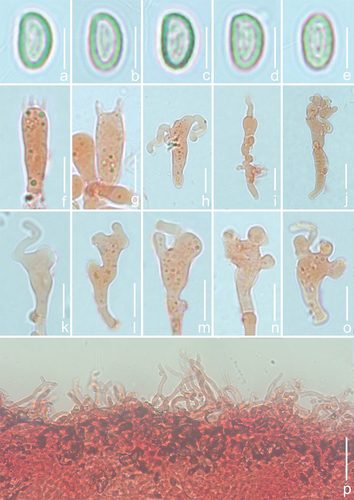
Helicogloea hangzhouensis F. Wu, L.R. Zhang & A. Tohtirjap, sp. nov.
Figure 37. Microscopic structures of Helicogloea hangzhouensis (holotype). (a,b) A vertical section of hymenium [basidia indicated by an arrow in (a) and basidiospores indicated by an arrow in (b)]. (c) Hyphae. (d) Probasidia indicated by an arrow. (e) Hyphidia indicated by an arrow. (f) Basidiospores. Scale bars: 10 μm.
![Figure 37. Microscopic structures of Helicogloea hangzhouensis (holotype). (a,b) A vertical section of hymenium [basidia indicated by an arrow in (a) and basidiospores indicated by an arrow in (b)]. (c) Hyphae. (d) Probasidia indicated by an arrow. (e) Hyphidia indicated by an arrow. (f) Basidiospores. Scale bars: 10 μm.](/cms/asset/cc299e4f-1eee-488a-83f5-44e2ae5a760b/tmyc_a_2316066_f0037_oc.jpg)
Figure 38. Basidiomes of Helicogloea hangzhouensis. (a) Wu 642 (holotype). (b) Wu 652. Scale bars: = 1 cm.

Fungal Names: FN 571651.
Etymology: hangzhouensis (Latin), refers to the species distributed in Hangzhou, China.
Diagnosis: Differs from H. septifera by shorter basidia, no tuberculate on hymenophoral surface, and never septate basidiospores.
Description: Basidiomes annual, gelatinous when fresh, hyaline to whitish, first postulate to cerebriform, then fusing together, effused, continuous, up to 3 cm long, 1 cm wide and 0.1 cm thick, almost invisible when dry. Hyphal system monomitic; simple septate, hyaline, thin-walled, 2.5–4.0 µm in diam. Probasidia abundant, saccate-clavate, more or less straight to somewhat sinuous, 30.2–39.5 × 8.1–10.5 µm, having basal cylindrical hyphidia. Basidia tubular-clavate, 4-celled, sterigmata straight to curved, transversally septate, thin-walled, 45.8–68.5 × 5.2–7.5 μm. Basidiospores germinating by germ tubes, cylindrical to broadly cylindrical, slightly or distinctly curved, hyaline, thin-walled, (10.8–)12.1–15.1(−18.2) × (5.8–)6.2 − 8.6(−9.2) μm, L = 13.82 μm, W = 7.23 μm, Q = 1.91 (n = 30/1).
Materials examined: China. Zhejiang Province, Hangzhou, Xiaoshan District, Shiniushan, on fallen angiosperm branch, 27 March 2023, Wu 642 (holotype in BJFC039975), Wu 652 (BJFC039976).
Notes: Helicogloea hangzhouensis is morphologically similar to H. microsaccata, but H. microsaccata differs by its thinner basidiomes (0.03–0.06 mm thick), and shorter probasidia (15–27 × 7–11 μm). The new species is closely related to H. septifera in our phylogeny (), but the ITS similarity between H. septifera (LE 253866, LE 260, and VS 11043) and H. hangzhouensis (Wu 642 and Wu 652) is only 92.81% of 583 bp, and H. septifera has longer basidia (56–101 × 6.8–9.0 μm), often tuberculate hymenophoral surface, and occasionally 1–2-septate basidiospores (Spirin et al. Citation2018). In addition, H. aseptata also resembles the new species by its whitish, gelatinous, and effused basidiomes, but H. hangzhouensis has larger basidiospores (10.3–13.8 × 5.8–8.1 μm in H. aseptata) and is distantly related to H. aseptata as a single lineage with high support in the phylogeny ().
Figure 39. Phylogenetic relationship among species of Helicogloea inferred from the combined dataset of ITS and nLSU regions. The topology is generated by the maximum likelihood algorithm. Bootstrap values and Bayesian posterior probabilities, when simultaneously above 50% and 0.8, respectively, are labelled at the nodes. The newly described species are in boldface.
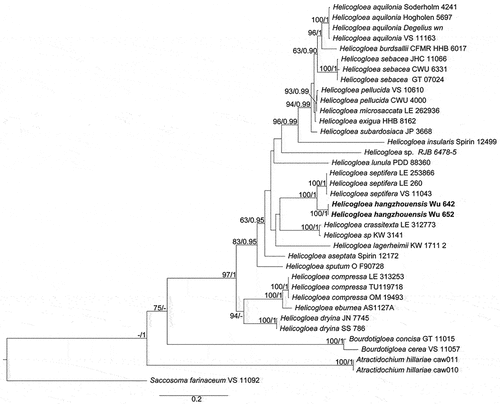
Pucciniomycetes
Pucciniales
Hyalopsoraceae P. Zhao and L. Cai, Mycology 10.1080/21501203.2022.2089262: 19.2022
Notes: Cummins and Hiratsuka (Citation1983, Citation2003) included Hyalopsora, Melampsoridium, and other seven genera in Pucciniastraceae, and Cummins and Hiratsuka (Citation2003) also considered Coleopuccinia as a synonym of Gymnosporangium (Gymnosporangiaceae) based on teliospore similarities. Phylogenetic results indicated that these three genera were allied but distinct from other members (especially the type genus) in the families Gymnosporangiaceae and Pucciniastraceae (Zhao et al. Citation2020). Based on phylogenetic results and detailed morphological comparisons, a new family Hyalopsoraceae was proposed to include Coleopuccinia, Hyalopsora, and Melampsoridium (Zhao et al. Citation2023).
Coleopuccinia Pat., Revue mycol., Toulouse 11(no. 41): 36.1889
Notes: The genus Coleopuccinia was established by Patouillard (Citation1889) with C. sinensis, which occurs on Cotoneaster species, as the type species. The second species, C. kunmingensis, was described by Tai (Citation1948) from Cotoneaster franchetii. Until recently, Coleopuccinia has only been reported in China and has only been described in the telial stage on plants of Cotoneaster. Cao et al. (Citation2018) treated C. kunmingensis as a synonym of C. sinensis due to their morphological similarities and revealed that Coleopuccinia should be treated as a separate genus from Gymnosporangium. In our previous studies, we assigned the genus Coleopuccinia to the family Hyalopsoraceae (Zhao et al. Citation2023).
Coleopuccinia yunnanensis P. Zhao and L. Cai, sp. nov.
Figure 40. Morphology of Coleopuccinia yunnanensis (holotype) designated in this study. (a–c) Telia on both sides of leaf. (d) Ultrastructure of telia. (e) Section of telia. (f) Ellipsoid or pyriform teliospores catenulate in gelatinous matrix. (g) 2-celled teliospores with transverse septa. Scale bars: d – f = 50 µm; g = 30 µm.
Fungal Names: FN 571661.
Etymology: yunnanensis (Latin), refers to the province where this new species was collected.
Diagnosis: Differing from C. sinensis by relatively larger teliospores.
Description: Spermogonia, aecia, and uredinia unknown. Telia hypophyllous, subepidermal, erumpent, with basally united, scattered, waxy in appearance, orange-yellow, 50–400 μm across; teliospores 2-celled by transverse septa, ellipsoid or fusiform, yellowish, catenulate in gelatinous matrix, 30–57 × 8–19 μm, walls smooth and evenly thickened.
Materials examined: China. Yunnan, Lijiang, III on Cotoneaster sp., 7 September 2014, Y.M. Li (ZP-R711, holotype in HMAS 352525); Yunnan, Lijiang, III on Cotoneaster sp., 7 September 2014, Y.M. Li, ZP-R6701 (HMAS).
Notes: Here, we found a new species Coleopuccinia yunnanensis, which is distinguishable from both C. sinensis and its synonym C. kunmingensis in the dimension of teliospores (30–57 × 8–19 μm vs. 17–45 × 7–19 μm).
Hyalopsora caprearum P. Zhao & L. Cai, sp. nov.
Figure 41. Morphology of Hyalopsora caprearum (holotype) designated in this study. (a–b) Uredinia on both sides of leaf. (c) Section of uredinia. (d) Ovoid or pyriform urediniospores with apparently thickened at the angles. Scale bars: a = 1 cm; b = 1 mm; c – d = 50 µm.
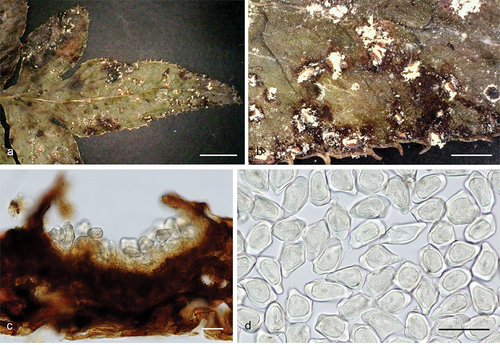
Fungal Names: FN 571662.
Etymology: caprearum (Latin), refers to urediniospores with apparently thickened apical walls.
Diagnosis: Differing from phylogenetically allied species H. nodispora by comparatively larger urediniospores with an apparently thickened apical wall.
Description: Spermogonia and aecia unknown. Uredinia amphigenous, scattered, 0.4–0.8 mm, urediniospores ovoid or pyriform, hyaline, 66–89 × 37–48 µm, wall smooth, angular, wall 5–10 µm thick, not evenly thickened, apparently thickened at the angles, up to 24 µm, no paraphyses. Telia not detected.
Materials examined: China. Yunnan Province, Diqing, Shangri-la, II on Pteris wallichiana, 7 September 2014, Y.M. Li (ZP-R2073, holotype in HMAS 352526); Yunnan Province, Diqing, Shangri-la, II on Pteris wallichiana, 7 September 2014, Y.M. Li, ZP-R9 (HMAS).
Notes: Hyalopsora caprearum is characterised by its relatively larger urediniospores with apparently thickened apical walls. It was phylogenetically close to H. nodispora (), but morphologically the dimension of urediniospores of H. caprearum is comparatively larger than H. nodispora (40–57 × 27–44 µm) (Sydow and Sydow Citation1915; Hiratsuka et al. Citation1992). In addition, the apical walls of urediniospores were relatively thick and uneven, which differs from all other Hyalopsora species present.
Figure 42. Phylogenetic relationship among species of morphologically related genera in the family Pucciniastraceae inferred from the combined dataset of ITS and nLSU regions. The topology is generated by the maximum likelihood algorithm. Bootstrap values and Bayesian posterior probabilities, when simultaneously above 75% and 0.75, respectively, are labelled at the nodes. The newly described species are in boldface.
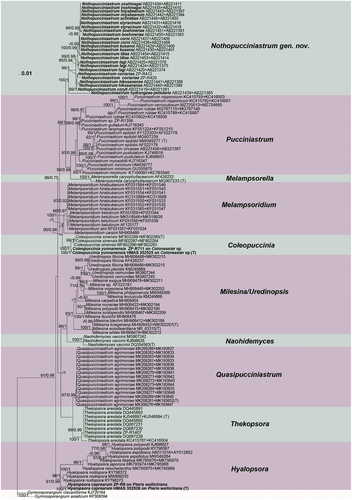
Nothopucciniastraceae P. Zhao and L. Cai, Mycology: 10.1080/21501203.2022.2089262, 20 (2022)
Notes: The traditionally defined Pucciniastraceae with nine genera (Cummins and Hiratsuka Citation1983, Citation2003) is highly polyphyletic (Zhao et al. Citation2020). Recent studies on Pucciniastraceae have reclassified several genera: Milesia, Naohidemyces, and Uredinopsis are now classified under Milesinaceae (Aime and McTaggart Citation2021), while Coleopuccinia, Hyalopsora, and Melampsoridium are now classified under Hyalopsoraceae, and Thekopsora is now classified under Thekopsoraceae (Zhao et al. Citation2023). Additionally, Pucciniastrum species have been found to form two distinct clades, and the presence of ostiolar peridial cells in uredinia differs between these two clades as observed in comprehensive morphological comparisons by Liang (Citation2006) and Yang (Citation2015). Based on morphological and molecular evidence, Zhao et al. (Citation2023) have proposed a new family called Nothopucciniastraceae.
Nothopucciniastrum P. Zhao and L. Cai, gen. nov.
Fungal Names: FN 571594.
Etymology: Notho = nothus in Greek, fake, close but different; pucciniastrum = pucciniastrum-like morphology.
Type species: Nothopucciniastrum tiliae (Miyabe) P. Zhao and L. Cai.
Description: Spermogonia Group I (types 2 and 3), subepidermal or subcuticular, determinate, with flat hymenia, bounding structures lacking. Aecia Peridermium-type, or Milesia-type, with well-developed peridia, aeciospores borne singly on pedicels, verrucose. Uredinia Milesia-type, with well-developed ostiolar cells, urediniospores borne singly, verrucose. Telia subepidermal or intraepidermal, not erumpent, consisting of laterally adherent teliospores one spore deep, teliospores sessile, aseptate or multiseptate, with vertical septa. Basidia external.
Nothopucciniastrum actinidiae (Hirats. f.) P. Zhao and L. Cai, comb. nov.
Fungal Names: FN 571595.
Basionym: Pucciniastrum actinidiae Hirats. f., J. Jap. Bot. 27: 111.1952.
Synonymy: Pucciniastrum actinidiae Hirats. f., Mem. Tottori Agric. Coll. 4: 279. 1936.
Nothopucciniastrum boehmeriae (Dietel) P. Zhao and L. Cai, comb. nov.
Fungal Names: FN 571596.
Basionym: Uredo boehmeriae Dietel, Bot. Jb. 28(3): 290. 1900.
Synonymy: Pucciniastrum boehmeriae (Dietel) Syd. and P. Syd., Ann. Mycol. 1(1): 19. 1903.
Nothopucciniastrum coriariae (Dietel) P. Zhao and L. Cai, comb. nov.
Fungal Names: FN 571597.
Basionym: Pucciniastrum coriariae Dietel, Bot. Jb. 28(3): 286.1900.
Nothopucciniastrum corni (Dietel) P. Zhao and L. Cai, comb. nov.
Fungal Names: FN 571598.
Basionym: Pucciniastrum corni Dietel, Bot. Jb. 34: 587. 1905.
Nothopucciniastrum coryli (Kom.) P. Zhao and L. Cai, comb. nov.
Fungal Names: FN 571606.
Basionym: Pucciniastrum coryli Kom., in Jaczewski, Komorov & tranzschel, Fungi Rossiae Exsicc.: no. 275.1899.
Nothopucciniastrum fagi (Dietel) P. Zhao and L. Cai, comb. nov.
Fungal Names: FN 571599.
Basionym: Pucciniastrum fagi G. Yamada, Bot. Mag., Tokyo 44: 280. 1930.
Nothopucciniastrum kusanoi (Dietel) P. Zhao and L. Cai, comb. nov.
Fungal Names: FN 571600.
Basionym: Pucciniastrum kusanoi Dietel, Bot. Jb. 32: 629. 1903.
Nothopucciniastrum hikosanense (Hirats. f.) P. Zhao and L. Cai, comb. nov.
Fungal Names: FN 571601.
Basionym: Pucciniastrum hikosanense Hirats. f., Ann. Phytopath. Soc. Japan 10: 154. 1940.
Nothopucciniastrum hydrangeae-petiolaris (Hirats. f.) P. Zhao and L. Cai, comb. nov.
Fungal Names: FN 571607.
Basionym: Pucciniastrum hydrangeae-petiolaris Hirats. f., J. Fac. agric., Hokkaido Imp. Univ., Sapporo 21(1): 27.1927.
Nothopucciniastrum miyabeanum (Hirats.) P. Zhao and L. Cai, comb. nov.
Fungal Names: FN 571602.
Basionym: Pucciniastrum miyabeanum Hirats., Bot. Mag., Tokyo 12: 3 (extr.). 1898.
Nothopucciniastrum styracinum (Hirats.) P. Zhao and L. Cai, comb. nov.
Fungal Names: FN 571603.
Basionym: Pucciniastrum styracinum Hirats., Bot. Mag., Tokyo 12: 2 (extr.). 1898.
Nothopucciniastrum tiliae (Miyabe) P. Zhao and L. Cai, comb. nov.
Fungal Names: FN 571604.
Basionym: Pucciniastrum tiliae Miyabe, in Hiratsuka, Bot. Mag., Tokyo 11: 47. 1897.
Nothopucciniastrum yoshinagae (Hirats.) P. Zhao and L. Cai, comb. nov.
Fungal Names: FN 571605.
Basionym: Pucciniastrum yoshinagae Hirats. f. [as “yoshinagai”], Trans. Tottori Soc. Agric. Sci. 2: 247. 1931.
Notes: According to Zhao et al (Citation2020, Citation2023), morphologically defined Pucciniastrum species show bifurcation into two phylogenetically distinct clades. Among these species, 13 species formerly classified under the genus Pucciniastrum, namely P. actinidiae, P. boehmeriae, P. coriariae, P. corni, P. coryli, P. fagi, P. hikosanense, P. hydrangeae-petiolaris, P. kusanoi, P. miyabeanum, P. styracinum, P. tiliae, and P. Yoshinaga, formed a distinct clade that received strong support, and it separated from the Pucciniastrum clade that comprised the type species P. epilobii (). Morphologically, these 13 species differed from Pucciniastrum and Melampsorella in their Milesia-type uredinia with ostiolar cells (Liang Citation2006; Yang Citation2015). This outcome aligns consistently with Qi et al. (Citation2019) and Zhao et al. (Citation2020). Based on the morphology and molecular findings, a novel family named Nothopucciniastraceae along with a new genus Nothopucciniastrum, and 10 new combinations have been proposed (Zhao et al. Citation2023). Here, we offered nomenclatural corrections and outlined the establishment of the new genus Nothopucciniastrum and 10 new combinations, with detailed information on basionyms, synonymies, and their registration numbers in the Fungal Names database. Moreover, we added three new combinations, Nothopucciniastrum coriariae, Nothopucciniastrum coryli, and Nothopucciniastrum hydrangeae-petiolaris in this study.
Protozoa
Incertae sedis
Myxomycetes G. Winter, Rabenhorst’s Kryptogamen-Flora, Pilze – Schizomyceten, Saccharomyceten und Basidiomyceten 1(1): 32 (1880)
Notes: Myxomycetes, the plasmodial or true slime moulds, is a monophyletic group within the Amoebozoa and is characterised by producing uninucleate amoeboflagellates, multinucleate plasmodia, and ornate spore-bearing fruiting bodies in its life cycle (Adl et al. Citation2012).
Physarales T. Macbr., The North American slime-moulds: 22 (1922)
Notes: This order is characterised by spores black, deep purplish, or violaceous brown in mass, lime in some and often all parts of the fructification except the spores, stalk subhapothallic, and assimilative stage a phaneroplasmodium.
Physaraceae Chevall., Flore Générale des Environs de Paris 1: 332 (1826)
Notes: This family is characterised by capillitium netted, limy, very rarely nearly limeless, peridium usually limy, and spores black, deep violaceous, or dark grey in mass, deep purplish brown to violaceous brown to pale violaceous by transmitted light.
Diachea Fr., Novitiae florae svecicae 5(2): 80 (1819)
Notes: Diachea is an important genus of Myxomycetes that was reported by Fries in 1825. Species of Diachea are common inhabitants of decaying wood, bark, leaves, and leaf litter in humid environments (Thind and Manocha Citation1964; Yamamoto Citation1987, Citation2007; Keller et al. Citation2004; Lado et al. Citation2022). The members of this genus are characterised by their always-stalked sporocarps, with a stalk and columella filled with lime granules, and a single, iridescent, limeless peridium (Kirk et al. Citation2008). Diachea formed a monophyletic clade in recent molecular phylogenetic studies, but the position of this genus remains unresolved (Prikhodko et al. Citation2023).
According to different authors, the genus Diachea currently comprises between 12 to 15 accepted species (Yamamoto Citation1987, Citation2007; Kirk et al. Citation2008; Lado et al. Citation2022; Lado Citation2023), of which five species, Diachea bulbillosa, D. leucopodia, D. splendens, D. subsessilis, and D. synspora have been reported in China (Li Citation1988).
A new species found on the surface of fallen leaves in Haitangshan National Nature Reserve, Liaoning Province, China, in September 2012 is described and illustrated below.
Diachea macroverrucosa D. Dai & B. Zhang, sp. nov.
Figure 43. Diachea macroverrucosa (holotype). (a) Sporocarps. (b–c) Capillitia. (d) Spores by TL. (e) Sporotheca by SEM. (f) Columella by SEM. (g) Capillitia tips by SEM. (h) Spores by SEM. Scale bars: a = 500 μm; b = 50 μm; c = 20 μm; d = 10 μm; e = 300 μm; f = 100 μm; g = 40 μm; h = 5 μm.
Fungal Names: FN 571653.
Etymology: macroverrucosa (Latin), refers to the large verrucose spores.
Diagnosis: Differing from D. bulbillosa by a conical columella and big capitate-warted spores.
Description: Sporocarps gregarious, not closely crowded, more often stipitate, 0.75–0.9 mm in total height (); sporotheca globose, 0.35–0.4 mm in diam., iridescent with blue tints; peridium membranous, fragile, iridescent, appearing translucent and smooth without spores by transmitted light, dehiscence occurring irregularly from apex when mature, usually with basal remnants adhering to the columella and stalk (); hypothallus membranous, colourless, no calcareous; stalk up to 1/2 of total height, white, glossy, stout, consisting of a translucent membrane densely packed with white, small, globose, lime granules, basally enlarged; columella a continuation of the stalk, white, absent to 1/2 the height of sporotheca, conical, tapering to a blunt point (); capillitium arise as stiff threads from all columella, smooth, forming an open network of branching and anastomosing threads, free of peridium, diminish towards the periphery (), dark brown under transmitted light, paler at tips and colourless at attachment to columella (); spores globose, free, dark brown to black in mass, dark brown under transmitted light, ornamentation appearing stout warts under light microscopy (), big warts with irregular, capitate ends under SEM (), 8–13 μm in diam.; Plasmodium not observed.
Materials examined: China. Liaoning Province, Fuxin Mongolia Autonomous County, Haitangshan National Nature Reserve, on a fallen leaf, 1 September 2012 (holotype in HMJAU M10067); Jilin Province, Chibei County, on a fallen leaf, 25 August 2013 (paratype in HMJAU M10027); Shanxi Province, Zhashui County, Niubeiliang Nature Reserve, on a fallen leaf, 21 July 2014 (paratype in HMJAU M10001).
Notes: Diachea macroverrucosa closely resembles D. bulbillosa (). The character of the spore ornamentation is striking in D. macroverrucosa, because the big capitate warts are special. Concerning species with white stalk in Diachea, it is somewhat similar to Diachea bulbillosa, D. leucopodia, D. radiata, D. splendens, D. subsessilis, and D. synspora. However, D. macroverrucosa differs from the above-mentioned speies as follows: D. bulbillosa develops a club-shaped columella and sparsely and irregularly warted spores (Penzig Citation1898); D. leucopodia produces broadly ovoid to cylindrical sporotheca and spinulose spores (Rostafiński Citation1874); D. synspora produces cylindrical sporotheca and clustered spores (Li Citation1988); D. subsessilis produces the distinctly reticulate spores (Peck Citation1878b); D. splendens produces spores marked with scattered tubercles, more or less connected by low ridges to form an imperfect reticulation (Peck Citation1878a); and D. radiata usually produces sessile, rarely short-stalked sporocarps and spinulose-reticulated spores (Farquharson and Lister Citation1916).
Figure 44. Phylogenetic relationship among species of Diachea inferred from the dataset of nSSU region. The topology is generated by the maximum likelihood algorithm. Bootstrap values and Bayesian posterior probabilities, when simultaneously above 50% and 0.8, respectively, are labelled at the nodes. The newly described species are in boldface.
Supplemental Material
Download MS Word (16.6 KB)Supplemental Material
Download MS Word (27.6 KB)Supplemental Material
Download MS Word (18 KB)Supplemental Material
Download MS Word (19.7 KB)Supplemental Material
Download MS Word (16.9 KB)Supplemental Material
Download MS Word (17.9 KB)Supplemental Material
Download MS Word (17.7 KB)Supplemental Material
Download MS Word (17.8 KB)Supplemental Material
Download MS Word (19.9 KB)Supplemental Material
Download MS Word (23.6 KB)Supplemental Material
Download MS Word (20.3 KB)Supplemental Material
Download MS Word (16.8 KB)Supplemental Material
Download MS Word (19.6 KB)Supplemental Material
Download MS Word (15.3 KB)Supplemental Material
Download MS Word (16.8 KB)Disclosure statement
No potential conflict of interest was reported by the author(s).
Supplemental data
Supplemental data for this article can be accessed online at https://doi.org/10.1080/21501203.2024.2316066.
Additional information
Funding
References
- Adl SM, Simpson AG, Lane CE, Lukeš J, Bass D, Bowser SS, Brown MW, Burki F, Dunthorn M, Hampl V, et al. 2012. The revised classification of eukaryotes. J Eukaryot Microbiol. 59(5):429–514. doi: 10.1111/j.1550-7408.2012.00644.x.
- Agerer R, Waller K. 1993. Mycorrhizae of Entoloma saepium: Parasitism or symbiosis? Mycorrhiza. 3(4):145–154. doi: 10.1007/BF00203608.
- Agretious TK, Prasanth KP. 2017. A new record of the genus Crepidotus (Crepidotaceae, fungi) from India. Kongunadu Res J. 4(1):73–74. doi: 10.26524/krj180.
- Aime MC. 1998. Generic concepts in the Crepidotaceae as inferred from nuclear large subunit ribosomal DNA sequences, morphology, and basidiospore dormancy patterns [ dissertation]. Blacksburg (VA): Virginia Polytechnic Institute and State University.
- Aime MC. 2006. Toward resolving family-level relationships in rust fungi (Uredinales). Mycoscience. 47:112–122. doi: 10.1007/s10267-006-0281-0.
- Aime MC, Baroni TJ, Miller OK. 2002. Crepidotus thermophilus comb. nov., a reassessment of Melanomphalia thermophila, a rarely collected tropical agaric. Mycologia. 94(6):1059–1065. doi: 10.1080/15572536.2003.11833161.
- Aime MC, McTaggart AR. 2021. A higher-rank classification for rust fungi, with notes on genera. Fungal Syst Evol. 7:21–47. doi: 10.3114/fuse.2021.07.02.
- Antibus RK, Croxdale JG, Miller OK, Linkins AE. 1981. Ectomycorrhizal fungi on Salix rotundifolia III. Resynthesized mycorrhizal complexes and their surface phosphatase activities. Can J Bot. 59(12):2458–2465. doi: 10.1139/b81-297.
- Antonín V, Mossebo DC. 2002. Two interesting Central African collections of Physalacria (Basidiomycetes, Agaricales): P. camerunensis sp. nov. And the first African record of P. tropica. Mycotaxon. 83:419–424.
- Bai M, Zhou LW, Tong YJ, Yin F. 2023. Risk assessment and warning system for strategic biological resources in China. Innovation Life. 1:100004. doi: 10.59717/j.xinn-life.2023.100004.
- Baker GE. 1936. A study of the genus Helicogloea. Ann Missouri Bot Gard. 23(1):69–128. doi: 10.2307/2399003.
- Baker GE. 1946. Addenda to the genera Helicogloea and Physalacria. Mycologia. 38:630–638. doi: 10.1080/00275514.1946.12024085.
- Bandoni RJ, Oberwinkler F. 1982. A taxonomic survey of the gasteroid, auricularioid heterobasidiomycetes. Can J Bot. 60(9):1726–1750. doi: 10.1139/b82-221.
- Bao DF, Hyde KD, Maharachchikumbura SSN, Perera RH, Thiyagaraja V, Hongsanan S, Wanasinghe DN, Shen HW, Tian XG, Yang LQ, et al. 2023. Taxonomy, phylogeny and evolution of freshwater Hypocreomycetidae (Sordariomycetes). Fungal Divers. 121:1–94. doi: 10.1007/s13225-023-00521-8.
- Baroni TJ, Angelini C, Bergemann SE, Lodge DJ, Lacey L, Curtis TA, Cantrell SA. 2020. Rhodocybe-clitopilus clade (Entolomataceae, Basidiomycota) in the Dominican Republic: New taxa and first reports of Clitocella, Clitopilus, and Rhodocybe for Hispaniola. Mycol Prog. 19(10):1083–1099. doi: 10.1007/s11557-020-01619-y.
- Barr ME. 1979. A classification of Loculoascomycetes. Mycologia. 71:935–957. doi: 10.1080/00275514.1979.12021099.
- Berthier J. 1985. Les Physalacriaceae du globe: (Hyménomycétales clavarioïdes). Bibl Mycol. 98:1–128.
- Boonmee S, Dsouza MJ, Luo ZL, Pinruan U, Tanaka K, Su HY, Bhat DJ, McKenzie EHC, Jones EBG, Taylor JE. 2016. Dictyosporiaceae fam. nov. Fungal Divers. 80:457–482. doi: 10.1007/s13225-016-0363-z.
- Boonyuen N, Chuaseeharonnachai C, Suetrong S, Sri-Indrasutdhi V, Sivichai S, Jones EBG, Pang KL. 2011. Savoryellales (Hypocreomycetidae, Sordariomycetes): a novel lineage of aquatic ascomycetes inferred from multiple-gene phylogenies of the genera Ascotaiwania, Ascothailandia and Savoryella. Mycologia. 103(6):1351–1350. doi: 10.3852/11-102.
- Bradshaw MJ, Carey J, Liu M, Bartholomew HP, Jurick WM, Hambleton S, Hendricks D, Schnittler M, Scholler M. 2023. Genetic time traveling: sequencing old herbarium specimens, including the oldest herbarium specimen sequenced from kingdom fungi, reveals the population structure of an agriculturally significant rust. New Phytol. 237(4):1463–1473. doi: 10.1111/nph.18622.
- Burdsall HH. 1985. A contribution to the taxonomy of the genus Phanerochaete (Corticiaceae, Aphyllophorales). Mycologia Memoirs. 10:1–165.
- Cao B, Tao SQ, Tian CM, Liang YM. 2018. Coleopuccinia in China and its relationship to Gymnosporangium. Phytotaxa. 347:235–242. doi: 10.11646/phytotaxa.347.3.4.
- Carbone I, Kohn LM. 1999. A method for designing primer sets for speciation studies in filamentous Ascomycetes. Mycologia. 91(3):553–556. doi: 10.1080/00275514.1999.12061051.
- Chang HS. 2001. Trichocladium anamorph of Ascotaiwania hsilio and monodictys-like anamorphic states of Ascotaiwania lignicola. Fungal Sci. 16:35–38.
- Chen CC, Chen CY, Wu SH. 2021. Species diversity, taxonomy and multi-gene phylogeny of phlebioid clade (phanerochaetaceae, irpicaceae, meruliaceae) of Polyporales. Fungal Divers. 111:337–442. doi: 10.1007/s13225-021-00490-w.
- Co-David D, Langeveld D, Noordeloos ME. 2009. Molecular phylogeny and spore evolution of Entolomataceae. Persoonia. 23(1):147–176. doi: 10.3767/003158509X480944.
- Consiglio G, Setti L. 2008. Il genere Crepidotus in Europa. Vicenza, Italy: A. M. B. Fondazione Centro Studi Micologici.
- Corner EJH. 1970. Supplement to a monograph of Clavaria and allied genera. Nova Hedwigia. 33:1–299.
- Crous PW, Boers J, Holdom D, Osieck ER, Steinrucken TV, Tan YP, Vitelli JS, Shivas RG, Barrett M, Boxshall AG, et al. 2022. Fungal planet description sheets: 1383–1435. Persoonia. 48(1):261–371. doi:10.3767/persoonia.2022.48.08.
- Crous PW, Shivas RG, Quaedvlieg WV, van der Bank M, Zhang Y, Summerell BA, Guarro J, Wingfield MJ, Wood AR, Alfenas AC, et al. 2014. Fungal planet description sheets: 214–280. Persoonia. 32(1):184–306. doi: 10.3767/003158514X682395.
- Crous PW, Wingfield MJ, Burgess TI, Carnegie AJ, GestJ H, Smith D, Summerell BA, Cano-Lira JF, Guarro J, Houbraken J, et al. 2017. Fungal planet description sheets: 625–715. Persoonia. 39:270–467. doi: 10.3767/persoonia.2017.39.11.
- Cummins GB, Hiratsuka Y. 1983. Illustrated genera of rust fungi. 2nd ed. MN, USA: St PaulAmerican Phytopathological Society Press.
- Cummins GB, Hiratsuka Y. 2003. Illustrated genera of rust fungi. 3rd ed. MN (USA): St PaulAmerican Phytopathological Society Press.
- Dayarathne MC, Maharachchikumbura SS, Jones EG, Dong W, Devadatha B, Yang J, Ekanayaka AH, De Silva W, Sarma VV, Al-Sadi AM, et al. 2019. Phylogenetic revision of Savoryellaceae and evidence for its ranking as a subclass. Front Microbiol. 10:840. doi: 10.3389/fmicb.2019.00840.
- Desjardin DE, Hemmes DE. 2001. Agaricales of the hawaiian islands 7. Notes on Volvariella, Mycena sect. Radiatae, Physalacria, Porpoloma and Stropharia. Harvard Pap Bot. 6:85–103.
- Dhanasekaran V, Jeewon R, Hyde KD. 2006. Molecular taxonomy, origins and evolution of freshwater ascomycetes. Fungal Divers. 23:351–390.
- Du HZ, Yang J, Liu NG, Cheewangkoon R, Liu JK. 2022. Morpho-Phylogenetic evidence reveals new species of Fuscosporellaceae and Savoryellaceae from freshwater habitats in Guizhou Province, China. J Fungi. 8(11):1138. doi: 10.3390/jof8111138.
- Edler D, Klein J, Antonelli A, Silvestro D, Matschiner M. 2021. raxmlGUI 2.0 beta: a graphical interface and toolkit for phylogenetic analyses using RAxML. Methods Ecol Evol. 12(2):373–377. doi: 10.1111/2041-210x.13512.
- Edwards RL, Jonglaekha N, Kshirsagar A, Maitland DJ, Mekkamol S, Nugent LK, Phosri C, Rodtong S, Ruchichachorn N, Sangvichien E, et al. 2003. The xylariaceae as phytopathogens. Recent Res Devel Plant Sci. 1(1):1–19.
- Farquharson C, Lister G. 1916. Notes on South Nigerian Mycetozoa. J Bot. 54:121–133.
- Fiore-Donno AM, Novozhilov YK, Meyer M, Schnittler M, Soldati T. 2011. Genetic structure of two protist species (Myxogastria, Amoebozoa) suggests asexual reproduction in sexual amoebae. PLoS One. 6(8):e22872. doi: 10.1371/journal.pone.0022872.
- Fournier J, Flessa F, Peršoh D, Stadler M. 2011. Three new Xylaria species from southwestern Europe. Mycol Prog. 10(1):33–52. doi: 10.1007/s11557-010-0671-8.
- Fournier J, Lechat C, Courtecuisse R. 2018. The genera Kretzschmariella and Nemania (Xylariaceae) in Guadeloupe and Martinique (French West Indies). Ascomycete Org. 10(1):1–47. doi: 10.25664/art-0226.
- Ge YP 2017. Taxonomy and molecular phylogeny of Crepidotaceae in China [ dissertation]. Changchun: Jilin Agricultural University.
- Ge YP, Bau T. 2020. Descriptions of six new species of Crepidotus from China. Mycosystema. 39(2):238–255. doi: 10.13346/j.mycosystema.190345.
- Ge YP, Yang SS, Bau T. 2017. Crepidotus lutescens sp. nov. (inocybaceae, agaricales), an ochraceous salmon colored species from northeast of China. Phytotaxa. 297(2):189–196. doi: 10.11646/phytotaxa.297.2.6.
- Glass NL, Donaldson GC. 1995. Development of primer sets designed for use with the PCR to amplify conserved genes from filamentous ascomycetes. Appl Environ Microb. 61(4):1323–1330. doi: 10.1128/aem.61.4.1323-1330.1995.
- Granmo A, Laessøe T, Schumacher T. 1999. The genus Nemania s.L. (xylariaceae) in Norden. Sommerfeltia. 27(1):1–96. doi: 10.2478/som-1999-0002.
- Greville RK. 1824. Flora Edinensis: or a description of plants growing at Edinburgh, arranged according to the Linnean system. Blackwood, Edinburgh.
- Guzman-Davalos L, Pradeep CK, Vrinda KB, Manoj Kumar A, Aime MC, Herrera M, Villalobos-Arámbula AR, Soytong K, Baroni TJ, Aime MC. 2017. A new stipitate species of Crepidotus from India and Thailand, with notes on other tropical species. Mycologia. 109(5):1–11. doi: 10.1080/00275514.2017.1401834.
- Hausknecht A. 2007. The genus entoloma (basidiomycetes, agaricales) of the mascarenes and Seychelles. Fungal Divers. 27:111–144.
- Hawksworth DL, Lücking R, Heitman J, James TY. 2017. Fungal diversity revisited: 2.2 to 3.8 million species. Microbiol Spectrum. 5(4):FUNK-0052–2016. doi: 10.1128/microbiolspec.FUNK-0052-2016.
- Hesler LR, Smith AH. 1965. North American species of Crepidotus. New York and London: Hafner Publishing Company.
- He X, Xue F. 1996. Physalacria, an unusual genus new to China. Acta Mycol Sin. 15:256–259. doi: 10.13346/j.mycosystema.1996.04.004.
- He MQ, Zhao RL, Hyde KD, Begerow D, Kemler PM, Yurkov A, McKenzie EHC, Raspé O, Kakishima M, Sánchez-Ramírez S. 2019. Notes, outline and divergence times of basidiomycota. Fungal Divers. 99(1):105–367. doi: 10.1007/s13225-019-00435-4.
- Hiratsuka N, Sato S, Katsuya K, Kakishima M, Hiratsuka Y, Kaneko S, Ono Y, Sato T, Harada Y, Hiratsuka T, et al. 1992. The rust flora of Japan. Tsukuba, Ibaraki, Japan: Tsukuba Shuppankai.
- Horak E, Desjardin DE, Hemmes DE. 1994. Reduced marasmioid and mycenoid agarics from Australasia. Aust Syst Bot. 7(2):153–170. doi: 10.1071/SB9940153.
- Hsieh HM, Ju YM, Rogers JD. 2005. Molecular phylogeny of Hypoxylon and closely related genera. Mycologia. 97(4):844–865. doi: 10.1080/15572536.2006.11832776.
- Hughes SJ, Pirozynski KA. 1972. Dicoccum Corda. Can J Bot. 50(12):2521–2534. doi: 10.1139/b72-323.
- Inderbitzin P, Desjardin DE. 1999. A new halotolerant species of Physalacria from Hong Kong. Mycologia. 91:666–668. doi: 10.1080/00275514.1999.12061066.
- Izhar A, Usman M, Khalid AN. 2021. Crepidotus iqbalii (Crepidotaceae, Agaricales) a new stipitate species, from Pakistan. Phytotaxa. 500(2):95–107. doi: 10.11646/phytotaxa.500.2.2.
- Jaklitsch WM, Réblová M. 2015. Savoryellaceae Jaklitsch & Réblová. Index Fungorum. 209:1.
- Jančovičová S, Adamčíková K, Caboň M, Adamčík S. 2020. How variable is Crepidotus variabilis? Phytotaxa. 449(3):253–264. doi: 10.11646/phytotaxa.449.3.4.
- Jančovičová S, Adamčíková S, Looney BP, Caboň M, Čaplovičová M, Kopáni M, Pennycook SR, Adamčíková K. 2017. Delimitation of European Crepidotus stenocystis as different from the North American species C. brunnescens (crepidotaceae, agaricales). Phytotaxa. 328(2):127–139. doi: 10.11646/phytotaxa.328.2.3.
- Jeewon R, Hyde KD. 2016. Establishing species boundaries and new taxa among fungi: recommendations to resolve taxonomic ambiguities. Mycosphere. 7(11):1669–1677. doi: 10.5943/mycosphere/7/11/4.
- Jian SP, Tolgor B, Zhu XT, Deng WQ, Yang ZL, Zhao ZW. 2020. Clitopilus, Clitocella, and Clitopilopsis in China. Mycologia. 112(2):371–399. doi: 10.1080/00275514.2019.1703089.
- Jones EBG, Hyde KD. 1992. Taxonomic studies on Savoryella Jones et Eaton (Ascomycotina). Bot Mar. 35(2):83–91. doi: 10.1515/botm.1992.35.2.83.
- Jones EBG, Suetrong S, Sakayaroj J, Bahkali AH, Abdel-Wahab MA, Boekhout T, Pang K-L. 2015. Classification of marine Ascomycota, Basidiomycota, Blastocladiomycota and Chytridiomycota, Basidiomycota, Blastocladiomycota and Chytridiomycota. Fungal Divers. 73(1):1–72. doi: 10.1007/s13225-015-0339-4.
- Jülich W. 1981. Higher taxa of Basidiomycetes. Bibl Mycol. 85:1–485.
- Ju YM, Rogers JD. 1996. A revision of the genus Hypoxylon. Mycologia memoir No.20. St. Paul (MN): APS Press.
- Ju YM, Rorgers JD. 1999. The Xylariaceae of Taiwan (excluding Anthostomella). Mycotaxon. 73:343–440.
- Karsten PA. 1890. Fragmenta mycologica XXIX. Hedwigia. 29:147–149.
- Keller HW, Skrabal M, Eliasson UH, Gaither TW. 2004. Tree canopy biodiversity in the Great Smoky Mountains National Park: ecological and developmental observations of a new myxomycete species of Diachea. Mycologia. 96(3):537–547. doi: 10.1080/15572536.2005.11832952.
- Khodaparast SA. 2012. Contribution to the knowledge of Hypoxylon and Annulohypoxylon in Guilan province (N Iran). Rostaniha.13: 197–206.
- Kirk PM, Cannon PF, Minter DW, Stalpers JA. 2008. Dictionary of the fungi. 10th ed. Wallingford (CT): CAB International; pp. 759–771.
- Kluting KL, Baroni TJ, Bergemann SE. 2014. Toward a stable classification of genera within the entolomataceae: a phylogenetic re-evaluation of the rhodocybe–clitopilus clade. Mycologia. 106(6):1127–1142. doi: 10.3852/13-270.
- Kobayasi Y. 1951. Notes on fungi 3. Revisions on the genus Physalacria of Japan. J Jap Bot. 28:311–316.
- Kohlmeyer J, Kohlmeyer E. 1979. Marine mycology: the higher fungi. New York, NY: Academic Press; p. 690.
- Kotlába F, Pouzar Z. 1972. Taxonomic and nomenclatural notes on some macromycetes. Ceská Mykologie. 26(4):217–222.
- Kumar AM, Aime MC, Vrinda KB, Pradeep CK. 2020. Two new species and a new record of crepidotus (Agaricomycetes) from India. Aust Syst Bot. 33:380–391. doi: 10.1071/sb19033.
- Kumar AM, Pradeep CK, Aime MC. 2022. New species and new records of Crepidotus (Crepidotaceae) from India. Mycol Prog. 21:311–326. doi: 10.1007/s11557-021-01751-3.
- Kumar AM, Vrinda KB, Pradeep CK. 2018. Two new species of Crepidotus (Basidiomycota, Agaricales) from peninsular India. Phytotaxa. 372(1):67–78. doi: 10.11646/phytotaxa.372.1.5.
- Lado C 2023. An online nomenclatural information system of Eumycetozoa. [accessed 2023 June 20]. https://eumycetozoa.com/data/index.php.
- Lado C, Treviño-Zevallos I, García-Martín JM, Wrigley de Basanta D. 2022. Diachea mitchellii: a new myxomycete species from high elevation forests in the tropical Andes of Peru. Mycologia. 114(4):798–811. doi: 10.1080/00275514.2022.2072140.
- Larsson KH. 1995. Taxonomy of Trechispora farinacea and proposed synonyms I. Species with a grandinioid or hydnoid hymenophore. Symb Bot Ups. 30:101–118.
- Larsson KH. 1996. New species and combination in Trechispora (Corticiaceae, Basidiomycotina). Nord J Bot. 16(1):83–98. doi: 10.1111/j.1756-1051.1996.tb00218.x.
- Li HZ. 1988. A new species of Diachea. Mycosystema. 7(2):99–101. doi: 10.13346/j.mycosystema.1988.02.007.
- Liang YM 2006. Taxonomic evaluation of morphologically similar species of Pucciniastrum in Japan based on comparative analyses of molecular phylogeny and morphology. [ dissertation]. Tsukuba (Japan): University of Tsukuba.
- Li Y, He SH, Chen CC, Nakasone KK, Ma HX. 2022. Global taxonomy and phylogeny of Irpicaceae (Polyporales, Basidiomycota) with descriptions of seven new species and proposals of two new combinations. Front Microbiol. 13:911978. doi: 10.3389/fmicb.2022.911978.
- Li GJ, Hyde KD, Zhao RL, Hongsanan S, Abdel-Aziz FA, Abdel-Wahab MA, Alvarado P, Alves-Silva G, Ammirati JF, Ariyawansa HA, et al. 2016. Fungal diversity notes; taxonomic and phylogenetic contributions to fungal taxa. Fungal Divers. 78:1–237. doi: 10.1007/s13225-016-0366-9.
- Li WL, Luo ZL, Liu JK, Bhat DJ, Bao DF, Su HY, Hyde KD. 2017. Lignicolous freshwater fungi from China I: Aquadictyospora lignicola gen. et sp. nov. And new record of Pseudodictyosporium wauense from northwestern Yunnan Province. Mycosphere. 8(10):1587–1597. doi: 10.5943/mycosphere/8/10/1.
- Linder DH. 1929. A monograph of the helicosporous fungi imperfecti. Ann Missouri Bot Gard. 16(3):227–388. doi: 10.2307/2394038.
- Linnaeus C. 1745. Flora svecica exhibens plantas per regnum Sueciaecrescentes. Salvii, Stockholm.
- Liu PG. 1995. Five new species of Agaricales from southern and southeastern Yunnan, China. Mycotaxon. 56:89–105.
- Liu M, Hambleton S. 2013. Laying the foundation for a taxonomic review of Puccinia coronata s.l. In a phylogenetic context. Mycol Prog. 12:63–89. doi: 10.1007/s11557-012-0814-1.
- Liu SL, He SH, Wang XW, May TW, He G, Chen SL, Zhou LW. 2022. Trechisporales emended with a segregation of Sistotremastrales ord. nov. (Basidiomycota). Mycosphere. 13(1):862–954. doi: 10.5943/mycosphere/13/1/11.
- Liu XY, Liu SL, Wei HW, Wang XW, Yu J, Shen S, Zhou LW. 2023. Preliminary species diversity and community phylogenetics of wood-inhabiting basidiomycetous fungi in the Dabie Mountains, Central China reveal unexpected richness. IMA Fungus. 14:23. doi: 10.1186/s43008-023-00130-9.
- Liu SL, Ma HX, He SH, Dai YC. 2019. Four new corticioid species in Trechisporales (Basidiomycota) from East Asia and notes on phylogeny of the order. MycoKeys. 48:97–113. doi: 10.3897/mycokeys.48.31956.
- Liu SL, Wei HW, Zhou LW. 2023. Xenasmatellalesord. nov. and Xenasmatellaceae fam. nov. for Xenasmatella (Agaricomycetes, Basidiomycota). Mycology. 14(3):175–189. doi: 10.1080/21501203.2023.2216213.
- Liu YJ, Whelen S, Hall BD. 1999. Phylogenetic relationships among ascomycetes: Evidence from an RNA polymerse II subunit. Mol Biol Evol. 16:1799–1808. doi: 10.1093/oxfordjournals.molbev.a026092.
- Li YC, Yang ZL. 2021. The boletes of China: tylopilus s.l. Singapore: Springer.
- Luo ZL, Hyde KD, Liu JK, Bhat DJ, Bao DF, Li WL, Su HY. 2018. Lignicolous freshwater fungi from China II: Novel Distoseptispora (Distoseptisporaceae) species from northwestern Yunnan Province and a suggested unified method for studying lignicolous freshwater fungi. Mycosphere. 9(3):444–461. doi: 10.5943/mycosphere/9/3/2.
- Luo K, Zhao C. 2022. A molecular systematics and taxonomy research on Trechispora (Hydnodontaceae, Trechisporales): concentrating on three new Trechispora species from East Asia. J Fungi. 8(10):1020. doi: 10.3390/jof8101020.
- Maharachchikumbura SSN, Ariyawansa HA, Wanasinghe DN, Dayarathne MC, Al-Saady NA, Al-Sadi AM. 2019. Phylogenetic classification and generic delineation of Hydeomyces desertipleosporoides gen. et sp. nov., (Phaeosphaeriaceae) from Jebel Akhdar Mountain in Oman. Phytotaxa. 391:28–38. doi: 10.11646/phytotaxa.391.1.2.
- Maharachchikumbura SSN, Hyde KD, Jones EBG, McKenzie EHC, Bhat J, Hawksworth DL, Dayarathne M, Huang SK, Norphanphoun C, Senanayake IC, et al. 2016. Families of Sordariomycetes. Fungal Divers. 79(1):1–317. doi:10.1007/s13225-016-0369-6.
- Maharachchikumbura SSN, Hyde KD, Jones EBG, McKenzie EHC, Huang SK, Abdel-Wahab MA, Daranagama DA, Dayarathne M, D’souza MJ, Goonasekara ID, et al. 2015. Towards a natural classification and backbone tree for Sordariomycetes. Fungal Divers. 72(1):199–301. doi:10.1007/s13225-015-0331-z.
- Ma HX, Li Y. 2018. Xylaria crinalis and X. betulicola from China—two new species with thread-like stromata. Sydowia. 70:37–49. doi: 10.12905/0380.sydowia70-2018-0037.
- Malysheva V, Spirin V, Schoutteten N, De LR, Pennanen J, Larsson KH. 2019. New and noteworthy species of Helicogloea (Atractiellomycetes, Basidiomycota) from Europe. Ann Bot Fenn. 57(1–3):1–7. doi: 10.5735/085.057.0101.
- Mao N, Lv JC, Xu YY, Zhao TY, Fan L. 2022. Two new Clitocella species from North China revealed by phylogenetic analyses and morphological characters. MycoKeys. 88:151–170. doi: 10.3897/mycokeys.88.80068.
- Matheny PB. 2005. Improving phylogenetic inference of mushrooms with RPB1 and RPB2 nucleotide sequences (Inocybe; Agaricales). Mol Phylogenet Evol. 35:1–20. doi: 10.1016/j.ympev.2004.11.014.
- Matheny PB, Curtis JM, Hofstetter V, Aime MC, Moncalvo JM, Ge ZW, Yang ZL, Slot JC, Ammirati JF, Baroni TJ. 2006. Major clades of Agaricales: A multilocus phylogenetic overview. Mycologia. 98(6):982–995. doi: 10.1080/15572536.2006.11832627.
- Matheny PB, Wang Z, Binder M, Curtis JM, Lim YW, Nilsson RH, Hughes KW, Hofstetter V, Ammirati JF, Schoch CL, et al. 2007. Contributions of rpb2 and tef1 to the phylogeny of mushrooms and allies (Basidiomycota, fungi). Mol Phylogenet Evol. 43(2):430–451. doi:10.1016/j.ympev.2006.08.024.
- Matsushima T. 1981. Matsushima mycological memoirs 2. Matsushima Mycol Mem. 2:1–68.
- McKenzie EHC. 2008. Two new dictyosporous hyphomycetes on Pandanaceae. Mycotaxon. 104:23–28.
- Moncalvo JM, Vilgalys R, Redhead SA, Johnson JE, James TY, Aime MC, Hofstetter V, Verduin SJW, Larsson E, Baroni TJ, et al. 2002. One hundred and seventeen clades of euagarics. Mol Phylogenet Evol. 23(3):357–400. doi:10.1016/S1055-7903(02)00027-1.
- Montecchio L, Roca S, Courty PE, Garbaye J. 2006. Entoloma nitidum quél. + carpinus betulus L. Descriptions Of Ectomycorrhizae. 9(10):33–38.
- Na Q, Liu ZW, Zeng H, Cheng XH, Ge YP. 2022. Crepidotus yuanchui sp. nov. and C. caspari found in subalpine areas of China. Mycoscience. 63:1–11. doi: 10.47371/mycosci.2021.10.004.
- Noordeloos ME. 2004. Entoloma s.l. Funghi Europaei, vol. 5a. Origgio, Italy: Candusso Edizioni.
- Noordeloos ME, Hausknecht A. 2007. The genus Entoloma (Basidiomycetes, Agaricales) of the Mascarenes and Seychelles. Fungal Divers. 27: 111–144.
- Ordynets A, Scherf D, Pansegrau F, Denecke J, Lysenko L, Larsson KH, Langer E. 2018. Short-spored Subulicystidium (Trechisporales, Basidiomycota): High morphological diversity and only partly clear species boundaries. MycoKeys. 35:41–99. doi: 10.3897/mycokeys.35.25678.
- Park KH, Kim C, Kim M, Kim NK, Park JK, Eimes JA, Cho HJ, Han SK, Lim YW. 2017. Three new recorded species of the Physalacriaceae on Ulleung Island, Korea. Mycobiology. 45(1):9–14. doi: 10.5941/MYCO.2017.45.1.9.
- Patouillard N. 1889. Le genre Coleopuccinia. Revue Mycologique. 11(41):35–36.
- Patouillard NT, de LG. 1892. Champignons de l’Equateur (Pugillus II). Bulletin de la Société Mycologique de France. 8(2):113–140.
- Peck CH. 1878a. Report of the Botanist 1876. Ann Rep of the NY St Mus. 30:23–78.
- Peck CH. 1878b. Report of the Botanist 1877. Ann Rep of the NY St Mus. 31:19–60.
- Peck CH. 1882. Fungi in wrong genera. Bull Torrey Bot Club. 9(1):1–4. doi: 10.2307/2476912.
- Penzig AJO. 1898. Die Myxomyceten der Flora von Buitenzorg. Leiden: Buchhandlung und Druckerei vormals.
- Petrini LE, Rogers JD. 1986. A summary of the hypoxylon serpens complex. Mycotaxon. 26:401–436.
- Poder R, Ferrari E. 1984. Crepidotus roseoornatus sp. n.—eine auffallend gefärbte Art auf Robinia pseudoacacia. Sydowia. 37:242–245.
- Posada D. 2008. jModelTest: Phylogenetic model averaging. Mol Biol Evol. 25:1253–1256. doi: 10.1093/molbev/msn083.
- Pouzar Z. 1985. (797)-(801) proposals for the conservation of five family names of fungi. Taxon. 34(4):709–712. doi: 10.2307/1222227.
- Prikhodko IS, Shchepin ON, Bortnikova NA, Novozhilov YK, Gmoshinskiy VI, Moreno G, López-Villalba Á, Stephenson SL, Schnittler M. 2023. A three-gene phylogeny supports taxonomic rearrangements in the family Didymiaceae (myxomycetes). Mycol Prog. 22(2):11. doi: 10.1007/s11557-022-01858-1.
- Qi XH, Cai L, Zhao P. 2019. Quasipucciniastrum agrimoniae, gen. et sp. nov. on Agrimonia (Rosaceae) from China. Mycology. 10(3):1–10. doi: 10.1080/21501203.2019.1610522.
- Qin J, Yang ZL. 2016. Three new species of Physalacria from China, with a key to the Asian taxa. Mycologia. 108(1):215–226. doi: 10.3852/15-166.
- Ranghoo VM, Hyde KD. 1998. Ascomycetes from freshwater habitats: Ascolacicola aquatica gen. et sp. nov. and a new species of Ascotaiwania from wood submerged in a reservoir in Hong Kong. Mycologia. 90(6):1055–1062. doi: 10.1080/00275514.1998.12027005.
- Ranghoo VM, Hyde KD, Liew ECY, Spatafora JW. 1999. Family placement of Ascotaiwania and Ascolacicola based on DNA sequences from the large subunit rRNA gene. Fungal Divers. 2:159–168.
- Réblová M, Hernández-Restrepo M, Fournier J, Nekvindová J. 2020. New insights into the systematics of Bactrodesmium and its allies and introducing new genera, species and morphological patterns in the Pleurotheciales and Savoryellales (Sordariomycetes). Stud Mycol. 95(1):415–466. doi: 10.1016/j.simyco.2020.02.002.
- Réblová M, Seifert KA, Fournier J, Štěpánek V. 2016. Newly recognised lineages of perithecial ascomycetes: The new orders Conioscyphales and Pleurotheciales. Persoonia. 37(1):57–81. doi: 10.3767/003158516X689819.
- Rehner SA, Buckley E. 2005. A beauveria phylogeny inferred from nuclear ITS and EF1- sequences: evidence for cryptic diversification and links to cordyceps teleomorphs. Mycologia. 97(1):84–98. doi: 10.3852/mycologia.97.1.84.
- Ronquist F, Teslenko M, van der Mark P, Ayres DL, Darling A, Höhna S, Larget B, Liu L, Suchard MA, Huelsenbeck JP. 2012. MrBayes 3.2: efficient Bayesian phylogenetic inference and model choice across a large model space. Syst Biol. 61:539–542. doi: 10.1093/sysbio/sys029.
- Rostafiński JT. 1874. Śluzowce (Mycetozoa) Monografia. Pamiętnik Towarzystwa Nauk Ścisłych w Paryżu. 5(1):1–215.
- Rusk SA, Spiegel FW, Lee SB. 1995. Design of polymerase chain reaction primers for amplifying nuclear ribosomal DNA from slime molds. Mycologia. 87(1):140–143. doi: 10.2307/3760957.
- Ryvarden L. 2002. A note on the genus Hydnodon Banker. Synopsis Fungorum. 15:31–33.
- San Martín GF, Rogers JD. 1989. A preliminary account of Xylaria of Mexico. Mycotaxon. 34:283–373.
- Schoutteten N, Roberts P, Van De Put K, Verbeken A. 2018. New species in Helicogloea and Spiculogloea, including a type study of H. graminicola (Bres.) G.E. Baker (Basidiomycota, Pucciniomycotina). Mycologie. 39(3):1–13. doi: 10.7872/crym/v39.iss3.2018.311.
- Senn-Irlet B. 1991. Crepidotus, Pellidiscus and Ramicola in Greenland (Agaricales: Crepidotaceae). Nord J Bot. 11(5):587–597. doi: 10.1111/j.1756-1051.1991.tb01268.x.
- Senn-Irlet B. 1992. Type studies in Crepidotus—I. Persoonia. 14(4):615–623.
- Senn-Irlet B. 1993. Type studies in Crepidotus—II. Persoonia. 15(2):155–167.
- Senn-Irlet B. 1995. The genus Crepidotus (Fr.) Staude in Europe. Persoonia. 16(1):1–80.
- Shen HW, Bao DF, Wanasinghe DN, Boonmee S, Liu JK, Luo ZL. 2022. Novel species and records of dictyosporiaceae from freshwater habitats in China and Thailand. J Fungi. 8(11):1200. doi: 10.3390/jof8111200.
- Shen S, Liu SL, Zhou LW. 2023. Taxonomy of Hyphodermella: a case study to show that simple phylogenies cannot always accurately place species in appropriate genera. IMA Fungus. 14:11. doi: 10.1186/s43008-023-00116-7.
- Silva CSD, Pereira MB, Pereira J. 2020. New accounts on Hypoxylaceae and Xylariaceae from Brazil. Rodriguésia. 71:1513. doi: 10.1590/2175-7860202071146.
- Singer R. 1949. The “Agaricales” (mushrooms) in modern taxonomy. Lilloa. 22:5–832.
- Singer R. 1986. The Agaricales in Modern Taxonomy. 4th ed. Germany: J. Cramer.
- Sivanesan A, Chang HS. 1992. Ascotaiwania, a new amphisphaeriaceous ascomycete genus on wood from Taiwan. Mycol Res. 96:481–484. doi: 10.1016/S0953-7562(09)81094-0.
- Sommai S, Pinruan U, Khamsuntorn P, Lueangjaroenkit P, Somrithipol S, Luangsa-Ard J. 2023. Three new species of Trechispora from northern and Northeastern Thailand. Mycol Prog. 22:95. doi: 10.1007/s11557-023-01886-5.
- Spirin W. 2003. Antrodiella romellii (Irpicaceae, basidiomycetes) in Russia. Mycena. 3:48–52.
- Spirin V, Malysheva V, Trichies G, Savchenko A, Pldmaa K, Nordén J, Miettinen O, Larsson KH. 2018. A preliminary overview of the corticioid Atractiellomycetes (Pucciniomycotina, Basidiomycetes). Fungal Syst Evol. 8:311–340. doi: 10.3114/fuse.2018.02.09.
- Spirin V, Volobuev S, Okun M, Miettinen O, Larsson KH. 2017. What is the type species of Phanerochaete (polyporales, Basidiomycota)? Mycol Prog. 16:171–183. doi: 10.1007/s11557-016-1267-8.
- Stadler M, Kuhnert E, Peršoh D, Fournier J. 2013. The xylariaceae as model example for a unified nomenclature following the “one fungus-one name (1F1N) concept. Mycol Int J Fungal Biol. 4(1):5–21. doi: 10.1080/21501203.2013.782478.
- Stamatakis A. 2014. RAxML version 8: a tool for phylogenetic analysis and post-analysis of large phylogenies. Bioinformatics. 30:1312–1313. doi: 10.1093/bioinformatics/btu033.
- Sutton BC. 1985. Notes on some deuteromycete genera with cheiroid or digitate brown conidia. Proc Indian Acad Sci. 94:229–244. doi: 10.1007/BF03053139.
- Sydow P, Sydow H. 1915. Monographia Uredinearum, vol III: Melampsoraceae, Zaghouaniaceae, Coleosporiaceae. Leipzig (Germany): Fratres Borntraeger.
- Tai FL. 1948. A new species of Coleopuccinia with remarks on the genus. Acta Agriculturae (China). 1(2):97–103.
- Thind KS, Manocha MS. 1964. The Myxomycetes of India-XVII. Mycologia. 56(5):712–717. doi: 10.1080/00275514.1964.12018160.
- Tian WH, Chen YP, Maharachchikumbura SSN. 2022. Neodigitodesmium, a novel genus of family Dictyosporiaceae from Sichuan Province, China. Phytotaxa. 559:176–184. doi: 10.11646/phytotaxa.559.2.6.
- Tsui CKM, Hyde KD. 2003. Freshwater mycology. In: Hyde K, editor. Fungal diversity research series. Vol. 10. Berlin, Germany: Springer; p. 1–350.
- Tulasne L, Tulasne C. 1863. Selecta fungorum carpologia II (English translation of W.B. Grove). New York (NY): Oxford University Press.
- Van DGK. 1995. Illustrations and descriptions of xylariaceous fungi collected in Papua New Guinea. Bull du Jardin botanique natl de Belgique / Bull van de Natl 2350 Plantentuin van België. 64(3/4):219–403. doi: 10.2307/3668386.
- Vialle A, Feau N, Allaire M, Didukh M, Martin F, Moncalvo J, Hamelin R. 2009. Evaluation of mitochondrial genes as DNA barcode for Basidiomycota. Mol Ecol Resour. 9:99–113. doi: 10.1111/j.1755-0998.2009.02637.x.
- Vilgalys R, Hester M. 1990. Rapid genetic identification and mapping of enzymatically amplified ribosomal DNA from several Cryptococcus species. J Bacteriol. 172:4238–4246. doi: 10.1128/jb.172.8.4238-4246.1990.
- Visser AA, Ros VID, De Beer ZW, Debets AJM, Haerog E, Kuyper TW, Læssøe T, Slippers B, Aanen DK. 2009. Levels of specificity of Xylaria species associated with fungus-growing termites: a phylogenetic approach. Mol Ecol. 18(3):553–567. doi: 10.1111/j.1365-294X.2008.04036.x.
- Vizzini A, Angelini C, Ercole E. 2012. A new Neopaxillus species (Agaricomycetes) from the Dominican Republic and the status of Neopaxillus within the Agaricales. Mycologia. 104(1):138–147. doi: 10.3852/10-345.
- Wang K, Cai L. 2023. Overview of the historical and current status of fungal taxonomy and diversity in China. Mycosystema. 42(1):50–62.
- Wang XW, Jiang JH, Zhou LW. 2020. Basidioradulum mayi and B. tasmanicum spp. nov. (Hymenochaetales, Basidiomycota) from both sides of Bass Strait, Australia. Sci Rep. 10:102. doi: 10.1038/s41598-019-57061-y.
- Wang XW, Liu SL, Zhou LW. 2023. An updated taxonomic framework of Hymenochaetales (Agaricomycetes, Basidiomycota). Mycosphere. 14(1):452–496. doi: 10.5943/mycosphere/14/1/6.
- Wang XW, May TW, Liu SL, Zhou LW. 2021. Towards a natural classification of Hyphodontia sensu lato and the trait evolution of basidiocarps within Hymenochaetales (Basidiomycota). J Fungi. 7(6):478. doi: 10.3390/jof7060478.
- Wani NA, Saini MK, Malik NA. 2021. Xerula radicata var. setosa var. nov. And three new records of the family Physalacriaceae. Agaricales From The Indian Subcontinent. J Fungal Biol. 11(1):248–262. doi: 10.5943/cream/11/1/18.
- Watling R, Aime MC. 2013. The genus Neopaxillus (Crepidotaceae). Mycotaxon. 126(1):83–90. doi: 10.5248/126.83.
- Wells K. 1990. An undescribed species of Helicogloea from Brazil. Mycol Res. 94(6):835–839. doi: 10.1016/S0953-7562(09)81388-9.
- Wendt L, Sir EB, Kuhnert E, Heitkämper S, Lambert C, Hladki IA, Romero AI, Luangsa-Ard JJ, Srikitikulchai P, Peršoh D, et al. 2018. Resurrection and emendation of the hypoxylaceae, recognised from a multigene phylogeny of the Xylariales. Mycol Prog. 17(1–2):115–154. doi:10.1007/s11557-017-1311-3.
- White TJ, Bruns T, Lee S, Taylor J, Innis MA, Gelfand DH, Sninsky J. 1990. Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In: Innis M, Gelfand D, Sninsky J White T, editors. PCR protocols: a guide to methods and applications. London (UK): Academic Press; pp. 315–322.
- Wijayawardene NN, Hyde KD, Dai DQ, Sánchez-García M, Goto BT, Saxena RK, Erdoğdu M, Selçuk F, Rajeshkumar KC, Aptroot A, et al. 2022. Outline of Fungi and fungus-like taxa – 2021. Mycosphere. 13(1):53–453. doi: 10.5943/mycosphere/13/1/2.
- Yamamoto Y. 1987. A new species of Diachea with clustered spores. J Jpn Bot. 62(11):346.
- Yamamoto Y. 2007. Notes on Japanese Myxomycetes (V)—A new species of Diachea found on moss covering an old concrete wall. Bull Natl Sci Mus, Ser B (Tokyo). 33(3–4):123–125.
- Yang T 2015. Phylogenetic and taxonomic studies of Pucciniastrum S.l. [ dissertation]. Beijing, China: Beijing Forestry University.
- Yang L, Bao DF, Luo ZL, Su XJ, Su HY. 2022. Neoascotaiwania aquatica sp. nov. from a freshwater habitat in Yunnan Province, China. Phytotaxa. 531(2):120–128. doi: 10.11646/phytotaxa.531.2.4.
- Yang CL, Xu XL, Wanasinghe DN, Jeewon R, Phookamsak R, Liu YG, Liu LJ, Hyde KD. 2019. Neostagonosporella sichuanensis gen. et sp. nov. (Phaeosphaeriaceae, Pleosporales) on Phyllostachys heteroclada (Poaceae) from Sichuan Province, China. MycoKeys. 46:119–150. doi: 10.3897/mycokeys.46.32458.
- Zhang B, Li Y. 2016. A new species, stemonitis sichuanensis, and a newly recorded species, Stemonitis marjana (myxogastria) from China. Phytotaxa. 258(2):195–199. doi: 10.11646/phytotaxa.258.2.10.
- Zhao P, Li Y, Li YJ, Liu F, Liang JM, Zhou X, Cai L. 2023. Applying early divergent characters in higher rank taxonomy of Melampsorineae (Basidiomycota, Pucciniales). Mycology. 14(1):11–36. doi: 10.1080/21501203.2022.2089262.
- Zhao P, Qi XH, Crous PW, Duan WJ, Cai L. 2020. Gymnosporangium species on Malus: species delineation, diversity and host alternation. Persoonia. 45:68–100. doi: 10.3767/persoonia.2020.45.03.
- Zhou LW, May TW. 2023. Fungal taxonomy: Current status and research agendas for the interdisciplinary and globalisation era. Mycology. 14:52–59. doi: 10.1080/21501203.2022.2103194.
- Zmitrovich IV. 2018. Conspectus systematis Polyporacearum v. 1.0. Folia Cryptogamica Petropolitana. 6:3–145.