Abstract
A study was conducted in 26 sites on agricultural landscapes in Central Uganda to collect baseline information about important drivers of butterfly richness and abundance. Data were collected for 1 year (2006) using line transects walk-and-counts, fruit-bait traps and handnets sampling methods. A total of 57,439 individuals belonging to 331 species were collected. Totals of 127, 131 and 299 species were recorded in transect counts, banana-bait and handnets, respectively. Of the 57,439 individuals registered, 75%, 19% and 6% were recorded in transect counts, handnet and banana-bait trap, respectively. Butterfly abundance and species richness were significantly (p < 0.05) affected by climatic factors (rainfall, temperature) in previous years (2004 and 2005) and richness and abundance of wild nectaring plants. Butterfly species richness (not the abundance) decreased with land-use intensity (p < 0.05) and was positively related to the cover of semi-natural habitats. Both butterfly species richness and abundance declined sharply with forest distance. Nearby forest remnants and high cover of semi-natural habitats are thus important for conservation of butterflies in coffee–banana agroforestry systems and farmers should be encouraged to protect such resources.
Introduction
Butterflies have been recognized as a useful biodiversity indicator group of tropical land-use systems because they are sensitive and react quickly to subtle changes in environmental and habitat conditions (Kremen Citation1994; Libert Citation1994; Brown Citation1997; Larsen Citation2008; Özden et al. Citation2008; Pozo et al. Citation2008). Due to their short life cycle, narrow niches and relatively low mobility, they are more sensitive to land-cover and land-use changes than long-lived animals. Butterflies are relatively easy to capture, manipulate and identify (Rogo and Odulaja Citation2001; Fitzherbert et al. Citation2006; Marin et al. 2009; Nyamweya and Gichuki Citation2010), which makes them important candidates for monitoring changes in habitat, biodiversity and environmental conditions (Kremen Citation1992; Howard et al. Citation2000; Cleary Citation2004; Cleary and Mooers Citation2004), including the impact of landscape and habitat management practices and disturbance regimes in terrestrial ecosystems (Stork et al. Citation2003; Öckinger and Smith Citation2008).
Butterfly activities are closely controlled by weather and many species are constrained by climate, mostly occupying a small part of the range of their host plants (Fitzherbert et al. Citation2006; Nyamweya and Gichuki Citation2010). Butterflies play a significant ecological role in agricultural landscapes. They perform essential ecosystem services (Rogo and Odulaja Citation2001; Schmidt and Roland Citation2006), especially in the recycling of nutrients (N, P, K) highly needed by crops. In their larval stage, butterflies feed on leaves of several wild plants found in the agricultural systems and therefore release faeces that contain large amounts of nutrients (Munyuli Citation2010). In addition, butterflies are food to birds and other predators and are hosts to several parasitoids that suppress crop pests (Summerville et al. Citation2001; Cardoso et al. Citation2008, 2009). Consequently, their conservation is essential to sustaining the productivity of natural and agricultural landscapes. Despite their diversity, ubiquity and ecological importance, butterflies remain relatively little studied, particularly with regard to their ecology, behaviour and functional role in farmland habitats (Marchiori and Romanowski Citation2006; Stireman et al. Citation2009). In agricultural systems, butterflies are suspected to be important pollinators of wild and cultivated crop species (Munyuli Citation2010). Roughly, 90% of butterfly species live in the tropics (Boriani et al. Citation2005; Bonebrake et al. Citation2010). However, knowledge of butterflies inhabiting farmland habitats is fairly good in Mediterranean regions compared with the sub-Saharan Africa. The relative scarcity of data on tropical butterfly populations hampers the ability to effectively conserve them, particularly as pollinating agents (Bonebrake et al. Citation2010) in agricultural systems.
Across sub-Saharan Africa, surveys of drivers of butterfly diversity in farmlands are rare in the literature. There are a few published papers quantitatively documenting the diversity of butterflies for some agricultural regions in East Africa. In Uganda, most of the works on butterflies have been carried out mainly in natural areas, forest ecosystems and in protected areas (Tumuhimbise et al. Citation1998; Howard et al. Citation2000; Molleman et al. Citation2006; Tushabe et al. Citation2006). There exist no published data describing extensively the diversity of butterflies found in agricultural landscapes in Uganda in relationship to climatic, regional, landscape and local drivers. However, such information is important for butterfly biodiversity conservation in the rural landscapes.
While several studies (Chay-Hernández et al. Citation2006; Kivinen et al. Citation2008; Pickens and Root Citation2008; Dover and Settele Citation2009) indicate that climatic, regional and landscape factors are important drivers for butterflies in farmland areas, other studies indicate that local factors (availability of nectaring resources) are of foremost importance in explaining the variation in species richness and total density of butterflies in farmland regions (Pöyry et al. Citation2009). It is not clear which local, landscape, regional and climatic factors are important predictors of butterfly species richness and abundance in Central Uganda.
The aim of this study was to explore the relationships between butterfly assemblages (species richness and abundance) and local, landscape, regional and climatic drivers.
Materials and methods
Study area and selection of study sites
This study was conducted in 26 different study sites in the banana–coffee system of Lake Victoria Arc, covering several districts of Central Uganda (Munyuli 2009a, 2009b, 2009c, Citation2011a, Citation2012; Munyuli et al. 2009). The Lake Victoria Arc is characterized by ferrisoils with high to medium fertility level and receives on average 1000–1800 mm of rains in a bimodal pattern (rainy seasons: March–May, September–November; semi-dry to dry seasons: June–August, December–February) with 22–28°C and 60–75% of mean annual temperature and relative humidity, respectively (Munyuli Citation2011b). Rainfall amounts and patterns are unpredictable. Several food and cash crops are grown in small-scale monoculture and/or polyculture fields that are integrated into the coffee–banana agroforest production systems where coffee and banana are the main crops (Munyuli Citation2011c). Rural Central Uganda is a mosaic landscape where ‘islands’ of patches of natural habitats (forest fragments, forest reserves, wetlands, woodlands) are found scattered within agricultural matrices dominated by linear and non-linear features of semi-natural habitats (fallows, hedgerows, grasslands, woodlots, rangelands). All study sites were situated at approximately 790–1000 m above sea level. A total of 26 sites were selected to represent a range of vegetation and habitat types of varying degrees of anthropogenic disturbance and management intensities. Selected study sites were grouped using human population density as a surrogate measure of agricultural intensity (Bolwig et al. Citation2006). Detailed vegetation, environmental and landscape characteristics of the 26 sites are presented in Munyuli (Citation2011a).
Butterfly sampling
Field sampling of butterflies was conducted in each of the 26 study sites. In each study site, an area of 1 km2 was selected and divided into five linear parallel transects 1000 m long and 200 m apart. Butterfly data were collected on one of the five transects per study site, and the selected transect was surveyed during five study site visits in 2006. Butterflies were sampled using three complementary methods (transects walk-and-counts, handnets and fruit-bait traps) universally recommended and extensively used to survey and monitor butterfly populations and communities (Pollard and Yates Citation1993; Raguso Citation1993; DeVries and Walla Citation2001; Kitahara and Sei Citation2001; Yahner Citation2001; Fermon et al. Citation2002; Kitahara Citation2004; Barlow et al. Citation2007; Kitahara et al. Citation2008; van Swaay et al. Citation2008; Marín et al. Citation2009; Vu Citation2009) in terrestrial ecosystems (Lehmann and Kioko Citation2005) in the tropics. These sampling methods have been applied in Uganda in previous studies (Owen Citation1971; Coe et al. Citation1999; Molleman et al. Citation2006; Akite Citation2008). Butterfly specimens were identified by consulting literature, nomenclature and coloured plates of butterflies and by using the reference collection available at Makerere University Zoology Museum. To avoid a mix up of the results, specimens collected by each of three sampling methods applied were identified separately. The taxonomic characteristics of butterflies were obtained from standard guides, including (i) Butterflies of Kenya (Larsen Citation1996), (ii) ‘Butterflies of West Africa’ (Larsen Citation2006) and (iii) ‘Butterflies of Uganda’ (Carder and Tindimubona Citation2002). Identification of all voucher specimens was confirmed by a butterfly taxonomist based at Makerere University Zoology Museum. All voucher specimens collected during the present research were deposited at Makerere University Zoology Museum.
Measurement of local, landscape, regional and climatic drivers
Local drivers
Local drivers (richness and abundance of floral nectaring resources) of importance for butterflies were measured following approaches described by Munyuli (Citation2010). These included the percent cover of flowering plants (trees, shrubs, herbs and weeds), the number of nectaring plant species and the percentage cover of cultivated floral resources (proportion of cultivated pollinator-dependent and non-pollinator-dependent crops) per 1 km2 area. Data on herbs were collected from 5 m × 5 m (25 m2) quadrats, while those on shrub and trees were collected from 10 m × 50 m (500 m2) quadrats. A total of 2025 m2 quadrats and 20,500 m2 quadrats were established per transect per study site.
Landscape drivers
The area size of different land-use types in each 1 km2 site was determined using a global positioning system (GPS) or using a tape measure for measurement of the dimensions of small fields (<50 × 50 m). Different land uses were grouped into types. Three landscape variables of high ecological importance for butterfly community studies in agricultural matrices were determined for each site: (i) the percentage of semi-natural habitat per square kilometre area; the term semi-natural habitats included all linear and non-linear semi-natural habitats such as fallows, hedgerows, field margins, grasslands, roadsides, woodlands, woodlots, track sides and stream edges. Because fallows play a particular role (as butterfly reservoirs and as foraging/breeding habitats) in the maintenance of many butterfly species in rural landscapes, the proportion of cover (%) of young fallows per site was calculated alongside the proportion of cover of semi-natural habitats; (ii) the percentage of the land area cultivated; and (iii) the forest distance, that is, the distance from a given study site to the nearest potential pollinators’ source. Distances up to 100 m were measured with a tape measure and distances greater than 100 m were measured with GPS (Garmin International, Olathe, KS, USA; corrected to ± 1 m accuracy with Pathfinder v 2.0).
Regional land-use intensity factors
Different land-use categories were obtained from the Makerere University Geographic Information Service that classifies land use around Lake Victoria into four categories: low, medium, high and very high. Land uses classified as low are areas where three-quarters of the land are maintained uncultivated compared with one-quarter that is under crop/animal production. Land uses classified as medium are managed habitat types where there is an almost equal amount of land cultivated and uncultivated, whereas land uses classified as high are areas dominated by crops or livestock and land uses classified as very high represent large monocultures (estates of tea, sugar, coffee, etc.).
Climatic drivers
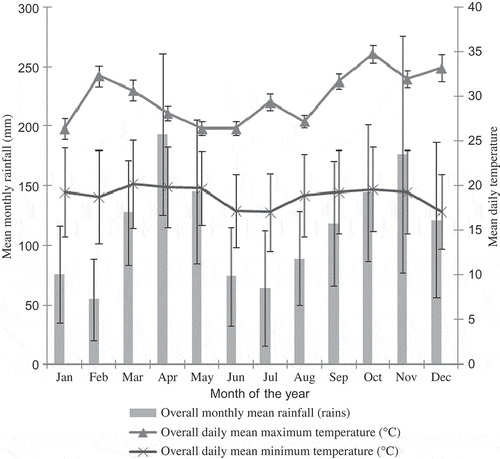
Data on regional climatic factors were obtained from meteorological stations located in the study area (Kamenyamigo, Entebbe, Jinja and Kiige meteorological stations). Using raw data from the stations, monthly means for 10 years (1997–2006) for temperature and rainfall () were calculated to detect trends in the rainfall patterns and temperature, since such oscillations may affect the patterns (trends in occurrence and activities) of butterfly species richness and abundance in rural landscapes. Later, other variables (of relevance to the study of patterns of butterfly communities in farmlands) were calculated: (i) the overall mean rains (means/month/10 years); (ii) the overall daily mean minimum and maximum temperatures (mean of 10 years); (iii) the mean monthly rainfall (2004, 2005, 2006); (iv) the mean monthly maximum temperature (2004, 2005, 2006); and (v) the mean monthly minimum temperature (2004, 2005, 2006).
Data analysis
Data from the three sources (transect walk-and-counts, banana-bait traps and handnets) were pooled to obtain total butterfly biodiversity per transect per study site per sampling round. Lumping data together helped to minimize variance associated with individual sampling methods (Bossart et al. Citation2006). Butterfly abundance was calculated as the total number of individuals recorded per transect per study site each sampling day, whereas butterfly species richness was calculated as the total numbers of butterfly species recorded per transect per study site each sampling round. To determine the suite of explanatory variables most closely related to butterfly species richness and abundance measurements, a correlation analysis was conducted. Thus, correlation among dependent variables (butterfly species richness and abundance) and independent variables (climatic, local, landscape and regional variables) characterizing the 26 study sites was tested using Pearson correlation after transformation (log10 and arcsine square root transformation) of the raw data (Munyuli 2009a, 2009b, 2009c; Munyuli et al. 2009). Based on the correlation matrix of all variables measured, only non-collinear independent variables that were significantly (p < 0.05) related to dependent variables were selected for further analyses in simple regression analyses. These also illustrated the trends and magnitude of the effects of independent factors on dependent variables. Scatter plots were used to illustrate the scale dependency of butterflies on the different drivers. For all simple regression models obtained, the coefficient of determinations (R2) were plotted to demonstrate the level of influence of the type of drivers that correlate with the response variable (abundance and species richness of butterflies). The value of the R2 was also used to identify variables with the most predictive effects. Thus, for all explanatory variables, the best regression model was selected based on the highest cross-validated R2.
To analyse the effects of land-use categories on butterfly abundance and diversity, a general linear model analysis of variance was conducted with butterfly community variables (abundance, species richness) as the dependent variables, and the regional land-use categorical variables (low, medium, high and very high) as fixed factors. The least significant difference (LSD) tests were used as post hoc tests for multiple comparisons of means.
Results
Characteristics of butterfly communities recorded during the surveys
In total, 331 species and 95 genera were registered comprising a total of 57,439 individuals in 26 study sites. Genera with the greatest number of species were Acraea (10.8%), Charaxes (8.9%), Bicyclus (7.6%) and Neptis (5.7%). The most rich tribes were Acraeni (15.5%), Pierini (14.5%), Charaxini (12.7%), Mycalesini (10.9%) and Limenitidini (8.6%). The dominant (>5% of total individuals recorded) butterfly species were Acraea acerata, Bicyclus safitza, Catopsilia florella and Junonia sophia. These dominant and widespread species could be found in all study sites. The most abundant genera were Acraea (17.6%), Junonia (17.4%), Bicyclus (11.1%), Catopsilia (7.9%), Eurema (7.7%), Belenois (6.0%) and Ypthima (5.1%), whereas the most abundant tribes included Junoniini (22.6%), Acraeini (22.4%), Mycalesini (14.2%), Pierini (10.2%), Polyommatini (7.4%) and Satyrini (5.2%). Around 91.2% (331 observed species against 360 expected species) of the species present in the study area were found. The results suggested that a complete inventory of the regional species pool was obtained. Fifteen species were identified as the most ecologically important species (‘characteristic species’) of the coffee–banana system butterfly assemblages, since they occurred with high constancy value (>50%). These were C. florella, J. sophia, B. safitza, A. acerata, Eurema hecabe, Ypthima albida, Zizula hylax, Acraea ventura, Eurema brigitta, Neptidopsis ophione, Junonia eonone, Zizeeria knysna, Cupidopsis cissus, Junonia chorimene and Acrae uvui. They should serve as reliable and efficient indicators of change in conditions in agroecological environments in further surveys. They may also be good candidates for monitoring the state of butterflies of the farmland habitats in East Africa.
Local drivers of butterfly richness and abundance
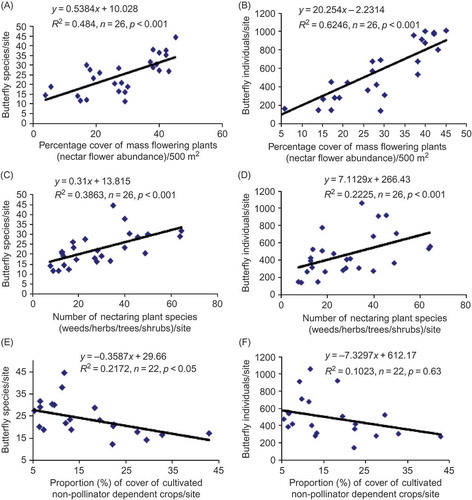
The results of simple linear and non-linear regressions indicated that mass flowering wild plant resources (nectar flower abundance) were significantly positively related to both species richness () and abundance () of butterflies. Overall, mass flowering plants explained 48% and 62% of variations in butterfly species richness and abundance, respectively. Similarly, the number of nectaring plant species (all weeds, herbs, shrubs, trees) was positively related to both species richness () and abundance () of butterflies. The number of nectaring plant species accounted for 38% and 22% of variations in butterfly species richness. This result suggests that diverse flowering plant species stimulated attraction of a species-rich flower-visiting butterfly community in the farm landscapes. Unexpectedly, cultivated pollinator-dependent floral resources were not related to butterfly species richness. Similarly, the relationship between cultivated pollinator-dependent crops and butterfly abundance was not significant (p > 0.05), and this indicated that some butterfly species could not visit animal-pollinated crop species. Apparently, butterflies do not benefit much from available mass flowering pollinator-dependent crops. However, cultivated non pollinator-dependent floral resources were significantly negatively related to butterfly species richness (). The percentage of cultivated non-pollinator crops explained 21% of the variation in butterfly species richness. Butterfly abundance was not significantly (p > 0.05) related to the proportion of pollinator-dependent crops, although there was a negative trend. These results indicate that the occurrence of butterflies in the agricultural landscapes of Central Uganda was largely influenced by wild floral resources and by cultivated non-pollinator-dependent crops rather than by cultivated pollinator-dependent floral resources.
Figure 2. Relationship between (i) nectar flower abundance (percentage of mass flowering plants), (ii) the abundance of cultivated floral resources (percentage of cultivated non-pollinator-dependent crops), (iii) the number of flowering plant species (weeds, herbs, trees, shrubs) and the number of butterfly species (A, C, E) and butterfly abundance (B, D, F) per 2 ha transect/study site. Note: Pollinator-dependent crops included all annual, biannual and perennial non-entomophilous crops potentially offering nectar to butterflies.
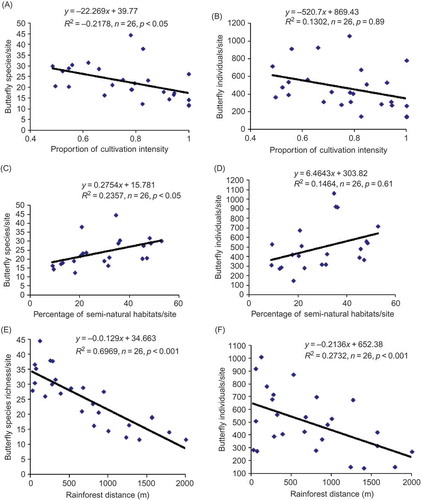
Landscape drivers of butterfly richness and abundance
Cultivation intensity was significantly negatively related to butterfly species richness (), but not to butterfly abundance (). Proportion of cultivation intensity explained 48% of the variation in butterfly species richness. Similarly, the proportion of semi-natural habitats in a 1 km2 area was significantly positively related to butterfly species richness () and not related to butterfly abundance (). Overall, the proportion of semi-natural habitats accounted for 23% of the variation in butterfly species richness. These results indicated that the increase in amount of semi-natural habitats in the landscape was likely to lead to increased species richness (not the abundance) of butterflies in agricultural matrices of Central Uganda. Forest distance was significantly negatively related to both butterfly abundance () and butterfly species richness (). Thus, the forest distance explained 69% and 27% of variations in butterfly species richness and abundance, respectively ().
Regional drivers of butterfly richness and abundance
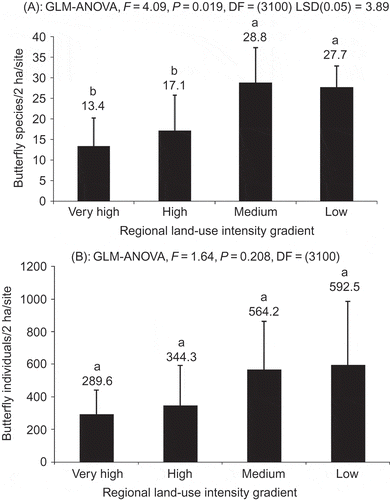
There were no significant variations in butterfly abundance among the four land-use intensity categories (p > 0.05, ), indicating that butterfly abundance was not very sensitive to the degree of agriculture intensification. However, there were significant effects of the land-use intensity on butterfly species richness (p < 0.05; ) that were significantly higher in low to medium land-use intensity categories than in high and very high land-use categories (). Study sites located in the low land-use category had almost double the number of species.
Figure 4. Effects of the regional land-use intensity categories on the species richness (A) and abundance (B) of butterflies collected from farmlands of Central Uganda in 2006. Notes: Means (x ± SE) followed by different letters are significant at p < 0.05 according to the LSD test. GLM, general linear model; ANOVA, analysis of variance.
Climatic drivers of butterfly richness and abundance
There were significant correlations between climatic variables and butterfly community parameters (). Rainfall of the year 2004 was negatively correlated to both species richness (r = –0.47, p < 0.05, n = 26) and abundance (r = –0.49, p < 0.05, n = 26) of butterflies. Similarly, the mean rainfall of the year 2005 was significantly negatively correlated to both species richness (r = –0.48, p < 0.001, n = 26) and abundance (r = –0.53, p < 0.05, n = 26) of butterflies. However, butterfly abundance and species richness were not significantly (p > 0.05) associated with the mean rainfall of the year 2006 or the overall mean rainfall of 10 years (). In addition, the mean maximum temperature of 10 years was positively correlated to both species richness (r = 0.51, p < 0.05, n = 26) and abundance (r = 0.48, p < 0.05, n = 26) of butterflies. In addition, the mean butterfly abundance was positively correlated to both the maximum temperature of the year 2004 (r = 0.51, p < 0.05, n = 26) and the maximum temperature of the year 2005 (r = 0.46, p < 0.05, n = 26) (). These results indicated that current butterfly abundance and/or species richness were likely being affected by past events. In other words, butterfly communities found in farmlands of Central Uganda may be sensitive to climate change. Although rainfall is expected to affect positively the number and cover of wild and cultivated flowering plant species, no significant (p > 0.05) correlations were found between the abundance (and the diversity) of cultivated and wild floral resources and the mean rainfall of 10 years, or the mean rainfall of the year 2004 or 2005 ().
Discussion
Local, landscape and regional drivers of butterfly abundance and diversity
Among local drivers, the abundance and species richness of wild flowering plants significantly affected butterfly abundance and species richness. Both the number and cover of wild blooming plant species were more important local drivers for butterflies than the abundance of cultivated floral resources in the coffee–banana agroforest systems in Central Uganda. It is likely that cultivated floral resources (pollinator-dependent and non-pollinator-dependent crops) are not the primary food plants for the majority of butterfly species inhabiting farmlands probably because locally grown plants are less rich in nectar, the main floral resource collected by most butterflies foraging in agricultural landscapes. A significant strong positive relationship between butterfly species richness and nectaring plant species richness was found in Central Uganda. This result is consistent with Simonson et al. Citation(2001) who found that butterfly species richness was positively correlated with total vascular plant species richness and native plant species richness in Colorado (USA). Worldwide, positive relationships between butterfly diversity and plant species richness are reported (e.g. Kumar et al. Citation2009): plant species serve as important food plants for larvae, flowers providing nectar resources for adult butterflies and the physical structure of plants create microhabitats providing shelter to numerous species of butterflies in rural landscapes. Thus, high plant species diversity is accepted to be an indicator of good habitat quality for generalist and specialist butterfly species (Kumar et al. Citation2009) inhabiting farmland habitats.
In this study, it was observed that butterfly species richness was sensitive to land-use intensity whereas the abundance was not, probably because very few adapted farmland species can easily be represented by a high number of individuals in the farmlands while the majority (>70%) of species may be ‘rare species’ represented by one to two individuals only. While studying the effect of agricultural intensification on the homogenization of Lepidoptera community in Finland, it was observed that the diversity of butterfly and day-active geometrid moth communities found within 134 differently fragmented landscapes decreased with increasing agricultural intensity with the consequence: promotion of habitat generalists in landscapes with moderate to intensive agriculture compared to region with low agriculture intensity (Ekroos et al. Citation2010).
Table 1. Cross-correlation matrix showing naïve multiple correlations of Lepidoptera (butterfly) variables with environmental, local and landscape variables
The results from Central Uganda revealed that forest distance was the most significant landscape driver followed by the proportion of semi-natural habitats. Both the abundance and species richness of butterflies declined linearly with increasing forest distance. This result is consistent with observations recorded in India where a study on adult butterfly communities visiting coffee plantations around a protected area in the Western Ghats found that both butterfly species composition and species richness declined with increasing forest distance (Dolia et al. Citation2008) up to 2000 m. Distance of up to 1700 m from mature forests did not have any significant effect on butterfly species richness in a mosaic landscape in Central Sulawesi in Indonesia (Veddeler et al. Citation2005), whereas in Central Uganda, butterfly species richness declined sharply up to a distance of 2000 m. The fact that forest distance was negatively related to butterfly species richness and abundance suggests that surrounding natural forests of agricultural matrices remain an irreplaceable habitat for several butterfly species belonging to different functional groups (e.g. breeding strategy) and life history traits in Central Uganda. In addition, this observation suggests that the ability of different agricultural land uses (human-dominated landscapes) to support Lepidoptera communities can be enhanced if tree plantations/crop fields are established in proximity to surrounding primary forests (Hawes et al. Citation2009). Obviously, a contiguous distribution of large secondary forest patches with many host trees is very important to conserve butterfly species in fragmented agricultural landscapes (Kobayashi et al. Citation2009). The observation made in Central Uganda about the effect of forest fragments is consistent with the conclusion of Koh Citation(2008) who clearly demonstrated that natural forests serve as an important population sources for butterfly species occurring in nearby agricultural matrices. In terms of habitat use, butterflies were found more in forest margins, forest fallows, wetlands, swampy habitats, woodlots, woodlands, grasslands and in adjacent fields (Munyuli Citation2011a) as compared with fields that were located far from such natural and semi-natural habitats. Forest is a primary factor dictating the occurrence of butterflies in agricultural landscapes of Central Uganda.
In this study, both the abundance and diversity of butterflies increased with increasing amount of semi-natural habitats per square kilometre. Similarly, in southeastern Sweden (Jonason et al. Citation2010), butterfly species richness (not the abundance) was found to be positively related to increasing tree cover in the farm landscape. Apart from land use and farm management methods or farming practices (Weibull and Östman Citation2003), vegetation structure, quality of the matrix surrounding an agricultural habitat (Binzenhöfer et al. Citation2008; Summerville et al. Citation2008), diversity and types of habitats (Dessuy and de Morris Citation2007; Ngai et al. Citation2008; Kumar et al. Citation2009), landscape heterogeneity and habitat connectivity (Davis et al. Citation2007) are important factors determining occurrence, movements, population dynamics, seasonality, persistence and long-term survival of Lepidoptera faunal communities in the agricultural landscapes (Dennis Citation2003; Greza et al. Citation2004; Chay-Hernández et al. Citation2006; Kivinen et al. Citation2008; Öckinger and Smith Citation2008; Pickens and Root Citation2008; Stasek et al. Citation2008; Dover and Settele Citation2009; Brückmann et al. Citation2010). Generally, agricultural matrices that are more resembling a nearby forest patch maintain higher butterfly diversity than matrices with lesser shade cover (Summerville et al. Citation2001; Kitahara and Watanabe Citation2003; Weibull and Östman Citation2003; Boriani et al. Citation2005; Aviron et al. Citation2007; Ohwaki et al. Citation2007, 2008; Barlow et al. Citation2008; Bergman et al. Citation2008; van Halder et al. Citation2008; Marín et al. Citation2009).
Climatic drivers of butterfly richness and abundance
It is generally accepted that climate factors regulate most insect species’ life cycles, including butterflies (Sparks and Yates Citation1997; Roy and Sparks Citation2000; Watt and McFarlane Citation2002). Climatic variables may therefore be very important in determining emergency (van Asch and Visser Citation2007; Saastamoinen and Hanski Citation2008), foraging, reproduction and breeding activities of many butterfly species in sub-Saharan Africa. In fact, seasonal flight activity, as well as species diversity and overall abundance vary greatly between sampling rounds (Munyuli Citation2010) in a study site. It is well established that temperature is a key factor increasing daily activities (flight, foraging movements) of butterflies, whereas rainfall affects indirectly butterfly through enhancing the availability of nectaring resources. Most butterfly species need resources like host plants (for larvae), nectar plants (for adults) and sites for resting, and both natural/semi-natural habitats and nectar plant resources are also affected by rainfall pattern (Öckinger et al. Citation2009; Rossi and van Halder Citation2010).
In this study, the abundance and species occurrence of butterflies were significantly related to rainfall and mean maximum temperature in previous years (2004, 2005), not the year of study (2006). The mean precipitation of the past 10 years was not significantly associated with any measure of richness and abundance of butterflies, but the mean maximum temperature in the same period was. The fact that butterfly community variables were related to mean maximum temperature and rainfall in previous years rather than current has also been found in temperate regions (e.g. Pollard Citation1988). In relation to rainfall, results obtained from Uganda coincide at least partially with results from Malaysia, where it was found (Brehm Citation2005) that monthly rainfall variation correlated with moth populations in previous months of the year, weather factors and plant phenology in the lowland dipterocarp forest. The intensity of tree flowering in the previous months was an important environmental factor that correlated positively with the numbers of species and individuals of moths that emerged in any subsequent month (Intachat et al. Citation2001). High rainfall in the previous 3 months led to an increase in moth abundance in the following month (perhaps by stimulating an increase in fresh plant material), whereas high rainfall and relative humidity thereafter served to decrease abundance, possibly by encouraging the spread and activity of pathogens that impacted on early life-stage survivorship (Intachat et al. Citation2001). It was concluded that the diversity of geometrid moths correlated more with weather parameters than with tree phenology (Intachat et al. Citation2001) in tropical regions, and important weather parameters that influenced moth abundance include monthly rainfall, relative humidity and minimum temperature in previous months. In addition, it is generally agreed that weather variables can have long-term effects on population fluctuations of different butterfly taxa (Sei-Woong Citation2003). Weather variables can help in detecting potential butterfly population changes following changes (Sei-Woong Citation2003) in climatic factors (changes in temperature regimes, rainfall patterns, relative humidity) that are believed to affect negatively/positively species richness, abundance and distribution of butterflies regionally as well as locally (Sei-Woong Citation2003; Kivinen et al. Citation2007). Although in Uganda the study was conducted on butterflies in agricultural systems, while in Malaysia the study was conducted on moths, the two studies were able to show that Lepidoptera biodiversity can be related to previous events (historical events) in weather factors.
Practically, weather factors affect the production of floral resources in current and previous years. High production of flowers in the previous wet months of the year may guarantee the availability of food for different butterfly taxa (larval and adult stages), and this in turn may tend to increase larval survivorship and therefore the abundance of adult butterflies. In some cases, the abundance of adult butterflies can increase with high abundance and diversity of flowering plants in the current and not in previous years. An increase in the number of host trees flowering would also mean an increased number of oviposition sites for the butterflies (particularly forest specialist species).
High rainfall in the months of the current year may deter butterflies from flying, thus resulting in a low catch in traps. High rainfall in the previous months of the year may also decrease the survivorship of the larvae, resulting in lower adult butterfly catch in the current month. During very rainy periods, larvae are more likely to be washed off their host plants. Combination of a wetter period and a high relative humidity in the months before adult emergence may also increase activities of disease microorganisms during that period, resulting in higher risk of larval mortality from disease and therefore reduction in adult abundance in the current month. Rainfall in the current month may also encourage disease at the pupal stage and cause mortality if heavy during the vulnerable eclosion period. High rainfall, on the other hand, can encourage blooming of wild and cultivated plants in most tropical agroecosystems for the benefit of butterflies (Munyuli Citation2010) and other Lepidoptera. Heavy blossoms in the previous months would encourage adult and larval survivorship and therefore increase the number of emergence adult butterflies in the following months of the following year.
In summary, if the annual and seasonal changes in rainfall and temperature continue to oscillate and if rainfall continue diminishing while temperature continue rising in Uganda (Munyuli Citation2010) this may, combined with already existing negative impacts of environmental degradation, lead to a high loss/decline in butterfly biodiversity while increasing the vulnerability of ecosystems relying on these keystone species to function properly.
Conclusion
Findings of this study indicated that the abundance and species diversity of butterflies in the coffee–banana agroforestry systems of Central Uganda were affected by local, landscape, regional and climate drivers. Plant diversity was the most influential local factor, whereas forest distance and cultivation intensity were the most influential landscape drivers. The presence of natural habitats (e.g. forest reserves, wetlands) and semi-natural habitats in the vicinity of agricultural matrices was related to high diversity of Lepidoptera. It was also found that butterfly communities were affected more by previous climatic events than current ones. Landscape managers should advocate for the maintenance of high-quality habitat matrix in agricultural mosaic farm landscapes to protect native butterfly faunal diversity and reduce their vulnerability to further environmental/climatic changes in the region. This could be supported by policies that enhance protection and restoration of forest fragments and promotion of interventions that enable farmers to adopt butterfly friendly conservation and farming practices, such as agroforestry systems with multipurpose tree species, to sustain butterfly communities and services in rural landscapes of Uganda.
To provide incentives to the conservation of butterfly fauna in agricultural landscapes in Uganda, further research is needed to understand the behavioural ecology of butterflies and to determine the pollination efficiency (Martins and Johnson Citation2009) of different butterfly species (as well as for other Lepidoptera taxa). Such studies will help in highlighting the importance of butterflies (and moths) as a group providing pollination services of high economic importance to both wild and cultivated plants in Uganda and in East and Central Africa.
Acknowledgements
I am very grateful to the Darwin Initiative (Defra, UK; project reference: 14-032; project title: Conserving biodiversity in modernized farm landscapes in Uganda) for funding this study. I am also very grateful to project leaders Dr Juliet Vickery (RSPB-Cambridge University, UK), Dr Phil Atkinson (British Trust for Ornithology, UK), Prof. Derek Pomeroy (Makerere University, Uganda), scientific supervisors Prof. Simon Potts (University of Reading, UK) and Prof. Philip Nyeko (Makerere University, Uganda). I am also grateful to Dr Bwinja M and to Mr Maurice Mutabazi (research assistant) for his assistance in the field. I am grateful to Akite Perpetra who helped during identification of butterfly to species level at Makerere University Zoology Museum. I am also grateful to anonymous reviewers of this journal for constructive criticisms and comments on earlier versions of this article.
References
- Akite P. 2008. Effects of anthropogenic disturbances on the diversity and composition of the butterfly fauna of sites in the Sango Bay and Iriiri areas, Uganda: implications for conservation. Afr J Ecol. 46(1):3–13.
- Aviron ST, Kindlmanna P, Burel F. 2007. Conservation of butterfly populations in dynamic landscapes: the role of farming practices and landscape mosaic. Ecol Model. 205(1–2):135–145.
- Barlow J, Araujo IS, Overal WL, Gardner TA, da Silva Mendes F, Lake IR, Peres CA. 2008. Diversity and composition of fruit-feeding butterflies in tropical Eucalyptus plantations. Biodivers Conserv. 17(5):1089–1104.
- Barlow J, Overal WL, Araujo IS, Gardner TA, Peres CA. 2007. The value of primary, secondary and plantation forests for fruit-feeding butterflies in the Brazilian Amazon. J Appl Ecol. 44(5):1001–1012.
- Bergman KO, Ask L, Askling J, Ignell H, Wahlman H, Milberg P. 2008. Importance of boreal grasslands in Sweden for butterfly diversity and effects of local and landscape habitat factors. Biodivers Conserv. 17(1):139–153.
- Binzenhöfer B, Biedermann R, Settele J, Schröder B. 2008. Connectivity compensates for low habitat quality and small patch size in the butterfly Cupido minimus. Ecol Res. 23(2):259–269.
- Bolwig S, Pomeroy D, Tushabe H, Mushabe D. 2006. Crops, trees, and birds: biodiversity change under agricultural intensification in Uganda’s farmed landscapes. Danish J Geogr. 106(2):115–130.
- Bonebrake TC, Ponisio LC, Boggs CL, Ehrlich PR. 2010. More than just indicators: a review of tropical butterfly ecology and conservation. Biol Conserv. 143(9):1831 –1841.
- Boriani L, Burgio G, Marini M, Genghini M. 2005. Faunistic study on butterflies collected in Northern Italy rural landscape. Bull Insectol. 58(1):49–56.
- Bossart JL, Opuni-Frimpong E, Kuudaar S, Krumah S. 2006. Richness, abundance, and complementarity of fruit-feeding butterfly species in relict sacred forests and forest reserves of Ghana. Biodivers Conserv. 15(1):333–359.
- Brehm G. 2005. Diversity and community structure of geometrid moths of disturbed habitat in a montane area in the Ecuadorian Andes. J Res Lepid. 38(1999):1–14.
- Brown JKS. 1997. Diversity, disturbance, and sustainable use of neotropical forests: insects as indicators for conservation monitoring. J Insect Conserv. 1(1):25–42.
- Brückmann SV, Krauss J, Steffan-Dewenter I. 2010. Butterfly and plant specialists suffer from reduced connectivity in fragmented landscapes. J Appl Ecol. 47(4):799–809.
- Carder N, Tindimubona L. 2002. Butterflies of Uganda. A field guide to butterflies and silk moths on the collection of the Uganda Society. Kampala (Uganda): The Uganda Society. 89 p.
- Cardoso P, Henriques SS, Gaspar C, Crespo LC, Carvalho R, Schmidt JP, Sousa P, Szűts T. 2009. Species richness and composition assessment of spiders in a Mediterranean Scrubland. J Insect Conserv. 13(1):45–55.
- Cardoso P, Scharff N, Gaspar C, Henriques SS, Carvalho R, Castro PH, Schmidt JB, Silva I, Szüts T, Castro AD, et al. 2008. Rapid biodiversity assessment of spiders (Araneae) using semi-quantitative sampling: a case study in a Mediterranean forest. Insect Conserv Diver. 1(2):71–84.
- Chay-Hernández DA, Delfín-González H, Parra-Tabla V. 2006. Ichneumonoidea (Hymenoptera) community diversity in an agricultural environment in the State of Yucatan, Mexico. Environ Entomol. 35(5):1286–1297.
- Cleary DFR. 2004. Assessing the use of butterflies as indicators of logging in Borneo at three taxonomic levels. J Econ Entomol. 97(2):429–364.
- Cleary DFR, Mooers A. 2004. Butterfly species richness and community composition in forests affected by ENSO-induced burning and habitat isolation in Borneo. J Trop Ecol. 20(4):359–367.
- Coe MJ, McWilliams NC, Stone GN, Packer MJ. 1999. Mkomazi: the ecology, biodiversity and conservation of a Tanzanian Savannah. London (UK): Royal geographical Society ( with the Institute of British Geographers). p. 608.
- Davis JD, Debinski DM, Danielson BJ. 2007. Local and landscape effects on the butterfly community in fragmented Midwest USA prairie habitats. Landsc Ecol. 22(9):1341–1354.
- Dennis P. 2003. Sensitivity of upland arthropod diversity to livestock grazing, vegetation structure and landform. J Food, Agric Environ. 1(2):301–307.
- Dessuy MB, de Morris ABB. 2007. Diversity of butterflies (Lepidoptera, Papilionoidea and Hesperioidea) in fragments of decidual seasonal forests in Santa Maria, Rio Grande do Sul State, Brazil. Rev Bras Zool. 24(1):108–120.
- DeVries PJ, Walla TR. 2001. Species diversity and community structure in neotropical fruit feeding butterflies. Biol J Linn Soc. 74(1):1–15.
- Dolia J, Devy MS, Aravind NA, Kumar A. 2008. Adult butterfly communities in coffee plantations around a protected area in the Western Ghats, India. Animal Conserv. 11(1):26–34.
- Dover J, Settele J. 2009. The influences of landscape structure on butterfly distribution and movement: a review. J Insect Conserv. 13(1):3–27.
- Ekroos J, Heliölä J, Kuussaari M. 2010. Homogenization of Lepidopteran communities in intensively cultivated agricultural landscapes. J Appl Ecol. 47(2):459–467.
- Fermon H, Waltert M, Mühlenberg M. 2002. Movement and vertical stratification of fruit-feeding butterflies in a managed West African rainforest. J Insect Conserv. 7(2):7–19.
- Fitzherbert E, Gardner T, Davenport TRB, Caro T. 2006. Butterfly species richness and abundance in the Katavi ecosystem of western Tanzania. Afric J Ecol. 44(3):353–362.
- Greza A, Zaviezo T, Reyes S. 2004. Short-term effects of habitat fragmentation on the abundance and species richness of beetles in experimental alfalfa micro-landscapes. Rev Chil Hist Nat. 77(3):547–558.
- Hawes J, Motta CDS, Overal WL, Barlow J, Gardner TA, Peres CA. 2009. Diversity and composition of Amazonian moths in primary, secondary and plantation forests. J Trop Ecol. 25(3):281–300.
- Howard PC, Davenport TRB, Kigenyi FW, Viskanic P, Baltzer MC, Dickinson CJ, Lwanga J, Matthews RA, Mupada E. 2000. Protected area planning in the tropics: Uganda’s national system of forest nature reserves. Conserv Biol. 14(3):858–875.
- Intachat J, Holloway JD, Staines H. 2001. Effects of weather and phenology on the abundance and diversity of geometroid moths in a natural Malaysian tropical rain forest. J Trop Ecol. 17(3):411–429.
- Jonason D, Milberg P, Bergman KO. 2010. Monitoring of butterflies within a landscape context in south-eastern Sweden. J Nat Conserv. 18(1):22–33.
- Kitahara M. 2004. Butterfly community composition and conservation in and around a primary woodland of Mount Fuji, central Japan. Biodivers Conserv. 13(5):917–942.
- Kitahara M, Sei K. 2001. A comparison of the diversity and structure of butterfly communities in semi-natural and human-modified grassland habitats at the foot of Mt. Fuji, central Japan. Biodivers Conserv. 10(3):331–351.
- Kitahara M, Watanabe M. 2003. Diversity and rarity hotspots and conservation of butterfly communities in and around the Aokigahara woodland of Mount Fuji, central Japan. Ecol Res. 18(5):503–522.
- Kitahara M, Yumoto M, Kobayashi T. 2008. Relationship of butterfly diversity with nectar plant species richness in and around the Aokigahara primary woodland of Mount Fuji, Central Japan. Biodivers Conserv. 17(11):2713–2734.
- Kivinen S, Luoto M, Heikkinen RK, Saarinen K, Ryttäri T. 2008. Threat spots and environmental determinants of red-listed plant, butterfly and bird species in boreal agricultural environments. Biodivers Conserv. 17(13):3289–3305.
- Kivinen S, Luoto M, Kuussaari M, Saarinen K. 2007. Effects of land cover and climate on species richness of butterflies in boreal agricultural landscapes. Agric Econ Environ. 122(4):453–460.
- Kobayashi T, Nakashizuka T, Kitahara M. 2009. Effects of fragmentation of secondary broadleaf deciduous forests on populations of the near-threatened butterfly, Sasakia charonda (Lepidoptera, Nymphalidae), in Central Japan. Ecol Res. 24(1):57–64.
- Koh LP. 2008. Can oil palm plantations be made more hospitable for forest butterflies and birds? J Appl Ecol. 45(4):1002–1009.
- Kremen C. 1992. Assessing the indicator properties of species assemblages of natural areas monitoring. Ecol Appl. 2(2):203–217.
- Kremen C. 1994. Biological inventory using target taxa: a case-study of the butterflies of Madagascar. Ecol Appl. 4(3):407–422.
- Kumar S, Simonson SA, Stohlgren TJ. 2009. Effects of spatial heterogeneity on butterfly species richness in Rocky Mountain National Park, CO, USA. Biodivers Conserv. 18(3):739–763.
- Larsen TB. 1996. The butterflies of Kenya and their natural history. Oxford (UK) : Oxford University Press. 453 p.
- Larsen TB. 2006. Butterflies of West Africa. Stockholm (Sweden): Apollo Publications. 612 p.
- Larsen TB. 2008. Forest butterflies in West Africa have resisted extinction … so far (Lepidoptera: Papilionoidea and Hesperioidea). Biodivers Conserv. 17(12):2833–2847.
- Lehmann I, Kioko E. 2005. Lepidoptera diversity, floristic composition and structure of three kaya forests on the south coast of Kenya. J East Afr Nat Hist. 94(1):121–163.
- Libert M. 1994. Monitoring of numbers and species composition of the butterfly community of two forested hills near Yaounde, Cameroun. Rev Ecol-Terre Vie. 25(2):151–175.
- Marchiori OM, Romanowski HP. 2006. Species composition and diel variation of a butterfly taxocene (Lepidoptera, Papilionoidea and Hesperioidea) in a restinga forest at Itapua State Park, Rio Grande de Sul, Brazil. Rev Bras Zool. 23(2):443–454.
- Marín L, León-Cortés JL, Stefanescu C. 2009. The effect of an agro-pasture landscape on diversity and migration patterns of frugivorous butterflies in Chiapas, Mexico. Biodivers Conserv. 18(4):4919–4934.
- Martins DJ, Johnson SD. 2009. Distance and quality of natural habitat influence hawkmoth pollination of cultivated papaya. Inter J Tropic Insect Sci. 29(3):114–123.
- Molleman F, Kop A, Brakefield PM, Devries PJ, Zwaan BJ. 2006. Vertical and temporal patterns of biodiversity of fruit feeding butterflies in a tropical forest in Uganda. Biodivers Conserv. 15(1):107–121.
- Munyuli TMB. 2009a. Is Pardosa pseudoannulata an effective predator agent of Aphis craccivora in Uganda and in Democratic Republic of Congo? Tunisian J Plant Protec. 4(1):91–98.
- Munyuli TMB. 2009b. Effects of native insect predators on population densities of Aphis craccivora and yields of Vigna unguiculata and Arachis hypogeae grown under various cropping systems, in Kivu province, eastern democratic republic of Congo. Tunisian J Plant Protec. 4(2):197–209.
- Munyuli TMB. 2009c. On-farm storages participatory evaluation and validation of the capability of native botanicals for control of bean bruchids (Acanthoscelides obtectus L., Coleoptera: Bruchidae) in South-Kivu province, eastern of Democratic Republic of Congo. Tropicultura. 27(3):174–183.
- Munyuli TMB. 2010. Pollinator biodiversity and economic value of pollination services in Uganda [PhD dissertation]. [Kampala (Uganda)]: Makerere University. 431 p.
- Munyuli TMB. 2011a. Pollinator biodiversity in Uganda and in Sub-Sahara Africa: landscape and habitat management strategies for its conservation. Inter J Biodivers Conserv. 3(11):551–609.
- Munyuli TMB. 2011b. Factors governing flower-visitation patterns and quality of pollination services delivered by social and solitary bee species to coffee in central Uganda. Afric J Ecol. 49(4):501–509.
- Munyuli TMB. 2011c. Farmers’ perceptions of pollinators’ importance in coffee production in Uganda. Agric Sci. 2(3):318–333.
- Munyuli TMB. 2012. Diversity of life-history traits, functional groups and indicator species of bee communities from farmlands of central Uganda. Jordan J Biol Sci. 5(1):1–14.
- Munyuli TMB, Kyamanywa S, Luther GC. 2009. Effects of cropping system and insecticide application on the incidence of arthropod parasitoids of cowpea insect pests in Uganda and Democratic Republic of the Congo. Tunisian J Plant Protec. 4(1):76–90.
- Ngai JT, Kirby KR, Gilbert B, Starzomski BM, Pelletier AJD, Conner JCR. 2008. The impact of land-use change on larval insect communities: testing the role of habitat elements in conservation. Ecoscience. 15(2):160–168.
- Nyamweya NH, Gichuki NN. 2010. Effects of plant structure on butterfly diversity in Mt. Marsabit Forest–northern Kenya. Afric J Ecol. 48(2):304–312.
- Öckinger E, Dannestam Å, Smith HG. 2009. The importance of fragmentation and habitat quality of urban grasslands for butterfly diversity. Landsc Urban Plan. 93(1):31–37.
- Öckinger E, Smith HG. 2008. Do corridors promote dispersal in grassland butterflies and other insects? Landsc Ecol. 23(1):27–40.
- Ohwaki A, Nakamura K, Tanabe SI. 2007. Butterfly assemblages in a traditional agricultural landscape: importance of secondary forests for conserving diversity, life history specialists and endemics. Biodivers Conserv. 16(5):1521–1539.
- Ohwaki A, Tanabe S-I, Nakamura K. 2008. Effects of anthropogenic disturbances on the butterfly assemblage in an urban green area: the changes from 1990 to 2005 in Kanazawa Castle Park, Japan. Ecol Res. 23(4):697–708.
- Owen DF. 1971. Tropical butterflies. The ecology and behavior of butterflies in the tropics with special reference to African species. Oxford (UK): Oxford Clarendon Press. 228 p.
- ÖzdenÖ, Ciesla WM, Fuller WJ, Hodgson DJ. 2008. Butterfly diversity in Mediterranean islands and in Pentadaktylos Pinus brutia forests of Cyprus. Biodivers Conserv. 17(12):2821–2832.
- Pickens BA, Root KV. 2008. Factors affecting host-plant quality and nectar use for the Karner blue butterfly: implications for oak savanna restoration. Nat Areas J. 28(3):210–217.
- Pollard E. 1988. Temperature, rainfall and butterfly numbers. J Appl Ecol. 25(3):819–828.
- Pollard E, Yates TG. 1993. Monitoring butterflies for ecology and conservation. Vol. 1. London (UK): Chapman & Hall.
- Pöyry J, Paukkunen J, Heliölä J, Kuussaari M. 2009. Relative contributions of local and regional factors to species richness and total density of butterflies and moths in semi-natural grasslands. Oecologia. 160(3):577–587.
- Pozo C, Luis-Martínez A, Lorente-Bousquets J, Salas-Suárez N, Vargas-Fernández I, Warren AD. 2008. Seasonality and phenology of the butterflies (Lepidoptera: Papilionoidea and Hesperioidea) of Mexico’s Calakmul region. Fla Entomol. 91(3):407–422.
- Raguso RA. 1993. Preliminary checklist and field observations of the butterflies of the Maquipucuna Field Station (Pichincha Province, Ecuador). J Res Lepid. 32(3):135–161.
- Rogo L, Odulaja A. 2001. Butterflies in two forest fragments at the Kenya coast. Afr J Ecol. 39(3):266–275.
- Rossi JP, van Halder I. 2010. Towards indicators of butterfly biodiversity based on a multi-scale landscape description. Ecol Indic. 10(2):452–458.
- Roy DB, Sparks TM. 2000. Phenology of British butterflies and climate change. Glob Change Biol. 6(4):407–416.
- Saastamoinen M, Hanski I. 2008. Genotypic and environmental effects on flight activity and oviposition in the Glanville fritillary butterfly. Am Nat. 171(6):701–712.
- Schmidt BC, Roland J. 2006. Moth diversity in a fragmented habitat: importance of functional groups and landscape scale in the boreal forest. Ann Entomol Soc Am. 99(6):1110–1120.
- Sei-Woong C. 2003. The relationship between local distribution and abundance of butterflies and weather factors. Korean J Ecol. 26(4):199–202.
- Simonson SE, Opler PA, Stohlgren TJ, Chong GW. 2001. Rapid assessment of butterfly diversity in a montane landscape. Biodivers Conserv. 10(8):1369–1386.
- Sparks TH, Yates TJ. 1997. The effect of spring temperature on the appearance dates of British butterflies 1883–1993. Ecography. 20(4):368–374.
- Stasek DJ, Bean C, Crist TO. 2008. Butterfly abundance and movements among prairie patches: the roles of habitat quality, edge, and forest matrix permeability. Environ Entomol. 37(4):897–906.
- Stireman JO, Greeney HF, Dyer LA. 2009. Species richness and host associations of Lepidoptera-attacking Tachinidae in the northeast Ecuadorian Andes. J Insect Sci. 9(39):1–19.
- Stork NE, Srivastava DS, Watt AD, Sen TB. 2003. Butterfly diversity and silvicultural practice in lowland rainforests of Cameroon. Biodivers Conserv. 12(3):387–410.
- Summerville KS, Dupont MM, Johnson AV, Krehbiel RL. 2008. Spatial structure of forest Lepidopteran communities in oak hickory forests of Indiana. Environ Entomol. 37(5):1224–1230.
- Summerville KS, Metzler EH, Crist TO. 2001. Diversity of Lepidoptera in Ohio forests at local and regional scales: how heterogeneous is the fauna? Ann Entomol Soc Am. 94(4):583–591.
- Tumuhimbise G, Okwakol MJN, Kangwagye TN. 1998. Species diversity of swallowtail butterflies (Papilionidae: Lepidoptera) in North Maramagambo Forest. Afr J Ecol. 39(4):113–115.
- Tushabe H, Kalema J, Byaruhanga A, Asasira J, Ssegawa P, Balmford A, Davenport T, Fjeldsa J, Friis I, Pain D, et al. 2006. A nationwide assessment of the biodiversity value of Uganda’s important bird areas network. Conserv Biol. 20(1):85–99.
- van Asch M, Visser ME. 2007. Phenology of forest caterpillars and their host trees: the importance of synchrony. Ann Rev Entomol. 52(2007):37–55.
- van Halder I, Barbaro L, Corcket E, Jactel H. 2008. Importance of semi-natural habitats for the conservation of butterfly communities in landscapes dominated by pine plantations. Biodivers Conserv. 17(5):1149–1169.
- van Swaay CAM, Nowicki P, Settele J, van Strien AJ. 2008. Butterfly monitoring in Europe: methods, applications and perspectives. Biodivers Conserv. 17(14):3455–3469.
- Veddeler D, Schultze C, Steffan-Dewenter I, Buchori D, Tscharntke T. 2005. The contribution of tropical secondary forest fragments to the conservation of fruit-feeding butterflies: effects of isolation and age. Biodivers Conserv. 14(14):3577–3592.
- Vu LV. 2009. Diversity and similarity of butterfly communities in five different habitat types at Tam Dao National Park, Vietnam. J Zool. 277(1):15–22.
- Watt AD, McFarlane AM. 2002. Will climate change have a different impact on different trophic levels? Phenological development of winter moth Operophtera brumata and its host plants. Ecol Entomol. 27(2):254–256.
- Weibull AC, Östman Ö. 2003. Species composition in agroecosystems: the effect of landscape, habitat, and farm management. Basic Appl Ecol. 4(4):349–361.
- Yahner RH. 2001. Butterfly communities in residential landscapes of central Pennsylvania. Northeast Nat. 8(1):113–118.