Abstract
Wetlands are impacted by surrounding land use and land cover (LULC), but little information is available specifically on headwater wetlands. A total of 30 headwater wetlands were examined in coastal Alabama, USA, representing a range of surrounding LULC. Percent cover by forest, agriculture, and impervious surface area (ISA) (i.e., urban land use) were quantified in each corresponding watershed. Principal component analysis (PCA) was used to eliminate collinearity within land use data and produce orthogonal variables that represented gradients of forest cover, agriculture, and urban land use. Canopy tree density, diameter, species cover, importance values, and soil value/chroma were measured in each wetland. Exotic species cover was significantly related to land use change with increases in exotic shrubs associated with decreases in watershed forest cover. Field observations and analysis suggest that extensive colonization by the exotic shrub Ligustrum sinense may inhibit tree recruitment and alter wetland forest structure. Increases in shrub/sapling prevalence index (a measure of hydrophytic vegetation) and soil chroma were positively correlated to increased watershed conversion to agriculture (and concurrent loss of forest cover). Strategies for conserving the services associated with these wetlands should include minimizing surrounding forest loss and maintaining groundwater inflow.
Introduction
Land use change associated with urbanization and agricultural practices is a leading cause of environmental degradation to aquatic systems, including wetlands (Faulkner Citation2004; Poff et al. Citation2006). For instance, agricultural practices leading to soil disturbance and various drainage techniques can change hydrologic conditions on the landscape, increase sediments and nutrients of run-off, and affect downstream water quality (DeLaney Citation1995; Zedler Citation2003). Similarly, urbanization results in large areas of land are being cleared, paved, and dramatically modified. Population growth in the coastal areas of the southeastern United States is expected to result in substantial increases in residential and housing development (and forest loss) in the region (Nagy, Lockaby, Helms, et al. Citation2011). Shifts in land use and land cover (LULC) from both urban and agricultural land use have been shown to have a significant effect on local hydrology, water chemistry, and morphology (Paul & Meyer Citation2001; Poff et al. Citation2006; Blann et al. Citation2009). There is a general understanding of how land use changes affect aquatic systems; however, most work has been related to streams (Allan Citation2004; Walsh et al. Citation2005; Blann et al. Citation2009). There are still important gaps in our understanding of other aquatic environments, particularly as it relates to headwater wetlands.
Based on their position in the landscape, headwater wetlands may be particularly susceptible to land use change. These wetlands are normally characterized by consistently high water tables which originate as subsurface flow from the surrounding landscape (Noble et al. Citation2007). Ditching or drainage tiles may alter the hydrology of wetland systems by enhancing drainage for improved agricultural production on adjacent lands (Blann et al. Citation2009). Similarly, greater impervious surface area (ISA) associated with urban land use has been shown to reduce infiltration, decrease ground water recharge, increase surface water flows, and increase erosion (Paul & Meyer Citation2001; Ehrenfeld et al. Citation2003; Chadwick et al. Citation2006). Drainage patterns are further disrupted by culverts and storm water conveyance which facilitate drainage of the landscape in order to lessen flooding in urban areas (Walsh et al. Citation2005). These alterations can have a major impact on wetland hydroperiod which over time may alter a variety of functional attributes including habitat for stream biota and riparian vegetation (Jones et al. Citation2000). Furthermore, increased run-off typically increases pollutants that have many direct and indirect effects on wetland environments such as shifts in vegetation, loss of wildlife biodiversity, and alterations to aquatic food webs (Baker Citation1992; Robb Citation1992; Gleason & Euliss Citation1998; Herliby et al. Citation1998; Zedler Citation2003).
As these environments are impacted hydrologically, species composition and forest structure may change (Faulkner Citation2004). For instance, the prevalence of obligate wetland species may increase due to artificial impoundments from roadways. Conversely, more facultative species may emerge where drainage is enhanced, subsurface water levels are lowered, and/or excessive sedimentation occurs (Baker Citation1992; Chadwick et al. Citation2006). Species shifts and changes to communities can also result in changes in productivity and dependent trophic webs (Brinson et al. Citation1981; Wallace et al. Citation1999). Under less disturbed conditions, these wetlands provide stable water supplies downstream while maintaining important habitat and aquatic biodiversity (Brinson Citation1996). With increasing land conversion, there is a tendency for greater forest fragmentation, which can contribute to physical changes to remnant wetlands and elicit invasive plant species (Ehrenfeld Citation2003). Increased roads on the landscape can directly and indirectly alter plant communities by providing preferential migration corridors and points of establishment for invasive plant species (von der Lippe & Kowarik Citation2007). Such non-native invasions have proven to be major threats to local diversity and other ecosystem functions (Lundgren et al. Citation2004; Mitchell et al. Citation2011).
The objective of this study was to examine how land use change may impact headwater wetlands within coastal Alabama. This is a crucial issue due to rapid land use change occurring in the region. For instance, from 1990 to 2000, Baldwin County, Alabama, had the second highest increase in population (43%) within the state (BCPZD Citation2005). Similarly, from 2000 to 2010, Baldwin County had a 24% increase in population (USCB Citation2010). This area has traditionally been impacted by agriculture, but increased population and urban land use have resulted in further forest loss. Examining wetlands along a gradient of increasing land use change, we expected to detect shifts in forest structure, species composition, and soil conditions with increasing forest conversion caused by agriculture and urbanization.
Methods
Study sites
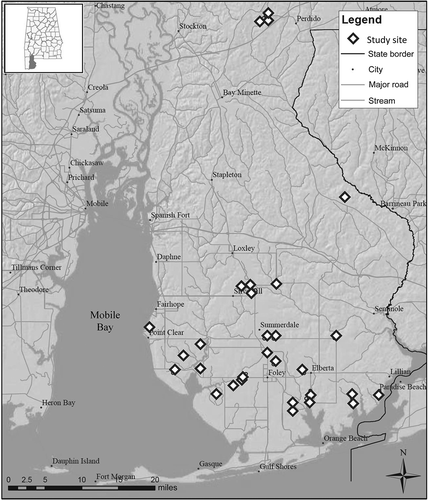
Headwater wetlands for this study were located in Baldwin County, Alabama, within the Southern Coastal Plain physiographic region in the southeastern United States (). The region is characterized by mild winters and hot and humid summers with mean annual temperatures ranging from 15ºC to 21ºC. Annual precipitation typically ranges from 125 to 180 cm and is fairly evenly distributed throughout the year (Noble et al. Citation2007). Headwater wetlands in coastal Alabama are groundwater driven and located at the headwater reaches of first-order streams (Noble et al. Citation2007). These wetlands are typically comprised of alluvial soils that are classified as ‘wet loamy alluvial lands’, while the surrounding uplands are generally sandy soils derived from marine deposits (McBride & Burgess Citation1964). This study began in June 2009 with the identification of approximately 60 headwater-slope wetlands as potential study sites. Wetlands were identified and delineated remotely with the use of topographic maps, aerial imagery, National Wetlands Inventory maps, and the county soil survey (McBride & Burgess Citation1964). Each wetland was visited to conduct a preliminary assessment, however, several properties could not be considered because owners declined permission for continued access. From this initial assessment, a total of 30 headwater-slope wetlands that displayed a range of surrounding LULC (e.g., urban, agriculture, forest) were selected for further examination. Wetlands selected were all forested (with evidence of characteristic tree species), located at the upper headwater reach, and retained evidence of wetland conditions. We also attempted to minimize differences in size (both wetland and watershed) to the greatest extent possible. The delineation of all 30 wetlands was confirmed in the field based on topography, wetland canopy vegetation (Magnolia virginiana was found in nearly all wetlands observed), and a normally clear vegetative transition to surrounding upland forest or pasture.
Watershed assessment and LULC classification
The associated watersheds for all 30 wetlands were delineated using the ArcSWAT 2009.93.6 model for Environmental Science Research Institute (ESRI) ArcMap 9.3 (ESRI Citation2009). Alterations in watershed area (increase or decrease) due to anthropogenic drainage features were considered from current aerial photography and confirmed by examining conditions in the field. Dominant LULC within corresponding wetland watersheds were evaluated with the use of ESRI ArcMap 9.3 (ESRI Citation2009). Aerial images from 2009 were used to manually delineate characteristic land uses (Anderson et al. Citation1976). For each watershed, LULC features were classed into pervious and impervious surfaces, which were further divided into subclasses to test between differing land use types. Pervious subclasses included forested lands, agricultural lands (row crops, pasture, etc.), lawns and green spaces, and unpaved roads and driveways. Impervious subclasses included building structures, roads, and other paved surfaces (driveways, patios, etc.). LULC classes and subclasses were then quantified per watershed for total percent forested land, agricultural land, and impervious surface area (ISA) to facilitate a better understanding and interpretation of land use data. Urban land use was represented by ISA as a more direct measure of hydrologic influence rather than discerning broad categorical measures (e.g., low-, medium-, and high-density residential).
Forested wetland structure, composition, and soils
Field assessments were conducted on all selected headwater wetlands. Four 100 m2 plots were evenly established along the length of each wetland (per Noble et al. Citation2007). Within each plot, canopy tree density (stems ha−1) and tree diameter at breast height (DBH-cm) were measured for all canopy individuals (DBH > 10.2 cm). Using these data, mean canopy basal area (m2 ha−1) was calculated for each wetland. Sapling and shrub layer was defined as woody plants with a height greater than 1 m and a DBH < 10.2 cm. Understory included all herbaceous and woody vegetation less than 1 m tall. Total cover for canopy, sapling/shrub, and understory species were visually estimated as % cover per plot and averaged per wetland. Exotic species (per Miller et al. Citation2010) cover was calculated based on cover data within each forest strata. To describe canopy tree species abundance and density, a point-quarter survey was conducted using a single random point within each wetland plot and measuring the four closet canopy species for distance to point and basal area. These combined data were used to estimate species density, dominance (% of total basal area), and frequency (% of plots detected). Importance values (IV) were calculated based on relative density, dominance, and frequency, resulting in a maximum possible IV score of 300 (IV300).
Prevalence index (PI), an index of hydrophytic vegetation occurrence, was calculated for all three strata within each wetland with use of species IVs (or percent cover for shrub/sapling and understory vegetation) and wetland indicator status (obligate [OBL], facultative wetland [FACW], facultative [FAC], facultative upland [FACU], and upland [UPL], Reed Citation1988). A hydrophytic index score was assigned to each wetland indicator status with OBL species receiving a value of 1, FACW species a value of 2, FAC species a value of 3, FACU species a value of 4, and UPL species a value of 5 (Wentworth et al. Citation1988). The cumulative IV/cover per plot of each indicator status was multiplied by its hydrophytic index score to calculate wetland PI (a weighted average per plot). Thus, wetlands with lower PI values comprised of more hydrophytic species.
Four soil samples of the top 15 cm of the soil profile were randomly sampled within each wetland plot. Soil chroma and value were averaged per wetland using a Munsell soil chart. A visual estimate of detritus (i.e., partially decomposed yet recognizable plant material) cover was recorded for each plot and averaged per wetland.
Statistical analysis
Data were analyzed to test for the relation of land use change on headwater wetland measures using R 2.12.2 software (R Development Core Team Citation2010). Due to inherent collinearity associated with land use data (King et al. Citation2005), principal component analysis (PCA) was used to generate independent variables (principal components) that best explain the variance within percent forest cover, agricultural land use and ISA data. Scatterplots of principal components and wetland forest structure, prevalence index per strata, species IV, soil chroma/value, and other measures were generated. Apparent relationships between wetland variables and principal components were tested through regression to examine the relation. A post-hoc analysis was also conducted to examine the relation between exotic shrub cover and canopy tree measures using linear regression. All measures met assumptions for tests based on the Shapiro–Wilk’s test for normality and Bartlett’s test of homoscedasticity. Statistical significance of all tests was set at p < 0.05 and was determined to be highly significant at p < 0.01.
Results
Watershed characteristics
Based on digital elevation models, watershed boundaries for all 30 headwater wetlands produced a mean (±SE) area of 89.5 ± 12.8 ha (). Manually adjusting watershed area for changes in surface water flow due to landscape modifications (e.g., roads and ditches) resulted in an adjusted mean area of 86.2 ± 13.0 ha. Mean wetland size for all study sites was 3.2 ± 0.7 ha with a mean ratio of watershed:wetland area of 8.2 ± 2.8.
Table 1. General characteristics of 30 headwater wetland study sites. Adjusted watershed area reflects hydrologic changes in landscape due to anthropogenic disturbances.
Mean forested land cover was 44.9% ± 5.4% and was evenly distributed between a range of 6% and 100% (). Mean agricultural land cover was 27.0% ± 4.4% (range: 0–82%) and mean ISA was 3.4% ± 1.0% (range: 0–24%). Watersheds with large percentages of land cover other than forest, agriculture and ISA () were generally composed of urban green spaces (e.g., lawns, landscaping) or open water. Based on principal component loadings (correlation coefficients) (), Comp. 1 explained 86.1% of the variation within land use data. Comp. 1 was highly correlated to forest cover while being negatively correlated to agricultural land use and no meaningful correlation to ISA. Comp. 2 explained 14.3% of the variation within land use data with a strong positive correlation to both agriculture and forested cover and a negative correlation to ISA, describing a mixed LULC of increasing forest and agricultural use with decreasing urban land use.
Table 2. Loadings (significant correlation coefficients, p < 0.05) of land use variables and principal components.
Wetland vegetation
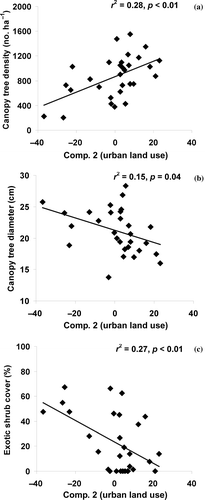
A wide range of measured forest structure, composition, and other environmental conditions were detected across the 30 headwater wetlands ( and ). Mean (±SE) canopy tree diameter, density, and basal area was 22.1 ± 1.2 cm, 864 ± 63 stems ha−1, and 30.5 ± 2.1 m2 ha−1, respectively. Mean cover of canopy-sized trees were 65.2% ± 3.2% across all sites. Most canopy tree measures were significantly correlated to Comp. 2. Mean canopy tree density and cover had a highly significant and positive relationship with Comp. 2 (a and ). Mean canopy tree diameter had a highly significant and negative relationship with Comp. 2 (b). Combined, these results suggest that wetlands had increasingly fewer but larger canopy trees as watersheds became less forested and more agricultural. Mean wetland basal area was not significantly related to either of the components.
Table 3. Mean, range, and SE (±1) for wetland variables and regression coefficients (r2) for independent variables explained by principal components (Comp. 1 and Comp. 2).
Figure 2. Scatterplot and linear regression results between (a) mean canopy tree density, (b) mean canopy tree diameter, and (c) exotic shrub cover tree diameter with Comp. 2.
Species composition also varied across study sites with a total of 51 individual species detected throughout all wetlands across strata. A total of 15 canopy tree species were detected in the study wetlands (), and based on mean IVs, the dominant canopy tree species included sweet bay (Magnolia virginiana), swamp tupelo (Nyssa biflora), red maple (Acer rubrum), slash pine (Pinus elliottii), and Chinese tallow (Triadica sebiferum). Correlation analysis indicated no significant relationships between components and any canopy tree species IV300 ().
Table 4. Mean, range, and SE (±1) of importance values (IV300) for 15 canopy species and the linear regression coefficient (r2) in relation to principal components (Comp. 1 and Comp. 2). No statistical significance was detected between species IV300 and principal components.
Mean shrub cover was 43.2% ± 3.6% and based on frequency of occurrence, the five most prevalent species were Chinese privet (Ligustrum sinense), buckwheat tree (Cyrilla racemiflora), wax myrtle (Morella cerifera), elderberry (Sambucus canadensis) and Virgina sweetspire (Itea virginica). There was a highly significant negative correlation between shrub cover and Comp. 2 (), indicating that shrub cover increased with increasing ISA and decreasing forest/agriculture cover. Mean understory cover was 36.3% ± 4.5% with various ferns and river cane (Arundinaria gigantea) being the most prevalent species. Ferns most commonly encountered included netted chain fern (Woodwardia areolata), cinnamon fern (Osmunda cinnamomea), and royal fern (Osmunda regalis).
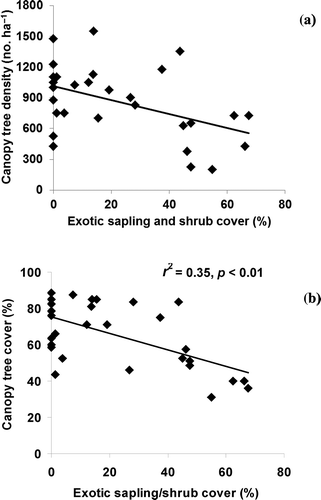
Exotic plant species were commonly encountered and related to both components. Exotic canopy cover was associated with T. sebiferum and negatively related to Comp. 1 (). Exotic canopy cover was uncommon (mean: 5.0% ± 2.5%) and this correlation was driven by few sites in agricultural settings where wetlands had substantial cover of T. sebiferum. Exotic shrub cover dominated by L. sinense was very common () and had a negative relationship with Comp. 2 (c), indicating that exotic shrubs increased with urban land use. Exotic shrub cover was also negatively related to Comp. 1 (), indicating that cover also increased with agricultural land use (and reduced forest cover) in the watershed. A post-hoc regression analysis indicated a significant relationship between exotic shrub cover with both canopy tree density and cover (a and b).
Figure 3. Scatterplot and linear regression results between (a) sapling/shrub prevalence index and (b) soil chroma (PI) with Comp. 1.
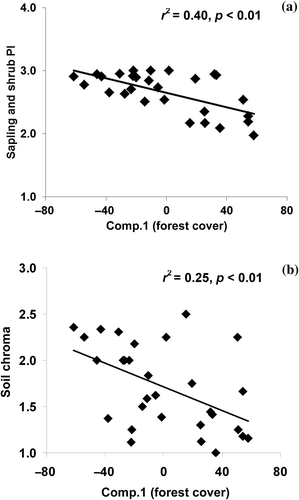
Figure 4. Scatterplot and linear regression results between canopy tree (a) density and (b) cover with exotic sapling/shrub cover.
Exotic ground cover (consisting of Commelina cummunis and/or Lygodium japonicum) was uncommon but also had a significant negative relationship with Comp. 1 (). Prevalence index (PI) was fairly consistent among strata and mean PI for the canopy, sapling/shrub, and understory was 2.1 ± 0.1, 2.6 ± 0.1 and 2.0 ± 0.1, respectively. A highly significant and negative correlation was detected between sapling/shrub PI and Comp. 1 ( and a). PI values of approximately 2 indicated primarily hydrophytic vegetation at the FACW level, while sapling/shrub PIs were more indicative of a mix of wetland and more facultative species.
Wetland soils, detritus cover, and hydrology
Mean soil chroma and value were 1.7 ± 0.1 and 2.8 ± 0.1, respectively, across wetland sites (). Soils were typically sandy loams with high organic content; however, we noticed shifts in soil texture and composition associated with LULC, as sand and silts appeared to have been displaced form surrounding uplands resulting from watershed conversion. Soil chroma displayed a significant and negative relationship with Comp. 1 (b), indicating that chroma increased with less watershed forest cover and more agricultural land use. Detritus cover ranged from 100% to as little as 25% but was not found to be significantly related to LULC.
Discussion
Results from this study suggest that wetland forest structure, cover by exotic species, and soil chroma were related to watershed land conversion. This is important because changes in forest structure and composition have been shown to represent fundamental changes in the ecosystem services and functions they provide (Sharitz & Mitsch Citation1993; Zedler & Kercher Citation2005). Changes in forest structure can represent substantial changes in wildlife habitat value (Semlitsch Citation2002) and nutrient cycling (Turner & Rabalais Citation2003). On average, headwater wetland forests in our study area were characteristic of early successional forests with high canopy tree density (864 ± 63 stems ha−1) and small diameters (22.1 ± 1.2 cm). These forests rarely met reference conditions for average canopy tree density (250–425 stems ha−1) or tree diameter (>30 cm DBH) based on a regional hydrogeomorphic (HGM) functional assessment guide (Noble et al. Citation2007). The early successional state of these forests is probably due to past harvesting and, to a lesser extent, fires (evidence of both was encountered); however, land use appears to be influencing the current trajectories of forest structure and composition. For instance, cover by L. sinense was the most extensive exotic species and was related to both increased urban and agricultural land use. The abundance of L. sinense is likely to be a result of increased edge effects and points of invasion, as have been noted for non-native species (von der Lippe & Kowarik Citation2007; Oneal & Rotenberry Citation2008; Niggeman et al. Citation2009). As forested environments become fragmented, these areas are also affected by increased light penetration, enabling the recruitment of less shade-tolerant species that would otherwise not become established in closed canopy forested systems. Several studies have made the linkage between land conversion (from increased agriculture and/or urban land use) and increased occurrence of exotic vegetation in wetlands and riparian areas (Galatowitsch et al. Citation2000; Moffatt et al. Citation2004; Miller et al. Citation2006). Houlahan et al. (Citation2006) found percent land use change adjacent (within 300 m) to wetlands in Ontario, Canada, predicted declines in species richness due to exotic species and emphasized the correlation between reduced forest cover and increases in the number of dispersal routes for exotic species into wetlands.
Increased exotic shrub cover has been commonly shown to decrease wetland biodiversity (Collier et al. Citation2002; Ehrenfeld Citation2003; Faulkner Citation2004); however, it may also alter forest structure and related ecosystem functions. Mitchell et al. (Citation2011) described riparian forests in the Georgia piedmont with lower basal areas and fewer canopy trees corresponding with areas of greater abundance of L. sinense. Likewise, Collier et al. (Citation2002) found that the Amur honeysuckle (Lonicera maackii), a common exotic shrub in northern and Midwest US forests, reduced tree seedling density of potential canopy trees by 68%. Greene and Blossey (Citation2012) found that an established L. sinense layer substantially reduced the survivorship of native seedlings in South Carolina floodplain forests and concluded that once this species is established and abundant, it becomes the primary driver for the loss of native plant species recruitment, including trees. Our results were consistent with these findings, as we saw evidence of reduced wetland tree density with increasing forest loss in the watershed (a and ). Examination of scatterplots showed that tree measures of canopy and density appeared to drop substantially after exotic sampling/shrub cover exceeded 40% (a and b). Over time, reduced tree recruitment may also explain increased canopy tree diameter. As canopy tree recruitment declines, it is expected that older trees represent more of the total canopy.
Both agriculture and urban land uses have the potential to alter drainage patterns to headwater wetlands, which may explain other trends observed in this study. For instance, results showed that wetland soil chroma increased with less forest cover and increased agricultural land use. This trend may be related to alterations of headwater wetland hydrology, increasing soil temperatures, and sedimentation. Chroma is considered to be a measure of the strength or purity of soil color and tends to be lowest when soils undergo prolonged anaerobic conditions. Under these conditions, iron/manganese becomes reduced and/or depleted and organic matter accumulation is enhanced (Galbraith & Vasilas Citation2004). Increased soil chroma in converted watersheds suggests that these wetlands may experience less soil saturation and flooding. Other studies have shown that as forested watersheds become increasingly converted (i.e., for urban or agricultural use), receiving streams and wetlands tend to become increasingly driven by surface water drainage and experience higher peak flows, lower base flows, and store less water (Ehrenfeld et al. Citation2003; Poff et al. Citation2006; Blann et al. Citation2009; Nagy, Lockaby, Kalin, et al. Citation2011). Groffman et al. (Citation2003) described this scenario as hydrologic drought in urban riparian soils and described how reduced base flows can increase riparian soil oxidation, diminish organic matter, and enhance soil color strength associated with mineral elements. Hydric soils are characterized by low redox conditions that tend to promote a chroma of ≤2, a threshold that has been used for the identification and delineation of wetlands (Mitsch & Gosselink Citation2000). It is noteworthy that only 77% of our sites had surface soils with chroma ≤2 (b). The deposition of upland sediments within wetland boundaries due to past land use practices may also be causing higher chroma values. The extent of historical erosion and sedimentation at each of our sites is uncertain; however, it was observed that several wetlands within highly converted watersheds had much less microtopography than reference wetlands, suggesting past deposition may have been significant.
Other evidence of hydrologic alteration included the increased PI for saplings/shrubs correlated to forest land conversion and increased agriculture land use. This effect was also influenced by the increasing abundance of L. sinense (a FAC species) and the concurrent displacement of more FACW and OBL shrubs. Shifts in PI values for other strata were expected but not observed. A natural shift in canopy vegetation to soil moisture has been demonstrated in southwest Alabama forested wetlands. Gemborys and Hodgkins (Citation1971) found increased importance of for Magnolia virginiana and Nyssa biflora (FACW and OBL wetland species, respectively) from the other more facultative species (Liquidamber styraciflua, Nyssa sylvatica, Quercus nigra) as soil moisture increased. The nature of canopy tree shifts is much slower and if tree recruitment is being suppressed by L. sinense (see above), then it is less likely that a corresponding shift in canopy tree composition will occur.
Forest structure, species composition, and soils are important features for the provision of ecosystem services. Increase in the presence of exotic species, decrease in hydrologic storage, and altered soil conditions from land use change all have a negative effect on ecosystem functions. These alterations in function reduce the services wetland ecosystems provide to local communities and larger watersheds. The shifts in ecosystem function and services are increasingly important as worldwide demographics change and more people immigrate to urban centers (~60% of the human population will live in urban environments by 2030 [Faulkner Citation2004]). The benefits forested systems provide, whether improved water quality, species richness, outdoor recreation, etc., are becoming increasingly important to urban communities. Determining the effect of land use on the future wetland ecosystem services can be difficult because there is often a time lag between land use change and associated changes in wetland function and service. However, given their importance, more attention is needed not only on the preservation of headwater wetlands but also on the surrounding lands. For these headwater wetlands in Alabama, it is clear that surrounding land conversion and subsequent changes in drainage will decrease wetland water storage and reduce retention of organic matter (Barksdale et al. Citation2013). Practices that promote gradual drainage from surrounding urban or agricultural lands into these wetlands will help support the numerous services the wetlands can provide. Ultimately, the services provided by these wetlands are dependent on maintaining these important processes and conditions (groundwater drainage, saturated soils, and native plant recruitment).
Conclusions
Using 30 headwater wetlands in coastal Alabama, we examined correlations between land conversion and forest structure/composition. Trends related to decreases in canopy tree density/cover and increases in exotic shrub cover suggest that these wetlands may fail to recruit characteristic species after surrounding land use changes. Increased presence and cover of L. sinense appears to be associated with increased forest fragmentation, edge effects, and disturbance, as measured by increased watershed disturbance. Decreases in forested land cover were also related to higher soil chroma and shrub/sapling PI, suggesting for reduced soil saturation that may result from changes in drainage. These wetlands are naturally driven by shallow groundwater inputs; however, the increased influence of surface water inputs via ditches and other landscape changes have likely altered wetland hydrology. The results of this study suggest that fundamental changes have occurred to most of the wetlands in the study area. These headwater wetlands may be particularly important for maintaining water quality in local estuaries and habitat for sensitive species. Further investigation of the capacity of these wetlands to provide important functions and their overall management and conservation are recommended.
Acknowledgments
Financial support for this project was provided by the Auburn University Center for Forest Sustainability and the Auburn University Water Resource Center.
References
- Allan JD. 2004. Landscapes and riverscapes: the influence of land use on stream ecosystems. Annu Rev Ecol Evol Syst. 35:257–284.
- Anderson JR, Hardy EE, Roach JT, Witmer RE. 1976. A land use and land cover classification system for use with remote sensor data. US Geological Survey Professional Paper 964:28. Reston (VA): USGS.
- Baker LA. 1992. Introduction to nonpoint source pollution in the United States and prospects for wetland use. Ecol Eng. 1:1–26.
- Baldwin County Planning and Zoning Department. 2005. The Baldwin county conservation plan-final summary document. Bay Minette (AL): Baldwin County Planning and Zoning Department.
- Barksdale WF, Anderson CJ, Kalin L. 2013. The influence of watershed run-off on the hydrology, forest floor litter and soil carbon of headwater wetlands. Ecohydrology. doi:doi:10.1002/eco.1404
- Blann KL, Anderson JL, Sands GR, Vondracek B. 2009. Effects of agricultural drainage on aquatic ecosystems: a review. Crit Rev Environ Sci Technol. 39:909–1001.
- Brinson MM. 1996. Assessing wetland function using HGM. National Wetland Newsletter. 18:10–16.
- Brinson MM, Lugo AE, Brown S. 1981. Primary productivity, decomposition, and consumer activity in freshwater wetlands. Annu Rev Ecol Evol Syst. 12:123–161.
- Chadwick MA, Dooberfuhl DR, Benke AC, Huryn AD, Suberkropp K, Thiele JE. 2006. Urbanization affects stream ecosystem function by altering hydrology, chemistry, and biotic richness. Ecol Appl. 16:1796–1807.
- Collier MH, Vankat JL, Hughes MR. 2002. Diminished plant richness and abundance below Lonicera maackii, an invasive shrub. Am Midl Nat. 147:60–71.
- DeLaney TA. 1995. Benefits to downstream flood attenuation and water quality as a result of constructed wetlands in agricultural landscapes. J Soil Water Conserv. 50:620–628.
- Ehrenfeld JG. 2003. Effects of exotic plant invasions on soil nutrient cycling processes. Ecosys. 6:503–523.
- Ehrenfeld JG, Cutway HB, Hamilton IVR, Stander E. 2003. Hydrologic description of forested wetland in Northeastern New Jersey, USA – and urban/suburban region. Wetlands. 23:685–700.
- [ESRI] Environmental Systems Research Institute. 2009. ArcGIS 9.3. Redlands (CA): Environmental Systems Research Institute.
- Faulkner S. 2004. Urbanization impacts on the structure and function of forested wetlands. Urban Ecosys. 7:89–106.
- Galatowitsch SM, Whited DC, Lehtinen R, Husveth J, Schik K. 2000. The vegetation of wet meadows in relation to their land-use. Environ Monit Assess. 60:124–144.
- Galbraith JM, Vasilas LM. 2004. Describing hydric soils. In: Vasilas LM, editor. A guide to the hydric soils in the mid-Atlantic region, Version 1.0. Morgantown (WV): USDA-NRCS.
- Gemborys SR, Hodgkins EJ. 1971. Forests of small stream bottoms in the coastal plain of Southwestern Alabama. Ecology. 52:70–84.
- Gleason RA, Euliss Jr NH. 1998. Sedimentation of prairie wetlands. Great Plains Res. 8:97–112.
- Greene BT, Blossey B. 2012. Lost in the weeds: Ligustrum sinense reduces native plant growth and survival. Biol Inv. 14:139–150.
- Groffman PM, Bain DJ, Band LE, Belt KT, Brush GS, Grove JM, Pouyat RV, Yesilonis IC, Zipperer WC. 2003. Down by the riverside: urban riparian ecology. Front Ecol Environ. 6:315–321.
- Herliby AT, Stoddard JT, Johnson CB. 1998. The relationship between stream chemistry and watershed land cover data in the mid-Atlantic region. US Water Air Soil Poll. 105:377–386.
- Houlahan JE, Keddy PA, Makkay K, Findlay CS. 2006. The effects of adjacent land use on wetland species richness and community composition. Wetlands. 26:77–96.
- Jones JA, Swanson FJ, Wemple BC, Snyder KU. 2000. Effects of roads on hydrology, geomorphology, and disturbance patches in stream networks. Conserv Biol. 14:76–85.
- King RS, Baker ME, Whigham DF, Weller DE, Jordan TE, Kazyak PF, Hurd MK. 2005. Spatial considerations for linking watershed land cover to ecological indicators in streams. Ecol Appl. 15:137–153.
- Lundgren MR, Small CJ, Dreyer GD. 2004. Influence of land use and sites characteristics on invasive plant abundance in the Quinebaug Highlands of Southern New England. Northeastern Nat. 11:313–332.
- McBride EH, Burgess LH. 1964. Soil survey of Baldwin County, Alabama. USDA-SCS Soil Survey Report 12:110. Washington (DC): USDA-SCS.
- Miller JH, Cambliss EB, Loewenstein NL. 2010. A field guide for the identification of invasive plants in southern forests. USDA-USFS, Southern Research Station, GTR SRS–119. Asheville (NC): USDA-USFS.
- Miller SJ, Wardrop DH, Mahaney WM, Brooks RP. 2006. A plant-based index of biological integrity (IBI) for headwater wetlands in central Pennsylvania. Ecol Ind. 6:290–312.
- Mitchell JD, Lockaby BG, Brantley EF. 2011. Influence of Chinese privet (Ligustrum sinense) on decomposition and nutrient availability in riparian forests. Inv Plant Sci Manage. 4:437–447.
- Mitsch WJ, Gosselink JG. 2000. Wetlands. 3rd ed. New York (NY): John Wiley and Sons.
- Moffatt SF, McLachlan SM, Kenkel NC. 2004. Impacts of land use on riparian forest along an urban–rural gradient in southern Manitoba. Plant Ecol. 174:119–135.
- Nagy RC, Lockaby BG, Helms B, Kalin L, Stoeckel D. 2011. Water resources and land use and cover in a humid region: the Southeastern United States. J Envt Qual. 40:867–878.
- Nagy RC, Lockaby BG, Kalin L, Anderson C. 2011. Effects of urbanization on stream hydrology and water quality: the Florida Gulf Coast. Hydrol Process. doi:doi:10.1002/hyp.8336
- Niggemann M, Jetzkowitz J, Brunzel S, Wichmann MC, Bialozyt R. 2009. Distribution of plants explained by human movement behavior. Ecol Model. 220:1339–1346.
- Noble CV, Wakeley JS, Roberts TH, Henderson C. 2007. Regional guidebook for applying the hydrogeomorphic approach to assessing the functions of headwater slope wetlands on the Mississippi and Alabama coastal plains. US Army Corps of Engineers ERDC/EL TR-07–9. Vicksburg (MS): US Army Corps of Engineers.
- Oneal AS, Rotenberry JT. 2008. Riparian plant composition in an urbanizing landscape in southern California. USA Lands Ecol. 23:553–567.
- Paul MJ, Meyer JL. 2001. Streams in the urban landscape. Annu Rev Ecol Evol Syst. 32:333–365.
- Poff NL, Bledsoe BP, Cuhaciayan CO. 2006. Hydrologic variation with land use across the contiguous United States: geomorphic and ecological consequences for stream ecosystem. Geomorph. 79:264–285.
- R Development Core Team. 2010. R: a language and environment for statistical computing, reference index version 2.12.12 [Internet]. [cited 2011 Jan]. Vienna: R Foundation for Statistical Computing, ISBN 3-900051-07-0. Available from: http://www.R-project.org
- Reed Jr PB. 1988. National list of plant species that occur in wetlands: national summary. US Fish and Wildlife Service Biological Report 88:24. Fort Collins (CO): US Fish and Wildlife Service.
- Robb DM. 1992. The role of wetland water quality standards in nonpoint source pollution control strategies. Ecol Eng. 1:143–148.
- Semlitsch RD. 2002. Critical elements for biologically based recovery plans of aquatic-breeding amphibians. Conserv Biol. 16:619–629.
- Sharitz RR, Mitsch WJ. 1993. Southern floodplain forests. In: Martin WH, Boyce SG, Echternacht AC, editors. Biodiversity of the Southeastern United States. Dordrecht:Springer.
- Turner RE, Rabalais NN. 2003. Linking landscape and water quality in the Mississippi River basin for 200 years. Bio-Sci. 53:563–572.
- US Census Bureau. 2010. 2010 Census data [Internet]. [cited 2011 Jul]. Available from: http://2010.census.gov/2010census/data/
- von der Lippe M, Kowarik I. 2007. Long-distance dispersal of plants by vehicles as a driver of plant invasions. Conserv Biol. 21:986–996.
- Wallace JB, Eggert SL, Meyer JL, Webster JR. 1999. Effects of resource limitation on detrital-based ecosystems. Ecol Mono. 69:409–442.
- Walsh CJ, Roy AH, Feminella JW, Cottingham PD, Groffman PM, Morgan II RP. 2005. The urban stream syndrome: current knowledge and the search for a cure. J N Am Bentho Soc. 24:706–723.
- Wentworth TR, Johnson GP, Kologiski RL. 1988. Designation of wetlands by weighted averages of vegetation data: a preliminary evaluation. J Am Water Res Assoc. 24:389–396.
- Zedler JB. 2003. Wetlands at your service: reducing impacts of agriculture at the watershed scale. Front Ecol Environ. 1:65–72.
- Zedler JB, Kercher S. 2005. Wetland resources: status, trends, ecosystem services, and restorability. Ann Rev Environ Resour. 30:39–74.