Abstract
The novel method developed for this study evaluates the impact of farming practices on farmland biodiversity, allowing for the assessment of the biodiversity potential of dairy farms at farm and product levels. We linked farming practices as pressure indicators to the species number and abundance of 11 indicator species groups (ISGs), evaluated semi-quantitatively by expert judgements. We calculated biodiversity potential based on food–web relationships between the ISGs, using Monte Carlo simulations for the analysis of uncertainty of expert assessments. We applied the assessment model to 8925 dairy farms from seven different Austrian regions, using official statistical data sets at farm level and interviews with farmers and experts. The results show that the approach can be used to identify differences in the biodiversity potential of farms and milk. Milk from organic farms received 4–79% higher biodiversity scores than milk from conventional farms in all regions. The application showed that in the case of Austrian dairy production, the approach can be used for assessments of both farms and products. However, the approach needs validation and, for product-level assessment, further development to cope with longer supply chains or compound products from different bio-geographic regions.
1. Introduction
The intensity of modern agricultural production systems has been increasing in the course of the twentieth century as a response to technical progress, productivity gains in other economic sectors, and the pressure of a growing world population with growing demand for food. This intensification has severely increased the pressure on natural resources such as land, air, and water (MA Citation2005).
Intensive modern agricultural production negatively affects biodiversity, in terms of genetic, species, and habitat diversity (Rockström et al. Citation2009; ten Brink et al. Citation2009). However, there are large variations in effects depending on farm management, production intensity, and region (OECD Citation2001). For example, agriculture may also have positive effects on biodiversity, such as when species and habitat diversity in agro-ecosystems are fostered by specific measures such as diverse crop rotations, banning synthetic weed and pest control, or reducing nitrate input into the system (Hole et al. Citation2005; Tscharntke et al. Citation2005).
Hole et al. extensively reviewed the impacts of organic farming and conventional agriculture on species numbers and diversity and reported that 87 of 123 studies found a positive impact of organic farming practices, 28 studies found no difference between organic and conventional agriculture, and the remaining eight studies concluded that there were negative impacts of organic farming (Hole et al. Citation2005). Organic farming practices were demonstrated to be most favourable for farmland birds, predatory insects, spiders, soil organisms, and the arable weed flora, while non-beneficial organisms (including pests) did not show different levels of abundance between farming systems (Bengtsson et al. Citation2005; Fuller et al. Citation2005; Hole et al. Citation2005). In Switzerland, differences between farming systems were more pronounced in arable land than on grassland (Schader et al. Citation2012), with prevalent differences between organic and non-organic farms found at species–group and at farm–structural level (Gibson et al. Citation2007; Schader et al. Citation2013). In addition to differences in biodiversity in cropped areas, Boutin et al. (Citation2008) identified higher plant species richness in semi-natural habitats on organic farms than on conventional farms.
Most of the above-mentioned studies determined species diversity in specific crops under different farming practices at field or habitat level and focused on certain groups of farmland species, such as vascular plants typical for field habitats, carabids, or butterflies. These studies provide valuable inputs about the impact of farming practices on farmland biodiversity, but most studies have assessed species diversity on field and farm scales. The only studies that have tended to focus at larger scales, such as the whole-farm scale, have assessed vertebrate species diversity.
It is not feasible to assess overall species diversity on entire farms, so models are needed that enable an evaluation of the biodiversity status of the whole agriculturally used area within a farm. However, such models are still scarce (Schader, Citation2009; von Haaren et al. Citation2012; Jenny et al. Citation2013). Furthermore, models are often requested that allow assessment of biodiversity impacts at the product level, particularly in the context of product declaration and food consumption as a growing number of processors, retailers, and consumers are interested in the environmental footprint of their purchase or consumption. A widely used method for assessing the environmental burdens of products is life cycle assessment (LCA) (International Organization for Standardization Citation2006a; Citation2006b). In contrast to other assessment methods, LCAs consider the whole life cycle of a product and relate the environmental impacts to a functional unit. These features make the LCA approach particularly useful in the consumption-related environmental assessment of products, although they have been a hindrance for the differentiated evaluation of biodiversity.
LCA practitioners mostly refer to eco-toxicity and land occupation as proxies for the biodiversity impacts of products (De Schryver et al. Citation2010). However, these indicators cannot represent biodiversity impacts in sufficient depth. Hence, there is currently no product-related biodiversity assessment method that is both broadly accepted, detailed enough to distinguish between agricultural production methods, and feasible in terms of data requirements to cover a large number of farms and produce representative results for products (Mila i Canals et al. Citation2007; Jeanneret et al. Citation2008). As a consequence, biodiversity aspects are seldom considered in product-related environmental assessments of agricultural products.
The aim of this paper is to address this methodological lack by presenting a novel, farm-level approach for assessing the farmland species diversity potential of different agricultural production systems and for extending the assessment to product level. A further aim is to demonstrate its application in a comparison between conventional and organic dairy farms in seven regions of Austria.
2. Methods
This segment of the paper is divided into two sections. Section 2.1 describes the derivation of a model to assess biodiversity potential at farm and product levels. Although the model can be readily adapted to other contexts, the weighting of several variables in the model is context-specific; so some parameters were weighted in consultation with experts in the particular context of this study. The method of weighing these variables is also described in this section. In Section 2.2, we describe how the model was applied in the case of Austrian dairy farms.
2.1. Biodiversity impact assessment approach
The general model framework was based on a biodiversity scoring system, which is a response-based whole-farm approach to assess wildlife-friendliness of agricultural production (Jenny et al. Citation2010, Citation2013) and which has already been implemented in the standards of integrated production systems in Switzerland. The framework for impact classification at species level was based on the procedures in Swiss Agricultural Life Cycle Assessments – Biodiversity (SALCA-BD) (Jeanneret et al. Citation2008), which assess the impacts of all land management interventions (tillage, plant protection, fertilisation, harvesting) on a set of indicator species groups (ISGs). The farm and regional aggregation procedures were based on an economic-ecological sector-level model called CH-FARMIS (the Swiss version of FARMIS) (Schader Citation2009). In addition to these three approaches, scientific peer-reviewed literature, data of national environmental evaluation programmes in Austria and Switzerland, and expert assessments were used for selecting and weighting parameters semi-quantitatively and for validating the model assumptions.
The model takes diverse agricultural habitats with optimal biodiversity management for enhancing species richness and abundance within farmlands as a reference state. A biodiversity potential of 100% is defined as this reference state, without specifying a particular abundance or number of species. Intensive agricultural production, in which the minimum legal environmental standards are followed, was used as the baseline (biodiversity potential = 0%) for the evaluation. Thus, land use activities that are a step towards the reference state from the legal minimum standards were included in the model (Table A1, Appendix). Model parameters were based on scientific literature (e.g. Hole et al. Citation2005) and/or the outcome of national evaluation programmes. The system boundaries were drawn at the farm level to encompass all variables that influence on-farm biodiversity and which explicitly include crop- and non-crop habitats, such as hedges or fallows, belonging to the farms. The method concentrates on agricultural pressure indicators assessing the pressure on or the promotion of biodiversity on the farm. By doing so, the method does not model biodiversity in a comprehensive sense since influences of landscape parameters on biodiversity beyond the farm scale are not included. The calculated biodiversity potential does not reflect an absolute state of biodiversity in terms of actual species numbers and abundances, but reflects the impact of different farming practices on farmland biodiversity on a relative scale.
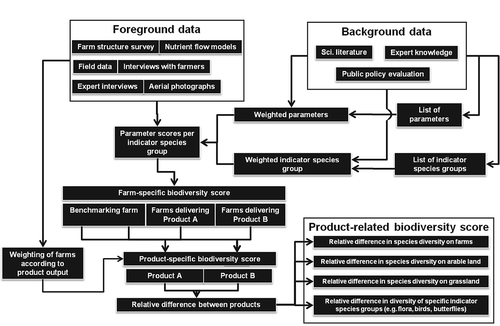
An overview of the approach used for deriving the biodiversity scores is shown in . Background data, such as expert knowledge, scientific literature, and policy evaluation reports, were used to select and weight parameters and ISGs. Foreground data, such as farm structure data and participation in agri-environmental programmes, were used to calculate the scores that a farm achieves for each parameter. These scores were then aggregated to the farm level and expressed as a proportion of the maximum possible score (normalisation), which enabled comparison between farms. If a product comparison is desired, such as comparing organic milk with conventional milk within a specific region, the normalised scores for each farm producing one product are aggregated and can then be compared with the aggregated score of the farms producing the other product.
2.1.1. System boundaries
System boundaries were set at the farm gate. This means that the biodiversity potential on the farm was exclusively taken into account, while biodiversity impacts of processing, retailing, and inputs, such as production and transport of mineral fertilisers, pesticides, or purchased fodder, were not considered in the biodiversity potential assessment.
2.1.2. Selection of ISGs
The potential for farmland species diversity and abundance on farms was calculated using the model, which includes the impact assessment of management practices and habitat structures (Table A1, Appendix) on 11 ISGs. The ISGs include soil fauna, soil microorganisms, vascular plants, birds, small mammals, amphibians, spiders, carabid beetles, butterflies, wild bees, and grasshoppers. These groups have been described in the literature as being suitable surrogate indicators for species diversity in farmland (Schloter et al. Citation2003; Jeanneret et al. Citation2006). Abundance was included since different farming practices do not necessarily result in different species numbers within a species group, but may lead to considerable differences in species abundances (Bengtsson et al. Citation2005).
2.1.3. Selection of model parameters
A list of land use parameters with impacts on biodiversity, subdivided into on-farm land uses (branches), was based on scientific literature, expert knowledge, and the results of national environmental evaluation programmes. These parameters include different parts of the farm and were classified according to the main cropping categories, such as arable land, vegetable growing, orchards, wine-growing, grassland, animal husbandry, and non-crop habitats (e.g. semi-natural). The parameters were formulated in such a way that only positive impacts needed to be considered. The full model consists of 91 parameters, which are classified and specified in Table A1 of the Appendix and were selected on the basis of the following criteria:
Impact on biodiversity: All the selected parameters need to have an impact on species diversity as reported in either scientific literature or public policy evaluations (e.g. agri-environmental schemes in Austria).
Data availability and verifiability: Farm-level data for the parameters need to be obtained from reliable sources. For example, most of the data in this study come from public databases that are used for the calculation of direct payment levels (Integrated Administration and Control System (IACS)). Data from interviews with farmers were verified and supplemented with field surveys and other data sources (see Section 2.2.2 for details).
2.1.4. Weighting of model parameters
Parameters were weighted according to their effectiveness in increasing the species number and abundance within the selected ISGs. The parameter weights (PWs) are defined as the sum of the effectiveness values (E) for each parameter in promoting each of the 11 ISGs, multiplied by the relative importance of the ISG (IW) (Equation (1)). By structuring the parameters and ISGs as a matrix, the impacts of each parameter on each ISG (Eij) were calculated. These could be expressed as the effectiveness of a parameter in promoting species diversity of the ISGs.
PW = Parameter weight
E = Effectiveness of a parameter in increasing species number and abundance in ISG
IW = Relative importance of ISG
i = Index of parameters
j = Index of ISG
The importance of the ISGs (IWj) and the effectiveness of parameters in improving species richness (Eij) were weighted by means of expert ratings by 23 indicator group-specific biologists, ecologists, and agronomists from Austria, Switzerland, and Germany. The experts were selected based on their expertise on a certain indicator species group and impacts on that group of farming practices in Austria and were provided with detailed information about the parameters. According to an expert assessment procedure, defined in Schader and Stolze (Citation2011) and based on the Nominal Group Technique (NGT) (Delbecq et al. Citation1975), experts were also asked to specify the level of uncertainty of their evaluation. Based on the assumption that the group of experts had full knowledge of the current state of research, the uncertainty levels were used for weighting the expert assessments in cases where the experts assessed the importance (IWj) and effectiveness (Eij) variables differently.
The 11 ISGs were weighted according to their relative importance in the food web (Equation (2)), whereas only direct feeding relationships between the ISGs were taken into account.
FSjm = Food share
j = Index of consumed ISGs
m = Index of consuming ISGs
The experts evaluated the relative food share of each indicator species group (FSj) as a source of food for the other ISGs (FSm). To calculate IWj, the food shares of each ISGm consuming ISGj were summed and divided by the total share of consumption in the food web. Finally, the relative importance of the ISGs was normalised to totals of 100% if expected values from Monte Carlo simulations (Rubinstein Citation2009) deviated from 100% (see Section 3.5).
Eij is defined as the product of the parameter’s ability to enhance the number of species (SNij) and the average abundance of individual species (ABij) (Equation (3)).
Table 1. Rating scales for species numbers (SNij) and abundance (ABij) for evaluations by experts.
Table 2. Rating scale for effectiveness scores (Eij) for evaluation by experts.
For instance, if an expert evaluated the effect of a parameter on species diversity with 2 (equivalent with a 33–67% increase in the number of species) and the abundance of individual species with 1 (equivalent to a 0–10% increase in the average abundance of individual species), the score for Eij becomes 2, which means this parameter is described, according to , as having a ‘minor positive effect’ on the species diversity in an ISG.
2.1.5. Aggregation to farm level
The performance (P) of a specific farm for each parameter was multiplied by its weight (PW) to calculate the farm-specific biodiversity score (S), which expresses the potential for farmland species diversity. Different farm branches (FBW) were weighted according to their areal proportion of the total agricultural land of the farm (Equation (4)). Farm branches considered are permanent grassland, arable crops, vegetable growing areas, fruit growing areas, vineyards, and semi-natural habitats. The weighting of farm branches allows for a fair comparison between structurally different farms. P was determined on the basis of the data sources A–F (see Section 2.2.2) and could be assigned values of 0%, 25%, 50%, 75%, or 100%.
S = Farm-specific biodiversity score
FBW = Farm branch weight
P = Performance of each farm with respect to a model parameter
PW = Effectiveness of parameter for biodiversity
i = Index of parameters
l = Index of farm branches
In a second step, the score was normalised with a benchmark score, which was the score the farm would have achieved if all of the parameters relevant for the specific farm had been implemented to 100%. Hence, each farm received a biodiversity score ranging from 0% to 100%.
2.1.6. Aggregation to product level
To apply the model to dairy products, data from all farms that delivered raw milk to a regional milk distributor were included in the model. According to the principle of mass allocation (derived from the raw milk quota of farms), the contribution of each farm to the final product was weighted (FW). This weight was multiplied with the farm-specific biodiversity score to derive the product-specific biodiversity score (PS) (Equation (5)).
PS = Product-specific biodiversity score
S = Farm-specific biodiversity score
FW = Farm weight, based on quantity of product delivered
k = Index of farms
2.2. Application of the approach to Austrian dairy production
We applied the approach to Austrian premium organic and conventional dairy production in the following seven regions in Austria: Kitzbühel (KB), Murau (MU), Walchsee (WS), Mühlviertel (MV), Ötscherland (OL), Steirisches Bergland (SB), and Waldviertel (WV). In addition to the official European regulation for organic agriculture, this premium organic milk has to fulfil private label regulations: soya bean feeding and the use of highly soluble organic fertilisers (e.g. horn meal) are forbidden, and producers must meet additional standards for animal welfare (Zurück zum Ursprung Citation2013).
For aggregation at the product level, all of the farms that delivered raw milk to a regional dairy were included in the analysis. According to the principle of mass allocation, farms were weighted according to the quantity of raw milk that each farm produced. The production practices of organic milk were compared with the average production practices of conventional milk in the seven different regions. The variable describing the production volume of conventional milk was defined as the average milk production from each of the same seven regions, which was aggregated as explained in Section 2.1.6, using the foreground data presented in Section 2.2.2.
2.2.1. Selection and identification of farms
For each organic farm, detailed information on land use, milk production, and participation in agri-environmental programmes was available from the administrators of the organic brand. This data was supplemented with more specific data on farming practices that was collected using interviews with a random sample of 152 organic farms that are representative of the farms in each region ( and Section 2.2.2). The sample was stratified according to the elevation of the farm. The conventional farms that were considered for each region were non-organic farms that possess a milk quota. A total of 8925 farms were analysed, including 1261 organic (575 hay-feeding and 686 silage-feeding farms) and 7664 conventional farms (1338 hay-feeding and 6326 silage-feeding farms). The number of organic and conventional farms per region that were included in the analysis is shown in .
Table 3. Selected farms in different regions investigated.
2.2.2. Foreground data of the farms
Seven different sources for foreground data from organic and conventional farms were used to provide sufficient data for a representative assessment.
Data from the Austrian Integrated Administration and Control System (IACS): For most parameters, input data were taken from IACS. The IACS database contains detailed, precise, and annually updated information at farm level for all farms in Austria that apply for subsidies. Data include information on parameters of farm size; farmland under different land uses, including which crops are planted; numbers of livestock; and special management practices that are subsidised by the Austrian agri-environmental programme (ÖPUL), which is a part of the Austrian Programme for Rural Development 2007–2013 (Lebensministerium, Citation2010). These agri-environmental measures make up a considerable proportion of the parameters used in the biodiversity potential assessment model (see Section 2.1). IACS data for conventional farms were taken from Hörtenhuber et al. (Citation2010) and from the results of analysis of aggregated IACS data.
If relevant foreground data were not found within the IACS data sets, the following sources were utilised:
Farmer interviews: Complementary data on production practices (e.g. mowing frequencies and techniques) and semi-natural habitats for the stratified random sample of 152 organic farms were collected via 35 face-to-face interviews and 117 telephone interviews with farmers. Data for conventional farms was based on the expert interviews (4), as experts were able to specify parameters for conventional farms but not for organic farms.
Field surveys: Habitat elements (semi-natural habitats, woodlands, extensive grasslands, etc.) were recorded on 35 sample farms, according to a methodology described in Jenny et al. (Citation2010).
Expert interviews: Interviews with five experts (agronomists) from universities, public research stations, and public agricultural offices yielded information on common agricultural practices in the studied regions (e.g. mowing dates, frequencies, and techniques).
Aerial photographs were used to estimate the area of semi-natural habitats on the farmland in the different regions.
Farm nutrient flow models were formulated to quantify levels of nitrogen (N)-import into the studied dairy production systems due to purchased concentrates, with nutrient flow models using IACS data based on Hörtenhuber et al. (Citation2010). The model determined the amounts of nitrogen required for the crops grown on-farm, which were differentiated by the regionally varying conventional and organic yields and feed qualities (expressed as crude protein with data collected from interviews and literature). Based on data for annual milk yields per cow and the amount of energy and protein that is provided with feed grown on-farm, the missing amount of nitrogen (and energy) was determined. This missing amount of nitrogen is needed from either purchased concentrates or from synthetic N-fertilisers. Synthetic N-fertilisers were not required for either organic or conventional grassland dairy farms due to the large amounts of concentrate feed that were purchased.
Table A1 (Appendix) shows which of the different data sources were used for which parameter. If available data and expert assessments did not justify the assumption that organic and conventional farms are different with respect to data from sources B–E, data values for organic and conventional farms were set as equal. For example, scientific literature and expert interviews yielded no evidence that organic and conventional farms differ with respect to the area of some kinds of semi-natural habitats, so region-specific values for these parameters were calculated from data sources B, C, and E, and this variable was set as equal for organic and conventional farms.
2.3. Sensitivity analysis
Sensitivity analysis was applied to the parameters and weights of the model, which were considered to be uncertain according to our data sources. In particular, this refers to
Monte Carlo simulations on the effects of parameters on ISGs: Experts had partly differing opinions about the effects of parameters on ISGs, so a model consisting of 1981 input variables was created to simulate the impacts of different scores. The software @RISK 6.0 (Palisade Corporation) was used for the simulations, which used Latin Hypercube sampling with 1500 iterations.
Different weights for aggregating the farms: As part of the sensitivity analysis, instead of weighting the farms according to their milk quota, other characteristics were used, such as the number of dairy cows and farm area.
3. Results
3.1. Weights for ISGs
The expected values based on the expert assessments, and the resulting total weights per ISG, are shown in . For example, the seventh column shows the importance of spiders for the other ISGs. While spiders have no relevance as a source of food for flora, they contribute about 12.3% of the food for birds and 23.9% of the food for amphibians. Meanwhile, the seventh row shows the importance of other ISGs as sources of food for spiders, with experts estimating the contribution of soil fauna to be 73.3% of the food for spiders.
Table 4. Food relationships between indicator species groups and total weights of indicator species groups for agro-ecosystems in Austria.
In the bottom row the scores are summed to the total weight of each ISG, which show that flora was evaluated as the most important species group: providing 29.6% of the feed sources for the other species groups. Soil fauna was seen as being most important, especially for spiders and carabid beetles, and received an overall weight of 20.4%. Soil microorganisms (total relative weight of 17.3%) are crucially important for flora (100%) and soil fauna (71.1%). The other indicator species received weights between 2.0% and 8.5%. Soil and epigeal fauna ISGs (soil fauna, spiders, and carabid beetles) were allocated a total relative weight of 33%.
3.2. Parameter weights
The weights allocated to ISGs were used to aggregate parameter effectiveness scores to a total PW for species diversity according to Equation (1). Values ranged from >0 to 4.4. Eight parameters were evaluated as having a strong effect on biodiversity (E ≥ 3): ‘diversity of semi-natural areas’, ‘ban of hydroponics’, ‘management of alpine meadows’, ‘no chemical steam sterilisation’, ‘conservation and management of ecologically valuable areas’, ‘conservation of meadow orchards’, ‘extensive pasture or wood pasture’, and ‘extensive grasslands only for bedding material’.
However, ‘ban of hydroponics’ and ‘no chemical steam sterilisation’ only had a strong effect on biodiversity on vegetable growing farms, which were only 12 of the organic and 25 of the conventional farms (i.e. 1.0% of organic and 0.3% of conventional farms).
Fourteen parameters received values ≤0.4 and thus were evaluated as having only a very minor effect. These were ‘cultivation of spring grains’, ‘number of different vegetable cultures’, ‘integrated production of vegetables’, ‘vineyards not irrigated’, ‘cultivation of rare vegetable varieties’, ‘use of bar mower on intensive meadows (instead of rotary mowers)’, ‘ban of mechanical choppers after mowing in intensive grassland’, ‘vegetable growing area without fleece’, ‘integrated production in greenhouses’, ‘small-scale improvement/upgrading with nesting aids and small artificial structures’, and ‘ban of hybrid seeds’. None of the parameters was evaluated as having a negative effect. Detailed evaluations of the parameters are shown in Table A2 (Appendix).
3.3. Biodiversity at farm level
Farm-level biodiversity scores were strongly determined by the farm structure of the farms. In this study, we did not attempt to match farms to enable comparison of structurally equal farms, but aimed to compare farms from the same region that delivers milk. In all regions, organic dairy farms were slightly larger than the average conventional dairy farm in the same region (). However, the number of milking cows on organic farms was lower than that of conventional farms in WS and OL. The proportion of arable land was similar on organic and conventional farms in all regions except in MU and OL, where the proportion of arable land was slightly higher on conventional farms.
Table 5. Overview of characteristics of the studied organic and conventional farms in the seven study regions.
Table 6. Key results of the Monte Carlo simulations of product-level biodiversity potential of conventional and organic milk from the seven study regions in Austria.
The farm-level biodiversity potential ranged from 7.6% to 51.5% and varied considerably within the farming systems. In all regions, farm-level biodiversity potential differed significantly between organic and conventional farms, although the ranges of organic and conventional farms overlapped broadly in most regions (). Highly significant differences (p < 0.001) between the farming systems were found in all regions, except in KB (p < 0.05).
Figure 2. Biodiversity potential at the farm level for organic and conventional farms in the seven study regions. Boxes show 25%-percentile, median and 75%-percentile; whiskers show 10%/90%-percentiles, dots show 5%/95%-percentiles. Mann–Whitney U-test for differences between organic and conventional farms of each region (*p < 0.05, ***p < 0.001). For abbreviations see .
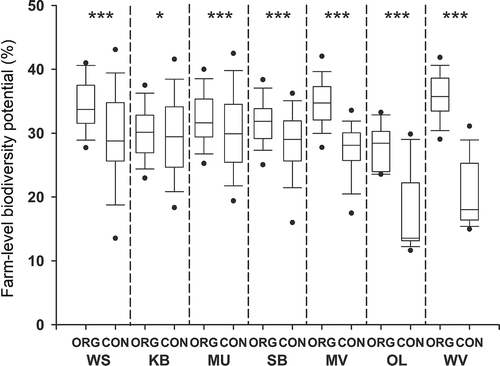
Due to the much higher number of conventional farms in each region, the overall highest and lowest scoring farms were conventional farms. However, in SB, MV, OL, and WV, the farm with the highest scores was an organic farm. Farms in WV, MU, and WS achieved the highest biodiversity scores, while conventional farms gained low biodiversity potential scores, especially in OL and WV, with median scores below 20%. The largest differences between organic and conventional farms were found between farms in OL, WV, and MV.
3.4. Biodiversity potential at product level
Calculating the farm biodiversity potential for raw milk led to biodiversity scores between 16% and 36%. The highest scores were achieved for organic milk from the WS, MV, and WV regions. The lowest scores were achieved for conventional milk from the OL and WV regions ().
Figure 3. Biodiversity potential of organic and conventional milk from different regions, depending on the type of aggregation. For abbreviations see .
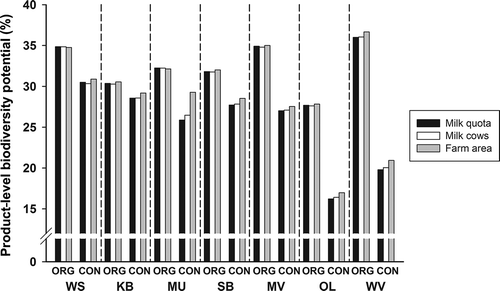
The differences between organic and conventional farms at the product level were generally lower in the regions where milk production is based on hay instead of grass silage (WS, KB, and MU). Farms in these regions are almost exclusively specialised dairy farms without other branches such as arable farming (). Differences were more pronounced in regions where arable farming made up a larger proportion of the farms (MV, OL, and WV).
Weightings for aggregating the farms according to farm area or number of cows, instead of according to milk quota, only marginally influenced product-level biodiversity scores (). Most differences were within the range of 1%. An exception is the conventional product from MU, which scored about 2.5% better when area aggregation was used.
The relative differences between the normalised product scores of organic and conventional milk (conventional milk in each region was set at 100%) were higher than 5% in all regions except in KB. In WS and SB, the differences in scores were between 10% and 15%, in MU and MV the differences were between 20% and 30%, and in OL and WV the differences were between 60% and 80% ().
Figure 4. Relative differences between biodiversity potential of conventional and organic milk from different regions. For abbreviations see .
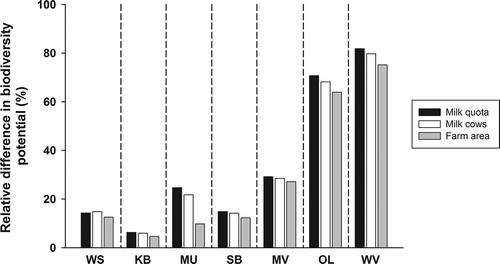
Due to the different reference scale, differences between aggregation procedures are more substantial and even accounted for more than 10% in one region (MU). In almost all regions, the highest differences were calculated if product aggregation was done according to the milk quota. The lowest differences between organic and conventional products resulted from aggregation according to area.
3.5. Uncertainty analysis
Apart from the aggregation procedure, uncertainty resulted from the expert assessments that were done to determine the impact of parameters on ISG and the feeding relationships. In , the uncertainty levels resulting from these expert assessments are shown for organic and conventional milk. The distribution of product scores, which were aggregated according to milk quota, is also shown in . A larger overlap of the curves indicates higher uncertainty in the difference between conventional and organic milk. The scores for all the conventional and organic milk farms show almost normal distribution curves. Each graph is split into three parts: the area between the two vertical dotted lines shows the range, with a likelihood of 90%, of the biodiversity potential scores for the conventional milk. The area beyond the left vertical dotted line shows the lowest 5% of biodiversity potentials in the Monte Carlo simulations. The area beyond the right vertical dotted line shows the highest 5% of biodiversity potentials in the Monte Carlo simulations. There are two rows of percentages shown above the distributions: the first row shows the percentages related to conventional milk, while the second row shows the percentages for organic milk. The values on top of the dotted lines show the biodiversity potentials at the point where these separating lines are located. Thus, for instance in WS, the likelihood that organic milk has a better biodiversity potential than the expected value of conventional milk is 99.7%. The likelihood of organic milk having a better biodiversity potential than even the 95% range of the conventional product is 63.6% (Table 6).
Figure 5. Results of the Monte Carlo simulations for biodiversity potential at product level. Vertical dotted lines and numbers in italic mark 5%/95%-percentiles of organic farms. Double row of percentages are percentage of organic (upper row) and conventional (lower row) farms below 5%-percentile, between 5%- and 95%-percentile, and above 95%-percentile of the organic farms.
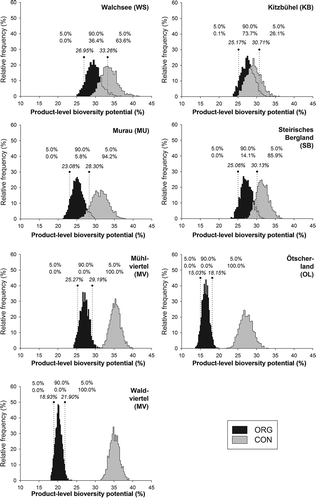
The average difference between the conventional and organic milk, according to Monte Carlo simulation, is highest in WV (+79%) and OL (+76%). Medium differences were calculated for MV (+32%) and MU (+28), while minor differences were calculated for SB (+18%), WS (+14%), and KB (+4%). According to the model results, the likelihood that organic milk will perform better than conventional milk from the same region is 99.7–100% in all regions except for KB, where this likelihood is about 78%. The likelihood that organic milk will have a biodiversity potential even higher than 95% of the range of conventional milk is between 26.1% (KB) and 100% (MV, WV, and OL) (Table 6).
4. Discussion
4.1. Applicability of the approach
The approach developed for this study can be used at two main levels: farm level and product level. Farm-level assessments can be used for monitoring the biodiversity potential and for comparing different farms or the same farm in different years. This allows for observation of differences and development in biodiversity conservation and promotion activities. The approach enables one to understand the implications of changes that farm management has on biodiversity. Hence, farm-specific list of measures for enhancing the biodiversity potential effectively can be generated. Also, comparison of management and biodiversity potential between single farms becomes possible. A typical application of farm-level results could be the allocation of direct payments (e.g. from agri-environmental programmes) to farms, based on the assessment results. However, the approach could also be used for capacity building of farmers with respect to optimising their biodiversity potential.
In addition to the farm-level assessments, the approach also includes a procedure for aggregating the biodiversity performance of several farms to product level. Although this impact assessment approach is not ISO14040 compliant, it allows for communication of the biodiversity potential of domestic products, such as milk and vegetables, in a comparative way. This can help processors and retailers in selecting suppliers or can help in conducting targeted capacity building for farmers. And aggregation to product level, regional, or sector-level assessments, such as for policy monitoring and evaluation, could also be generated with a similar procedure.
An enabling factor for these multiple applications is the flexibility of the approach with respect to data availability. If data is available, each of the 91 parameters can be specified separately to provide a comprehensive overview of a single farm. However, if a large number of farms are to be assessed, a set of selected parameters can be specified per farm, while others can be analysed for sample farms only. However, if data for the latter does not show significant differences, values for two compared products should be kept constant so as not to overestimate differences.
4.2. Relevance of the results
A main advantage of this approach is the ability to compare the biodiversity potential of farms using a standardised and transparent procedure that is adaptable to other countries and regions. The approach gains a comprehensive view by taking relevant indicators that influence biodiversity in terms of species diversity into account. In this study, 11 key species groups were taken as indicators for the entire range of wildlife species in agricultural and semi-natural habitats in Austria.
If most production steps of the life cycle of agricultural goods are performed on single farms, as is the case for milk in Austria, the farm-level approach can produce plausible results for the biodiversity potential at product level. However, there was a bias in this study due to off-farm-produced fodder components, such as concentrates, because the impact of these purchased fodder components on biodiversity was not included in our analysis. This bias increases with increasing proportions of off-farm-produced inputs, which are usually greater on conventional farms.
The assumptions underlying this model are mainly concerned with parameter selection and parameter weighting. It is unlikely that fundamental biases occurred because experts reviewed the parameter weighting, and cases where experts were uncertain about a rating, or had differing views, were taken into account with the uncertainty analysis. The stochastic model built for analysing biodiversity with Monte Carlo simulations proved to be a powerful tool to illustrate how different assumptions affect statements at farm and product levels.
A main improvement of the approach used in this study over detailed field- or farm-specific assessments is its representativeness, as the assessments were made for all organic farms delivering milk, which were then compared to all non-organic farms delivering milk. However, not all data were available for each and every farm, so a sample of regional farms was analysed to provide supplementary data. Where there was no justification from our sampled data or from the literature for the assumption that organic and conventional farms would differ, values for both the farming systems were assumed to be identical.
Our results are in line with a large number of biodiversity assessments of organic and conventional practices. Existing meta-studies show substantial benefits of organic practices (Bengtsson et al. Citation2005; Fuller et al. Citation2005; Hole et al. Citation2005). While the meta-studies were built on measurements of biodiversity at single field or farm level, representative studies from other countries in the alpine region show even larger differences between organic and conventional dairy farms at larger scale (Schader et al. Citation2013). Schader et al. (Citation2013) showed, by linking a crop and farm-level biodiversity model (Jeanneret et al. Citation2008) to an economic sector model, that especially structural differences, such as the different uptake rates of agri-environmental measure between organic and conventional farms, are a substantial driver for differences in biodiversity potential between the two farming systems.
However, the assumptions behind the product-level results need to be discussed in detail. The approach does not aim to calculate the impacts on biodiversity along the life cycle of a product-related functional unit in compliance with ISO 14040. Instead, it analyses the level of species diversity with reference to a species-rich agricultural landscape on an average farm delivering a certain product.
Therefore, longer supply chains, and especially those with parts of the products coming from other agri-ecological zones, would be more difficult to compare. Additional expert assessments and indicators would be necessary for covering such regions. For example, the impact on biodiversity of the production of imported concentrates that are used in conventional dairy production was not considered, although their increasing use is related to deforestation of rainforests in other regions of the world. Inclusion of such concentrates in an analysis would even increase the relative difference between organic and conventional products because the rules of organic farming do not allow the use of such concentrates. The results presented in this study are therefore rather conservative estimates of the differences between the two farming systems.
Biodiversity potential at product level was assessed on the basis of all operations on the farm. For example, a farm-level assessment of a farm that produces both vegetables and milk will include the biodiversity potential resulting from vegetable production as part of the product-level aggregation for milk. While this is unusual compared to other product-related approaches, we argue that all farm branches are interrelated, and on-farm biodiversity is also influenced by operations that are not directly linked to dairy production. This explicitly includes the management of semi-natural habitats. Furthermore, our approach focuses on impacts within the production system, i.e. the farm, including soil, while ISO14040-compliant approaches explicitly do not include these impacts.
As there is currently no approach that delivers biodiversity impact assessments that are compliant with ISO14040 (Tuomisto et al. Citation2012), most biodiversity assessments at a larger scale are based on indicator sets (EEA Citation2005; Paracchini et al. Citation2008). Therefore, our method is a step towards a more comprehensive and detailed assessment of biodiversity potential.
4.3. Need for further research
Although many of the indicators used in this study have been tested in other projects, further research is needed to validate the approach in practice. Since a validation for biodiversity over all ISGs is not practicable, testing should include whether the species number and abundance of selected species of the ISGs indeed correlate with the aggregated expert-based ratings. Subsequently, optimisation approaches, such as a cross-entropy procedure (Golan et al. Citation1996), could help to calibrate the model according to these measurements.
5. Conclusions
The approach developed for this study is able to evaluate farming practices, with respect to species number and abundance of 11ISGs, which allows to derive conclusions on the potential of farm species diversity. Aggregation to total PWs of farming practices was done on the basis of feeding relationships between the ISGs in Austrian agricultural ecosystems. Despite the given uncertainties with respect to the weighting of parameters and ISGs, the approach enables the calculation of aggregated species diversity scores and differentiation between farms and farming systems.
The organic dairy production systems assessed at farm and product levels showed significantly higher biodiversity potentials than did the conventional systems. Based on a large, representative farm sample, the study also showed that organic dairy systems have a higher biodiversity potential than conventional products with biodiversity scores from organic farms at product level ranging from 4% to 79% above the biodiversity scores of conventional farms. Particularly large differences between conventional and organic dairy products were found in WV and ÖL, while differences in KB were particularly low. KB was also the only region where farm-level biodiversity potential was not highly significantly different from conventional farms.
We demonstrated that this method is an applicable and complementary approach to life cycle assessments for making statements about the biodiversity potential at farm and product levels in cases where products are predominantly produced at specific farms. In these cases, the biodiversity potential can serve as a proxy for biodiversity impacts of products.
Acknowledgements
The authors are grateful for the support of this study by the interviewed experts (Theo Blick, Wolfgang Büchs, Andreas Fliessbach, Günter Gollmann, Patrick Gros, Werner Haberl, Ilse Hoffmann, Erwin Meyer, Norbert Milasovszky, Manfred Pendl, Martin Pollheimer, Guido Reiter, Leo Sachslehner, Norbert Sauberer, Heimo Schedl, Ulrich Schneppat, Angela Sessitsch, Norbert Teufelbauer, Barbara Thurner, Thomas Wrbka, Johann G. Zaller, Peter Zulka, and Thomas Zuna-Kratky), and farmers, and to Robert Home for editing the manuscript. Furthermore, we thank Zurück zum Ursprung as well as the Hofer KG for financial and logistical support of this study.
References
- Bengtsson J, Ahnström J, Weibull AC. 2005. The effects of organic agriculture on biodiversity and abundance: a meta-analysis. J Appl Ecol. 42:261–269.
- Boutin C, Baril A, Martin PA. 2008. Plant diversity in crop fields and woody hedgerows of organic and conventional farms in contrasting landscapes. Agric Ecosyst Environ. 123:185–193.
- De Schryver AM, Goedkoop MJ, Leuven RS, Huijbregts MA. 2010. Uncertainties in the application of the species area relationship for characterisation factors of land occupation in life cycle assessment. Int J Life Cycle Assess. 15:682–691.
- Delbecq AL, Van de Ven AH, Gustafson DH. 1975. Group techniques for program planning: a guide to nominal group and Delphi processes. Glenview (IL): Longman Higher Education.
- EEA. 2005. Agriculture and environment in EU-15 – the IRENA indicator report, EEA Report No 6/2005. Copenhagen : EEA.
- Fuller RJ, Norton LR, Feber RE, Johnson PJ, Chamberlain DE, Joys AC, Mathews F, Stuart RC, Townsend MC, Manley WJ, et al. 2005. Benefits of organic farming to biodiversity vary among taxa. Biol Lett. 1:431–434.
- Gibson RH, Pearce S, Morris RJ, Symondson WOC, Memmott J. 2007. Plant diversity and land use under organic and conventional agriculture: a whole-farm approach. J Appl Ecol. 44:792–803.
- Golan A, Judge G, Miller D. 1996. Maximum entropy econometrics. Robust estimation with limited data Chichester. New York (NY): Wiley.
- Hole DG, Perkins AJ, Wilson JD, Alexander IH, Grice PV, Evans AD. 2005. Does organic farming benefit biodiversity? Biol Conserv. 122:113–130.
- Hörtenhuber S, Lindenthal T, Amon B, Markut T, Kirner L, Zollitsch W. 2010. Greenhouse gas emissions from selected Austrian dairy production systems – model calculations considering the effects of land use change. Renew Agric Food Syst. 25: 1–14.
- International Organization for Standardization. 2006a. Environmental management – life cycle assessment – principles and framework. Geneva: International Organization for Standardization.
- International Organization for Standardization. 2006b. Environmental management – life cycle assessment – requirements and guidelines. Geneva: International Organization for Standardization.
- Jeanneret P, Baumgartner D, Freiermuth R, Gaillard G. 2006. Méthode d’évaluation de l’impact des activités sur la biodiversité. Zürich: Agroscope Reckenholz Tänikon (ART).
- Jeanneret P, Baumgartner D, Freiermuth-Knuchel R, Gaillard G. 2008. A new LCIA method for assessing impacts of agricultural activities on biodiversity (SALCA-biodiversity). In: Nemecek T, Gaillard G, edtiors. Proceedings of the 6th International Conference on Life Cycle Assessment in the Agri-Food Sector Towards a Sustainable Management of the Food-Chain; 2008 Nov 12–14. Zürich: Agroscope Reckenholz Tänikon (ART).
- Jenny M, Fischer J, Pfiffner L, Birrer S, Graf R. 2010. Leitfaden für die Anwendung des Punktesystems. Frick (Sempach): Forschungsinstitut für biologischen Landbau (FiBL), Schweizerische Vogelwarte.
- Jenny M, Zellweger-Fischer J, Balmer O, Birrer S, Pfiffner L. 2013. The credit point system: an innovative approach to enhance biodiversity on farmland. Aspects Appl Biol. 118:23–30.
- Lebensministerium. 2010. Evaluierungsbericht 2010. Wien: Halbzeitbewertung des Österreichischen Programms für die Entwicklung des ländlichen Raums.
- MA. 2005. Ecosystems and human well-being: biodiversity synthesis. Washington (DC): Millenium Ecosystem Assessment (MEA), World Resources Institute.
- Mila i Canals L, Romanya J, Cowell S. 2007. Method for assessing impacts on life support functions (LSF) related to the use of ‘fertile land’ in Life Cycle Assessment (LCA). J Clean Prod. 15:1426–1440.
- OECD. 2001. Agriculture and biodiversity – developing indicators for policy analysis. Paris: Organisation for Economic Co-operation and Development (OECD).
- Paracchini ML, Petersen J-E, Hoogeveen Y, Bamps C, Burfield I, van Swaay C. 2008. High nature value farmland in Europe. An estimate of the distribution patterns on the basis of land cover and biodiversity data. Luxemburg: Office for Official Publications of the European Communities.
- Rockström J, Steffen W, Noone K, Persson Å, Chapin FS III, Lambin E, Lenton TM, Scheffer M, Folke C, Schellnhuber H, et al. 2009. Planetary boundaries: exploring the safe operating space for humanity. Ecol Soc. [Internet]. [cited 2011 Oct 24]; 14. Available from: http://www.ecologyandsociety.org/vol14/iss2/art32/
- Rubinstein RY. 2009. Simulation and the Monte Carlo method. Hoboken (NJ): Wiley-Interscience.
- Schader C. 2009. Cost-effectiveness of organic farming for achieving environmental policy targets in Switzerland [PhD thesis]. Aberystwyth University, Aberystwyth, Wales. Frick (Switzerland): Research Institute of Organic Farming (FiBL).
- Schader C, Lampkin N, Christie M, Nemecek T, Gaillard G, Stolze M. 2013. Evaluation of cost-effectiveness of organic farming support as an agri-environmental measure at Swiss agricultural sector level. Land Use Policy. 31:196–208.
- Schader C, Stolze M. 2011. Bewertung der Nachhaltigkeit der biologischen Landwirtschaft in der Schweiz durch Experten. In: Leithold G, Becker K, Brock C, Fischinger S, Spiegel A-K, Spory K, Wilbois K-P, Williges U, edtiors. Proceedings of the 11 Wissenschaftstagung Ökologischer Landbau; 2011 Mar 15–18; Gieβen.
- Schader C, Stolze M, Gattinger A. 2012. Environmental performance of organic agriculture. In: Boye JI, Arcand Y, editors. Green technologies in food production and processing. New York (NY): Springer; p. 183–206.
- Schloter M, Dilly O, Munch J. 2003. Indicators for evaluating soil quality. Agric Ecosyst Environ. 98:255–262.
- ten Brink P, Berghöfer A, Sröter-Schlaack C, Sukhdev P, Vakrou A, White S, Wittmer H. 2009. TEEB – the economics of ecosystems and biodiversity for national and international policy makers. Geneva: United Nations Environment Programme (UNEP).
- Tscharntke T, Klein AM, Kruess A, Steffan‐Dewenter I, Thies C. 2005. Landscape perspectives on agricultural intensification and biodiversity–ecosystem service management. Ecol Lett. 8:857–874.
- Tuomisto H, Hodge I, Riordan P, Macdonald D. 2012. Does organic farming reduce environmental impacts? A meta-analysis of European research. J Environ Manage. 112:309–320.
- von Haaren C, Kempa D, Vogel K, Rüter S. 2012. Assessing biodiversity on the farm scale as basis for ecosystem service payments. J Environ Manage. 113:40–50.
- Zurück zum Ursprung. 2013. Acht Grundwerte. Zurück zum Ursprung (ZZU) [Internet]. [cited 2013 Nov 13]. Available from: http://www.wernerlampert.com/pruef-nach/acht-grundwerte/
Appendix
Table A1. Overview of model parameters, scales, origin of parameters, and data sources by farm branch.
Table A2. Distribution of parameter weights for aggregated biodiversity after Monte Carlo simulations.