Abstract
The management of natural vegetation and forested habitat within agricultural areas can be important for bolstering ecosystem services. In coffee agro-ecosystems, proximate forested habitat can provide resources for Hymenoptera that contribute to crop pollination and biological control of pests. We conducted a field study on 12 coffee farms in the highlands of Tarrazú, Costa Rica over a period of 3 years during both dry (flowering) and rainy (nonflowering) seasons. We compared the abundance of Hymenoptera observed around coffee plants on farms that were either isolated from or immediately adjacent to substantial forest habitat. Our results demonstrated a seasonally dependent response, with higher numbers of Hymenoptera observed in adjacent farms than in isolated farms during dry seasons, but no difference in the numbers in isolated farms and adjacent farms during rainy seasons. We discuss the implications of these findings with respect to the potential pollination and biological control benefits associated with preserving forest/noncrop habitat within coffee agro-ecosystems.
Introduction
The sustainable management of ecosystem services within agricultural habitats has been identified as a critical need in the conservation of biological diversity and the stability of food systems (Allen-Wardell et al. Citation1998; Kremen et al. Citation2002; Klein et al. Citation2003a, Citation2003b; Mas & Dietsch Citation2004; Ricketts et al. Citation2004; Blanche et al. Citation2006; Brittain et al. Citation2013; Vanbergen & The Insect Pollinators Initiative Citation2013). The importance of arthropod exchanges between forest and crop habitat has received much attention in the past decade; forest habitat may play an important role in providing resources for pollinators and natural enemies (Banks Citation2000, Citation2004; De Marco & Coelho Citation2004; Naughton-Treves & Salafsky Citation2004; Ricketts Citation2004; Perfecto et al. Citation1996; Banks et al. Citation2007; Priess et al. Citation2007; Klein et al. Citation2008; Jha & Vandermeer Citation2010). Many pollinators in agricultural landscapes in the Mesoamerican tropics are in the order Hymenoptera, including eusocial native bees (e.g., Meliponini, Halictidae) as well as introduced honeybees (e.g., Apis mellifera). Likewise, many important natural enemies in agro-ecosystems are also hymenopterans – especially parasitoid wasps (e.g., Braconidae, Ichneumonidae). Tropical agricultural landscapes composed of a patchwork of crop and natural vegetation represent an opportunity for assessing both yields and conservation schemes as both pollinators and parasitoid wasps in agro-ecosystems often rely on noncrop habitat (e.g., in-field vegetation, hedgerows, weedy margins, and adjacent forest) for supplemental resources and shelter (Kremen et al. Citation2002; Mas & Dietsch Citation2004; Klein et al. Citation2008; Ricketts et al. Citation2008; Peters et al. Citation2012). Coffee agro-ecosystems are a prime example of this sort of landscape heterogeneity, as they often consist of a mixture of coffee and forest habitat, especially in steep tropical environments where coffee is often grown. Although coffee is self-pollinating, cross-pollination by bees from nearby forest habitat can significantly bolster yields (Klein et al. Citation2003a; Ricketts, Citation2004; Vergara & Badano Citation2009; Garibaldi et al. Citation2013). Furthermore, forest habitat adjacent to coffee can influence the overall abundance and diversity of Hymenoptera in coffee plantations – including both bees and wasps important for biological control (Banks et al. Citation2013). Understanding the role that forest remnants play within agro-ecosystems is critical to prescribing best management practices in coffee-producing areas, especially as managed and feral bees decline worldwide (Kremen et al. Citation2002; National Research Council Citation2007; Brittain et al. Citation2010; Potts et al. Citation2010; Lebuhn et al. Citation2013).
We describe here a field experiment, set in a mosaic of coffee farms and small patches of forest habitat in the central highlands of Costa Rica, aimed at better understanding the relationships between forest remnants, coffee management, and ecosystem services. In particular, we set out to explore how the proximity of forest fragments to coffee fields affects populations of hymenopteran pollinators and biological control agents. Over a 2-year period across rainy and dry seasons, we compared the abundance of Hymenoptera observed around coffee plants on farms that were either isolated from or adjacent to nearby forest habitat.
Material and methods
Hymenoptera around coffee plants were observed on 12 coffee farms in the Tarrazú region in the central highlands of Costa Rica. All of the farms were within the domain of CoopeTarrazú, a growers’ cooperative consisting of more than 2500 farmers. Individual coffee farms were selected based on proximity to forest – i.e., either directly adjacent to or isolated from substantial forest fragments. The Tarrazú region receives an average of 2500 mm of rainfall per year and has distinct dry (December to April) and rainy (May to November) seasons during which temperatures remain fairly constant. Coffee flowering in the region occurs during the dry season, typically from February to April. Coffee plants may flower between one and four times, depending on the rainfall and other environmental factors (e.g., elevation, aspect) (N. Ureña, personal communication). To compare seasonal variation of Hymenoptera abundance within coffee farms, observations were conducted in both dry (flowering) and rainy (nonflowering) seasons: three times in 2010 (twice during the dry season and once during the rainy season), once in 2011 (rainy season), and once in 2012 (dry season). Farms ranged from 0.15 to 5.7 ha in size and were between 1330 masl to 1810 masl in elevation. Each farm was classified as either ‘adjacent’ (directly next to a forest habitat patch at least 0.5 ha in size) or ‘isolated’ (>100 m from any forest habitat at least 0.5 ha in size). Sampling areas on different farms were separated by a minimum of 900 m, except in the case of two farm samples that were only 500 m apart. Positions of sampling transects were determined using a Trimble Geo XM® GPS receiver coupled with a Hurricane® antenna receiver. The spatial distribution between sampling transects was verified using Generate Near Table analysis in ArcGIS 10.1 (ESRI Citation2012).
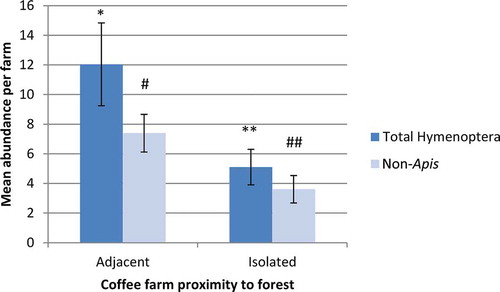
Figure 1. Mean number of total Hymenoptera and non-Apis Hymenoptera observed during dry (flowering) seasons for adjacent vs. isolated farms (n = 18, error bars are ±SE). Differences between adjacent and isolated for both groups were statistically significant (t = 2.28, p = .03 for total Hymenoptera; t = 2.40, p = .02 for non-Apis). Different number of asterisks/pound signs denotes significant difference.
At each farm, three observers each chose a coffee plant (one plant per observer) randomly located within a 20 × 20-m area that was mapped out 50 m away from the edge of the farm or the adjacent forest habitat. Observers recorded all Hymenoptera coming within 10 cm of the plant surface of their focal coffee plant within a 20-min observation period to measure the presence and abundance of bees and wasps in coffee plantations regardless of season. They further noted whether the observed Hymenoptera were the introduced European honeybee (A. mellifera) or non-Apis Hymenoptera. The mean number of Hymenoptera recorded per plant was calculated for each farm. Mean abundances of Hymenoptera observed on each farm type were compared between dry and rainy seasons using t-test. Furthermore, differences in the mean number of hymenopteran observed between adjacent and isolated farms across all seasons were analyzed using fixed-effects ANOVA, with season and proximity to forest as main effects. Following the observation period, observers spent 10 min using butterfly nets to capture hymenopteran specimens flying near the focal coffee plants to create a representative voucher collection. Specimens were later identified to species where possible at the University of Costa Rica.
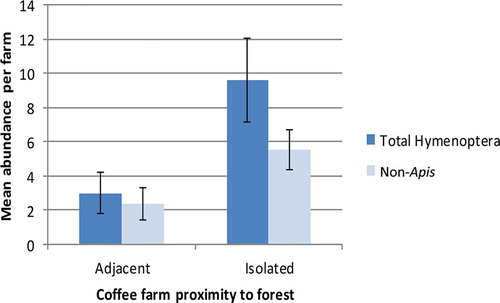
Figure 2. Mean number of total Hymenoptera/non-Apis Hymenoptera observed during rainy (nonflowering) seasons for adjacent vs. isolated farms (n = 12, error bars are ±SE). Differences between adjacent and isolated for neither group were statistically significant (t = −1.97, p = .06 for total Hymenoptera; t = −1.72, p = .10 for non-Apis).
Ground cover within each farm was characterized using three 1-m2 quadrats randomly placed within the previously established 20 × 20-m experimental areas. Percent cover of live vegetation and percent cover of flowering plants were recorded for each quadrat; means of these ground cover metrics were calculated per farm for each sampling period.
Table 1. Analysis of variance of total hymenopteran observations, with season (dry vs. rainy) and isolation of farm from forest (isolated vs. adjacent) as main effects.
Results
Analysis of our data revealed that an interaction between season and farm isolation explained a significant amount of the variation in total hymenopteran visitation to coffee farms (). During the dry season, the mean total number of Hymenoptera observed around coffee plants on adjacent farms was more than twice that of total Hymenoptera visiting plants on isolated farms (t-test, n = 36, df = 34, t = 2.28, p = .03; ). Additionally, non-Apis Hymenoptera observation rates were nearly double in coffee farms adjacent to forest habitat than isolated farms during this time frame (t-test, n = 36, df = 34, t = 2.40, p = .02; ). During the rainy season, there were more total Hymenoptera and non-Apis Hymenoptera around plants in isolated farms than in adjacent farms – though these differences were not statistically significant (t-test, n = 24, df = 22, t = −1.97, –1.72, respectively; p = .06, .10, respectively; ). For both seasons, there was no statistically significant difference in A. mellifera observed among adjacent and isolated farms (dry season: t-test, n = 36, df = 34, t = 1.79, p = .08; rainy season: t-test, n = 24, df = 22, t = −1.60, p = .12).
Non-Apis Hymenoptera captured post-observation in and around coffee farms included representatives from Meliponini, a tribe of stingless bees that are common pollinators; furthermore, high numbers of parasitoid wasps (e.g., Ichneumonidae, Braconidae) were also captured in the rainy season (). For ground cover, there were no differences in percent flowering plants between adjacent and isolated farms, between dry and rainy seasons, nor in the interaction between these two factors (ANOVA, p > .05). Furthermore, there were no differences in percent live vegetation between adjacent and isolated farms – but there was a significantly higher percent of live vegetation overall in the rainy season across all farms (ANOVA, n = 60, df = 1, F = 23.76, p < .001).
Discussion
The utility of natural vegetation and forested habitat in and around agro-ecosystems for a variety of ecosystem services, including biological control and pollination, has been a focus of many recent theoretical and field-based studies (Klein et al. Citation2002; Ricketts Citation2004; Potts et al. Citation2010; Clough et al. Citation2011; Tscharntke et al. Citation2012). In several recent studies, increasing distances from forest habitat have been associated with declines in hymenopteran pollinators (Ricketts Citation2004; Ricketts et al. Citation2008; Farwig et al. Citation2009) as well as parasitoid wasp populations (Klein et al. Citation2006). Our results indicate that in the Tarrazú coffee highlands of Costa Rica, the presence of forest habitat adjacent to cultivated areas can be associated with increased numbers of Hymenoptera. In other studies, bee densities have been positively correlated with increases in pollination and yield (Veddeler et al. Citation2008). Likewise, a high diversity of parasitoid wasps has been positively correlated with improved biological control (Tylianakis et al. Citation2006). Taken together, our results support the growing body of work suggesting that the conservation of forest habitats can result in both economic and environmental benefits (Priess et al. Citation2007; Ricketts & Lonsdorf Citation2013).
Table 2. Non-Apis Hymenoptera captured in coffee farms during observation periods, by season.
Recent field studies have shown that pollinator response to landscape management varies with the species composition of pollinators and spatial scale of available resources (Veddeler et al. Citation2006; Jha & Vandermeer Citation2009a, Citation2009b). For the farms in the current study, nearby forest fragments may play an important role for native bees such as Meliponini by providing appropriate nesting habitat (e.g., tree cavities) and noncrop foraging resources (Ricketts et al. Citation2008). Recent work has also demonstrated that both native and exotic bees in neotropical coffee agro-ecosystems prefer to forage in areas with high densities of trees (Jha & Vandermeer Citation2009a), where native bee diversity and visitation rates are influenced by changes in local flowering resources at relatively small spatial scales (Jha & Vandermeer Citation2009b). We found that the number of A. mellifera visits were not significantly affected by proximity to forest in either season, which may reflect the long-range foraging abilities of A. mellifera that allow the species to respond dynamically to shifts in floral resources at larger landscape scales (Jha & Vandermeer Citation2009b). The potential synergistic interactions between native and nonnative Hymenoptera (e.g., introduced honeybees), which has been shown elsewhere to positively influence crop yields (Brittain et al. Citation2013; Garibaldi et al. Citation2013), merits further study in coffee agro-ecosystems.
The seasonal pattern we obtained (reflected in the significant isolation × season interaction, ) suggests a complex relationship between forest management and Hymenoptera. That we found higher numbers of Hymenoptera in adjacent farms than in isolated farms during the coffee blooming period in the dry season is not particularly surprising as bees foraging from a forest base will naturally encounter nearby farms (e.g., adjacent farms) first and may overlook isolated farms when flowering is widespread across the landscape. This pattern changed during the rainy season when the number of visiting Hymenoptera on adjacent farms was not statistically different from the number visiting isolated farms. This may reflect a shift in resource availability during the post-coffee flowering season; although our data revealed no differences in flowering plants or live ground vegetation between adjacent and isolated plants, we did record significantly higher levels of live ground vegetation overall for all farms during the rainy season than during the dry season.
Typical coffee management in the Tarrazú region consists of shade tree pruning activity following the harvest during the rainy season. Increased pruning and subsequent increased sunlight on the ground might be responsible for an increase in live ground cover during the rainy season. Recent studies have shown that while social bees decline with distance for forest fragments, solitary bee diversity increases with increased sunlight (Klein et al. Citation2003c). Farms isolated from forest habitat may support some Hymenoptera during the rainy season equally as well as farms adjacent to forest habitat since increased sunlight and ground cover within coffee farms may provide resources for pollinators and parasitoids throughout the months following the mass-flowering of the coffee crop (Priess et al. Citation2007). Thus, we might expect that the availability of widespread resources could drive a more uniform distribution of Hymenoptera across farms. This ‘dilution effect’ phenomenon is especially evident in some non-Apis pollinators that have a tendency to distribute their foraging activities across patches of high resource availability while bypassing isolated or relatively small areas of flowering plants (Greenleaf et al. Citation2007; Jha & Vandermeer Citation2009a). More diverse ground cover vegetation throughout coffee farms in the region may enable Hymenoptera to be less reliant upon forest resources during the rainy season and therefore more likely to generate similar observation frequencies for both adjacent and isolated farms. More information about species-specific habitat requirements (e.g., vegetation, microclimate, and water resource needs) is necessary to fully understand differences in seasonal habitat use.
Overall, we found that the mean number of hymenopteran visitors varies seasonally in each type of farm, suggesting that there may be times when the Tarrazú coffee farms undergo a pollinator deficit. As pollinator dynamics in coffee agro-ecosystems are driven by the availability of total flowering resources (Jha & Vandermeer Citation2010; Karanja et al. Citation2010; Peters et al. Citation2013), a more comprehensive picture of those resources could shed a more mechanistic light on the patterns we describe here. More detailed analysis of hymenopteran residence time and movement among the different habitats is necessary to develop a better understanding of the community dynamics across spatial and temporal scales. Furthermore, a better understanding of the effects of shade pruning and herbicide use on ground cover vegetation is needed to prescribe optimal management practices for the preservation of Hymenoptera in coffee agro-ecosystems. Our data suggest that preservation of forest habitat in and around coffee agro-ecosystems has a beneficial effect on the number of Hymenoptera visiting nearby coffee farms particularly during the dry season when coffee experiences its mass-flowering period.
Acknowledgements
The authors would like to acknowledge the coffee farmers and members of CoopeTarrazú R.L. that generously allowed access to their farms for our research and the CoopeTarrazú management for logistical support and a facility to use as a laboratory. This research would not have been possible without the financial support of Ernst & Young LLP and the volunteerism of the Earthwatch-Ernst & Young Global Ambassadors, who gave their time and effort to observe Hymenoptera and assist with data collection. We would like to thank Earthwatch scientists Sebastian Castro-Tanzi and Natalia Ureña-Retana who were instrumental in developing and maintaining critical relationships with participating farmers as well as providing logistical support during the course of this project. A special thank you is due to the Starbucks Coffee Company and Arthur Riggs’ Emerging Scientist Fellowship for their generous support of the Earthwatch Tarrazú field center and the Earthwatch research program. The authors owe a debt of gratitude to the teams of volunteers who helped collect data and to Dr. Paul Hanson of the Universidad de Costa Rica for hymenopteran identification and helpful comments. Finally, the authors thank two anonymous reviewers for helpful comments and suggestions that greatly improved the manuscript.
References
- Allen-Wardell G, Bernhardt P, Bitner R, Burquez A, Buchmann S, Cane J, Cox PA, Dalton V, Feinsinger P, Ingram M, et al. 1998. The potential consequences of pollinator declines on the conservation of biodiversity and stability of food crop yields. Conserv Biol. 12:8–17. doi:10.1046/j.1523-1739.1998.97154.x.
- Banks JE. 2000. Natural vegetation in agro-ecosystems: pattern and scale of heterogeneity. In: Ekbom B, Irwin M, Robert Y, editors. Interchanges of insects between agricultural and surrounding landscapes. Dordrecht: Kluwer Press; p. 215–229.
- Banks JE. 2004. Divided culture: integrating agriculture and conservation biology. Front Ecol Environ. 2:537–545. doi:10.1890/1540-9295(2004)002[0537:DCIAAC]2.0.CO;2
- Banks JE, Hannon L, Hanson P, Dietsch T, Castro S, Urena N, Chandler M. 2013. Effects of proximity to forest habitat on hymenoptera diversity in a Costa Rican coffee agroecosystem. Pan Pac Entomol. 89:60–68. doi:10.3956/2012-28.1.
- Banks JE, Sandvik PJ, Keesecker L. 2007. Beetle (Coleoptera) and spider (Araneae) diversity in a mosaic of farmland, edge, and tropical forest habitats in western Costa Rica. Pan Pac Entomol. 83:152–160. doi:10.3956/0031-0603-83.2.152.
- Blanche KR, Ludwig JA, Cunningham SA. 2006. Proximity to rainforest enhances pollination and fruit set in orchards. J Appl Ecol. 43:1182–1187. doi:10.1111/j.1365-2664.2006.01230.x
- Brittain C, Williams N, Kremen C, Klein AM. 2013. Synergistic effects of non-Apis bees and honey bees for pollination services. Proc Royal Soc B Biol Sci. 280:20122767. doi:10.1098/rspb.2012.2767
- Brittain CA, Vighi M, Bommarco R, Settele J, Potts SG. 2010. Impacts of a pesticide on pollinator species richness at different spatial scales. Basic Appl Ecol. 11:106–115. doi:10.1016/j.baae.2009.11.007.
- Clough Y, Barkmann J, Juhrbandt J, Kessler M, Wanger TC, Anshary A, Buchori D, Cicuzza D, Darras K, Putra DD, et al. 2011. Combining high biodiversity with high yields in tropical agroforests. Proc Natl Acad Sci. 108:8311–8316. doi:10.1073/pnas.1016799108.
- De Marco P, Coelho FM. 2004. Services performed by the ecosystem: forest remnants influence agricultural cultures’ pollination and production. Biodivs Conserv. 13:1245–1255. doi:10.1023/B:BIOC.0000019402.51193.e8.
- Environmental Systems Resource Institute. 2012. ArcGIS 10.1. Redlands, CA.
- Farwig N, Bailey D, Bochud E, Herrmann JD, Kindler E, Reusser N, Schüepp C, Schmidt-Entling MH. 2009. Isolation from forest reduces pollination, seed predation and insect scavenging in Swiss farmland. Landscape Ecol. 24:919–927. doi:10.1007/s10980-009-9376-2
- Garibaldi LA, Steffan-Dewenter I, Winfree R, Aizen MA, Bommarco R, Cunningham SA, Kremen C, Carvalheiro LG, Harder LD, Afik O, et al. 2013. Wild pollinators enhance fruit set of crops regardless of honey bee abundance. Science 339:1608–1611.
- Greenleaf SS, Williams NM, Winfree R, Kremen C. 2007. Bee foraging ranges and their relationship to body size. Oecologia 153:589–596. doi:10.1007/s00442-007-0752-9.
- Jha S, Vandermeer JH. 2009a. Contrasting bee foraging in response to resource scale and local habitat management. Oikos 118:1174–1180. doi:10.1111/j.1600-0706.2009.17523.x.
- Jha S, Vandermeer JH. 2009b. Contrasting foraging patterns for Africanized honeybees, native bees and native wasps in a tropical agroforestry landscape. J Trop Ecol. 25:13–22. doi:10.1017/S026646740800566X
- Jha S, Vandermeer JH. 2010. Impacts of coffee agroforestry management on tropical bee communities. Biol Conserv. 143:1423–1431. doi:10.1016/j.biocon.2010.03.017.
- Karanja RHN, Njoroge GN, Gikungu MW, Newton LE. 2010. Bee interactions with wild flora around organic and conventional coffee farms in Kiambu District, Central Kenya. J Poll Ecol. 2:7–12.
- Klein AM, Cunningham SA, Bos M, Steffan-Dewenter I. 2008. Advances in pollination ecology from tropical plantation crops. Ecology 89:935–943. doi:10.1890/07-0088.1.
- Klein AM, Steffan-Dewenter I, Buchori D, Tscharntke T. 2002. Effects of land-use intensity in tropical agroforestry systems on coffee flower-visiting and trap-nesting bees and wasps. Conserv Biol. 16:1003–1014. doi:10.1046/j.1523-1739.2002.00499.x
- Klein AM, Steffan-Dewenter I, Tscharntke T. 2003a. Bee pollination and fruit set of Coffea arabica and C. canephora (Rubiaceae). Am J Bot. 90:153–157. doi:10.3732/ajb.90.1.153
- Klein AM, Steffan-Dewenter I, Tscharntke T. 2003b. Pollination of Coffea canephora in relation to local and regional agroforestry management. J Appl Ecol. 40:837–845. doi:10.1046/j.1365-2664.2003.00847.x.
- Klein AM, Steffan-Dewenter I, Tscharntke T. 2003c. Fruit set of highland coffee increases with the diversity of pollinating bees. Proc Royal Soc B Biol Sci. 270 :955–961. doi:10.1098/rspb.2002.2306.
- Klein AM, Steffan-Dewenter I, Tscharntke T. 2006. Rain forest promotes trophic interactions and diversity of trap-nesting Hymenoptera in adjacent agroforestry. J Anim Ecol. 75:315–323. doi:10.1111/j.1365-2656.2006.01042.x.
- Kremen C, Williams NM, Thorp RW. 2002. Crop pollination from native bees at risk from agricultural intensification. Proc Natl Acad Sci. 99:16812–16816. doi:10.1073/pnas.262413599.
- Lebuhn G, Droege S, Connor EF, Gemmill-Herren B, Potts SG, Minckley RL, Griswold T, Jean R, Kula E, Roubik DW, et al. 2013. Detecting insect pollinator declines on regional and global scales. Conserv Biol. 27:113–120. doi:10.1111/j.1523-1739.2012.01962.x.
- Mas AH, Dietsch TV. 2004. Linking shade coffee certification to biodiversity conservation: butterflies and birds in Chiapas, Mexico. Ecological Appl. 14:642–654. doi:10.1890/02-5225.
- National Research Council. 2007. Status of pollinators in North America. Washington (DC): National Academies Press.
- Naughton-Treves L, Salafsky N. 2004. Wildlife conservation in agroforestry buffer zones: opportunities and conflict. In: Schroth G, da Fonseca AB, Harvey CA, Gascon C, Vasconcelos HLIzac AN, editors. Agroforestry and biodiversity conservation in tropical landscapes. Washington (DC): Island Press; pp. 319–445.
- Perfecto I., Rice RA, Greenberg R, Vandervoort ME. 1996. Shade coffee: a disappearing refuge for biodiversity. Bioscience. 46:598–608. doi:10.2307/1312989
- Peters VE, Carroll CR. 2012. Temporal variation in coffee flowering may influence the effects of bee species richness and abundance on coffee production. Agroforestry Syst. 85:95–103. doi:10.1007/s10457-011-9476-2
- Peters VE, Carroll CR, Cooper RJ, Greenberg R, Solis M. 2013. The contribution of plant species with a steady-state flowering phenology to native bee conservation and bee pollination services. Insect Conservation Divers. 6:45–56. doi:10.1111/j.1752-4598.2012.00189.x
- Potts SG, Biesmeijer JC, Kremen C, Neumann P, Schweiger O, Kunin KE. 2010. Global pollinator declines: trends, impacts and drivers. TREE. 25:345–353.
- Priess JA, Mimler M, Klein A-M, Schwarze S, Tscharntke T, Steffan-Dewenter I. 2007. Linking deforestation scenarios to pollination services and economic returns in coffee agroforestry systems. Ecol Appl. 17:407–417. doi:10.1890/05-1795.
- Ricketts TH. 2004. Tropical forest fragments enhance pollinator activity in nearby coffee crops. Conserv Biol. 18:1262–1271. doi:10.1111/j.1523-1739.2004.00227.x
- Ricketts TH, Daily GC, Ehrlich PR, Michener C. 2004. Economic value of tropical forest to coffee production. Proc Natl Acad Sci. 101:12579–12582. doi:10.1073/pnas.0405147101.
- Ricketts TH, Lonsdorf EV. 2013. Mapping the margin: comparing marginal values of tropical forest remnants for pollination services. Ecological Appl. 23:1113–1123. doi:10.1890/12-1600.1.
- Ricketts TH, Regetz J, Steffan-Dewenter I, Cunningham SA, Kremen C, Bogdanski A, Gemmill-Herren B, Greenleaf SS, Klein AM, Mayfield MM, et al. 2008. Landscape effects on crop pollination services: are there general patterns? Ecol Lett. 11:499–515. doi:10.1111/j.1461-0248.2008.01157.x.
- Tscharntke T, Clough Y, Wanger TC, Jackson L, Motzke I, Perfecto I, Vandermeer J, Whitbread A. 2012. Global food security, biodiversity conservation and the future of agricultural intensification. Biol Conserv. 151:53–59. doi:10.1016/j.biocon.2012.01.068.
- Tylianakis J, Tscharntke T, Klein AM. 2006. Diversity, ecosystem function, and stability of parasitoid–host interactions across a tropical habitat gradient. Ecology. 87:3047–3057. doi:10.1890/0012-9658(2006)87[3047:DEFASO]2.0.CO;2.
- Vanbergen AJ, The Insect Pollinators Initiative. 2013. Threats to an ecosystem service: pressures on pollinators. Front Ecol Environ. 11:251–259. doi:10.1890/120126.
- Veddeler D, Klein AM, Tscharntke T. 2006. Contrasting responses of bee communities to coffee flowering at different spatial scales. Oikos 112:594–601. doi:10.1111/j.0030-1299.2006.14111.x.
- Veddeler D, Olschewski R, Tscharntke T, Klein AM. 2008. The contribution of non-managed social bees to coffee production: new economic insights based on farm-scale yield data. Agroforestry Syst. 73:109–114. doi:10.1007/s10457-008-9120-y.
- Vergara CH, Badano EI. 2009. Pollinator diversity increases fruit production in Mexican coffee plantations: the importance of rustic management systems. Agr Ecosyst Environ. 129:117–123. doi:10.1016/j.agee.2008.08.001.
