Abstract
There is increasing interest in home gardens (HGs) as biodiversity hot spots. However, knowledge on how sociocultural characteristics and environment influence knowledge and management of HG species is still limited. Eliciting these links helps illustrate how HG could conserve biodiversity. This study addressed the following hypotheses: (i) age and gender shape the knowledge of HG species; (ii) knowledge on HG species varies across phytochorological zones; (iii) use values (UVs) of HG species are correlated to their ecological importance and (iv) HG species is mostly used for food and medicinal purposes. Data were collected from 285 HGs, across three phytochorological zones of Benin, using semi-structured interviews. Quantitative analyses were performed using ethnobotanical indexes and statistical tests. Our results confirmed our assumptions except for hypothesis (i). Gender and age did not determine knowledge on HG species. Nevertheless, noticeable differences were encountered among the zones regarding species, knowledge and use types. UV and ecological importance were highly correlated. Our results support the point that HGs sustain food and medicine supply while contributing to conservation of local biodiversity. However, with modern mutations, HGs are unlikely to be preserved if they are not actively mainstreamed in production and conservation policies.
1. Introduction
Tropical ecosystems have undergone drastic changes in terms of habitat loss, fragmentation and conversion to agriculture (Laurance Citation2007). To curb this trend, the Conference of the Parties to the Convention on Biological Diversity (COP VI/26) committed to achieving a significant reduction in the rate of biodiversity loss by 2010. There is increasing evidence that traditional agroforestry systems (on-farm conservation) stand as a promising option to achieve this goal (Gardner et al. Citation2009; Teklay et al. Citation2013). Such systems can help mitigate ecosystem degradation while providing food and economic opportunities to rural people (Brandt et al. Citation2012). Among these traditional agroforestry systems, home gardens (HGs) are receiving increasing attention.
An HG refers to a small fenced plot close to a farmer’s homestead, where annual, biennial and/or perennial cultivated species are grown in beds (Vogl & Vogl-Lukasser Citation2003; Galluzi et al. Citation2010). In many places across the world, home gardening is a traditional conservation system, where some key versatile plant species are grown by local farmers near their houses (Galluzi et al. Citation2010). Many studies have focused on HGs, investigating their potential to host biodiversity or to alleviate poverty (Reyes-Garcia et al. Citation2010; Fraser et al. Citation2011; Salako et al. Citation2014). The role of HGs as repositories of biological diversity has been acknowledged through a comprehensive and interdisciplinary investigation of their agro-biodiversity. However, it is not well known whether local people still have the necessary knowledge to preserve this system.
Traditional knowledge is important in the course of a broad range of questions related to the relationship between humans and nature (Souto & Ticktin Citation2012). Traditional knowledge is not a static entity for any set of skills in any culture, and people often change their techniques when easier methods become available. Different groups of people in various parts of the world perceive and interact with nature differently and have different traditions of environmental knowledge (Nakashima et al. Citation2012). In the tropics, small-scale farmers rely on HGs for invaluable livelihood services, such as food security, medicinal plants and market alternatives (Camou-Guerrero et al. Citation2008; Souto & Ticktin Citation2012). Customs, traditions and aesthetic preferences are instrumental in determining the overall aspect of the gardens (Smith et al. Citation2006; Brandt et al. Citation2012). Indeed, different crops or varieties are often maintained because of the significance of each in a family’s traditions or preferences. For instance, Italian gardeners insist that a species has a better taste than another or is more suited for preparing a certain time-honoured recipe or because they fulfil aesthetic requirements (Portis et al. Citation2004). Since different cultures live in different environment, one may expect their knowledge and use of HG species to differ.
Reyes-Garcia et al. (Citation2010) found that household members generally share HG responsibilities and that many HG characteristics vary with the distribution of gardening tasks. Men and women also differ in how they use garden products, with women favouring household consumption versus sale or gifting (Reyes-Garcia et al. Citation2010). Thus, the influence of gender on the choice of species maintained in a garden has been shown. Yet, a comprehensive investigation of how age categories of owners affect HG species choice is still lacking. A study investigating leafy vegetable management by farmers showed that the richness and composition of species managed by households are shaped by the age of household-heads and land ownership by women (Avohou et al. Citation2012). Leafy vegetables have also been illustrated as the most prominent species in HGs (Salako et al. Citation2014). Thus, gender and age could also shape the knowledge of HG species.
HGs are also found in West African countries, where some key species are kept by local farmers near their houses. The literature on their importance, diversity and management is however relatively poor. Much of the available literature focused on the biodiversity they host and their potential to conserve threatened species and crop wild relatives (Salako et al. Citation2014). Investigating traditional knowledge on HGs and how this knowledge shapes the choice of species across gender and age could help better illustrate how HGs could sustainably conserve biodiversity.
Benin, like most West African countries, is covered by three contrasting phytochorological zones: the Guineo-Congolean zone, the Sudano-Guinean transition zone and the Sudanian zone (White Citation1983; Akoègninou et al. Citation2006). The following sociolinguistic groups are the most prominent in these zones: Fon and Holli in the Guineo-Congolean zone; Anii, Itcha and Nago in the Sudano-Guinean zone; Ditamari, Gourmantche and Waama in the Sudanian zone (Assogbadjo et al. Citation2012; Avohou et al. Citation2012).
The present study aimed at testing the following hypotheses. Based on the evidence that choice of leafy vegetables depends on gender and age (see Avohou et al. Citation2012), we hypothesized that gender and age would shape the knowledge of HG species. Provided that different sociolinguistic groups and HG species are found in different places (Salako et al. Citation2014), we assumed that knowledge on HG species would vary across phytochorological zones. Since the exploitation of species depends on their availability (Gilmore et al. Citation2013), we hypothesized that use values (UVs) of HG species would be highly correlated to their ecological importance. As people tend to keep genetic resources in their vicinity mainly for food and medicinal uses (Achigan-Dako et al. Citation2011; Horn et al. Citation2012), we assumed that HG species would be mostly used for foods and medicinal purpose.
2. Materials and methods
2.1. Study area
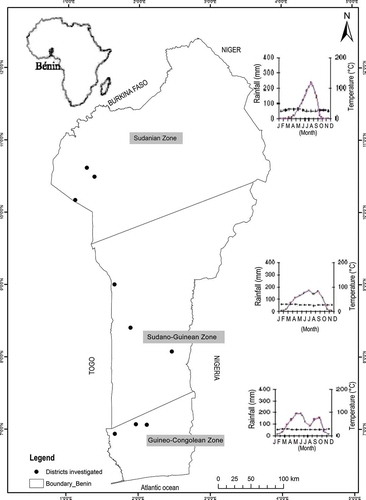
The study was conducted in Benin, a West African country located between 6°20’ and 12°25’N and 1° and 3°40’E (White Citation1983). Biogeographically, Benin is subdivided into three contrasting phytochorological zones (): the Guineo-Congolean zone, the Sudano-Guinean transition zone and the Sudanian zone (Akoègninou et al. Citation2006). Rainfall regime is bimodal in the Guineo-Congolean zone. Above this zone northwards, rainfall distribution becomes unimodal. Human activities have resulted in widespread degradation of vegetation. In the southern part, where the population density is high, vegetation is composed of fallows and small forest patches of less than 5 ha (Sinsin et al. Citation2004). Woodlands are prominent in the transition zone while trees and shrubs savannas represent the typical vegetation in the Sudanian zone.
2.2. Sampling and data collection
The data collection phase took place between June and September 2011. Before starting the survey, we obtained prior informed consent from local leaders and households in the target zones. Detailed information of the study and its importance were provided to local people. An exploratory survey was conducted in three districts randomly chosen in each phytochorological zone: Tanguieta, Toucoutouna and Boukombé in the Sudanian zone; Bassila, Bantè and Dassa in the Sudano-Guinean zone and Aplahoué, Agbangnizoun and Zogbodomey in the Guineo-Congolean zone. In each zone, 100 informants were asked if they possess a HG. The proportion (p) of positive answers was used to compute the numbers (n) of the individuals to be surveyed following Dagnelie (Citation1998):
is the value of the normal random variable corresponding to a probability value of 1 − α/2. For a probability value of 0.975 (or α = 0.05),
≈1.96; d is the margin error of the estimation of any parameter to be computed from the survey and a value of 8% was considered (Assogbadjo et al. Citation2011).
Values of n were rounded to 75 in the Sudanian zone and to 80 in the Guineo-Congolean and Sudano-Guinean zones. So, 80 owners of HGs were considered in the Guineo-Congolean and Sudano-Guinean zones while 75 were surveyed in the Sudanian zone. Each informant had only one HG. Therefore, 235 HGs were studied overall. In each sampled HG, the informant knowledge on the usage types and organs used was recorded. Data were collected using semi-structured interviews. Interviews were conducted orally with sometimes the assistance of a local translator.
2.3. Data analysis
2.3.1. Assessment of local knowledge on HG species across phytochorological zones
All data were arranged in a matrix including ethnobotanical and sociolinguistic importance data for species inside a HG as reported by each informant. A quantitative analysis including the computation of indexes (three different parameters, see ) was employed in order to measure (i) how diversified the use of HG species was among the informants across the phytochorological zones and (ii) how evenly different species contribute to their livelihood. These parameters also indicated how species are used and how knowledge about their uses is distributed within the community. The informants were grouped into classes by gender (men and women) and age (age <30 years; 30 <age <60 years; age >60 years) (Assogbadjo et al. Citation2008) to compare knowledge between men and women and to investigate how these uses are distributed according to age. Details on the indexes used and their application can be found in Byg and Balslev (Citation2001) and Monteiro et al. (Citation2006). Statistical tests were applied to evaluate gender- and age-biased differences. Since the collected data were not normally distributed (Ryan–Joiner test of normality), the non-parametric Kruskal–Wallis test was performed. In addition, a one-way analysis of variance (ANOVA) was run to reveal the possible differences among phytochorological zones regarding values of the indexes. All analyses were performed in SAS 9.2. (Citation2007)
Table 1. Measures of home garden species uses among informants in a phytochorological zone (adapted from Byg and Balslev Citation2001; Monteiro et al. Citation2006).
2.3.2. Assessment of sociolinguistic importance of HG species across phytochoria
Sociocultural characteristics of the informants (gender, age, sociolinguistic groups and level of education) were used to assess the variability of sociolinguistic characteristics among them. Similarities (with respect to HG species by sociolinguistic group) among the three phytochorological zones were assessed using the similarity index of Jaccard (Citation1912) (Equation (2)).
a is the number of shared or common species between phytochorological zones i and h (positive matches); b is the number of species which are exclusive to the phytochorological zone i (e.g. h absence mismatches); c is the number of species which are exclusive to the phytochorological zone h (e.g. i absence mismatches).
The total UV of each species in each phytochorological zone was computed using the following formula (Equation (3)):
Sij is the score given to a category of uses j by the informant i (Sij = 1 if the informant mentions a given category of uses j and Sij = 0 if the informant does not mention it). ncu is the total number of category of uses in a given zone and N the total number of informants in the phytochorological zone considered.
The importance value index (IVI) (Curtis and McIntosh Citation1951) was computed to measure the ecological importance of each HG species in each phytochorological zone. For a given species i of a given phytochorological zone, the IVI was computed as follows (Equation (4)):
RDi is the relative density of the species i:
, where p is the total number of species recorded in the phytochorological zone and Ni is the mean density of the species i in that phytochorological zone.
RFi is the relative frequency of the species i:
, with
, where fi is the frequency of the species i; ji is the number of HGs in which the species i was counted and k is the total number of HGs (k = 80 or 75).
RDo is the relative dominance of the species i:
, Doi is the mean dominance of the species i in the phytochorological zone.
, ai is the area covered by species i in a HGs of area A.
The IVI value is referred to as the importance percentage. It gives an overall estimation of the level of importance of a species in the HGs of a given phytochorological zone.
To investigate whether the most ecologically important species were also the most valued (used) species, the Pearson correlation between species ecological value importance (IVI) and their UV in each phytochorological zone was computed.
Finally, the most used organs across species and the specific uses were inferred from the database by phytochorological zone.
3. Results
3.1. Sociolinguistic characteristics of HG owner
HG owners sampled in each phytochorological zone allied to the three predominant sociolinguistic groups (see ). The main sociolinguistic groups were Fon, Itcha and Ditamari, respectively, in the Guineo-Congolean, the Sudano-Guinean and the Sudanian zones. In the three phytochoria, informants showed similar distributions of age, but most of them were between 30 and 60 years old (61%, n = 80 in the Guineo-Congolean zone; 58%, n = 80 in Sudano-Guinean zone and 75 %, n = 75 in Sudanian zone). There were more men with no education in the Guineo-Congolean zone than anywhere else, whereas the Sudanian zone showed the highest number of illiterate women ().
Table 2. Sociolinguistic characteristics of the informants.
3.2. Ethnobotanical knowledge on HG species across phytochorological zones
3.2.1. Diversity and distribution of uses among informants
Overall, total equitability values (IEs) were low (less than 0.5) for men and women within all phytochorological zones. There were significant differences neither in terms of diversity of uses (ID) (H = 0.01, p > 0.05; H = 0.9, p > 0.05 and H = 6.44, p > 0.05) nor in terms of IE (H = 0.01, p > 0.05; H = 0.07, p > 0.05 and H = 6.44, p > 0.05) between men and women in the Sudanian, the Sudano-Guinean and the Guineo-Congolean zones (see ). Knowledge about the use of the HG species appeared to be evenly distributed among informants across gender and age in all zones. However, the average total IEs were respectively 0.47, 0.79 and 0.68. This would suggest that, knowledge was widely shared among informants from the Sudanian and the Sudano-Guinean zones while a relatively small group of informants were more knowledgeable than the others in the Guineo-Congolean zone.
Table 3. Measures of the local use and knowledge on HG species across phytochorological zone.
3.2.2. Informant consensus value for types of use
Usage types of the species grown in HGs are represented by the consensus value for the types of use (CTU). Nine usage types were recorded for the Guineo-Congolean zone while six were encountered in the Sudano-Guinean zone and seven usage types in the Sudanian zone (). There were no little differences between usage types described by men and women within the same phytochorological zone. However, two of the usage types (cultural and fence) were specific to the Guineo-Congolean zone while one (spice) was specific to the Sudano-Guinean zone. No specific usage type had the greatest value simultaneously in all phytochorological zones. Medicinal use was the predominant usage type in the Guineo-Congolean and Sudanian zones (0.21 and 0.25, respectively), followed by trading and fodder uses (0.20 and 0.22). In the Sudano-Guinean zone, food use had the greatest value (0.26), followed by fodder and ornamental uses (0.22 and 0.21, respectively).
Table 4. Consensus value for the usage types among informants.
3.3. Sociolinguistic significance of HG species across phytochorological zones
The assessment of similarities among sociolinguistic groups with respect to hosted species () showed the highest similarities between Fon and Holli in the Guineo-Congolean zone, Anii and Nago in the Sudano-Guinean zone and Ditamari and Gourmantche in the Sudanian zone. They shared, respectively, 53, 55 and 51 species. Anii and Adja, Anii and Waama and Holli and Waama showed the highest dissimilarity with the lowest number of common species (14, 11 and 18 species, respectively). In addition, adult and old people knew more than young people in all phytochoria (). Women were more knowledgeable on food, trading, ornamental, dye and spice uses of the HG species than men in all phytochorological zones ().
Figure 2. Usage types per age category across phytochorological zones.
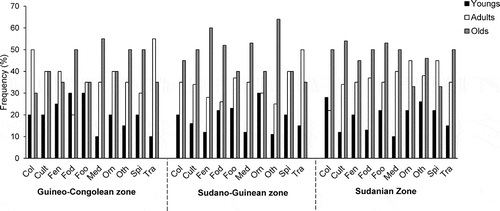
Figure 3. Usage types per gender across phytochorological zones.
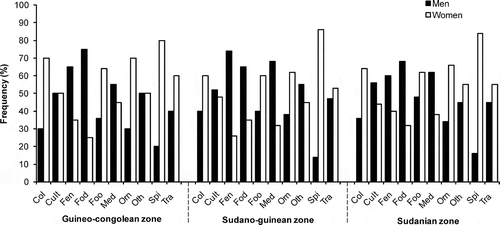
Table 5. Similarities index of Jaccard (with respect to hosted species by sociolinguistic groups) among the three phytochoria.
Significant (p < 0.05) positive (r > 0) correlations were found between species ecological importance and their use value, indicating that, in general, the most ecologically important species were also the most used species (). This relationship was stronger in the Sudanian zone than in the Sudano-Guinean zone and Guineo-Congolean zone ().
Figure 4. Relationships between species ecological importance value index (IVI) and their use value (UV) across phytochorological zones. (a) Guineo-Congolean zone; (b) Sudano-Guinean zone; (c) Sudanian zone.
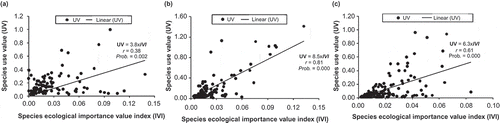
Nevertheless, because the relationships between ecological importance of species and their UV were not perfect (i.e. Pearson correlation = 1), the species ranking according to IVI values and UV values was not identical. shows the list of the top 20 species according to IVI and UV in each phytochorological zone.
Table 6. Top 20 species according to ecological importance value (IVI) and use values (UV) in each phytochorological zone.
3.4. Most used species and organs across phytochorological zone
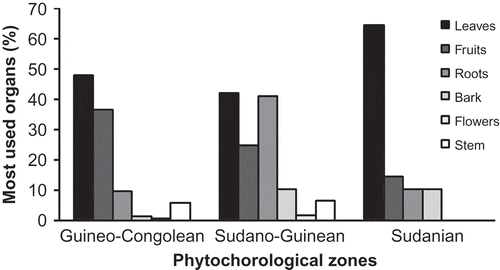
Six organs (leaves, fruits/seeds, roots/tubers, barks, stem and flowers) were exploited by the informants. All these organs were used in the Guineo-Congolean and Sudano-Guinean zones while only four organs were targeted in the Sudanian zone (). Leaves appeared to be the most used organs across phytochorological zones (47.89%, n = 96; 42.16%, n = 181 and 64.58%, n = 133, respectively, in the Guineo-Congolean, the Sudano-Guinean and the Sudanian zone).
Flowers of the HG species were not used by the informants in the Sudanian zone and also appeared to be the least used plant organ in the Guineo-Congolean and Sudano-Guinean zones.
4. Discussion
4.1. Knowledge similarity among informants
Our findings highlight knowledge sharing among people. There were no significant differences in citation in terms of either ID or IE between men and women within phytochorological zones. This would suggest that men and women are considered equally within target communities as far as sharing and transferring knowledge on HG species is considered (Brosi et al. Citation2007; Arya et al. Citation2010; McMillen Citation2012). Furthermore, no significant discrepancies were observed between age categories within phytochorological zone. The results do not support the expectation that knowledge on HG species will be influenced by gender and age. This departs from the observations of Avohou et al. (Citation2012) but is congruent with some other previous investigations (da Silva Sousa et al. Citation2012). Some reasons could explain our findings. First, most participants were between the age of 30 and 60 years. Thus, it is possible that among surveyed communities, the maximum of the knowledge on HG species is acquired before the age of 30, leading to little difference in knowledge among people aging 30 years and above. Furthermore, since most surveyed people were illiterate, they could have remained much close to their culture. As such, knowledge could have been transferred through generations easily and with little erosion. There were no differences between usage types of men and women within the same phytochorological zone. However, noticeable differences were observed among phytochorological zones with respect to usage types and species hosted in HGs. For instance, only HG owners from the Guineo-Congolean zone use species for cultural applications and fence. This confirms the hypothesis which assumes that traditional knowledge on HGs differs among phytochorological zones. Because of its proximity to the coast and the fact that it hosts the capital of the country, this phytochorological zone has experienced thorough human migrations (INSAE Citation2013). In addition, due to a weak enforcement of the national land policy, many difficulties are customarily encountered by people willing to acquire land in this zone. Therefore, people tend to keep species necessary for their cultural need in their HGs, as reported elsewhere (Poot-Pool et al. Citation2012; Abbasi et al. Citation2013), and use them to secure their land. The observed differences among phytochorological zones would suggest that resilience of local knowledge systems could be affected by cultural oscillations (Begossi et al. Citation2002).
4.2. Ecological importance and species shared among groups
The results support the expectation that the most ecologically important species are the most used in HGs. The results of the present study are consistent with those reported by Bye (Citation1995) and Maldonado et al. (Citation2013). Other studies demonstrated that versatile species are often culturally more important (Phillips & Gentry Citation1993; Haarmeyer et al. Citation2013). Local people could have found appropriate ways to guarantee the survival of these species as they play key role in their daily needs. The top 20 most ecologically important and used species differed across phytochorological zones, although some of these species were shared by two or the three phytochorological zones. The differences may be linked to specificity of the native vegetation of each zone, while the observed slight similarly may relate to the propagation of some species through human migrations (i.e. Assogbadjo et al. Citation2012).
From our findings, it also appeared that geographically closed sociolinguistic groups share similar species. In the Guineo-Congolean zone, Fon and Holli had the highest similarity value, whereas Anii and Nago and Ditamari and Gourmantché share more species respectively in the Sudano-Guinean zone and the Sudanian zone. This suggests that culture, cultural link and environment influence selection and resource use. Indeed, prominent sociolinguistic groups of each phytochorological zone share secular linkages. For instance, wedding arrangements are customary between Fon and Holli people. This could have facilitated knowledge sharing across these sociolinguistic groups.
4.3. Most used organ per species and prominent uses
Overall six organs are used by the informants. Our observations are congruent with the fact that people conserve plant species to make use of specific organs (Avohou et al. Citation2012). Food and medicinal uses were the most important across zones as hypothesized. The leaves appear to be the most used organs in all phytochorological zones. This is not surprising since many species are grown in the HGs for food needs. Plant leaves are a very important part of the diet in Benin (Dansi et al. Citation2008; Achigan-Dako et al. Citation2011). Local people may hold strong knowledge of harvest impacts, which prevents them from exploiting species beyond sustainable levels in their HGs. Leaf harvest is moderately harmful to species as long as it allows individual trees to rejuvenate. For example, the observation that pruning improves foliage quantity is consistent with the theory of compensatory growth of plants after defoliation (Bruna & Nogueira-Ribeiro Citation2005; Gaoue & Ticktin Citation2009). However, chronic defoliation may lead to a reallocation of nitrogen from leaves to perennial organs (Fornar & du Toit Citation2007). In contrary, flowers of the HG species are not used by the informants in the Sudanian zone and appear to be the least used plant organ in the Guineo-Congolean and Sudano-Guinean zones. The main reason could be either lack of knowledge on use of this plants part or well-known potential negative impact of its harvest on the reproduction of species.
4.4. Implications and limits
This study has some key implications. It reveals how HG owners conserve important species in their ecosystem for daily needs. Our findings support the point that HGs can critically contribute to conservation of biodiversity while sustaining food and medicine supply in local communities. In addition, there were no discrimination in knowledge of HG species with regard to gender and age. This could ensure long-term conservation of the body of knowledge on these species. Despite their contribution to conservation, nutrition and medicine, HGs are unlikely to be preserved in the context of modern mutations (urbanization, poor traditional knowledge transfer) and modern cropping systems if they are not actively mainstreamed in formal conservation and production strategies and policies. They also need to be integrated into environmental education programs targeting the young generation. Sustainability of HG will also depend upon other factors including pro-local people value chain development and capacity building in local women for creating micro-industries to process and package extracted products.
The present study has some limitations. When matching the data collected through individual interviews with the botanical identification, we were obliged in some areas to operate with a local translator. This carried the usual risk of poor or inaccurate translation and enumerator error. Nevertheless, the wide geographical and sociolinguistic coverage of the study represents an important step towards understanding the role and usage of HGs. It also provides a solid foundation for further research.
5. Conclusion
This study showcases knowledge of local people on HGs and provides basic information for future conservation actions. Knowledge on HG species was not influenced by gender and age at phytochorological level. Our results would suggest knowledge, use types and HG species to vary across the surveyed zones. The ecologically most important species were the most used species, and the leaves were the most used organs. Further research could explore how knowledge on HG is transferred across generation. The determinants of rights and access to HG products could also be explored to enhance our understanding of traditional conservation and production systems. These will provide insights into how to account for HG in modern systems.
Acknowledgements
We are very grateful to all the participants and especially local informants for their help during our fieldwork. We also thank anonymous reviewers for their relevant contribution to the improvement of this manuscript. B. Fandohan received a postdoctoral research fellow grant from the Chinese Academy of Sciences [Grant No 2012Y1ZA0009] which provided him an excellent working environment to contribute to the manuscript.
References
- Abbasi AM, Khan MA, Shah MH, Shah MM, Pervez A, Ahmad M. 2013. Ethnobotanical appraisal and cultural values of medicinally important wild edible vegetables of Lesser Himalayas-Pakistan. J Ethnobiology Ethnomedicine. 9:66. doi:10.1186/1746-4269-9-66
- Achigan-Dako EG, N’Danikou S, Assogba-Komlan F, Ambrose-Oji B, Ahanchede A, Pasquini MW. 2011. Diversity, geographical, and consumption patterns of traditional vegetables in sociolinguistic communities in Benin: implications for domestication and utilization. Econ Bot. 65:129–145. doi:10.1007/s12231-011-9153-4
- Akoègninou A, van Der Burg WJ, van Der Maesen LJG, Adjakidjè V, Essou JP, Sinsin B, Yèdomonhan H. 2006. Flore analytique du Bénin. Cotonou & Wageningen: Backuys Publishers.
- Arya D, Tewari A, Shah S. 2010. Erosion of biodiversity knowledge between younger and older generation regarding plant identification and their uses in oak and pine dominated zone of Garhwal Himalaya. New York Sci J. 3:108–111.
- Assogbadjo AE, Fandohan B, Glèlè Kakaï R, Kyndt T, Hardy OJ, Gheysen G, Sinsin B. 2012. Genetic evidence of the contribution of ethnic migrations to the propagation and persistence of the rare and declining scrambling shrub Caesalpinia bonduc L. Hum Ecol. 40:117–128. doi:10.1007/s10745-011-9442-7
- Assogbadjo AE, Glèlè Kakaï R, Adjallala FH, Azihou AF, Vodouhê GF, Kyndt T, Codjia JTC. 2011. Ethnic differences in use value and use patterns of the threatened multipurpose scrambling shrub (Caesalpinia bonduc L.) in Benin. J Med Plant Res. 5:1549–1557.
- Assogbadjo AE, Glèlè Kakaï R, Chadare FJ, Thomson L, Kyndt T, Sinsin B, Van Damme P. 2008. Folk classification, perception, and preferences of baobab products in West Africa: consequences for species conservation and improvement. Econ Bot. 62:74–84. doi:10.1007/s12231-007-9003-6
- Avohou HT, Vodouhe SR, Dansi A, Bellon M, Kpeki B. 2012. Ethnobotanical factors influencing the use and management of wild edible plants in agricultural environments in Benin. Ethnobotany Res Appl. 10:571–592.
- Begossi A, Hanazaki N, Tamashiro JY. 2002. Medicinal plants in the Atlantic Forest (Brasil): knowledge, use and conservation. Hum Ecol. 30:281–299. doi:10.1023/A:1016564217719
- Brandt R, Zimmermann H, Hensen I, Castro JCM, Rist S. 2012. Agroforestry species of the Bolivian Andes: an integrated assessment of ecological, economic and socio-cultural plant values. Agroforest Syst. 86:1–16. doi:10.1007/s10457-012-9503-y
- Brosi BJ, Balick MJ, Wolkow R, Lee R, Kostka M, Raynor W, Gallen R, Raynor A, Raynor P, Ling DL. 2007. Cultural erosion and biodiversity: canoe-making knowledge in Pohnpei, Micronesia. Conservation Biol. 21:875–879. doi:10.1111/j.1523-1739.2007.00654.x
- Bruna EM, Nogueira-Ribeiro MBN. 2005. The compensatory responses of an understory herb to experimental damage are habitat dependent. Am J Bot. 92:2101–2106. doi:10.3732/ajb.92.12.2101
- Bye R. 1995. Ethnobotany of the Mexican dry tropical forests. In: Bullock SH, Mooney HA. Medina E, editors. Seasonally dry tropical forests. 1st ed. Cambridge: Cambridge University Press; p. 423–438.
- Byg A, Balslev H. 2001. Diversity and use of palms in Zahamena, Eastern Madagascar. Biodivers Conserv. 10:951–970. doi:10.1023/A:1016640713643
- Camou-Guerrero A, Reyes-García V, Martínez-Ramos M, Casas A. 2008. Knowledge and use value of plant species in a rarámuri community: a gender perspective for conservation. Hum Ecol. 36:259–272. doi:10.1007/s10745-007-9152-3
- Curtis JT, McIntosh RP. 1951. An upland forest continuum in the prairie forest border region of Wisconsin. Ecology. 32:476–496. doi:10.2307/1931725
- Dagnelie P. 1998. Statistiques théoriques et appliquées. Brussels: De Boeck et Larcier.
- Dansi A, Adjatin A, Vodouhè R, Adéoti K, Adoukonou-Sagbadja H, Faladé V, Yedomonhan H, Akoègninou A, Akpagana K. 2008. Biodiversité des légumes feuilles traditionnels consommés au Bénin. Bibliothèque nationale, Bénin; 183p.
- da Silva Sousa R, Hanazaki N, Lopes JB, de Barros RFM. 2012. Are gender and age important in understanding the distribution of local botanical knowledge in fishing communities of the Parnaiba Delta environmental protection areas? Ethnobotany Res & Appl. 10:551–559.
- Fornar DA, du Toit JT. 2007. Browsing lawns? Responses ofacacia nigrescensto ungulate browsing in an African Savanna. Ecology. 88:200–209. doi:10.1890/0012-9658(2007)88[200:BLROAN]2.0.CO;2
- Fraser JA, Junqueira AB, Clement CR. 2011. Homegardens on amazonian dark earths, non anthropogenic upland, and floodplain soils along the Brazilian middle Madeira river exhibit diverging agrobiodiversity. Econ Bot. 65:1–12. doi:10.1007/s12231-010-9143-y
- Galluzzi G, Eyzaguirre P, Negri V. 2010. Home gardens: neglected hotspots of agro-biodiversity and cultural diversity. Biodivers Conserv. 19:3635–3654. doi:10.1007/s10531-010-9919-5
- Gaoue OG, Ticktin T. 2009. Fulani knowledge of the ecological impacts of Khaya senegalensis (Meliaceae) foliage harvest in Benin and its implications for sustainable harvest. Econ Bot. 63:256–270. doi:10.1007/s12231-009-9091-6
- Gardner TA, Barlow J, Chazdon R, Ewers RM, Harvey CA, Peres CA, Sodhi NS. 2009. Prospects for tropical forest biodiversity in a human-modified world. Ecol Lett. 12:561–582. doi:10.1111/j.1461-0248.2009.01294.x
- Gilmore MP, Endress BA, Horn CM. 2013. The socio-cultural importance of Mauritia flexuosa palm swamps (aguajales) and implications for multi-use management in two Maijuna communities of the Peruvian Amazon. J Ethnobiology Ethnomedicine. 9:29. doi:10.1186/1746-4269-9-29
- Haarmeyer DH, Schumann K, Bernhardt-Römermann M, Wittig R, Thiombiano A, Hahn K. 2013. Human impact on population structure and fruit production of the socio-economically important tree Lannea microcarpa in Burkina Faso. Agroforest Syst. 87:1363–1375. doi:10.1007/s10457-013-9644-7
- Horn CM, Gilmore MP, Endress BA. 2012. Ecological and socio-economic factors influencing aguaje (Mauritia flexuosa) resource management in two indigenous communities in the Peruvian Amazon. For Ecol Manag. 267:93–103. doi:10.1016/j.foreco.2011.11.040
- INSAE. 2013. Recensement Général de la Population et de l’Habitat (RGPH4): Résultat provisoires; 35p.
- Jaccard P. 1912. The distribution of the flora in the alpine zone. New Phytol. 11:35–50.
- Laurance WF. 2007. Have we overstated the tropical biodiversity crisis? Trends Ecol Evol. 22:65–70. doi:10.1016/j.tree.2006.09.014
- Maldonado B, Caballero J, Delgado-Salinas A, Lira R. 2013. Relationship between use value and ecological importance of floristic resources of seasonally dry tropical forest in the Balsas River Basin, México. Econ Bot. 67:17–29. doi:10.1007/s12231-013-9222-y
- McMillen H. 2012. Ethnobotanical knowledge transmission and evolution: the case of medicinal markets in Tanga, Tanzania. Econ Bot. 66:121–131. doi:10.1007/s12231-012-9201-8
- Monteiro JM, Albuquerque UP, Lins Neto EMF, Araújo EL, Amorim ELC. 2006. Use patterns and knowledge of medicinal species among two rural communities in Brazil’s semi–arid northeastern region. J Ethnopharmacology. 105:173–186. doi:10.1016/j.jep.2005.10.016
- Nakashima DJ, Galloway McLean K, Thulstrup HD, Ramos Castillo A, Rubis JT. 2012. Weathering uncertainty: traditional knowledge for climate change assessment and adaptation. Paris and Darwin: UNESCO and UNU.
- Phillips O, Gentry AH. 1993. The useful plants of Tambopata. Peru: I. Statistical hypotheses tests with a new quantitative technique. Econ Bot. 47:15–32. doi:10.1007/BF02862203
- Poot-Pool WS, Van Der Wal H, Flores-Guido S, Pat-Fernández JM, Esparza-Olguín L. 2012. Economic stratification differentiates home gardens in the Maya village of Pomuch, Mexico. Econ Bot. 66:264–275. doi:10.1007/s12231-012-9206-3
- Portis E, Acquadro A, Comino C, Lanteri S. 2004. Effect of farmers’ seed selection on genetic variation of a landrace population of pepper (Capsicum annuum L.) grown in North-West Italy. Genet Resour Crop Evol. 51:581–590.
- Reyes-García V, Vila S, Aceituno-Mata L, Calvet-Mir L, Garnatje T, Jesch A, Lastra JJ, Parada M, Rigat M, Vallès J, Pardo-De-Santayana M. 2010. Gendered homegardens: a study in three mountain areas of the Iberian Peninsula. Econ Bot. 64:235–247. doi:10.1007/s12231-010-9124-1
- Salako VK, Fandohan B, Kassa B, Assogbadjo AE, Idohou AFR, Gbedomon RC, Chakeredza S, Dulllo ME, Glele Kakai R. 2014. Home gardens: an assessment of their biodiversity and potential contribution to conservation of threatened species and crop wild relatives in Benin. Genet Resour Crop Evol. 61:313–330.
- SAS. 2007. SAS/STAT user’s guide, Version 9.2. Cary (NC): SAS Institute Inc.
- Sinsin B, Eyog Matig O, Assogbadjo AE, Gaoué OG, Sinadouwirou T. 2004. Dendrometric characteristics as indicators of pressure of Afzelia africana Sm. dynamic changes in trees found in different climatic zones of Benin. Biodivers Conserv. 13:1555–1570. doi:10.1023/B:BIOC.0000021328.56517.46
- Smith RM, Thompson K, Hodgson JG, Warren PH, Gaston KJ. 2006. Urban domestic gardens (IX): composition and richness of the vascular plant flora, and implications for native biodiversity. Biol Conservation. 129:312–322. doi:10.1016/j.biocon.2005.10.045
- Souto T, Ticktin T. 2012. Understanding interrelationships among predictors (age, gender, and origin) of local ecological knowledge. Econ Bot. 66:149–164. doi:10.1007/s12231-012-9194-3
- Teklay A, Abera B, Giday M. 2013. An ethnobotanical study of medicinal plants used in Kilte Awulaelo District, Tigray Region of Ethiopia. J Ethnobiology Ethnomedicine. 9:65. doi:10.1186/1746-4269-9-65
- Vogl CR, Vogl-Lukasser B. 2003. Tradition, dynamics and sustainability of plant species composition and management in homegardens on organic and non-organic small scale farms in alpine Eastern Tyrol, Austria. Biol Agric Hortic. 21:349–366. doi:10.1080/01448765.2003.9755278
- White F. 1983. The vegetation of Africa, a descriptive memoire to accompany the UNESCO/AETFAT/UNSO.UNESCO. Nat Resour Res. 20:1–356.