Abstract
Wetlands play an important role in carbon cycling. Perturbation of these ecosystems by human activities causes changes in the soil carbon storage and carbon gaseous emissions. These changes might have important repercussions for global warming. The aim of this study was to investigate whether the conversion of freshwater forested wetlands (FW) to flooded grasslands (FGL) has affected soil carbon cycling. Soil carbon pools and soil organic carbon (SOC) fractions (water-soluble carbon (WSC), hot-water-soluble carbon (HWSC), and HCl/HF soluble carbon (HCl/HF-SC)) were compared between FW and FGL. Additionally, the seasonal dynamic of methane (CH4) and carbon dioxide (CO2) fluxes were monitored in both ecosystems located in the coastal plain of Veracruz State Mexico. In FW, soil organic matter (SOM) concentrations were significantly (P ≤ 0.05) higher than FGL. Soil bulk density (BD) was slightly higher in FGL than FW but it was not significantly different (P ≥ 0.05). The average of WSC and HWSC in FW were not significantly (P ≤ 0.05) different. Total carbon pools (44 cm deep) were not significantly different (P = 0.735). During the dry season, CO2 fluxes (26.38 ± 4.45 g m−2 d−1) in FGL were significantly higher (P = 0.023) than in FW (14.36 ± 5.77 g m−2 d−1). During the rainy and windy seasons, both CH4 and CO2 fluxes were significantly higher (P = 0.000 and P = 0.001) in FGL compared with FW. It was concluded that converting FW to FGL causes loss of SOC and increases carbon gaseous fluxes.
1. Introduction
Wetlands are the interface between terrestrial and aquatic components of the landscape (Mitsch & Gosselink Citation2007). They are widely recognized for providing several ecosystem services such as flood control, aquifer recharge, and nutrient removal (Hansson et al. Citation2005). However, their contribution to the global cycling of atmospheric gases and their important role as carbon sinks is less recognized. Wetlands, despite occupying relatively small areas of the earth’s surface (2–6%), contain a large proportion of the world’s carbon stored in terrestrial soil reservoirs (Whiting & Chanton Citation2001; Mitra et al. Citation2005; Lal Citation2008). Soil organic carbon (SOC) pool is complex; based on resistance to mineralization, it has been divided into labile, intermediate, and recalcitrant organic carbon pools (Cheng et al. Citation2007). Soil labile and intermediate C pools have a mean residence time of years to several decades while recalcitrant C pools have a mean residence time of hundreds to thousands years (Zou et al. Citation2005; Cheng et al. Citation2007; Silveira et al. Citation2008). Labile and intermediate fractions of organic carbon can respond rapidly to environmental change; therefore, they are more sensitive indicators of the effects of land use than total SOC (von Lützow et al. Citation2002; Zhang et al. Citation2007; He et al. Citation2008).
In wetlands ecosystems, flooding conditions not only allow accumulating significant amounts of carbon, but also promote the production and release of methane (CH4), a powerful greenhouse gas (GHG). Wetlands are a major source of CH4 in the atmosphere (Whalen Citation2005) contributing 23–40% of the annual terrestrial CH4 emissions and comprising 77–83% of natural sources (IPCC Citation2001). In addition to CH4, carbon dioxide (CO2) is produced in wetlands soils under both aerobic and anaerobic conditions (Smith et al. Citation2003; Coles & Yavitt Citation2004; Elberling et al. Citation2011). CH4 emissions to the atmosphere are an environmental concern because global warming potential (GWP) for CH4 is 25 times than GWP for CO2 (Solomon et al. Citation2007). Therefore, small increase of CH4 concentration in the atmosphere might have an important impact on global warming.
Human activities can alter the carbon stocks in wetlands and the exchange of GHG with the atmosphere (Roulet Citation2000). For example, it has been reported that livestock grazing, significantly reduced the above-ground biomass, net primary productivity, and enhanced CH4 emissions in wetlands on the Qinghai-Tibetan plateau in China (Hirota et al. Citation2005). According to Solomon et al. (Citation2007), global increases in CO2 concentration are due primarily to land-use change and fossil-fuel consumption. Based on measured CO2 fluxes using satellite observations and emission inventories in China, Wang et al. (Citation2011) reported almost five times more CO2 emissions in slope grasslands compared with swamp soils, considering similar areas. However, despite the potential importance, few studies are available to assess the effects of livestock grazing on the GHGs emissions in tropical wetland ecosystems.
In Mexico, wetlands are located mainly on the coast (Contreras-Espinosa & Warner Citation2004), and these areas are among the most transformed ecosystems in the country (Moreno-Casasola Citation2008). Coastal wetlands in the Mexican state of Veracruz are threatened mainly by cattle ranching, petrochemical activities, and urbanization. Because of this, most freshwater wetlands show significant changes in their ecology, such as invasion of exotic species, siltation, pollution, and changes in their hydrology (López-Rosas et al. Citation2006; Moreno-Casasola et al. Citation2009).
In order to assess the impact of human activity on the carbon sequestration service that wetlands provide; the objective of this study was to compare soil organic matter (SOM), soil carbon pools, and the seasonal carbon gaseous fluxes (CO2 and CH4) in both coastal freshwater swamps and areas that have been converted to flooded grasslands (FGL). We hypothesize that carbon pools in flooded grasslands will be lower than in forested wetlands (FW) due to the decrease of carbon inputs and higher mineralization rates. The opposite will occur with carbon gaseous fluxes, that is, higher fluxes in FGL due to higher carbon mineralization.
2. Materials and methods
2.1. Study site
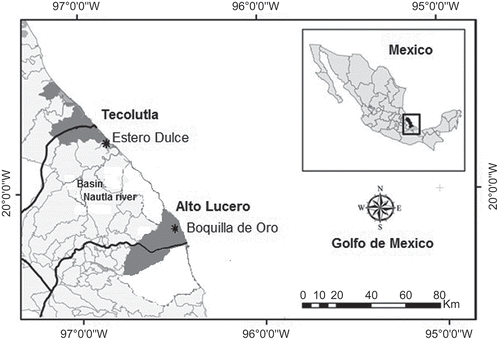
The study was carried out in two freshwater FW and two adjacent flooded grasslands, located on the coastal plain of the Gulf of Mexico in the state of Veracruz. The study sites located from north to south were Estero Dulce (20º17ʹ53ʺN, 96º52ʹ19ʺW) and Boquilla de Oro (19º49ʹ47ʺN, 96º26ʹ59ʺW) (). Flooded grasslands were established in FW 15–20 years ago. Natural wetlands have been transformed to support cattle ranching as economic activity. The transformation included cutting native wetland trees to allow the growth of native and introduced flood tolerant grasses. Drainage of these areas has not been performed. However, the introduction of exotic grasses causes that flooded grasslands experience shorter hydroperiods than natural wetlands (López-Rosas et al. Citation2006). The studied FW are fenced to exclude grazing, while flooded grasslands experience from moderate (1.8 animals per ha) to heavy (3 animals per ha) grazing (Girma et al. Citation2007). These areas are grazed from March to early August, and afterward animals are moved to uplands because of the rainy season. Flooded grasslands are not fertilized with chemicals; and tillage is not performed in these sites. A detailed description of the study sites is shown in .
Table 1. Characteristics of the studied wetlands in the coastal plain of Veracruz, Mexico.
The climate of the coastal plain of the Gulf of Mexico has three seasons: rainy season (July to October), windy season (November to February) that has cold fronts with strong winds and rain, and the dry season (March to June). The annual precipitation mean fluctuates between 1200 and 1650 mm. The mean annual temperature varies between 17°C and 37°C. A detailed description of the study sites is shown in .
2.2. Soil sampling
In each type of wetland (FW or FGL), three random sampling plots (1 m2) were established. In these plots, four soil cores (0.48 m deep × 0.05 m diameter) were taken using a Russian peat borer. This borer has thin sharp-edged walls, providing a core without compaction, distortion, or disturbance. Each core was sectioned off with a blade at intervals of 4 cm. One of the four cores in each sampling plot was used for analysis of bulk density (BD). The soil layers were placed in aluminum pans having a predetermined dry weight, and transported to the laboratory where they were stored below 4ºC until they were dried in an oven.
Composite samples were made with three wet soil samples taken from the same depth in each sampling plot. Each of the mixed wet composted samples were packed in containers and stored below 4ºC until they were dried at room temperature and analyzed for carbon content.
2.3. Soil analysis
In the laboratory, wet soil composite samples were mixed again to reduce soil heterogeneity and visible residues of vegetation were removed. Composite samples were dried at room temperature, pulverized, and sieved (2 mm). For quantifying the organic matter, approximately 2 g of dried soil samples were pretreated with 10 M HCl to avoid possible carbonate interferences (Hernandez & Mitsch Citation2007). After this, SOM was quantified by loss on ignition at 450ºC for 4 hours (Craft et al. Citation1988; Bernal & Mitsch Citation2008).
BD was obtained by drying a known volume of sediment at 105ºC (19.64 cm−3); then, it was weighed until a constant weight was reached. The values obtained were used in the formula BD (g cm−3) = Mass/volume.
For the purpose of carbon pool calculations, the organic carbon percentage was calculated as a portion of organic matter, using Van Bemmelen’s factor (0.58) which has been used for several wetland soils including these tropical wetlands (Wang et al. Citation2003; Hernandez & Mitsch Citation2007; Marín-Muñiz et al. Citation2014). The carbon pool was calculated in kg C m−2, according to the following equation (Moreno et al. Citation2002; Cerón-Bretón et al. Citation2011):
Total carbon storage to a 44 cm depth was calculated by adding the carbon stored in each one of the soil layers (Bernal & Mitsch Citation2012).
2.4. Soluble organic carbon fractions
Extractions of water-soluble carbon (WSC), hot-water-soluble carbon (HWSC), and HCl/HF soluble carbon (HCl/HF-SC) were carried out according to Hernandez and Mitsch (Citation2007). Soluble organic carbon concentrations in each of the extracts were analyzed in a Total Organic Carbon analyzer (Torch, Teledyne Tekmar).
2.5. Gas measurements and flux calculations
Fluxes of CH4 were measured in situ once every 2 months starting in August 2010 until February 2012, and the CO2 from February 2011 to February 2012, using the closed chamber technique (Altor & Mitsch Citation2006; Hernandez & Mitsch Citation2006; Nahlik & Mitsch Citation2010). The closed chamber consisted of two parts: a base and a removable cap, each made of polyvinyl chloride (PVC) pipe (15 cm diameter). The bases were permanently installed in the swamps in February 2010 and in June 2010 in the flooded grasslands (four chambers in each type of wetland at the two sites, n = 8 for each type of wetland). The bases were 30 cm high and inserted approximately 5 cm into the wetland soils; the base had an open bottom and a collar, 5 cm from the top. The removable cap includes a gray butyl sampling port and an alcohol-type thermometer in the top. Every time gas fluxes were measured, the cover was put on the base collar, and water was added to ensure a gas-tight seal between the base and the cap. Chambers were closed, and every 5 minutes internal gas samples were taken for the next 45 minutes and the internal temperature registered. Gas samples (25 ml) were taken using 60-ml propylene syringes (TERUMO) having a one-way stopcock (Lieur). Gas samples were injected through rubber septa into pre-evacuated 20-ml glass vials. Septa were boiled before use for 30 minutes to eliminate potential gas leaking. All samples were taken between 10:00 h and 16:00 h (local time) and were analyzed within 72 h after collection.
Gas concentrations were analyzed on a Perkin Elmer Clarus 500 gas chromatograph equipped with a flame ionization detector (FID) for CH4 and also equipped with a methanizer to detect levels of CO2. For sample separation, a stainless steel column packed with Poropak Q (80/100 mesh), 6 ft in length, and 2-mm ID was used. The temperatures for oven, injector, and detector were at 40, 95, and 200°C for CH4, and 60, 80, and 350°C for CO2, respectively. Nitrogen (7 ml min−1) was used as a carrier gas. CH4 and CO2 were quantified separately. Matheson gas standards balanced with N2 were used to perform calibration curves. All the individual analyzed gas values (ppm CH4 and CO2) were corrected using the ideal gases law (pv = nRT) according to the formula (Duan et al. Citation2009; Nahlik & Mitsch Citation2010):
The normalized gas concentrations were used to calculate gas flux rates (Hernandez & Mitsch Citation2006) according to the following equation:
For each chamber measurement, gas sample concentration values were plotted versus sample time. Microsoft ExcelTM was used to calculate linear regressions on each flux rate. Results were included only if R2 was greater than 0.85 (Altor & Mitsch Citation2006).
2.6. Conversion to CO2-equivalents
A GWP factor of 25 for CH4 (Solomon et al. Citation2007) was used to convert CH4 emissions to CO2-equivalents for comparing their contributions to the global radiative impact.
2.7. Physical and chemical analysis
2.7.1. Water level
When surface water was present, the water level was recorded using a measuring stick. When no surface water was present, a sensor connected to a multimeter (Steren) was used to detect the water level in four monitoring wells, located in each type of wetland at the three study sites. Monitoring wells were made from PVC pipe (13 mm ID), 3 m in length (inserted 1.5 m in the soil), installed in each studied wetland (Infante et al. Citation2012).
2.7.2. Redox potential
Soil redox potential (Eh) was measured within a 30 cm diameter around each chamber, at a soil depth between 0 and 5 cm using a platinum rode and one calomel reference electrode (Corning 476,340), both connected to a digital multimeter. Platinum electrodes were calibrated in situ before every monitoring with quinhydrone (Aldrich) 50 mgl−1 in a pH 4.0 buffer solution (Bohn Citation1971).
2.8. Statistical analysis
All statistical analyses were performed with SPSS version 18 for Windows. A Kolmogorov–Smirnov test was used to check normality. Physical–chemical variables and carbon in soil data fit normal distributions. One-way analysis of variance (ANOVA) was used to find out whether the type of ecosystem had an effect on the average of soil carbon concentration, BD, WSC, HWSC, and HCl/HF-SC. These parameters were compared at the same depth between FW and FGL using a t-test for paired samples. To detect differences in carbon pools between two types of ecosystems, a t-test was used. Two-way ANOVA with Tukey comparison was used to determine whether climatic season and ecosystem types had an effect in Eh, water levels, and soil temperature. Data for GHGs failed to meet criteria for normal distribution (P < 0.001); therefore, non-parametric statistical tests were used such as Kruskal-Wallis and Mann-Whitney to compare CH4 and CO2 emissions between the type of ecosystem and climatic season. A non-parametric t-test for paired samples was used to compare the emissions of CH4 or CO2 between FW and FGL during the same months of sampling. A p-value = 0.05 was used to reveal the statistical significance.
3. Results
3.1. Soil organic matter concentration, soil bulk density, and carbon pools
The SOM concentration decreased in the studied FW soils according to the depth from 382 to 300 g kg−1 (), while in the FGL, SOC decreased from 172 to 97 g kg−1. When SOM concentration was compared between FW and FGL at the same depth, they were significantly higher (P ≤ 0.05) in FW, for all depths. Average SOM in FW (284.25 ± 15.2 g kg−1) was also significantly higher (P = 0.001) than average SOM in FGL (134.41 ± 6.33 g kg−1).
Soil BD in FGL varied from 0.44 to 0.77 g cm−3, increasing with depth, while BD in the FW varied from 0.41 to 0.57 g cm−3; and BD did not increase with depth. Although we observed a trend of higher BD in FGL, when it was compared to FW, no significant differences were found (P ≥ 0.05) neither at any depth, nor in the average between FGL (0.63 ± 0.02 g cm−3) and FW (0.49 ± 0.02 g cm−3).
When the total carbon pools were calculated to 44 cm, the results were 30.63 ± 5.23 kg C m−2 for FGL and 28.14 ± 4.87 kg C m−2 for FW, showing no significant difference (P = 0.735).
3.2. Water-soluble C (WSC), hot-water-soluble C (HWSC), and HCl/HF soluble C
In FGL soils, WSC decreased with depth from 0.70 to 0.27 g C kg −1, while in FW soils, WSC increased after 17 cm from 0.50 to 0.90 g C kg −1. In the top layer, WSC was slightly higher in FGL than FW but not significantly different (P = 0.094). Average WSC in the whole soil profile of FGL (0.74 ± 0.08 g C kg −1) was also not significantly different (P = 0.094) from FW (0.54 ± 0.09 g C kg −1) ().
Figure 2. Profile of soil organic matter (a) and bulk density (b) in the FW (gray lines) and FGL (black lines) soils. Each point in the graph is the mean of six composite cores at 4 cm of depth. Horizontal bars represent standard errors.
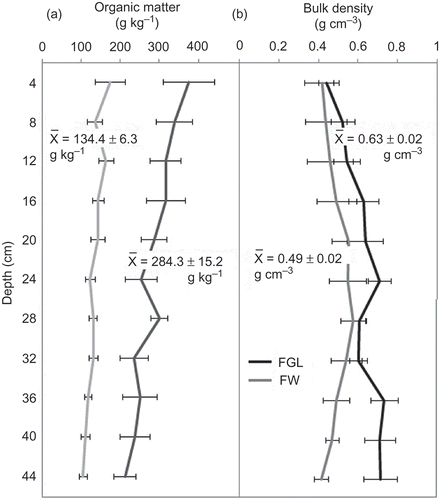
Figure 3. Carbon fractions in the FW wetland (gray lines) and FGL (in black lines) soils. Horizontal bars represent standard errors.
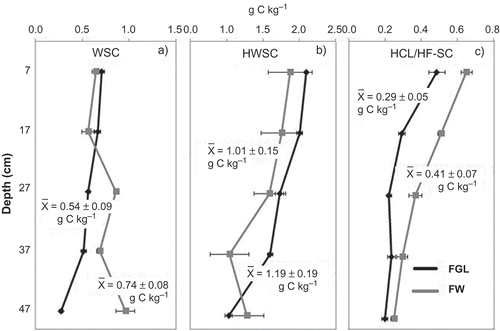
HWSC decreased with depth in both ecosystems; FGL showed higher HWSC than FW in the whole profile except in the deepest layer. The average of HWSC in the whole profile of FGL was 1.19 ± 0.19 g C kg −1, while in FW it was 1.01 ± 0.15 g C kg −1 being not significantly different (P = 0.309).
Organic carbon extracted by HCl/HF decreased significantly with depth in both types of wetlands (P < 0.05). HCl/HF-SC in FW was higher than in FGL in the entire profile. Average of HCl/HF-SC in the entire profile of FGL (0.29 ± 0.05 g C kg −1) was significantly lower (P = 0.006) than in FW (0.41 ± 0.07 g C kg −1).
3.3. Water level, redox potential, and soil temperature dynamics
Water level in the studied wetlands ranged from −70 to 10 cm () in FW and from −45 to 10 cm in FGL, without significant differences (; P = 0.998). When water level values were averaged per each season, significant differences were observed (P = 0.021) with higher values for the rainy (4.86 ± 2.30 and 1.18 ± 3.89 cm in FGL and FW, respectively) and the windy seasons (−4.30 ± 3.99 and 3.53 ± 1.79 cm in FGL and FW, respectively), compared with water levels observed during the dry season (−33.75 ± 10.78 cm in FGL and −37.99 ± 24.24 in FW).
Figure 4. Water levels (a–b), redox potential (c–d), and soil temperature (e–f), in FGL (○) and FW (●) measured bimonthly (left) and averaged by climatic season (right); white bars are flooded grasslands, gray bars are FW. Vertical lines on bars and circles values represent standard error, and different letters indicate significant difference.
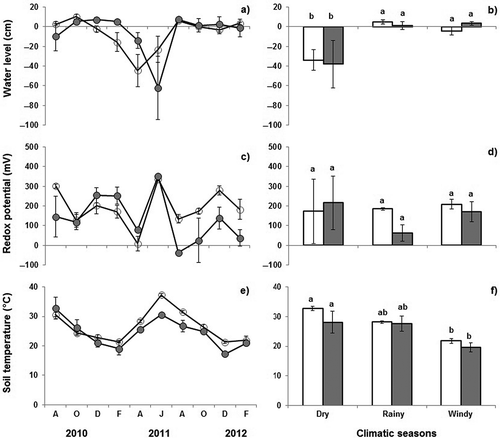
Eh values in the soil oscillated from −37 to 350 mV in FW and from 0 to 337 mV in FGL (), and no significant differences were observed (; P = 0.613). When Eh values were averaged by season, Eh average in FW decreased from dry (216.26 ± 136.83 mV) to rainy (63.07 ± 42.16 mV) and windy–rainy seasons (170.98 ± 52.13 mV), while in the FGL, Eh values did not decrease (173.03 ± 164.09, 185.12 ± 6.17 and 209.22 ± 24.45 mV, respectively). In both types of wetlands Eh values were not significantly different among the seasons (P = 0.695).
Soil temperatures in the freshwater wetlands ranged from 19 to 37°C in both types of wetlands (e–f). Soil temperatures in FGL were 1–2°C higher than in FW during the study period with few exceptions. However, no significant differences were found between the two types of wetlands (P = 0.271). Seasons had a significant effect (P = 0.020) on soil temperature with lower temperatures during the windy season (19–21ºC) as compared to rainy (27–28ºC) and dry seasons (28–32ºC).
3.4. Methane and carbon dioxide emissions
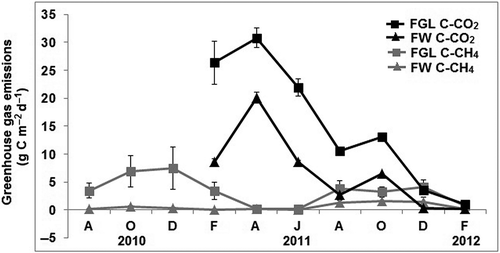
Both CH4 and CO2 fluxes were significantly influenced by the season (P = 0.0001, P = 0.0001) with higher CH4 emissions during the rainy (August–October) and windy (December–February) seasons compared with the dry season (April–June) (). For CO2, the opposite occurred; high fluxes were observed during the months of dry season and low fluxes during rainy and windy seasons. Additionally, both gas emissions were significantly affected by the type of ecosystem. During the dry season, average emissions of CO2 (26.38 ± 4.45 g m−2 d−1) were significantly higher (P = 0.023) in FGL compared with FW (14.36 ± 5.77 g m−2 d−1), while CH4 emissions were low and similar in both types of ecosystems (150.14 ± 75.22 and 145.68 ± 30.47 mg m−2 d−1 in FW and FGL, respectively; P = 0.224). During the rainy season, significantly higher CH4 (P = 0.000) and CO2 emissions (P = 0.001) were found in FGL (4349.03 ± 853.46mg m−2 d−1 and 11.82 ± 1.24 g m−2 d−1, respectively) than in FW (869.01 ± 314.27mg m−2 d−1 and 4.59 ± 1.87 g m−2 d−1, respectively). Also in the windy season, significantly higher CH4 (P = 0.001) and CO2 (P = 0.014) emissions were found in FGL (3912.01 ± 1378.30mg m−2 d−1 and 10.3 ± 8.07 g m−2 d−1, respectively) than in FW (481.66 ± 324.92mg m−2 d−1 and 2.98 ± 2.77 g m−2 d−1, respectively).
3.5. Global warming potential
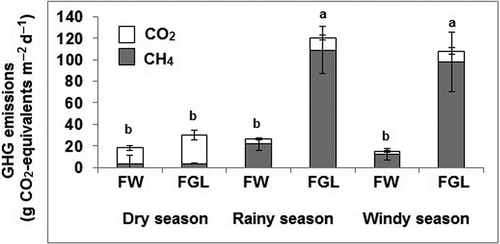
We converted seasonal average emission of CH4 into CO2-equivalents to compare its cumulative contributions to global radiative balance (). During the dry season the main component of GHG fluxes was CO2 flux for both types of ecosystems. For rainy and windy seasons the main component of GHG fluxes was CH4 for both types of ecosystems. FGL had statistically higher (P ≤ 0.05) radiative balance in all rainy and windy seasons (120 and 108 g m−2 d−1, respectively) than FW (26 and 15 g m−2 d−1, respectively). On the other hand, during dry season the radiative balance was not significantly (0.985) different between FGL (30 g m−2 d−1) and FW (18 g m−2 d−1).
Figure 6. Total emissions of methane and carbon dioxide expressed as CO2-equivalents according to GWP (CH4:25 and CO2:1; Solomon et al. Citation2007).
4. Discussion
In the areas converted to FGL, SOC was only 47% of the observed in FW. This is probably due to the decrease in the carbon inputs to the soils and changes in hydrology. Litterfall in these tropical FW has been described as high as 9–15 ton ha per year−1 (Infante et al. Citation2012) and a large part of the carbon remains in the flooded soils. However, parts of these FW were transformed to FGL at least 15 years ago (). The transformation included clearing trees that allowed the growth of native and exotic flood-tolerant grasses to support cattle grazing. Channelization to drain FGL has not been performed in these sites; however, changes in vegetation cover have induced shorter hydroperiods in FGL than in FW (). Even though water levels were not significantly different, FW were flooded during windy–rainy season while the FGL experienced some dry periods. Shorter hydroperiods might stimulate high C mineralization as it was confirmed by observed high carbon fluxes in this study. The loss of organic carbon in degraded wetlands due to changing land use has been described in other tropical wetlands. Sigua et al. (Citation2009) found that natural wetlands in South Florida had 180 g C kg−1 while altered pastures (wetlands converted to pastures for 45 years) had only 5.4 g C kg−1 and after 6 years of wetland restoration SOC increased to 10.7 g C kg−1. On the other hand, in temperate wetlands, Shang et al. (Citation2013) described the term ‘grasslandification’ as the process where wetlands are drained and converted to pasture with dominant plants of grasses. They described that grasslandification for 50 years in Chinese alpine wetlands had reduced vegetation quality and increased the degree of drought and reduced the C, N, and P content of soils. Their observations are similar to the findings in transformed FW to FGL for at least 15 years in Veracruz, Mexico. There are few studies of soil carbon in Mexican wetlands (Campos et al. Citation2011; Marín-Muñiz et al. Citation2014), and this study is the first report comparing SOC in disturbed wetlands. In other Mexican ecosystems such as the upland tropical forest ‘La selva Lacandona’, land-use change to pasture has decreased soil carbon pools by approximately 50% (De Jong et al. Citation2000). In the Brazilian Amazonia, conversion of forest to pastures over several decades also caused a decrease of SOC (Fearnside & Barbosa Citation1998).
Despite of low organic carbon content in FGL, carbon pools were similar to FW. This happens because carbon pools were calculated using SOC content and BD, and the latter were higher in FGL than the observed in FW. Higher BD in FGL might be caused by compaction due to cattle hoof action and shorter hydroperiods (Howe et al. Citation2009; Teuber et al. Citation2013). Similar results were obtained in Zoige alpine wetlands in China; degraded wetlands (flooded meadows) had higher carbon pools than pristine FW despite the latter had higher carbon concentrations but lower BD (Huo et al. Citation2013).
The simplest methods to measure available C substrates or labile carbon in both agricultural and wetland soils are WSC and HWSC. Land-use changes cause soil degradation, and sometimes these carbon fractions are more sensitive than total organic carbon to such degradation (Ghani et al. Citation2003; Dodla et al. Citation2012; Uchida et al. Citation2012). We measured WSC and HWSC to investigate whether the transformation of FW to FGL has affected the carbon cycling. However, no clear differences were observed. A trend of higher WSC in the top layer of FGL was found but in deeper layers FW had higher concentrations of WSC. The high concentration of WSC in deeper layers in wetland soil is due to leaching of WSC from the top layer due to flooding conditions (Dodla et al. Citation2012). In this study, FGL showed shorter hydroperiods which might have limited the leaching of WSC. Although not statistically different, HWSC also showed a trend of higher concentrations in the whole profile of FGL than in FW. HWSC consists of a labile pool of SOM which includes microbial biomass as well as soluble soil carbohydrates and amines (Ghani et al. Citation2003). The fact that this type of carbon was higher in FGL and might also due to shorter hydroperiods that enhance less reduced conditions and higher activity of aerobic microorganisms that hydrolyze SOM releasing HWSC. We found that HWSC were three to four times higher than WSC, and this occurs because hot water dissolves more complex carbon compounds such as microbial biomass C, root exudates, amino acids, and C bound to soil enzymes. The results of this study are similar to the values found in coastal wetland soils of the Mississippi River deltaic plain where HWSC was 4–13 higher than WSC (Dodla et al. Citation2012). Also, Ghani et al. (Citation2003) found in uplands soils that WSC constituted only approximately 3–6% of HWSC and this type of carbon has been correlated positively with soil respiration (Uchida et al. Citation2012). In this study, the results showed a higher content of HWSC in FGL than in FW, and the former has the higher carbon gaseous fluxes. On the other hand, the carbon fraction – HCl/HF-SC was significantly higher in FW than in FGL. This fraction represents carbon closely associated with soil minerals (Al and Fe) and it is considered less available for microorganisms than WSC and HWSC (Stevenson Citation1982; Nguyen Citation2000). Other studies in upland soils have indicated that Al- and Fe-bound organic matter fractions were subjected to depletion during the harvesting and pasturing (Murata et al. Citation1995).
We found a strong seasonal influence on carbon gaseous fluxes in both FW and FGL. When water tables dropped during the dry season both types of wetlands showed low CH4 emissions and high CO2 emissions. In contrast, when soils were flooded (rainy and windy seasons), higher CH4 emissions and lower CO2 emissions were observed. This finding agrees with several studies that have described that CH4 emissions are favored when soils are flooded (Altor & Mitsch Citation2006; Nahlik & Mitsch Citation2010; Morse et al. Citation2012). However, despite both types of wetlands showed the same seasonal trend in carbon gaseous fluxes, the magnitude of CH4 and CO2 fluxes in FGL was higher than in FW. This agrees with results found in disturbed wetlands. Hirota et al. (Citation2005) found that livestock grazing stimulated CH4 emissions from alpine wetlands in Tibet, compared with wetlands without grazing. Also, Oates et al. (Citation2008) observed greater CH4 emissions under grazing conditions in spring-fed wetlands of a California oak savanna. In this study, the explanation for higher CH4 emissions in FGL compared with FW might be due to several factors, including the physical soil disturbances by hoof action of cattle, changes in vegetation cover, different hydroperiods, and changes in the soil’s chemistry due to deposition of cattle excreta on soils during grazing.
Cattle disturb soil porosity and break up the stratification of surface and sub-surface water, which contain O2 and methanotrophic bacteria. It has been described that livestock grazing and agricultural practices may have an effect on the soil’s ability to consume CH4 by altering the distribution of pore space, thereby reducing CH4 diffusion rates through the soil profile, and slowing transport to sites of CH4 oxidizing bacteria (Boeckx & Cleemput Citation1997). Compaction leads to a reduction of aerobic microsites and consequently the decrease of CH4 oxidation by oxidizing bacteria (Sitaula et al. Citation2000). Our study found greater compaction in FGL than in FW and is potentially a major influence on reduction of CH4 consumption. Besides these physical factors, the change from native wetland trees to grasses in FGL might also have decreased CH4 oxidation, because wetland plants supply oxygen to the rhizosphere, which enhances areas of potential CH4 oxidation in the soil (Brix et al. Citation1996; Frenzel & Rudolph Citation1998).
Hydrology is one of the factors controlling Eh which influences biogeochemical process in wetlands soils (Mitsch & Gosselink Citation2007). The sediment Ehs in the studied sites were moderately reducing (−100 to 250 mV) (Bohn Citation1971). Methanogenesis is such an obligate anaerobic process that it would not be expected to occur in sediments until the Eh is at least −150 mV (Wang et al. Citation1993; Kludze & DeLaune Citation1994). However, authors such as Huang et al. (Citation2005) and Wang et al. (Citation1993) also have found that methanogenic activities are still active at values close to −100 mV. Chapelle et al. (Citation1996) described that although Eh measurements are easy to do in the field, they do not always indicate with accuracy the anoxic biogeochemical process in the soils; and this is one possible explanation to the results in this study.
Paradoxically, higher CH4 emissions were found in FGL, which have shorter hydroperiods and although not statistically a distinguishable high Eh compared with FW. This might be due to more wet and dry cycles that FGL experience in comparison with FW, especially during rainy and windy seasons (). Badiou et al. (Citation2011) described that CH4 emissions in the restored wetlands of the Canadian prairie pothole region increased dramatically just as the wetland basin was becoming dry. The same trend was observed by Pennock et al. (Citation2010) in an ephemeral wetland in Saskatchewan, Canada. Badiou et al. (Citation2011) described that the transition period causes the release of a massive pulse of CH4 due to the fact that the wetland sediment is still saturated and anoxic favoring methanogenesis. Additionally, the sediment surface would warm dramatically as water levels decrease, thereby increasing rates of methanogenesis which are known to increase with soil temperature (Bartlett & Harriss Citation1993). Lastly, the decrease in water column depth above the sediment–water interface would facilitate the transfer of CH4 from the sediments to the atmosphere while reducing the potential for CH4 consumption within the water column. The more frequent wet and dry cycles in FGL might also be responsible for higher CO2 emissions than FW during the rainy and windy seasons. Wilson et al. (Citation2011) found that carbon mineralization and therefore CO2 emissions increased significantly after flooding occurred in riparian wetland soils.
Soil fertilization caused by cattle excreta deposition on FGL soil is another factor that might explain the higher CO2 and CH4 emissions in these sites. Studies in uplands soils have shown that manure addition to soils increase CO2 emissions because it promotes the bioavailable pool of organic carbon (Zhai et al. Citation2011). In this study, we found a trend of higher WSC in the upper layers of FGL soils compared with FW. In rice paddies, it has been described that nitrogen fertilization increases CH4 emissions because it enhances soil carbon inputs decreases CH4 oxidation due to substrate switch from CH4 to ammonia by methanotrophs (Banger et al. Citation2012). Recently, it has been uncovered that ammonia inhibits the expression of particulate CH4 monooxygenase genes in aerobic methanotrophs (Dam et al. Citation2014).
Regarding GWP, we found in both types of wetlands that CO2 was the main gas contributing to radiative balance during the dry season, while during the rainy and windy seasons, it was CH4. These results are similar to those found in restored FW in the southeastern US coastal plain by Morse et al. (Citation2012). In dry wetland areas, they found CO2 as the main contributor to the radiative balance, while in flooded wetland areas, the main contributor was CH4. In this study, during dry season the sum of GWP was twice higher in FGL than FW, while during rainy and windy seasons, it was six and five times higher, respectively. Hirota et al. (Citation2005) described similar trends in disturbed alpine wetlands in Tibet. The sum of GWP, estimated from CO2 and CH4 fluxes, was 6–11-fold higher under grazing conditions than under non-grazing conditions.
5. Conclusions
Soil carbon concentration decreased in areas converted from FW to FGL due to decreases in carbon inputs, physical disturbances, and shorter hydroperiods which enhance higher CO2 and CH4 emissions. However, carbon pools did not decrease in FGL due to an increase in soil BD. Carbon sequestration in wetlands soils is an important environmental service that is negatively affected by changing land use of FW in the flood plains of Veracruz, Mexico. Considering that high CO2 and CH4 emissions increase global temperature; if large areas of FW wetlands are transformed to FGL, then the impacts of these land-use changes might have repercussions for global warming. Therefore, better policies and law enforcement for freshwater wetland protection, conservation, and restoration are needed in Mexico to avoid this positive feedback to global warming.
Acknowledgements
Funding for this work was provided by the Mexican National Council for Science and Technology – CONACYT – through Sector fund CONACYT-SEMARNAT Grant # 107887 and the Basic Science Grant # 081942. The authors thank Alejandro Hernández, Monserrat Vidal, J. Alejandro Marín, and Carmelo Maximiliano for their help in the field work. We are also grateful to the local guides who accompanied us throughout the field work: Tomas León Rodríguez and Eduardo Lauranchet.
References
- Altor A, Mitsch WJ. 2006. Methane flux from created riparian marshes: relationship to intermittent versus continuous inundation and emergent macrophytes. Ecol Eng. 28:224–234. doi:10.1016/j.ecoleng.2006.06.006
- Badiou P, McDougal R, Pennock D, Clark B. 2011. Greenhouse gas emissions and carbon sequestration potential in restored wetlands of the Canadian prairie pothole region. Wetlands Ecol Manage. 19:237–256. doi:10.1007/s11273-011-9214-6
- Banger K, Tian H, Lu C. 2012. Do nitrogen fertilizers stimulate or inhibit methane emissions from rice fields? Global Change Biol. 18:3259–3267. doi:10.1111/j.1365-2486.2012.02762.x
- Bartlett KB, Harriss RC. 1993. Review and assessment of methane emissions from wetlands. Chemosphere. 26:261–320. doi:10.1016/0045-6535(93)90427-7
- Bernal B, Mitsch WJ. 2008. A comparison of soil carbon pools and profiles in wetlands in Costa Rica and Ohio. Ecol Eng. 34:311–323. doi:10.1016/j.ecoleng.2008.09.005
- Bernal B, Mitsch WJ. 2012. Comparing carbon sequestration in temperate freshwater wetland communities. Global Change Biol. 18:1636–1647. doi:10.1111/j.1365-2486.2011.02619.x
- Boeckx P, Cleemput O. 1997. Methane emission from a freshwater wetland in Belgium. Soil Sci Soc Am J. 61:1250–1256. doi:10.2136/sssaj1997.03615995006100040035x
- Bohn HL. 1971. Redox potentials. Soil Sci. 112:39–45. doi:10.1097/00010694-197107000-00007
- Brix H, Sorrell BK, Schierup H-H. 1996. Gas fluxes achieved by in situ convective flow in Phragmites Australis. Aquat Bot. 54:151–163. doi:10.1016/0304-3770(96)01042-X
- Campos A, Hernández ME, Moreno-Casasola P, Cejudo E, Robledo A, Infante D. 2011. Soil water retention and carbon pools in tropical forested wetlands and marshes of the Gulf of Mexico. Hydrolog Sci J. 56:1388–1406. doi:10.1080/02626667.2011.629786
- Cerón-Bretón JG, Cerón-Bretón RM, Rangel-Marrón M, Muriel-García M, Cordoba-Quiroz AV, Estrella-Cahuich A. 2011. Determination of carbon sequestration rate in soil of a mangrove forest in Campeche, Mexico. Int J Energ Environ. 3:328–336.
- Chapelle F, Haack S, Adriens PA, Henry M, Bradley A. 1996. Comparison of Eh and H2 measurements for delineating redox processes in a contaminated aquifer. Environ Sci Technol. 30:3565–3569. doi:10.1021/es960249+
- Cheng L, Leavitt SW, Kimball BA, Pinter Jr PJ, Ottman MJ, Matthias A, Wall GW, Brooks T, Williams DG, Thompson TL. 2007. Dynamics of labile and recalcitrant soil carbon pools in a sorghum free-air CO2 enrichment agroecosystem. Soil Biol Biochem. 39:2250–2263. doi:10.1016/j.soilbio.2007.03.031
- Coles JRP, Yavitt JB. 2004. Linking below ground carbon allocation to anaerobic CH4 and CO2 production in a forested peatland, New York state. Geomicrobiol J. 21:445–455. doi:10.1080/01490450490505419
- Contreras-Espinosa F, Warner BG. 2004. Ecosystem characteristics and management considerations for coastal wetlands in Mexico. Hydrobiologia. 511:233–245. doi:10.1023/B:HYDR.0000014097.74263.54
- Craft CB, Broome SW, Seneca ED. 1988. Nitrogen, phosphorus and organic carbon pools in natural and transplanted marsh soils. Estuaries. 11:272–280. doi:10.2307/1352014
- Dam B, Dam S, Kim Y, Liesack W. 2014. Ammonium induces differential expression of methane and nitrogen metabolism-related genes in Methylocystis sp. strain SC2. Environ Microbiol. doi:10.1111/1462-2920.12367
- De Jong BHJ, Ochoa-Gaona S, Castillo-Santiago MA, Ramirez-Marcial N. 2000. Carbon flux and patterns of land-use/ land-cover change in the Selva Lacandona, Mexico. AMBIO. 29(8):504–511.
- Dodla SK, Wang JJ, DeLaune R. 2012. Characterization of labile organic carbon in coastal wetland soils of the Mississippi River deltaic plain: relationships to carbon functionalities. Sci Total Environ. 435–436:151–158. doi:10.1016/j.scitotenv.2012.06.090
- Duan X, Wang X, Ouyang Z. 2009. Influence of common reed (Phragmites australis) on CH4 production and transport in wetlands: results from single-plant laboratory experiments. Water Air Soil Poll. 197:185–191. doi:10.1007/s11270-008-9802-0
- Elberling B, Askaer L, Jørgensen C, Joensen H, Kühl M, Glud R, Lauritsen F. 2011. Linking soil O2, CO2, and CH4 concentrations in a wetland soil: implication for CO2 and CH4 fluxes. Environ Sci Technol. 45:3393–3399. doi:10.1021/es103540k
- Fearnside PM, Barbosa RI. 1998. Soil carbon changes from conversion of forest to pasture in Brazilian Amazonia. Forest Ecol Manag. 108:147–166. doi:10.1016/S0378-1127(98)00222-9
- Fenchel T, Blackburn TH. 1979. Bacteria and mineral cycling. London: Academic Press.
- Frenzel P, Rudolph J. 1998. Methane emission from a wetland plant: the role of CH4 oxidation in eriophorum. Plant Soil. 202:27–32. doi:10.1023/A:1004348929219
- Ghani A, Dexter M, Perrott KW. 2003. Hot-water extractable carbon in soils: a sensitive measurement for determining impacts of fertilisation, grazing and cultivation. Soil Biol Biochem. 35:1231–1243. doi:10.1016/S0038-0717(03)00186-X
- Girma T, Don P, Asfaw H, Yilma J, Wagnew A. 2007. Effect of livestock grazing on soil micro-organisms of cracking and self-mulching vertisol. Ethiop Vet J. 11:141–150.
- Hansson L, Bronmark C, Anders Nilsson P, Abjornsson K. 2005. Conflicting demands on wetland ecosystem services: nutrient retention, biodiversity or both? Freshwater Biol. 50:705–714. doi:10.1111/j.1365-2427.2005.01352.x
- He Y, Xu ZH, Chen CR, Burton J, Ma Q, Ge Y, Xu JM. 2008. Using light fraction and macroaggregate associated organic matters as early indicators for management-induced changes in soil chemical and biological properties in adjacent native and plantation forests of subtropical Australia. Geoderma. 147:116–125. doi:10.1016/j.geoderma.2008.08.002
- Hernandez ME, Mitsch WJ. 2006. Influence of hydrologic pulses, flooding frequency, and vegetation on nitrous oxide emissions from created riparian marshes. Wetlands. 26:862–877. doi:10.1672/0277-5212(2006)26[862:IOHPFF]2.0.CO;2
- Hernandez ME, Mitsch WJ. 2007. Denitrification potential and organic matter as affected by vegetation community, wetland age, and plant introduction in created wetlands. J Environ Qual. 36:333–342. doi:10.2134/jeq2006.0139
- Hirota M, Tang Y, Hu Q, Kato T, Hirata S, Mo W, Cao G, Mariko S. 2005. The potential importance of grazing to the fluxes of carbon dioxide and methane in an alpine wetland on the Qinghai-Tibetan plateau. Atmos Environ. 39:5255–5259. doi:10.1016/j.atmosenv.2005.05.036
- Howe AJ, Rodríguez JF, Saco PM. 2009. Surface evolution and carbon sequestration in disturbed and undisturbed wetland soils of the Hunter estuary, southeast Australia. Estuar Coast Shelf S. 84:75–83. doi:10.1016/j.ecss.2009.06.006
- Huang GH, Li XZ, Hu YM, Shi Y, Xiao DN. 2005. Methane (CH4) emission from a natural wetland of northern China. J Environ Sci Heal. 40:1227–1238. doi:10.1081/ESE-200055666
- Huo L, Chen Z, Zou Y, Lu X, Guo J, Tang X. 2013. Effect of Zoige alpine wetland degradation on the density and fractions of soil organic carbon. Ecol Eng. 51:287–295. doi:10.1016/j.ecoleng.2012.12.020
- Infante D, Moreno-Casasola P, Madero-Vega C. 2012. Litterfall of tropical forested wetlands of Veracruz in the coastal floodplains of the Gulf of Mexico. Aquat Bot. 98:1–11. doi:10.1016/j.aquabot.2011.11.006
- IPCC. 2001. Climate change 2001: synthesis report. In: Watson, R.T. and The Core Writing Team editors. A contribution of working groups I, II, and III to the third assessment report of the intergovernmental panel on climate change. Cambridge (UK) and New York (NY): Cambridge University Press.
- Kludze HK, DeLaune RD. 1994. Methane emissions and growth of Spartina patens in response to soil redox intensity. Soil Sci Soc Am J. 58:1838–1845. doi:10.2136/sssaj1994.03615995005800060037x
- Lal R. 2008. Carbon sequestration. Philos Trans R Soc B: Biol Sci. 363:815–830. doi:10.1098/rstb.2007.2185
- López-Rosas H, Moreno-Casasola P, Mendelssohn I. 2006. Effects of experimental disturbances on a tropical freshwater marsh invaded by the African grass Echinochloa pyramidalis. Wetlands. 26:593–604. doi:10.1672/0277-5212(2006)26[593:EOEDOA]2.0.CO;2
- Marín-Muñiz JL, Hernández ME, Moreno-Casasola P. 2014. Comparing soil carbon sequestration in coastal freshwater wetlands with various geomorphic features and plant communities in Veracruz, Mexico. Plant Soil. doi:10.1007/s11104-013-2011-7
- Mitra SR, Wassmann R, Vlek P. 2005. An appraisal of global wetland area and its organic carbon stock. Curr Sci. 88:25–35.
- Mitsch WJ, Gosselink JG. 2007. Wetlands. 4th ed. New York (NY): John Wiley and Sons.
- Moreno E, Guerrero A, Gutiérrez M, Ortiz C, Palma D. 2002. Los manglares de Tabasco, una reserva natural de carbono. Madera Bosques. 8:115–128.
- Moreno-Casasola P. 2008. Los humedales en México, tendencias y oportunidades. Cuadernos Biodiversidad. 28:10–18.
- Moreno-Casasola P, López-Rosas H, Infante D, Peralta LA, Travieso-Bello AC, Warner BG. 2009. Environmental and anthropogenic factors associated with coastal wetland differentiation in La Mancha, Veracruz, Mexico. Plant Ecol. 200:37–52. doi:10.1007/s11258-008-9400-7
- Morse JL, Ardón M, Bernhardt ES. 2012. Greenhouse gas fluxes in southeastern U.S. coastal plain wetlands under contrasting land uses. Ecol Appl. 22:264–280. doi:10.1890/11-0527.1
- Murata T, Nguyen ML, Goh KM. 1995. The effects of long-term superphosphate application on soil organic matter content and composition from an intensively managed New Zealand pasture. Eur J Soil Sci. 46:257–264. doi:10.1111/j.1365-2389.1995.tb01834.x
- Nahlik AM, Mitsch WJ. 2010. Methane emissions from created riverine wetlands. Wetlands. 30:783–793. doi:10.1007/s13157-010-0038-6
- Nguyen LM. 2000. Organic matter composition, microbial biomass and microbial activity in gravel-bed constructed wetlands treating farm dairy wastewaters. Ecol Eng. 16:199–221. doi:10.1016/S0925-8574(00)00044-6
- Oates L, Jackson R, Allen-Diaz B. 2008. Grazing removal decreases the magnitude of methane and the variability of nitrous oxide emissions from spring-fed wetlands of a California oak savanna. Wetlands Ecol Manage. 16:395–404. doi:10.1007/s11273-007-9076-0
- Pennock D, Yates T, Bedard-Haughn A, Phipps K, Farrell R, McDougal R. 2010. Landscape control on N2O and CH4 emissions from freshwater mineral soil wetlands of the Canadian prairie Photole region. Geoderma. 155:308–319. doi:10.1016/j.geoderma.2009.12.015
- Peters V, Conrad R. 1995. Methanogenic and other strictly anaerobic bacteria in desert soils and other oxic soils. Appl Envir Microbiol. 61:1673–1676.
- Roulet NT. 2000. Peatlands, carbon storage, greenhouse gases, and the kyoto protocol: prospects and significance for Canada. Wetlands. 20:605–615. doi:10.1672/0277-5212(2000)020[0605:PCSGGA]2.0.CO;2
- Shang ZH, Feng QS, Wu GL, Ren GH, Long RJ. 2013. Grasslandification has significant impacts on soil carbon, nitrogen and phosphorus of alpine wetlands on the Tibetan plateau. Ecol Eng. 58:170–179. doi:10.1016/j.ecoleng.2013.06.035
- Sigua G, Coleman S, Albano J. 2009. Beef cattle pasture to wetland reconversion: impact on soil organic carbon and phosphorus dynamics. Ecol Eng. 35:1231–1236. doi:10.1016/j.ecoleng.2009.05.004
- Silveira ML, Comerford NM, Reddy KR, Cooper WT, El-Rifai H. 2008. Characterization of soil organic carbon pools by acid hydrolysis. Geoderma. 144:405–414. doi:10.1016/j.geoderma.2008.01.002
- Sitaula BK, Hansen S, Sitaula J, Bakken LR. 2000. Methane oxidation potentials and fluxes in agricultural soil: effects of fertilization and soil compaction. Biogeochemistry. 48:323–339. doi:10.1023/A:1006262404600
- Smith K, Ball T, Conen F, Dobbie K, Massheder J, Rey A. 2003. Exchange of greenhouse gases between soil and atmosphere: interactions of soil physical factors and biological processes. Eur J Soil Sci. 54:779–791. doi:10.1046/j.1351-0754.2003.0567.x
- Solomon S, Qin D, Manning M, Alley RB, Berntsen T, Bindoff NL, Chen Z, Chidthaisong A, Gregory JM, Hegerl GC, et al. 2007. Technical summary. In: Solomon S, Qin D, Manning M, Chen Z, Marquis M, Averyt KB, Tignor M, Miller HL, editors. Climate change 2007: the physical science basis. Contribution of working group I to the fourth assessment report of the intergovernmental panel on climate change. Cambridge (UK): Cambridge University Press.
- Stevenson FJ. 1982. Humus chemistry. New York (NY): Wiley.
- Teuber LM, Hölzel N, Fraser LH. 2013. Livestock grazing in intermountain depressional wetlands – effects on plant strategies, soil characteristics and biomass. Agr Ecosyst Environ. 175:21–28. doi:10.1016/j.agee.2013.04.017
- Uchida Y, Nishimura S, Akiyama H. 2012. The relationship of water-soluble carbon and hot-water-soluble carbon with soil respiration in agricultural fields. Agr Ecosyst Environ. 156:116–122. doi:10.1016/j.agee.2012.05.012
- von Lützow M, Leifeld J, Kainz M, Kögel-Knabner I, Munch JC. 2002. Indications for soil organic matter quality in soils under different management. Geoderma. 105:243–258. doi:10.1016/S0016-7061(01)00106-9
- Wang K, Jiang H, Zhang X, Zhou G. 2011. Analysis of spatial and temporal variations of carbon dioxide over China using SCIAMACHY satellite observations during 2003–2005. Int J Rem Sens. 32:815–832. doi:10.1080/01431161.2010.517805
- Wang S, Tian H, Liu J, Pan S. 2003. Pattern and change of soil organic carbon storage in China: 1960–1980s. Tellus B. 55:416–427. doi:10.1034/j.1600-0889.2003.00039.x
- Wang Z, Delaune RD, Patrick Jr WH, Masscheleyn PH. 1993. Soil redox and pH effects on methane production in a flooded rice soil. Soil Sci Soc Am J. 57:382–385. doi:10.2136/sssaj1993.03615995005700020016x
- Whalen SC. 2005. Biogeochemistry of methane exchange between natural wetlands and the atmosphere. Environ Eng Sci. 22:73–94. doi:10.1089/ees.2005.22.73
- Whiting GJ, Chanton JP. 2001. Greenhouse carbon balance of wetlands: methane emission versus carbon sequestration. Tellus B. 53:521–528. doi:10.1034/j.1600-0889.2001.530501.x
- Wilson JS, Baldwin DS, Rees GN, Wilson BP. 2011. The effects of short term inundation on carbon dynamics, microbial community structure and microbial activity in floodplain soil. River Res Appl. 27:213–225. doi:10.1002/rra.1352
- Zhai LM, Liu HB, Zhang J, Huang JZ, Wang BR. 2011. Long-term application of organic manure and mineral fertilizer on N2O and CO2 emissions in a red soil from cultivated maize-wheat rotation in China. Agr Sci China. 10:1748–1757. doi:10.1016/S1671-2927(11)60174-0
- Zhang JB, Song CC, Yang WY. 2007. Land use effects on the distribution of labile organic carbon fractions through soil profiles. Soil Sci Soc Am J. 70:660–667.
- Zou XM, Ruan HH, Fu Y, Yang XD, Sha LQ. 2005. Estimating soil labile organic carbon and potential turnover rates using a sequential fumigation–incubation procedure. Soil Biol Biochem. 37:1923–1928. doi:10.1016/j.soilbio.2005.02.028