Abstract
We characterized and compared the variability and spatial patterns of denitrification and soil properties in two natural and two restored depressional wetlands in northern Indiana, USA. Soil properties included soil moisture content, bulk density, plant-available N (NO3-N, NH4-N), soil organic carbon (C), total nitrogen (N), and C:N ratio. Restored wetlands had greater denitrification and higher spatial variability than natural wetlands. Further, restored wetlands had greater bulk density and C:N ratios. In contrast, natural wetlands had greater soil moisture, plant-available N, organic C, and total N. Similar to denitrification, soil moisture, bulk density, NO3-N, total N, and C:N ratios had greater variance in restored wetlands than in natural wetlands. Denitrification and several soil properties exhibited positive global spatial autocorrelation, though trends differed between the individual wetlands for soil properties. Variogram analysis suggested little spatial structure in variables at the chosen observational scale. Denitrification hot spots were detected in natural and restored wetlands, though these hot spots did not correspond to hot spots of any of the other soil variables. Overall, spatial patterns of denitrification and soil properties differed between natural and restored wetlands and should be considered when assessing effectiveness of restored wetlands at providing ecosystem services, such as N removal and C storage.
Introduction
Wetlands exist at the interface of upland and aquatic systems and provide many ecosystem services such as carbon (C) sequestration, nutrient (nitrogen [N] and phosphorus [P]) retention and transformation, water storage, and biodiversity support (Mitsch et al. Citation2001; Zedler Citation2003; Fennessy & Craft Citation2011). Wetlands in agricultural landscapes are uniquely positioned to intercept nutrients from agricultural fields, limiting the potential for downstream eutrophication. In particular, wetlands are effective at removing excess NO3-N from surface and groundwater draining agricultural fields (Woltemade Citation2000; Mitsch et al. Citation2001; Zedler Citation2003; Kovacic et al. Citation2006).
Wetland restoration aims to reintroduce ecosystem services to the landscape. This is particularly true in the agricultural landscapes of Midwestern United States where extensive wetland loss has occurred due to the expansion of row-crop agriculture (Mitsch et al. Citation2001; Zedler Citation2003; Fennessy & Craft Citation2011). Water quality improvement through N removal is one such ecosystem service that is often identified as justification for restoring wetlands (Osborne & Kovacic Citation1993; Woltemade Citation2000; Rabalais et al. Citation2002; Zedler Citation2003; Fennessy & Craft Citation2011). However, restored wetlands may not perform equally to their natural counterparts. The effectiveness of restored wetlands in removing excess NO3-N depends upon NO3-N load, connectivity to adjacent waters, water residence time, soil anaerobiosis, microbial communities, and soil texture (Mitsch et al. Citation2001; Bruland et al. Citation2003, Citation2006; Ullah & Faulkner Citation2006).
The spatial structure of soil properties and processes adds an additional level of complexity to the comparison of natural and restored wetlands. Spatial heterogeneity can influence above- and belowground biodiversity, which in turn can affect biogeochemical cycling of C and N (Schlesinger et al. Citation1996; Ettema et al. Citation1998; Ettema & Wardle Citation2002). Wetland functions also can exhibit strong spatial patterns over multiple scales, ranging from centimeters to kilometers (Ettema et al. Citation1998; King et al. Citation2004). Determining the spatial variation in the nutrient status and biogeochemical processes in wetlands will allow managers to determine whether certain locations of a wetland are more likely to be a nutrient source or sink (Grunwald et al. Citation2004, Citation2007). Hydrologic flowpaths into and along the wetlands influence nutrient, particularly NO3-N, delivery to macrophytes and above- and belowground production, which in turn affects distribution of soil organic matter and microbial communities (Anderson et al. Citation2005). Further, different species and different distributions of plants can interact with the heterogeneous distribution of resources in the soil matrix, thereby providing some degree of spatial structure in soil biota (Ettema & Wardle Citation2002). By understanding how factors at multiple spatial scales influence wetland functions, particularly C, N, and P biogeochemical cycling, wetland conservation and restoration can be optimized to improve the overall performance and delivery of ecosystem services (Schlesinger et al. Citation1996; Bruland et al. Citation2006; Grunwald et al. Citation2007).
Denitrification can be a significant pathway of N removal in wetland and riparian buffers, though rates can differ between natural and restored systems based on factors such as soil organic C content, NO3-N concentrations, soil texture, and soil moisture (Hanson et al. Citation1994; Hunter & Faulkner Citation2001; Burgin & Hamilton Citation2007; Orr et al. Citation2007; Peralta et al. Citation2010). However, denitrification and the controlling factors are not distributed homogenously throughout the wetlands and riparian areas and often exhibit high spatial variability (Ettema et al. Citation1998; Bruland & Richardson Citation2004, Citation2005; King et al. Citation2004; Bruland et al. Citation2006). For example, Harms et al. (Citation2009) found that water vectors from nearby streams influenced distributions of C and N in a semiarid floodplain; in one of their two sites, C and N spatial patterns were good predictors of denitrification.
Spatial variability of soil properties and processes can be influenced by biotic and abiotic factors such as vegetation distributions, soil invertebrate and microbial communities, surface and subsurface hydrology, nutrient loading, and sedimentation (Ettema et al. Citation1998; Ettema & Wardle Citation2002; King et al. Citation2004; Bruland et al. Citation2006). Denitrification in natural and restored riverine and non-riverine wetlands in North Carolina was best predicted by NO3-N, soil organic C, and soil moisture, although the strength of relationships varied among sites (Bruland et al. Citation2006). Further, both means and variability of NO3-N, NH4-N, and denitrification differed between restored and natural wetlands. Natural wetlands had greater variability in NO3-N and denitrification than restored or created wetlands, whereas NH4-N exhibited similar variability between sites. Similarly, Gallardo (Citation2003) also observed that flooding influences larger scale spatial patterns of soil nutrients in floodplain forests, though vegetation also influenced soil properties, particularly C and N, at smaller scales. Schlesinger et al. (Citation1996) also found strong spatial patterns in soil N in desert soils, with spatial variation resulting from distributions of different shrubs and grasses. Conversely, Burke et al. (Citation1999) showed that topography, not vegetation, influenced soil C pools in a shortgrass steppe, with C pools greater at toeslope positions. Collectively, these processes interact to lead to the creating of microsite variability within the soil profile.
Often, however, comparisons of ecosystem services between natural and restored wetlands ignore their underlying spatial structure. Failing to take into account the spatial variability of soil properties and processes increases the difficulty in identifying factors controlling ecosystem services such as C sequestration, nutrient accumulation, and denitrification. We investigated the effects of restoration on the spatial variability of denitrification in two restored and two natural depressional wetlands. Our hypotheses were (1) natural wetlands would have greater variability of denitrification and associated soil properties than restored wetlands, and (2) denitrification and soil properties would be spatially autocorrelated in natural wetlands, whereas no spatial structure would be present in restored wetlands. Previous homogenization of the soil profile via cultivation in the restored wetlands, combined with a relatively short time following restoration, would likely result in lower variability and spatial structure of denitrification and associated soil properties relative to the reference depressional wetlands (Bruland et al. Citation2006; Orr et al. Citation2014).
Methods
Site description
We sampled two natural and two restored wetlands in Newton County in northwestern Indiana (). Restored wetlands were on the Kankakee Sands Prairie Restoration macrosite owned by The Nature Conservancy and were restored in 2001 under the Wetlands Reserve Program by filling in the surrounding drainage ditches (C. O’Leary, personal communication). Prior to restoration, the land was used for row-crop agriculture. The wetlands and surrounding prairies are actively managed by seeding, invasive removal, and prescribed fires every 2–3 years. These wetlands are depressional, precipitation-fed systems, and the soils are mapped as Granby loamy fine sand (sandy, mixed, mesic Typic Endoaqualls). The surrounding land-use is a mixture of restored mesic prairie and row-crop agriculture. Dominant vegetation in the wetlands consists of Schoenoplectus pungens (Vahl) Palla, Polygonum pensylvanicum L., Eleocharis erythropoda Steud., Juncus brachycephalus (Engelm.) Buchenau, Scirpus cyperinus (L.) Kunth, Leersia oryzoides (L.) Sw., Phalaris arundinacea L., and Solidago altissima L.
Figure 1. Map of study sites in northwestern Indiana. Polygon represents property boundary of Kankakee Sands Nature Preserve.
Natural wetlands were located at the Willow Slough Fish and Wildlife Area owned and managed by the Indiana Department of Natural Resources. Similar to the restored wetlands, natural wetlands are depressional, precipitation-fed wetlands underlain by Adrian drained muck (sandy, mixed, euic, mesic Terric Haplosaprists) and dominated by Calamagrostis canadensis (Michx.) P. Beauv. and Scirpus cyperinus (L.) Kunth.
Soil sampling
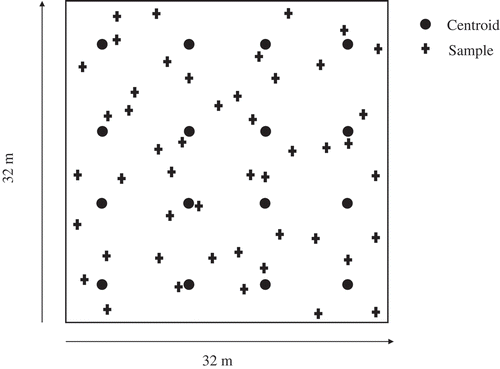
We established a 32 × 32 m sampling grid in each wetland in August 2010. Each grid was partitioned into four rows and four columns for a total of 16 subgrids in each site (). The centroid of the each subgrid was marked with polyvinyl chloride pipe, and three randomly selected soil cores (8.5 cm diameter × 5-cm deep) were collected around each centroid for a total of 48 soil cores per wetland (192 cores total). Initially, 15-cm-deep cores were taken and sectioned into 0- to 5-cm and 5- to 15-cm sections; however, only the 0- to 5-cm samples were analyzed to keep the sample size manageable. Cores were collected with a stainless-steel piston corer, placed into resealable plastic bags, and returned to the laboratory on ice. All soils were collected over 2 days in late August after at least 1 week of no rain. Restored wetlands were sampled on the first day and natural wetlands were sampled on the following day. All soil samples were processed within 48 hr of collection. Following the soil collection, surface water was collected in 20-L carboys, returned to the laboratory, and filtered through 0.20-µm mesh for use in denitrification assays.
Soil analyses
Field-moist soils were analyzed for soil moisture content, plant-available N (NO3-N, NH4-N), and denitrification. Soil moisture content was determined by measuring the change in mass of a 5-g sample after drying to a constant weight. Plant-available N was extracted from soil samples using 2N KCl (Mulvaney Citation1996) and analyzed using a Lachat Quickchem (Lachat Instruments, Loveland, CO, USA). Unamended denitrification was measured with the acetylene-inhibition method (Tiedje Citation1994). Twenty-five grams of field-moist soil were placed into 125-mL Wheaton bottles with screw caps equipped with gray butyl septa. Each bottle received 50 mL of filtered site water amended with chloramphenicol (0.21 µM) to inhibit microbial growth. The bottles were flushed for 5 minutes with ultra-high-purity N2 gas, and the headspace was adjusted to 10% atm with acetylene to block the reduction of N2O to N2. Incubations were conducted for 90 minutes at 25°C and a 5-mL sample was collected after 30, 60, and 90 minutes and stored in 2-mL evacuated Wheaton vials sealed with aluminum crimp tops and gray butyl septa. The bottles were vigorously shaken by hand for 30 seconds prior to the collection to equilibrate N2O between the soil slurry and headspace. After collection, a 5-mL mixture of N2 and acetylene (9:1) was added to maintain constant pressure. Nitrous oxide concentrations were measured using a gas chromatograph (SRI Instruments, Menlo Park, CA, USA) with an electron capture detector, and concentrations were corrected for dilution through multiple sample collections. Denitrification rates were determined by regressing N2O concentrations against time. All denitrification rates were expressed on both a dry weight basis by correcting for the soil moisture content and an area basis by multiplying dry weight rates by the sampling depth and bulk density.
Following analyses of field-moist soils, remaining soils were dried, ground, and passed through a 2-mm mesh sieve to determine bulk density, organic C, and total N. Carbonates were removed by placing subsamples in a dessicator with a beaker of concentrated HCl for 24 hr (Hedges & Stern Citation1984). Organic C and total N were determined from these subsamples using a Perkin-Elmer 2400 CHN Analyzer (Perkin-Elmer, Waltham, MA, USA). Bulk density was calculated by dividing the total dry weight of the soil sample by the volume of the core (Blake & Hartge Citation1986). All results were expressed on a dry gram basis.
Geostatistics and data analysis
A priori, soil cores within each wetland were not considered independent samples due to their close proximity to one another (Bruland et al. Citation2006). All variables exhibited weak but significant positive spatial autocorrelation (see below) at least in one wetland. Data were tested for normality using the Kolmogorov–Smirnov test (α = 0.05) and, when necessary, were natural-log-transformed to achieve a normal distribution (IBM SPSS, Armonk, NY, USA). Means and standard errors were calculated for each soil property, and the variance between natural and restored wetlands was compared using Levene’s test (α = 0.01). Due to the weak observed autocorrelation, means were compared with a one-way ANOVA with Tukey’s post hoc test.
Omnidirectional variograms were generated for denitrification and associated soil properties to evaluate their actual spatial structure in each of the four wetlands. Variograms describe how data are correlated with distance, typically in a specified direction; omnidirectional variograms essentially combine all directional variograms into a single plot, indicating overall spatial continuity or structure in empirical data. They indicate mean variability between multiple pairs of points across all lag distances in each possible direction (Eastman Citation2009). In order to test for the presence of spatial autocorrelation among soil properties in each wetland, global Moran’s I was calculated. Moran’s I tests for autocorrelation among samples by comparing the variation of paired points within a specified lag distance (spatial covariation) to the total variance (Moran Citation1950; Legendre & Fortin Citation1989). The significance of the test is estimated by generating a z-score from a randomly permutated distribution (Moran Citation1950; Legendre & Fortin Citation1989). Values range from −1 indicating that the neighboring values are dissimilar (negative spatial autocorrelation) to +1 indicating that neighboring values are similar (positive spatial autocorrelation). The Moran’s I test was performed over a range of lag distances from the lowest value at each site up to 20 m. The lowest lag distance is the shortest distance between points such that each location has at least one neighbor. The global Moran’s I test is a ‘global’ statistic of spatial autocorrelation, meaning that clusters of high values (hot spots) and low values (cold spots) are not identified (Moran Citation1950). The Getis–Ord Gi* test (Getis & Ord Citation1992) was used to detect hot spots of denitrification in each wetland. Hot spots (and cold spots) are identified as clusters with significantly greater (or lower) values relative to surrounding points. Omnidirectional variograms were generated using IDRISI Taiga (Clark Labs, Worcester, MA, USA), and all other geostatistical analyses were performed using GeoDa (Luc Anselin, University of Illinois, Urbana-Champaign, Urbana, IL, USA; Anselin et al. Citation2006).
Results
Denitrification was greater in restored wetlands (30 ng N2O g−1 hr−1) than in natural wetlands (1.1 ng N2O g−1 hr−1) and was most strongly correlated to C:N ratios (rho = 0.50, p < 0.01). Due to the differences in bulk density between the natural and restored wetlands, denitrification was corrected for the specific bulk density and sampling depth to express rates based on area. When expressed on a mass basis, the denitrification between the two natural wetlands significantly differed (); however, no differences were detected between these two sites when expressed on an area basis (). Denitrification patterns were comparable between the two restored wetlands regardless of units. Because rates and patters were comparable regardless of units, rates based on the dry weight were used for geostatistical analysis.
Figure 3. Boxplots of denitrification (natural-log transformed) from the two natural and restored wetlands (n = 48). Different letters indicate significant differences based on Tukey’s test. Variance was significantly greater in restored wetlands based on Levene’s test (p < 0.05).
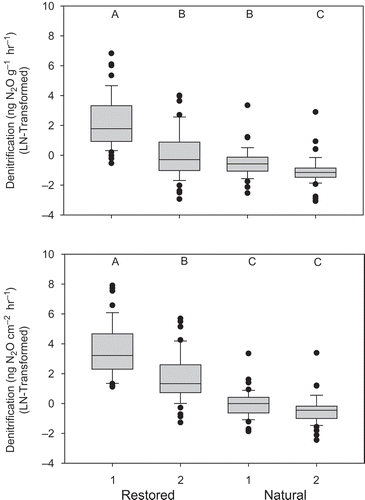
Restored wetlands also had greater bulk density and C:N ratios, whereas natural wetlands had greater soil moisture, plant-available N (NO3-N, NH4-N), organic C, and total N (). Levene’s test (α = 0.01) indicated significantly greater variation in denitrification, soil moisture, bulk density, total N, and C:N ratios () in restored wetlands than in natural wetlands. Natural wetlands, on the other hand, had greater variance of NO3-N. Plant-available NH4-N and organic C had comparable variation between natural and restored wetlands.
Table 1. Site means (±1 SE) of soil properties at each of the sampled wetlands.
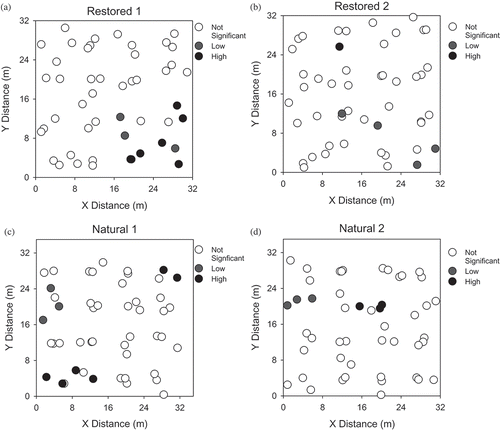
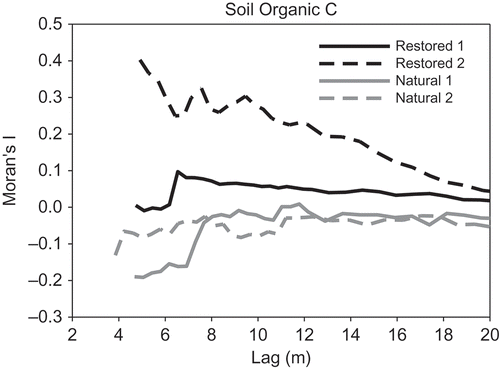
Overall, omnidirectional variograms did not indicate marked spatial trends in denitrification or other measured variables across the individual natural or restored wetlands (data not shown). Global Moran’s I analyses revealed a wide range of spatial autocorrelation among soil properties and the four wetlands, at variable lag distances. Denitrification exhibited positive spatial autocorrelation in both natural wetlands between 4 and 5 m and up to 16 m in restored wetland 1; no spatial autocorrelation was detected at any lag distance in restored wetland 2 (, ). Organic C was autocorrelated in restored wetland 2 only up to 20 m, whereas no spatial autocorrelation was found in natural wetlands or restored wetland 1 (, ). Conversely, NO3-N was spatially autocorrelated in both natural wetlands, but only in one of the two restored wetlands (). The two natural wetlands exhibited similar patterns for both denitrification and soil organic C ( and , respectively). However, denitrification in restored wetland 2 had a comparable pattern to the natural wetlands (), whereas soil organic C in restored wetland 1 mimicked the natural wetlands ().
Table 2. Lag distances (m) with significant spatial autocorrelation in the two natural and two restored depressional wetlands based on Moran’s I analysis.
Figure 4. Moran’s I values for denitrification across lag distances in natural and restored wetlands.
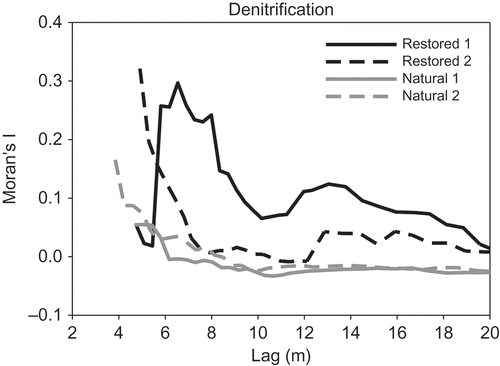
Figure 5. Moran’s I values for soil organic C across lag distances in natural and restored wetlands.
Getis–Ord Gi* results indicated significant hot spots of denitrification in all four wetlands (). Restored wetland 1 had 6 significant hot spots and restored wetland 2 only had one. Six hot spots were detected in natural wetland 1 and only three hot spots were detected in natural wetland 2. Hot spots and cold spots were also identified for other measured soil properties, though denitrification hot spots did not correspond to those of NO3-N, soil moisture, or organic C in any of the wetlands.
Discussion
Higher denitrification in restored wetlands relative to natural wetlands was an unexpected result. Other studies have found greater denitrification in natural wetlands than in restored wetlands in multiple hydrogeomorphic settings. Hunter and Faulkner (Citation2001) measured greater denitrification rates in natural bottomland wetlands (657 ng N2O g−1 hr−1) compared to restored bottomland wetlands (167 ng N2O g−1 hr−1), and Bruland et al. (Citation2006) found greater denitrification in natural riverine and non-riverine wetlands compared to created and restored wetlands. In both studies, positive correlations were detected between denitrification and soil moisture. Using a larger set of wetlands (n = 20), including the four used in this study, Marton et al. (Citation2014) measured greater ambient and potential denitrification in natural wetlands than in restored wetlands, with denitrification rates positively correlated to soil NO3-N and moisture. In the current study, denitrification was positively correlated to C:N ratios (rho = 0.50, p < 0.01) and negatively correlated to both NO3-N (rho = −0.50, p < 0.01) and soil organic C (rho = −0.34, p < 0.01). One possible explanation is that the N2O produced during incubations in the current study was the result of dissimilatory nitrate reduction to ammonia (DNRA) rather than denitrification, which can occur in substrates with high C:N ratios and produces N2O as a by-product (Burgin & Hamilton Citation2007). In created freshwater wetlands in Texas, Scott et al. (Citation2008) found DNRA occurring simultaneously with denitrification, though DNRA frequently accounted for a small portion (<5%) of total NO3-N removal. However, they did find that DNRA accounted for up to 36% of NO3-N removal in one location characterized by low overlying water NO3-N concentrations. In a review of aquatic N-cycling processes, Burgin and Hamilton (Citation2007) discuss the variability of DNRA in total NO3-N removal across freshwater and marine systems, and that this process may be less important in freshwater wetlands. The magnitude of DNRA in the current study, while not known, could have implications for the overall delivery of ecosystem services. If, for example, DNRA is a more important NO3-N removal pathway than denitrification, then N2O production may exceed N2 production, which is a less favorable outcome as N2O is a highly potent greenhouse gas.
Soil organic C was greater in natural wetlands relative to restored wetlands. Development of soil organic matter is a slow process (Craft et al. Citation1999; Ballantine & Schneider Citation2009), and restored wetlands in the current study were only 10 years old. Further, the prairies surrounding the restored wetlands are burned every 2–3 years. The high sand content of the restored wetlands (91% sand, Marton et al. Citation2014) promotes drainage to groundwater, thereby allowing fires to burn through the wetlands, which may inhibit the buildup of soil organic C (Neff et al. Citation2005). Anderson et al. (Citation2005) found that soil organic matter concentrations increased over 10 years in two created riverine marshes in Ohio, while also increasing in spatial variability due to variability in inundation, vegetation, and sediment deposition.
For most soil properties, restored wetlands had greater variance () and greater significant lag distances (), though causal processes behind the observed spatial trends are not entirely clear. Bruland and Richardson (Citation2005) also measured significant spatial trends in created, restored, and natural riverine and non-riverine wetlands. They suggested that overbank flooding from the adjacent river could have led to increasing soil organic matter and decreasing sand content with increasing distance from the stream. Bruland and Richardson (Citation2005) also found linear and non-linear trends in soil properties from non-riverine wetlands, which they hypothesized to be related to variations in unmeasured properties such as topography, above- and belowground biomass, and soil biota (Schlesinger et al. Citation1996; Ettema & Wardle Citation2002). These study sites were depressional systems, likely having minimal horizontal movement of surface water. In contrast to our study, Bruland et al. (Citation2006) found lower variation in denitrification and NO3-N in restored non-riverine wetlands compared to natural wetlands. Conversely, they found equal or greater variation of soil moisture and NH4-N in restored non-riverine wetlands. Their restored wetlands were former agricultural fields, and they reported that surface soils were homogenized during restoration. However, their results were not consistent between riverine and non-riverine wetlands. In one of their riverine sites, spatial variability of denitrification was greater in the restored wetland than in the natural wetland. Orr et al. (Citation2014) also found greater denitrification rates in a restored floodplain wetland relative to a natural floodplain wetland. They found greater variability and no spatial structure in denitrification at the restored site, consistent with our findings. However, denitrification exhibited a strong spatial structure in their natural site. The lack of a significant spatial structure in the current study suggests that means and variances of denitrification and soil properties across the sampled natural and restored wetlands are not spatially dependent or vary at spatial scales greater or smaller than the scale at which these measurements were made.
Another possibility is the complexity of wetland ecosystems, with intra-site variability, soil and hydrological dynamics, and disturbance regimes make it difficult to accurately parse out the spatial variability in these soil properties and processes. Several factors, alone or acting in concert, can influence the spatial distribution and structure of soil properties, which in turn can influence the spatial structure and magnitude of denitrification. Following the end of cultivation, vegetation communities can go through successional stages, which in turn can influence soil properties such as C and N pools, soil moisture, oxygen content, and ultimately denitrification (Gross et al. Citation1995; Rotkin-Ellman et al. Citation2004; Diekmann et al. Citation2007). Bachand and Horne (Citation1999) found greater rates of NO3-N removal in a constructed wetland with a mixed vegetation community of Typha spp. and Scirpus spp. relative to individual monocultures. Gross et al. (Citation1995) found greater spatial variability in soil N in mid-successional fields relative to a newly abandoned field and a forest, while Rotkin-Ellman et al. (Citation2004) showed that different tree species lead to differences in soil organic matter patches, which can be hot spots of denitrification. Soil texture between the natural and restored wetlands in the current study was relatively comparable (Marton et al. Citation2014), though there soil organic matter was greater in natural wetlands relative to restored wetlands. Though not statistically greater, the restored wetlands had greater plot scale (1 m2) and site-level species richness relative to the natural wetlands (Hopple & Craft Citation2013). The greater plant diversity, coupled with differences in organic matter and slight variations in soil texture, could have led to the observed differences in magnitude and spatial structure of denitrification and other soil properties (e.g., soil organic C) between and within natural and restored wetlands.
The observed spatial variance in soil properties from natural and restored wetlands was not entirely the result of random processes. Significant positive spatial autocorrelation for denitrification and several other soil properties ( and , ) suggested that, in addition to the plot-scale directional trends, significant spatial variability was present as well. Though the Moran’s I analysis shows whether variables are globally autocorrelated over space, the test does not provide insight into the causality of the spatial patterns. Future work could incorporate greater sample sizes and attempt explanatory models, such as spatially weighted regressions. Spatial patterns in vegetation, hydrodynamics, and soil biota have been found to influence soil nutrient pools and processes (Schlesinger et al. Citation1996; Ettema & Wardle Citation2002; King et al. Citation2004). Schlesinger et al. (Citation1996) found that available N (NO3-N, NH4-N) was strongly autocorrelated within 20 cm of the perennial bunchgrass Bouteloua eriopoda in desert soils, resulting from belowground nutrient cycling by the shrubs and associated soil microorganisms. In a restored riparian wetland in Georgia, Ettema et al. (Citation1998) found that soil properties (NO3-N, soil moisture) exhibited strong spatial autocorrelation. Plant-available NO3-N was autocorrelated at distances up to 84 m, whereas in the current study, NO3-N was autocorrelated at distances up to 8 m. One possible explanation for the large discrepancy between distances in the two studies is the influence of hydrology. Ettema et al. (Citation1998) measured soil variables in a riparian wetland that received periodic pulses of water from the adjacent river, distributing sediment and nutrients over greater distances. Conversely, our study sites were precipitation-fed depressional wetlands, which received little in the way of allochthonous inputs. King et al. (Citation2004) measured the variation and relationships between environmental factors and vegetation communities along a 10-km transect of wetlands in the Everglades. They found that nutrients (K, N, and P) and hydrology influenced vegetation patterns at the wetland scale, whereas P loading controlled vegetation community patterns along the 10-km transect.
Denitrification hot spots occur where reactants and conditions suitable for denitrification are disproportionately higher than the surrounding areas (McClain et al. Citation2003). Important factors controlling denitrification rates are NO3-N, labile C, and anoxic conditions (Hunter & Faulkner Citation2001; McClain et al. Citation2003; Bruland et al. Citation2006; Ullah & Faulkner Citation2006; Bruland et al. Citation2009). Generally, hot spots of denitrification and controlling factors should correspond. In the current study, however, denitrification was not correlated with NO3-N, soil organic C, or soil moisture. Further, denitrification hot spots did not overlap with variables required for denitrification (e.g., soil moisture, NO3-N, organic C), suggesting that other factors may have been influencing denitrification. Plausible factors that may explain denitrification include differences in microbial communities, soil texture, or interactions between NO3-N, organic C, and moisture (Hanson et al. Citation1994; Bruland et al. Citation2006; Peralta et al. Citation2010). Also, because denitrification is a microbial process, our scale of measurement at the nearest tenth of a meter may have been too coarse to detect relationships between denitrification and associated soil properties.
Other studies have focused on identifying hot spots of denitrification and soil properties, though hot spots were identified visually either based on interpolated maps or based on parametric statistics that ignore spatial autocorrelation among samples (Rotkin-Ellman et al. Citation2004; Bruland & Richardson Citation2005; Bruland et al. Citation2006). Our study differed in that we used the Getis–Ord Gi* test to statistically verify hot spots of denitrification. Ignoring the spatial aspect of soil properties and processes in attempting to estimate hot spots artificially inflates the significance of the statistical tests by treating each sample as an independent observation, though due to their proximity in space, the samples cannot truly be considered independent (Moran Citation1950; Legendre & Fortin Citation1989; Goovaerts Citation1998).
The comparable, and in some cases greater, spatial variability in denitrification and soil properties in restored wetlands relative to natural wetlands was surprising. Previous research has suggested that prior land use affects soil properties, particularly agriculture that homogenizes soil structure both vertically and horizontally (Bruland et al. Citation2003). We found the opposite trend in that denitrification in restored wetlands exhibited stronger spatial variability and a comparable numbers of hot spots relative to natural wetlands. These results suggest that, despite prior cultivation and homogenization of soil profiles, restoration can result in systems with comparable ranges of biogeochemical functioning.
There are both advantages and disadvantages of our sampling design. Our sample plots were only 32 × 32 m which prevented us from adequately sampling the entire wetland and thereby capturing site-scale variability. However, we were able to sample four wetlands in total allowing us to make comparisons both between and within natural and restored depressional wetlands. For example, though spatial patterns of denitrification and soil organic C were comparable between the two natural wetlands, the patterns differed between the two restored sites. Though it cannot be determined why these differences exist, it is possible that differences in plant density and diversity between the two restored sites are influencing belowground dynamics.
With a smaller extent and finer grain, we were able to investigate patterns in denitrification and the associated soil properties over shorter spatial scales. Changing either the extent or the grain of the study area would ultimately change the variability, and thus the predictability, of a given parameter (Wiens Citation1989). This combined with the fact that we were unable to fit a reliable semivariogram to any of our variables implies an inherent difficulty and potential inapplicability in scaling up our results to the wetland scale. Though these results may not be effectively scaled to the wetland scale, findings from this and other similar studies (Bruland & Richardson Citation2004, Citation2005; Orr et al. Citation2014) can provide useful information guiding wetland restoration and post-restoration monitoring and assessment. For example, reestablishment of hydrology, native vegetation, and organic matter amendments will help a restored system have comparable or greater denitrification potential when compared to natural reference sites. Further, these results also show that a high number of samples can provide a good estimation of the variability of soil properties and processes, though the sampling extent would have to cover the majority of the wetland to fully understand the spatial structure and to appropriately scale those findings. While it is not feasible to conduct spatially intensive investigations of denitrification in most cases, more studies that are conducted with a spatial sampling design will increase our understanding of the controls on spatial structuring of ecosystem services and how this structure changes over time.
Incorporating spatial patterns provides greater understanding of how individual and multiple environmental factors influence the distribution and development of soil properties and processes. This is particularly true for restoring wetlands, in which the primary goal is to return some ecosystem service to the landscape. Our results showed that, despite lower concentrations of soil C and N, restored wetlands had more complex spatial variability in denitrification, soil moisture, bulk density, total N, and C:N ratios compared to natural wetlands and provided a greater range of biogeochemical transformations. Increased knowledge of the spatial structure of soil properties will further reveal factors controlling processes such as denitrification at multiple spatial scales in restored wetlands. By understanding the underlying factors driving denitrification and other soil processes (e.g., accretion, sedimentation, P sorption), wetland restoration can be improved to maximize the delivery of ecosystem services.
Acknowledgments
We would like to thank Chip O’Leary and Stephanie Frischie at The Nature Conservancy at the Kankakee Sands Preserve and the Indiana Department of Natural Resources for access to their property. We would also like to thank Ellen Herbert, Anya Hopple, and Bri Richards for help with the collection, preparation, and analysis of samples, and Maggie Marton for editorial assistance. This study was funded by the US Department of Agriculture, Natural Resources Conservation Service, Conservation Effects Assessment Program through The Great Lakes-Northern Forest Cooperative Ecosystem Studies Unit, Cooperative Agreement Number 68-7482-9-516.
References
- Anderson C, Mitsch W, Nairn R. 2005. Temporal and spatial development of surface soil conditions at two created riverine marshes. J Environ Qual. 34:2072–2081.
- Anselin L, Syabri I, Kho Y. 2006. GeoDa: an introduction to spatial data analysis. Geogr Anal. 38:5–22.
- Bachand P, Horne A. 1999. Denitrification in constructed free-water surface wetlands: II. Effects of vegetation and temperature. Ecol Eng. 14:17–32.
- Ballantine K, Schneider R. 2009. Fifty-five years of soil development in restored freshwater depressional wetlands. Ecol Appl. 19:1467–1480.
- Blake G, Hartge K. 1986. Bulk density. In: Klute A, editor. Methods of soil analysis, part 1. Physical and mineralogical methods: agronomy monograph no. 9. 2nd ed. Madison (WI): American Society of Agronomy; p. 363–375.
- Bruland G, Hanchey M, Richardson C. 2003. Effects of agriculture and wetland restoration on hydrology, soils, and water quality of a Carolina bay complex. Wetl Ecol Manage. 11:141–156.
- Bruland G, Richardson C. 2004. A spatially explicit investigation of phosphorus sorption and related soil properties in two riparian wetlands. J Environ Qual. 33:785–794.
- Bruland G, Richardson C. 2005. Spatial variability of soil properties in created, restored, and paired natural wetlands. Soil Sci Soc Am J. 69:273–284.
- Bruland G, Richardson C, Daniels W. 2009. Microbial and geochemical responses to organic matter amendments in a created wetland. Wetlands. 29:1153–1165.
- Bruland G, Richardson C, Whalen S. 2006. Spatial variability of denitrification potential and related soil properties in created, restored, and paired natural wetlands. Wetlands. 26:1042–1056.
- Burgin A, Hamilton S. 2007. Have we overemphasized the role of denitrification in aquatic ecosystems? A review of nitrate removal pathways. Front Ecol the Environ. 5:89–96.
- Burke I, Lauenroth W, Riggle R, Brannen P, Madigan B, Beard S. 1999. Spatial variability of soil properties in the Shortgrass Steppe: the relative importance of topography, grazing, microsite, and plant species in controlling spatial patterns. Ecosystems. 2:422–438.
- Craft C, Reader J, Sacco J, Broome S. 1999. Twenty-five years of ecosystem development of constructed Spartina alterniflora (Loisel) marshes. Ecol Appl. 9:1405–1419.
- Diekmann L, Lawrence D, Okin G. 2007. Changes in the spatial variation of soil properties following shifting cultivation in a Mexican tropical dry forest. Biogeochemistry. 84:99–113.
- Eastman J. 2009. IDRISI Taiga: guide to GIS and image processing. Worcester (MA): Clark Labs, Clark University.
- Ettema C, Coleman D, Vellidis G, Lowrance R, Rathbun S. 1998. Spatiotemporal distributions of bacterivorous nematodes and soil resources in a restored riparian wetland. Ecology. 79:2721–2734.
- Ettema C, Wardle D. 2002. Spatial soil ecology. Trends Ecol Evol. 17:177–183.
- Fennessy S, Craft C. 2011. Agricultural conservation practices increase wetland ecosystem services in the Glaciated Interior Plains. Ecol Appl. 21:S49–S64.
- Gallardo A. 2003. Spatial variability of soil properties in a floodplain forest in Northwest Spain. Ecosystems. 6:564–576.
- Getis A, Ord J. 1992. The analysis of spatial association by use of distance statistics. Geogr Anal. 24:189–206.
- Gittins R. 1968. Trend-surface analysis of ecological data. J Ecol. 56:845–869.
- Goovaerts P. 1998. Geostatistical tools for characterizing the spatial variability of microbiological and physico-chemical soil properties. Biol Fertil Soils. 27:315–334.
- Gross K, Pregitzer K, Burton A. 1995. Spatial variation in nitrogen availability in three successional plant communities. J Ecol. 83:357–367.
- Grunwald S, Reddy K, Newman S, DeBusk W. 2004. Spatial variability, distribution and uncertainty assessment of soil phosphorus in a south Florida wetland. Environmetrics. 15:811–825.
- Grunwald S, Reddy K, Prenger J, Fisher M. 2007. Modeling of the spatial variability of biogeochemical soil properties in a freshwater ecosystem. Ecol Modell. 201:521–535.
- Hanson G, Groffman P, Gold A. 1994. Denitrification in riparian wetlands receiving high and low groundwater nitrate inputs. J Environ Qual. 23:917–922.
- Harms TK, Wentz EA, Grimm NB. 2009. Spatial heterogeneity of denitrification in semi-arid floodplains. Ecosystems. 12:129–143.
- Hedges J, Stern J. 1984. Carbon and nitrogen determinations of carbonate-containing solids. Limnol Oceanogr. 29:657–663.
- Hopple A, Craft C. 2013. Managed disturbance enhances biodiversity of restored wetlands in the agricultural Midwest. Ecol Eng. 61:505–510.
- Hunter R, Faulkner S. 2001. Denitrification potentials in restored and natural bottomland hardwood wetlands. Soil Sci Soc Am J. 65:1865–1872.
- King R, Richardson C, Urban D, Romanowicz E. 2004. Spatial dependency of vegetation-environment linkages in an anthropogenically influenced wetland ecosystem. Ecosystems. 7:75–97.
- Kovacic DA, Twait RM, Wallace MP, Bowling JM. 2006. Use of created wetlands to improve water quality in the Midwest-Lake Bloomington case study. Ecol Eng. 28:258–270.
- Legendre P, Fortin M. 1989. Spatial pattern and ecological analysis. Vegetatio. 80:107–138.
- Marton JM, Fennessy MS, Craft CC. 2014. Functional differences between natural and restored wetlands in the Glaciated Interior Plains. J Environ Qual. 43:409–417.
- McClain M, Boyer E, Dent C, Gergel S, Grimm N, Groffman P, Hart S, Harvey J, Johnston C, Mayorga E, et al. 2003. Biogeochemical hot spots and hot moments at the interface of terrestrial and aquatic ecosystems. Ecosystems. 6:301–312.
- Mitsch W, Day J, Wendell Gilliam J, Groffman P, Hey D, Randall G, Wang N. 2001. Reducing nitrogen loading to the Gulf of Mexico from the Mississippi river basin: strategies to counter a persistent ecological problem. BioScience. 51:373–388.
- Moran P. 1950. Notes on continuous stochastic phenomena. Biometrika. 37:17–23.
- Mulvaney R. 1996. Nitrogen- inorganic forms. In: Sparks DL, editor. Methods of soil analysis, part 3: chemical methods. Madison (WI): Soil Science Society of America; p. 1123–1184.
- Neff J, Harden J, Gleixner G. 2005. Fire effects on soil organic matter content, composition, and nutrients in boreal interior Alaska. Can J For Res. 35:2178–2187.
- Orr C, Stanley E, Wilson K, Finlay J. 2007. Effects of restoration and reflooding on soil denitrification in a leveed Midwestern floodplain. Ecol Appl. 17:2365–2376.
- Orr CH, Predick KI, Stanley EH, Rogers KL. 2014. Spatial autocorrelation of denitrification in a restored and a natural floodplain. Wetlands. 34:89–100.
- Osborne L, Kovacic D. 1993. Riparian vegetated buffer strips in water-quality restoration and stream management. Freshw Biol. 29:243–258.
- Peralta A, Matthews J, Kent A. 2010. Microbial community structure and denitrification in a wetland mitigation bank. Appl Environ Microbiol. 76:4207–4215.
- Rabalais NN, Turner RE, Scavia D. 2002. Beyond science into policy: Gulf of Mexico hypoxia and the Mississippi River. BioScience. 52:129–142.
- Rotkin-Ellman M, Addy K, Gold A, Groffman P. 2004. Tree species, root decomposition and subsurface denitrification potential in riparian wetlands. Plant Soil. 263:335–344.
- Schlesinger W, Raikes J, Hartley A, Cross A. 1996. On the spatial pattern of soil nutrients in desert ecosystems. Ecology. 77:364–374.
- Scott JT, McCarthy MJ, Gardner WS, Doyle RD. 2008. Denitrification, dissimilatory nitrate reduction to ammonium, and nitrogen fixation along a nitrate concentration gradient in a created freshwater wetland. Biogeochemistry. 87:99–111.
- Tiedje J. 1994. Denitrifier enzyme activity. In: Weaver RW, Angle S, Bottomley P, editors. Methods of soil analysis, part 2: microbiological and biochemical properties. Madison (WI): Soil Science Society of America; p. 256–257.
- Ullah S, Faulkner S. 2006. Denitrification potential of different land-use types in an agricultural watershed, lower Mississippi valley. Ecol Eng. 28:131–140.
- Wiens J. 1989. Spatial scaling in ecology. Funct Ecol. 3:385–397.
- Woltemade C. 2000. Ability of restored wetlands to reduce nitrogen and phosphorus concentrations in agricultural drainage water. J Soil Water Conserv. 55:303–309.
- Zedler JB. 2003. Wetlands at your service: reducing impacts of agriculture at the watershed scale. Front Ecol Environ. 1:65–72.