ABSTRACT
In natural forests of Central Africa, several studies indicate a dramatic decrease in commercial trees, including species of concern for conservation. Enrichment planting with these species will favor both the long-term recovery of their populations and biodiversity conservation in logged forests. In this study, we analyzed the survival and growth of 23 species in plantations. Fourteen 0.2–1.1 ha mixed species plantations consisting of single-species 15 × 15 m blocks were studied for 5 years in a logging concession of southeastern Cameroon. The plantation design considered both species light requirements and sensitivity to damage by pests. To identify the best species for enrichment planting, we assessed both species performance and plantation costs. We also tested for relationships between species traits and species performance. Mean annual diameter growth increments ranged from 1.67 to 42.9 mm. No significant relationship was found between growth and survival. Herbivory by wild Bovidae was the main cause of mortality and should be carefully considered in rehabilitation efforts. We found a significant negative relationship between wood density and maximum growth rate. The other traits tested were not good predictors of species performance in plantations. The two best-performing species, Triplochiton scleroxylon and Terminalia superba, could reach the minimum cutting diameter during a 30-year cutting cycle. Costs were high and mechanized site preparation is suggested to reduce them. Widespread adoption of such plantations will only occur if financial incentives or national regulations for assuring regeneration are implemented.
EDITED BY Nicholas Brokaw
1. Introduction
More than 400 million hectares of tropical forest are designated for timber production. Most of them have been or will be selectively logged. In their meta-analysis, Putz et al. (Citation2012) emphasized that although 85–100% of species of mammals, birds, invertebrates, and plants remain after selective logging, timber volumes decline by about 65% after the first harvest if the same species are again harvested. This depletion of logged species is of growing concern and many commercial species are now listed in the International Union for Conservation of Nature (IUCN) red list (e.g., Bourland et al. Citation2012). Post-logging silvicultural treatments, including planting of high-value species, would need to be applied in order to reestablish or conserve the timber biodiversity of forest stands. This paper analyzes the survival and growth of such high-value species in simple mixture in plantations in Central Africa.
Central African moist forests cover around 180 million hectares, and 26% of this area is licensed for commercial use by logging companies (De Wasseige et al. Citation2012). In most countries, logging companies must comply with an updated legal framework, including a detailed management plan based on tree inventories, estimates of recovery rates, planned felling cycles, and site-specific minimum cutting diameters (MCDs) (Bayol & Borie Citation2004; Fargeot et al. Citation2004). In the Congo basin, logging is very selective, with only six logged species accounting for 75% of total timber volume (Ruiz Pérez et al. Citation2005). Timber extraction rates per unit area are low (on average 6 m3 and less than two trees per hectare), associated damages are about 10%, and vegetation recovery is rather fast (Ruiz Pérez et al. Citation2005).
In spite of the logging regulatory framework and limited extraction, the continuity of supply of key timber species in Central Africa is in question. Although biomass can recover quickly after logging (i.e., 20 years), regaining exploitable wood volume is much slower (Chazdon Citation2003). In a long-term study at the M’Baïki silvicultural experiment in the Central African Republic, trees available for exploitation decreased dramatically after a first cutting cycle of 24 years (Gourlet-Fleury et al. Citation2013). For two important timber species, modeling the populations dynamics under a repeated felling regime showed a decrease in exploitable stock linked to decline of the target populations, indicating that the traditional logging regime is far from sustainable (Karsenty & Gourlet-Fleury Citation2006).
In Central Africa, forest managers face two major issues: (i) the difficulty of recovering the timber stock 20–30 years after logging (i.e., after a complete cutting cycle) and (ii) the lack of regeneration in the pioneer species that make up most of the harvest because of natural decline of their populations with increasing stand age. According to Gourlet-Fleury et al. (Citation2013), reducing the felling intensity or even doubling the length of the cutting cycle will not ensure recovery of these pioneer species, but only hasten their decline due to further natural reductions in regeneration.
Silviculture may help recover timber stock. In a 24-year experiment, Gourlet-Fleury et al. (Citation2013) assessed the effect of thinning (mean of 21 trees thinned ha−1, and 6.6 m2 ha−1 of basal area removed) on forest structure and dynamics. They concluded that thinning combined with logging significantly increased gain in biomass, but it had no effect on gain in timber stock at the end of the first felling cycle. However, these treatments should promote gains in timber stock in subsequent cycles. In the same experiment, Ouédraogo et al. (Citation2011) explained that thinning enhanced the growth and survival of nonpioneer light demanders or shade-bearers, to the detriment of pioneer species.
If thinning fosters the growth of nonpioneer saplings, other techniques are necessary to regenerate pioneer timber species, which probably establish after larger perturbations such as cultivation and subsequent abandonment (Doucet Citation2003; Van Gemerden et al. Citation2003; Biwolé et al. Citation2015). In some areas, late secondary growth rather than pristine forests are indicated by abundant large pioneer tree species such as Pericopsis elata and Triplochiton scleroxylon, which were included in our study (Gillet & Doucet Citation2013; Biwolé et al. Citation2015; Bourland et al. Citation2015). Management techniques such as enrichment planting have been recommended to promote these species (Fayolle et al. Citation2014; Ouédraogo et al. Citation2014).
Planting success was a major preoccupation for foresters in the mid-twentieth century in Africa. Many trials were based on a few or even a single species (e.g., Khaya spp., Tarrietia utilis, Dupuy & Koua Citation1993; Dupuy & Chézeaux Citation1994) sometimes planted on huge areas (e.g., Aucoumea klaineana in Gabon, Brunck et al. Citation1990). In spite of good results, these plantations were abandoned because of labor costs and because management plans were considered sufficient to ensure sustainable forest management. Today, increasing pressure on tropical forests and global concerns about the maintenance of ecosystem services (including timber production, biodiversity, and carbon), again focus attention on yield, and also on restoring biodiversity in production forests (Parrotta et al. Citation1997). Approaches to restoring tropical forest ecosystems vary depending on the amount of forest and soil degradation, residual vegetation, and desired restoration outcomes (Chazdon Citation2008).
Stanturf et al. (Citation2014) proposed the term ‘rehabilitation’ for restoring desired species composition, structure, or processes to an existing but degraded ecosystem. There are several methods of rehabilitation including enrichment planting or planting after partial overstory removal. In such planting, species mixtures are desirable for both improving biodiversity and the range of goods and services as well as limiting pest-induced damages (Piotto et al. Citation2004; Potvin & Gotelli Citation2008). Different kinds of mixtures exist. Simple mixtures consist of two or more species planted in single-species blocks or rows (Stanturf et al. Citation2014). Such mixtures are useful on sites with distinct gradients in environmental factors such as drainage or light. Species must thus be selected according to the site conditions (Stanturf et al. Citation2014).
However, there is little data on site tolerance and growth in Africa as emphasized in the meta-analysis of Piotto (Citation2008). The use of functional traits could help to predict species performance in plantations. Plant functional traits, in particular specific leaf area (SLA), seed size, wood density, and tree height at maturity (Hmax), are often good predictors of tree growth rates within communities (Poorter et al. Citation2008). For example, functional traits could predict tree growth and survival in plantings in Mexico (Martínez-Garza et al. Citation2013). While the relationships may not hold over a broad area, among tree species in a local area, those with high SLA, small seeds, low wood density, and high Hmax tend to have fast growth rates (Poorter et al. Citation2008; Paine et al. 2015).
In this study, we test a forest rehabilitation method using simple mixtures of high-value timber species planted after manual removal of the understory in degraded forests in southeastern Cameroon. In 2014, we analyzed the survival and growth of 23 species planted between 2009 and 2012 in 14 mixed-species plots. We addressed the following questions: (i) How do the selected species perform in simple mixtures? (ii) Is it possible to use plant functional traits to select the best candidates for mixed plantation? (iii) What is the cost of these plantations?
2. Methods
2.1. Study area
The study area is located in the province of Eastern Cameroon (between 3°01′ N and 3°44′ N; 13°20′ E and 14°31′ E). The study plantations were established in Forest Management Units 10–041, 10–042, 10–044, 10–039, 10–030, and 10–031, managed by the FSC-certified Pallisco Company. According to Worldclim (Citation2015), annual rainfall is ca. 1640 mm with two distinct rainy seasons (August–November and March–June), and the mean annual temperature is 23.1°C (Hijmans et al. Citation2005). The topography is undulating with elevation varying between 500 and 650 m. The geological substrate consists of volcanic intrusions and metamorphic rocks, and soils are classified as Ferralsols (Jones et al. Citation2013). The forest is mostly semi-deciduous and has been classified as Celtis forest by Fayolle et al. (Citation2014). It is spatially heterogeneous in species composition and degraded by recent (<50 years) or long-term (>200 years) human disturbances (Morin-Rivat et al. Citation2014). Due to the lack of regeneration of commercial light-demanding species, enrichment plantings have been recommended for this forest by Fayolle et al. (Citation2014). The host forest company has included enrichment plantings in its environmental policy since 2009 in order to promote the regeneration of the logged species in the framework for certification.
2.2. Study species
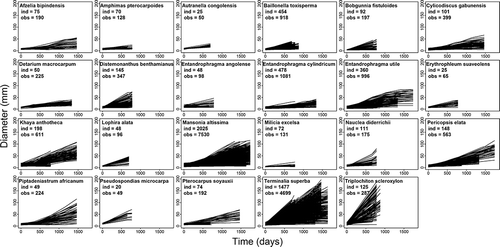
We selected 23 high-value species for planting (), based on their use as high-value timber or nontimber forest products (fruits, edible caterpillars, medicine, etc.). Many of them are considered as threatened by the IUCN (http://:www.iucnredlist.org/), and one is included in CITES Appendix II (P. elata, https://www.cites.org/eng/app/appendices.php). We gathered information on qualitative traits (deciduousness, regeneration guild, dispersal) from Hawthorne (Citation1995) and Meunier et al. (Citation2015). We extracted information on quantitative traits from several databases. Seed mass was obtained from Seed Information Database Version 7.1 (available from: http://data.kew.org/sid/). Wood density was extracted from the Dryad global wood density database (Chave et al. Citation2009; Zanne et al. Citation2009). Maximum height and diameter were computed from Meunier et al. (Citation2015). If data were not available at the species level, we used the most frequent or mean value at the genus level (Slik et al. Citation2008). Most of the focal species (20 out of 23) were deciduous, pioneer or nonpioneer light demanders, and dispersed by wind or animals. Among the study species, seed mass varied between 2 (Nauclea diderrichii) and 9613 mg (Detarium macrocarpum). Wood density ranged from 0.33 (T. scleroxylon) to 0.88 g·cm−3 (Lophira alata), with an average of 0.63 g·cm−3. Maximum diameter at breast height (dbh) ranged from 0.6 (Pseudospondias microcarpa) to 3.0 m (Baillonella toxisperma) and maximum height ranged from 20 (P. microcarpa) to 60 m (B. toxisperma, Cylicodiscus gabunensis, Entandrophragma cylindricum, Entandrophragma utile, Khaya anthotheca, Piptadeniastrum africanum).
Table 1. Characteristics and functional traits of the species used in this study.
2.3. Experimental design
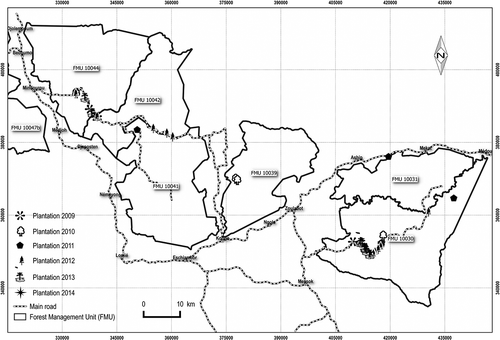
From 1 January 2009 to 31 December 2014, during the wet season, the forest company planted 53 plots (totaling 44.4 ha) with seedlings grown in nurseries. These plots were established in the most degraded areas along principal or secondary roads (). These locations facilitated plot maintenance and preserved forest cover in less disturbed areas.
Figure 1. Location of the enrichment plantings between 2009 and 2014 in the Pallisco management units, southeastern Cameroon.
The planting design was a ‘simple mixture, multiple species, single cohort planting’ as defined by Stanturf et al. (Citation2014). Planting followed these four steps. First, the high-value species and all other species with a dbh >50 cm were identified and protected. Second, the understory was clear cut by a team of 10 workers using machetes or a chainsaw. Third, the species planted were selected based on their shade tolerance (P, NPLD sensu Hawthorne Citation1995, ), their availability in the nurseries, and canopy openness at the sites. Fourth, for each plot, species were established in blocks according to the visually evaluated canopy cover by the team supervisor following the recommendations of Hawthorne (Citation1995) and Meunier et al. (Citation2015). Planting was done in 15 × 15 m single-species blocks using 25 seedlings per species planted 3 m apart in a block (). Planted seedlings were around 50 cm high.
Figure 2. Example of a multispecies plot consisting of 21 single-species blocks. Each block includes 25 seedlings planted 3 m apart. Each seedling is identified by a combination of a letter (X axis) and a distance (Y axis). Bt, Baillonella toxisperma; Db, Distemonanthus benthamianus; Ea, Entandrophragma angolense; Ec, Entandrophragma cylindricum; Eu, Entandrophragma utile; Ka, Khaya anthotheca; Ma, Mansonia altissima; Me, Milicia excelsa; Nd, Nauclea diderrichii; Ts, Terminalia superba.
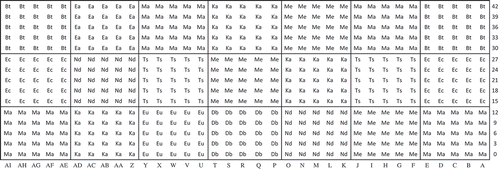
Within each plot, species blocks were alternated to minimize the effects of parasites and predation. Milicia excelsa, Entandrophragma spp., and K. anthotheca are extremely sensitive to parasites (gall-forming insects and/or shootborers) (Bosu et al. Citation2006; Opuni-Frimpong et al. Citation2008). In the first year, maintenance cleaning was done every four months. In the second year, two cleanings were performed every six months, and one cleaning was done at the end of the third year. Future thinning will be necessary to promote the best tree in each species block, the width of a block (15 m) being roughly the diameter of one mature crown.
We analyzed the performance of the 23 species in 14 plots planted between 2009 and 2012, out of the 53 original plots (). The size of the selected plots ranged from 0.2 to 1.1 ha, and the number of species varied from 2 to 10 per plot. At plantation establishment, canopy cover was estimated above each planted seedling with a clinometer. The presence (1) or absence (0) of vegetation in the following height intervals – 0–10 m, 10–20 m, and >20 m – was visually assessed. The sum was calculated to obtain a canopy cover index in a range from 0 (full open) to 3 (full cover). After plantation establishment, the proportion of seedlings planted in fully open areas (total cover index = 0) was 75% (SD = 18%), including 81% for pioneer and 62% for nonpioneer species.
Table 2. Size, openness, planting date, and species combinations (number of planted seedlings) for experimental plots.
The survival and diameter of each seedling was recorded each year. For 2–5 years, depending on the plot, diameters of 6540 tagged seedlings were measured at 10 cm above the stem base with a caliper. At the end of the experiment, the crown exposure of each seedling was classified following Dawkins (Citation1958). Code 1 was assigned to fully shaded understory trees, 2 to upper understory trees partly exposed to direct light, 3 to lower canopy trees partly exposed to direct light, 4 to canopy trees fully exposed to light from above, and 5 to fully emergent with no other vegetation in an inverted vertical cone of 45°. All field measurements were performed by the same team.
Plantations yields and costs were estimated for the 53 plots using data from the logging company between 2009 and 2014. The costs for planted and maintained areas were calculated each year. Costs were classified into wages, transport (fuel, maintenance, and depreciation), and materials (machetes, chainsaw, etc.). Costs were also divided into the main operation stages (seedling production, site preparation, planting, and maintenance).
2.4. Data analysis
To quantify tree survival, we estimated the survival function S(t) over time using the nonparametric Kaplan–Meier estimator (Harrell Citation2001), which gives the probability of an individual seedling surviving to time t, the time since the beginning of the experiment:
where ti is the time interval, di is the number of deaths that occur in the interval ti, ni is the number of seedlings that are alive at the end of the interval ti, and Π is the product operator across all cases less than or equal to t. Since the time from plantation establishment varied among plots and species from 690 to 1740 days, survival at t = 690 days was determined for all species.
A total of 17,794 diameter measurements were made on the 4621 trees alive at the end of the monitoring period. The diameter growth was modeled with a linear mixed model. The time period (up to ca. 5 years) was not long enough to justify the use of a nonlinear model (Paine et al. Citation2012). Because measures of tree diameter were repeated through time, these observations are dependent and correlated with each other. Consequently, including random effects to account for individual tree variability was required. It was achieved using a mixed modeling procedure. Models with fixed and random effects, including a random intercept (α) and a random slope (β), were tested. The best model was selected based on Akaike information criterion comparison of models, and the significance of the difference between pairs of models was tested with the likelihood ratio test. We first identified the best random structure (random slope) and then the best fixed effects (species and species–time interaction) in accordance with Zuur et al. (Citation2009). The equation that was found to give the best mixed linear model, based on the diameter (D) of the seedling (i) belonging to species (s) at the time (t) was
with as and cs as fixed parameters, βis as a random parameter, and εist as the error.
We then extracted the fixed model parameters for each species corresponding to the mean growth value and the best linear unbiased estimate of the random effects (BLUPS) for each individual in order to compute the mean of the growth rate of the best-performing seedlings (i.e., best 10% of stems).
Species performance was represented by mean growth rates (all stems), maximum growth rates (average of best 10% of stems), and mortality rates (all stems).
We tested for significant relationships between species traits and species performance using Pearson correlation for quantitative traits and Kruskal–Wallis analysis for qualitative traits. All statistical analyses were performed within the open source R environment (R version 2.14.1). The ‘lme4’ package was used for fitting mixed models (Bates et al. 2015).
3. Results
3.1. Species survival and growth
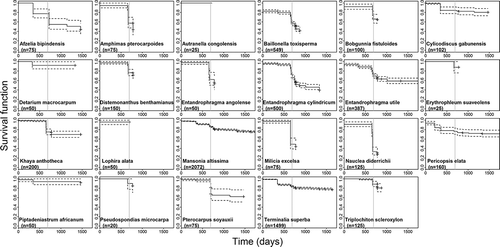
By the end of the experiment, some species experienced high mortality, while others had high survival (). The probability of survival at 690 days () ranged from 31.2% for Nauclea diderrichii (88 dead, 37 living seedlings) to 100% for Autranella congolensis (0 dead, 25 living seedlings). After 690 days, the survival rate stabilized (e.g., P. elata, at ca. 70%) or decreased further (e.g., E. cylindricum).
Table 3. For each species, number of observations (N), average canopy cover index (from 0, full open, to 3, full cover), average Dawkins index (from 1, lower understory, to 5, emergent), proportion (%) of emergent stems (fifth category of Dawkins), survival at 690 days (% of stems alive), maximum and mean diameter growth rates (in mm year−1), minimum cutting diameter (Cameroon national regulation), and time to reach the minimum cutting diameter given the mean diameter growth rate.
Figure 3. Kaplan–Meier survival estimates over time, with 95% confidence bounds for the 23 studied species. The vertical gray line indicates survival at t = 690 days for comparison among species.
In the first years, growth trajectories tended to be linear for the 23 study species (). The mean annual diameter growth increment (cs) ranged from 1.67 mm for M. excelsa to 42.90 mm for T. scleroxylon (). Although both were planted according to their shade tolerance guild under low canopy cover, the proportion of fully emergent stems (fifth category of Dawkins) was 3% for M. excelsa and 61.3% for T. scleroxylon (). For all species considered together, the relationship between survival and growth was positive but not significant (r = 0.291, p = 0.178).
3.2. Relationships between species performance and traits
We observed only weak correlations between species performance and most functional traits (). However, we found a significant relationship between the wood density and the maximum growth rate, with fast-growing species tending to have lighter wood. If the fastest growing species (T. scleroxylon) was removed from the analysis, the relationship was no longer significant. We also identified a weak, nonsignificant, correlation between leaf phenology and survival, with deciduous species tending to have a better survival rate than evergreen species. There were however only two evergreen species among the 23 studied species, N. diderrichii and Bobgunnia fistuloides, with probabilities of survival of 31.2% and 66.0%, respectively, at t = 690 days, below the 73.7% average of the 23 studied species.
Table 4. Relationship between the performance of 23 species (mean and maximum growth, and survival) in plantations and species functional traits.
3.3. Costs
The forest planting team consisted of 13 people, including two nurserymen and one supervisor. This team was only in charge of the silvicultural activities. As a consequence, the cost per hectare was specific to this team. The team was estimated to be able to plant 10 ha per year and to maintain ca. 60 ha per year (three cleanings on 10 ha the first year following planting, two cleanings on 10 ha in the second year, and the last cleaning on 10 ha in the third year). The total estimated cost per hectare was $7038 (€5585) distributed as follows: wages 69%, vehicles 26% (including depreciation, fuel, and maintenance), and materials 5%. If the total cost was split among the main operation stages, the results were as follows: seedling production 11%, site preparation 53%, planting 8%, and maintenance 28%.
Because of the plantation design, the number of expected crop trees was 44 per hectare (44 blocks per hectare with one crop tree per block remaining after thinning). Therefore, the total cost for raising a mature tree from seed was approximated at $160 (€127) in 2014. This figure is probably underestimated because thinning costs were not included. In Cameroon, the MCD ranges from 50 to 100 cm, depending on the species (). Any projections about the time needed for trees to reach this diameter are imprecise. But if the average observed growth rates could be maintained with thinning (see Section 4), the time to the MCD would be ca. 19 years for the best-performing species (T. scleroxylon) and between 30 and 160 years for most other species.
4. Discussion
4.1. Identification of the best species for enrichment planting
Many plantation methods have been tested since the early twentieth century, but few data are available on species performance in young plantations, even for important timbers like Entandrophragma spp. (Dupuy & Mille Citation1993) or species of concern for conservation like P. elata (on CITES Appendix II), A. congolensis, and B. fistuloides (classified CR and EN in the IUCN red list, respectively). Most of the quantitative information available is from projections of early species growth derived from older trees in plantations of a known age, but with little information on plantation maintenance (Ndongo et al. Citation2009; Ebuy et al. Citation2011). Moreover, to avoid bias due to environmental and time variations, multispecies comparison in the same site during the same period has been needed, as was carried out in this study.
We found promising growth rates and high survival rates for some species (e.g., T. scleroxylon, T. superba, and L. alata). In comparison to other plantations of the same age (maximum 10 years), our estimates of mean annual diameter increment were (i) higher for T. scleroxylon (Lapido et al. Citation1951; Dupuy & Koua Citation1993), (ii) similar for T. superba (Tariel & Groulez Citation1958; Appiah Citation2012), and (iii) slightly higher for L. alata (Biwolé et al. Citation2012). For most of the other species, the values we observed were similar or slightly lower than those reported in other studies (Dupuy & Koua Citation1993; Koumba Zaou et al. Citation1998; Onyekwelu Citation2007; Addo-Danso Citation2010).
The positive (but nonsignificant) relationship between survival and growth was not always consistent with results from other studies. For example, Beckage and Clark (Citation2003) found that species with the highest mortality rates outperformed the other species. On the other hand, in our study, for some species (e.g., M. excelsa and Entandrophragma spp.), low growth increments were associated with high mortality rates. Attempts to grow M. excelsa in plantations have generally failed due to attacks by Phytolyma lata, a gall-forming insect. Shade during the first 12–18 months minimizes the development of galls and associated dieback, but repeated attacks in open areas have frequently resulted in high mortality (Nichols et al. Citation1999; Bosu et al. Citation2006). Fayolle et al. (Citation2015) have recently recommended planting M. excelsa in logging gaps rather than in open areas. Similarly, Hypsipyla shoot borers often hamper plantation success for Meliaceae (Khaya spp. and Entandrophragma spp.) in open areas. In our study, planting K. anthotheca under light shade () allowed for good growth and reduced mortality, confirming the results obtained by Opuni-Frimpong et al. (Citation2008) in Ghana.
The high mortality rates obtained for the other Meliaceae species (Entandrophragma spp.) were not due to shoot borer attack but rather the result of shoots being browsed by wild Bovidae (mainly Tragelaphus spekii and Cephalophus spp.) (personal observation). Such damage in fallow forests with canopy structure very similar to young plantations has also been reported by Hall (Citation2008). Due to their low re-sprouting capacity, the growth of the injured trees was probably reduced by competing, overtopping vegetation (). The tradeoffs among survival, growth, and herbivory present a serious challenge for these species (Goodale et al. Citation2014).
4.2. Relationships between functional traits and species performance
The lack of significant correlations between qualitative functional traits and performance measures might be due to several factors. Variation in regeneration guild was limited; most of the species used were deciduous and light-demanding (either pioneer or nonpioneer), only two species were evergreen, and none was a shade bearer. Martínez-Garza et al. (Citation2013) found that regeneration guild could be used to predict tree growth, but they also emphasized that some nonpioneer species performed nearly as well as pioneers. Functional guilds are not discrete because there is a continuum in such traits as light requirements (Agyeman et al. Citation1999). The maximum growth of nonpioneer species also occurs in partial to full vertical illumination (Martínez-Garza et al. Citation2005). Nursery-grown seedlings of nonpioneer species can cope with high light levels and a low water supply, and they grow faster in the open conditions than in shade (Fayolle et al. Citation2015). The type of dispersal () was also a poor predictor of species growth, perhaps because the main dispersal categories can be found in both fast- and slow-growing tropical tree species (Agyeman et al. Citation1999).
The weak relationship between quantitative functional traits () and growth is more surprising because wood density, seed mass, and adult stature are usually considered to be significant predictors of tree growth and mortality for trees with diameter above 10 cm (Poorter et al. Citation2008). Wood density was negatively correlated with maximum growth rate, but its relationship with mean growth rate was not significant, possibly because too few species were sampled (Poorter et al. Citation2008).
Growth and mortality rates are often negatively correlated with seed mass. In our experiment, the relationship, although negative, was not statistically significant. Seed size affects survival and indirectly growth since small-seeded species have limited reserves and must quickly deploy roots and leaves to become autotrophic (Poorter et al. Citation2008). Many small-seeded species have photosynthetic cotyledons and a high SLA, which boosts juvenile growth. But correlation between seed size and growth disappears over time as the initial SLA differences are reduced.
Variation in SLA can be a main driver of interspecific variation in seedling growth rate (Poorter & Bongers Citation2006), although SLA is only a good predictor at a low irradiance level and not in open sites (Martínez-Garza et al. Citation2013). Other leaf traits, like leaf dry mass content (LDMC), could be better predictors for open sites, at least for nonpioneer species, because a high LDMC is an adaptation to survive in dry open area conditions (Martínez-Garza et al. Citation2013). Leaves with higher LDMC have higher moduli of elasticity and thicker and more rigid lignified cell walls than species with lower LDMC. These characteristics allow them to maintain leaf turgor in dry conditions (Martínez-Garza et al. Citation2013). For the species studied, however, LDMC may not predict growth well due to the overlap in LDMC values between the fast-growing and slow-growing species used in this study. Vanhal (Citation2013) reported LDMC values of 0.51, 0.31, and 0.36 g·g−1 for fast-growers T. scleroxylon, T. superba, and L. alata, respectively, versus LDMC values of 0.30, 0.33, and 0.36 g·g−1, for slow growers Afzelia bipindensis, E. cylindricum, and Pterocarpus soyauxii, respectively.
Maximum size reached by the studied species was also a poor predictor of performance, probably because the relationship between height and growth depends on the ontogenic stage. Light increases with height in forest canopies, and the crowns of taller species receive more light than those of shorter species (Poorter et al. Citation2008). Nearly all the studied species were able to reach the canopy and the variance in sizes was probably too small to detect any relationship between height and growth. Another architectural feature, such as crown length, might have been a better predictor. Long crown species have a larger leaf area and are more self-shading at midday, thus minimizing photoinhibition due to high leaf temperature (Martínez-Garza et al. Citation2013).
4.3. Perspectives on supply and costs
Without long-term monitoring, any growth projection is imprecise, but useful estimates can be made. If unthinned, growth for any of the studied species may not be linear over a long time period (Paine et al. Citation2012). Obiang et al. (Citation2014) showed that growth rates of two species (A. klaineana and L. alata) decrease when their stems reach 50–70 cm in diameter. However, in mixed plantations, Dupuy and Koua (Citation1993) observed a linear growth rate of Khaya spp. until at least 50 cm in diameter. In 50-year-old unthinned plantations, diameter growth rates for the best-performing stems of Distemonanthus benthamianus (at 825 surviving stems per hectare (SSH)), E. cylindricum (800 SSH), L. alata (1375 SSH), Mansonia altissima (300 SSH), and T. scleroxylon (800 SSH) were 10, 7, 9, 10, and 14 mm·year−1, respectively (Ndongo Citation2006). Given such high growth rates after 50 years, we can assume that the average growth rates we observed can be maintained up to the legal cutting diameter () if thinning is performed. The possible time to reach this diameter ranged from 19 years (T. scleroxylon) to 599 years (M. excelsa). This latter value can be explained by the low growth rate of M. excelsa under repeated attacks by P. lata in open areas.
In Central Africa, forests remain the property of the State and concessions are licensed to private logging companies for a cutting cycle. In Cameroon, the duration of the cutting cycle is 30 years. Over this period, only two of the studied species would probably reach the MCDs: T. scleroxylon and T. superba (). Their average volumes at the MCDs should be 6.3 and 2.8 m3, if calculated with the volume tables used by the government, V = 0.000209D2,35 and V = 0.000252D2,28, respectively (Fayolle et al. 2013).
The per tree cost we estimated for raising a mature tree (US$160) is roughly equivalent to the market sales price for 1 m3 of round wood: average log FOB (Free On Board) prices $163 for T. scleroxylon (ITTO Citation2012) and $128 for T. superba (http://database.prota.org). The FOB prices for other species can be much higher (e.g., ca. $350 for P. elata, http://database.prota.org) but their growth rates are lower (0.8 m3 for P. elata after 30 years if calculated with the volume table recommended by Fayolle et al. 2013). While our estimates of production costs are rough, they help explain the current financial disincentive for implementing silvicultural operations, as shown in Amazonia by Schulze (Citation2008). The high cost of planting has always been cited as one of the main reasons for the abandonment of this practice in tropical regions. Mechanization could reduce the expensive preparation of the sites (53% of the total cost) and should be tested. Alternatively, gap enrichment planting is relatively inexpensive (Doucet et al. Citation2009; Schulze Citation2008). Planting in logging gaps should be favored for some species that have shown good performance in this environment, such as M. excelsa or B. toxisperma (Fayolle et al. Citation2015).
5. Conclusions
Simple mixtures could be an effective way to restore biodiversity of timber species and to maintain a long-term supply of goods and services from Central African moist logged forests. Although long-term monitoring is needed to confirm the 5-year results from this study, some native species with high growth and survival rates may perform as well in plantations as exotic species (Dupuy et al. Citation1999), but the quality of their wood needs to be monitored because the effect of fast growth is unknown (Bhat Citation2000).
Management costs, and a legal framework for plantations that does not address regeneration, limit plantation establishment. Long-term concessions and lower taxes for logging companies that replant harvested species are prerequisites for employing silviculture both at the larger scale and for day-to-day management.
Acknowledgments
The authors thank the Cameroonian authorities, particularly the MINFOF and the MINRESI, for allowing this work. They are grateful to the Pallisco Company, and specifically Michel Rougeron, Loïc Douaud, Richard Fétéké, and the technical crew for silvicultural research and operations, for access to the field, for the seed collection, the raising of seedlings in the nursery, the plantation, and the regular monitoring of seedlings in plantations. Acknowledgments are also due to the asbl Nature+, specifically to Michèle Federspiel, Benjamin Cerisier, and Ezana Semereab, for logistic and scientific support. The authors thank Mark Horvat for checking the English. Finally, they thank the anonymous reviewers and Nicholas Brokaw for helpful comments on the manuscript.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Addo-Danso SD 2010. Survival and growth in a moist-semi deciduous forest in Ghana: comparison of monoculture and mixed-species plantations [Thesis]. Freiburg, Germany: Faculty of Forest and Environmental Science, Albert-Ludwigs University.
- Agyeman VK, Swaine MD, Thompson J. 1999. Responses of tropical forest tree seedlings to irradiance and the derivation of a light response index. J Ecol. 87:815–827.
- Appiah M. 2012. Changes in plant species composition within a planted forest in a deciduous agroecosystem in Ghana. Agrofor Syst. 85:57–74.
- Bates D, Maechler M, Bolker B. 2012. Fitting Linear Mixed-Effects Models Using lme4. J Stat Soft.67:1–48
- Bayol N, Borie JM. 2004. Itinéraires techniques d’aménagement des forêts de productions en Afrique centrale. Bois For Trop. 281:35–48.
- Beckage B, Clark JS. 2003. Seedling survival and growth of three forest tree species: the role of spatial heterogeneity. Ecology. 84:1849–1861.
- Bhat KM. 2000. Timber quality of teak from managed tropical plantations with special reference to Indian plantations. Bois For Trop. 263:6–16.
- Biwolé A, Bourland N, Daïnou K, Doucet JL. 2012. Définition du profil écologique de l’azobé, Lophira alata, une espèce ligneuse africaine de grande importance: synthèse bibliographique et perspectives pour des recherches futures. Biotech Agron Soc Env. 16:217–228.
- Biwolé AB, Morin-Rivat J, Fayolle A, Bitondo D, Dedry L, Dainou K, Doucet JL. 2015. New data on the recent history of the littoral forests of southern Cameroon: an insight into the role of historical human disturbances on the current forest composition. Plant Ecol Evol. 148:19–28.
- Bosu PP, Cobbinah JR, Nichols JD, Nkrumah EE, Wagner MR. 2006. Survival and growth of mixed plantations of Milicia excelsa and Terminalia superba 9 years after planting in Ghana. For Ecol Manag. 233:352–357.
- Bourland N, Cerisier F, Daïnou K, Livingstone Smith A, Hubau W, Beeckman H, Brostaux Y, Fayolle A, Biwolé AB, Fétéké F, et al. 2015. How tightly linked are pericopsis elata (Fabaceae) patches to anthropogenic disturbances in Southeastern Cameroon? Forests. 6:293–310.
- Bourland N, Kouadio YL, Fétéké F, Lejeune P, Doucet J-L. 2012. Ecology and management of Pericopsis elata (Harms) Meeuwen (Fabaceae) populations: A review. Biotechnol Agron Soc Environ. 16:486–498.
- Brunck F, Grison F, Maitre HF. 1990. L’Okoumé: Monographie. Montpellier: Centre Technique Forestier Tropical (CIRAD-Forêt).
- Chave J, Coomes D, Jansen S, Lewis SL, Swenson NG, Zanne AE. 2009. Towards a worldwide wood economics spectrum. Ecol Lett. 12:351–366.
- Chazdon RL. 2003. Tropical forest recovery: legacies of human impact and natural disturbances. Perspect Plant Ecol Evol Syst. 6:51–71.
- Chazdon RL. 2008. Beyond deforestation: restoring forests and ecosystem services on degraded lands. Science. 320:1458–1460.
- Dawkins HC. 1958. The management of natural tropical high - forest with special reference to Uganda, Institute paper. Oxford: Imperial forestry institute, University of Oxford.
- De Wasseige C, De Marcken P, Bayol N, Hiol Hiol F, Mayaux P, Desclée B, Billand A, Nasi R. 2012. Les Forêts du Bassin du Congo - Etat des Forêts 2010. Luxembourg: Office des publications de l’UE.
- Doucet J-L 2003. L’alliance délicate de la gestion forestière et de la biodiversité dans les forêts du centre du Gabon [dissertation]. Faculté Universitaire des Sciences Agronomiques de Gembloux.
- Doucet JL, Kouadio YL, Monticelli D, Lejeune P. 2009. Enrichment of logging gaps with moabi (Baillonella toxisperma Pierre) in a Central African rain forest. For Ecol Manag. 258:2407–2415.
- Dupuy B, Chézeaux E. 1994. La sylviculture du niangon en plantation. Bois For Trop. 239:9–22.
- Dupuy B, Koua MB. 1993. Les plantations d’acajou d’Afrique. Leur sylviculture en forêt dense humide ivoirienne. Bois For Trop. 236:25–42.
- Dupuy B, Maître HF, NGuessan Kanga A. 1999. Table de production du teck (Tectona grandis): L’exemple de la Côte d’Ivoire: La sylviculture du teck. Bois For Trop. 261:7–16.
- Dupuy B, Mille G. 1993. Timber plantations in the humid tropics of Africa. FAO forestry paper 98. Rome: FAO.
- Ebuy J, Lokombe JP, Ponette Q, Sonwa D, Picard N. 2011. Allometric equation for predicting aboveground biomass of three tree species. J Trop For Sci. 23:125–132.
- Fargeot C, Forni E, Nasi R. 2004. Réflexions sur l’aménagement des forêts de production dans le bassin du Congo. Bois For Trop. 281:19–34.
- Fayolle A, Ouédraogo DY, Ligot G, Daïnou K, Bourland N, Tekam P, Doucet JL. 2015. Differential performance between two timber species in forest logging gaps and in plantations in Central Africa. Forests. 6:380–394.
- Fayolle A, Picard N, Doucet J-L, Swaine M, Bayol N, Bénédet F, Gourlet-Fleury S. 2014. A new insight in the structure, composition and functioning of central African moist forests. For Ecol Manag. 329:195–205.
- Fayolle A, Rondeux J, Doucet JL, Ernst G, Bouissou C, Quevauvillers S, Bourland N, Fétéké R, Lejeune P. 2013. Réviser les tarifs de cubage pour mieux gérer les forêts du Cameroun. Bois For Trop. 317:35–49.
- Gillet JF, Doucet JL. 2013. The abundance of charcoal fragments emphasizes the assumption of huge palaeofires in the mixed moist semi-evergreen rainforest of the northern republic of Congo. In: Damblon F, editor. Proceedings of the Fourth International Meeting of Anthracology; 2008 September 8–13; Brussels. BAR International Series 2486. Rome: Archaeopress.
- Goodale UM, Berlyn GP, Gregoire TG, Tennakoon KU, Ashton MS. 2014. Differences in survival and growth among tropical rain forest pioneer tree seedlings in relation to canopy openness and herbivory. Biotropica. 46:183–193.
- Gourlet-Fleury S, Mortier F, Fayolle A, Baya F, Ouédraogo D, Bénédet F, Picard N. 2013. Tropical forest recovery from logging: a 24 year silvicultural experiment from Central Africa. Philos Trans R Soc B Biol Sci. 368:20120302.
- Hall JS. 2008. Seed and seedling survival of African mahogany (Entandrophragma spp.) in the Central African Republic: implication for forest management. For Ecol Manag. 255:292–299.
- Harrell FE. 2001. Regression modeling strategies: with applications to linear models, logistic regression, and survival analysis. New York: Springer-Verlag.
- Hawthorne WD. 1995. Ecological profiles of Ghanaian forest trees. Tropical forestry papers 29. Oxford: Oxford Forestry Institute.
- Hijmans RJ, Cameron SE, Parra JL, Jones PG, Jarvis A. 2005. Very high resolution interpolated climate surfaces for global land areas. Int J Climatol. 25:1965–1978.
- ITTO. 2012. Annual review and assessment of the world timber situation. Yokohama: ITTO.
- Jones A, Breuning-Madsen H, Brossard M, Dampha A, Deckers J, Dewitte O, Gallali T, Hallett S, Jones R, Kilasara M, et al. 2013. Soil atlas of Africa. Luxembourg: Publications Office of the European Union.
- Karsenty A, Gourlet-Fleury S. 2006. Assessing sustainability of logging practices in the Congo Basin’s managed forests: the issue of commercial species recovery. Ecol Soc. 11:26.
- Koumba Zaou P, Nze Nguema S, Mapaga D, Deleporte P. 1998. Croissance de 13 essences de bois d’œuvre plantées en forêt gabonaise. Bois For Trop. 256:21–33.
- Lapido DO, Leakey RRB, Grace J. 1951. Clonal variation in a four-year-old plantation of Triplochiton scleroxylon K. Schum. and its relation to the predictive test for branching habit. Silvae Genet. 40:130–135.
- Martínez-Garza C, Bongers F, Poorter L. 2013. Are functional traits good predictors of species performance in restoration plantings in tropical abandoned pastures? For Ecol Manag. 303:35–45.
- Martínez-Garza C, Pena V, Ricker M, Campos A, Howe HF. 2005. Restoring tropical biodiversity: leaf traits predict growth and survival of late-successional trees in early-successional environments. For Ecol Manag. 217:365–379.
- Meunier Q, Moumbogou C, Doucet JL. 2015. Les Arbres Utiles du Gabon. Belgique: Les presses Agronomiques de Gembloux.
- Morin-Rivat J, Fayolle A, Gillet JF, Bourland N, Gourlet-Fleury S, Oslisly R, Bremond L, Bentaleb I, Beeckman H, Doucet JL. 2014. New evidence of human activities during the holocene in the lowland forests of the Northern Congo basin. Radiocarbon. 56:209–220.
- Ndongo PAO. 2006. Evaluation de la potentialité des plantations forestières au Centre-Sud Cameroun : résultats des mesures effectuées dans l’arboretum de Mbalmayo et des enquêtes menées en périphérie de sa réserves. France: Cirad.
- Ndongo PAO, Peltier R, Linjouom I, Louppe D, Smektala G, Beligne V, Njoukam R, Tieche B, Temgoua L. 2009. Plantations de bois d’oeuvre en zone équatoriale africaine: Cas de l’arboretum de l’Enef de Mbalmayo au sud du Cameroun. Bois For Trop. 299:37–48.
- Nichols JD, Ofori DA, Wagner MR, Bosu P, Cobbinah JR. 1999. Survival, growth and gall formation by Phytolyma lata on Milicia excelsa established in mixed-species tropical plantations in Ghana. Agric For Entomol. 1:137–141.
- Obiang NLE, Ngomanda A, Hymas O, Chézeaux E, Picard N. 2014. Diagnosing the demographic balance of two light-demanding tree species populations in central Africa from their diameter distribution. For Ecol Manag. 313:55–62.
- Onyekwelu JC. 2007. Growth, biomass yield and biomass functions for plantation-grown Nauclea diderrichii (de wild) in the humid tropical rainforest zone of south-western Nigeria. Bioresour Technol. 98:2679–2687.
- Opuni-Frimpong E, Karnosky DF, Storer AJ, Cobbinah JR. 2008. Silvicultural systems for plantation mahogany in Africa: Influences of canopy shade on tree growth and pest damage. For Ecol Manag. 255:328–333.
- Ouédraogo DY, Beina D, Picard N, Mortier F, Baya F, Gourlet-Fleury S. 2011. Thinning after selective logging facilitates floristic composition recovery in a tropical rain forest of Central Africa. For Ecol Manag. 262:2176–2186.
- Ouédraogo D-Y, Fayolle A, Daïnou K, Demaret C, Bourland N, Lagoute P, Doucet J-L. 2014. Enrichment of logging gaps with a high conservation value species (Pericopsis elata) in a Central African moist forest. Forests. 5:3031–3047.
- Paine CE, Marthews TR, Vogt DR, Purves D, Rees M, Hector A, Turnbull LA. 2012. How to fit nonlinear plant growth models and calculate growth rates: an update for ecologists. Methods Ecol Evol. 3:245–256.
- Paine CE, Amissah L, Auge H, Baraloto C, Baruffol M, Bourland N, Bruelheide H, Daïnou K, de Gouvenain RC, Doucet JL, Doust S, Fine PVA, Fortunel C, Haase J, Holl KD, Jactel H, Li X, Kitajima K, Koricheva J, Martínez-Garza C, Messier C, Paquette A, Philipson C, Piotto D, Poorter L, Posada JM, Potvin C, Rainio K, Russo SE, Ruiz-Jaen M, Scherer-Lorenzen M, Webb CO, Wright SJ, Zahawi RA, Hector A. 2015. Globally, functional traits are weak predictors of juvenile tree growth, and we do not know why. J Ecol. 103:978–989.
- Parrotta JA, Turnbull JW, Jones N. 1997. Catalyzing native forest regeneration on degraded tropical lands. For Ecol Manag. 99:1–7.
- Piotto D. 2008. A meta-analysis comparing tree growth in monocultures and mixed plantations. For Ecol Manag. 255:781–786.
- Piotto D, Vıquez E, Montagnini F, Kanninen M. 2004. Pure and mixed forest plantations with native species of the dry tropics of Costa Rica: a comparison of growth and productivity. For Ecol Manag. 190:359–372.
- Poorter L, Bongers F. 2006. Leaf traits are good predictors of plant performance across 53 rain forest species. Ecology. 87:1733–1743.
- Poorter L, Wright SJ, Paz H, Ackerly DD, Condit R, Ibarra-Manríquez G, Harms KE, Licona JC, Martínez-Ramos M, Mazer SJ, et al. 2008. Are functional traits good predictors of demographic rates? Evidence from five neotropical forests. Ecology. 89:1908–1920.
- Potvin C, Gotelli NJ. 2008. Biodiversity enhances individual performance but does not affect survivorship in tropical trees. Ecol Lett. 11:217–223.
- Putz FE, Zuidema PA, Synnot T, Peña- Claros M, Pinard MA, Sheil D, Vanclay JK, Sist P, Gourlet-Fleury S, Griscom B, Palmer J, Zagt R. 2012. Sustaining conservation values in selectively logged tropical forests: the attained and the attainable. Cons Lett. 5:296–303.
- Ruiz Pérez M, Ezzine De Blas D, Nasi R, Sayer JA, Sassen M, Angoué C, Gami N, Ndoye O, Ngono G, Nguinguiri JC, et al. 2005. Logging in the Congo Basin: A multi-country characterization of timber companies. For Ecol Manag. 214:221–236.
- Schulze M. 2008. Technical and financial analysis of enrichment planting in logging gaps as a potential component of forest management in the eastern Amazon. For Ecol Manag. 255:866–879.
- Slik JWF, Bernard CS, Van Beek M, Breman FC, Eichhorn KAO. 2008. Tree diversity, composition, forest structure and aboveground biomass dynamics after single and repeated fire in a Bornean rain forest. Oecologia. 158:579–588.
- Stanturf JA, Palik BJ, Dumroese RK. 2014. Contemporary forest restoration: A review emphasizing function. For Ecol Manag. 331:292–323.
- Tariel J, Groulez J. 1958. Les plantations de limba au Moyen-Congo. Bois For Trop. 61:9–25.
- Van Gemerden BS, Olff H, Parren MPE, Bongers F. 2003. The pristine rain forest? Remnants of historical human impacts on current tree species composition and diversity. J Biogeogr. 30:1381–1390.
- Vanhal E 2013. Stratégies démographiques et fonctionnelles des arbres d’Afrique Centrale [dissertation]. University of Liège.
- Worldclim. 2015. Global climate data [Internet]; [cited 2015 Sep 29]. Available from: http://www.worldclim.org/
- Zanne AE, Lopez-Gonzalez G, Coomes DA, Ilic J, Jansen S, Lewis SL, Miller RB, Swenson NG, Wiemann MC, Chave J 2009. Global wood density database. Dryad. Identifier; [cited 2015 Dec 14]. Available from: http://hdl.handle.net/10255/dryad.235
- Zuur A, Ieno EN, Walker N, Saveliev AA, Smith GM. 2009. Mixed effects models and extensions in ecology with R. New York (NY): Springer Science & Business Media.