ABSTRACT
Understanding biodiversity in homegardens embedded in landscapes dominated by commercial monoculture agriculture is critical for sustainable management of agrobiodiversity and meeting rural households’ needs in the face of global changes. We assessed agrobiodiversity in the 120 homegardens and its contribution to rural household livelihood strategies within a commercial monoculture sugarcane cultivation land matrix in eastern Uganda. We recorded a total of 68 plant species from 46 genera representing 27 families. Species richness spanned 6 to 19 species, and α-diversity (H’) ranged from 0.6 to 2.3; with 86.67% of the homegardens having H’ >1. Species composition differed significantly (global RANOSIM = 0.153, p < 0.001) among the villages. The most important and commonly maintained plants were those that provided food, fuelwood and money income and included Zea mays L., Manihot esculenta, Phaesolus spp., Coffea sp., Musa spp., Ipomea batatus and Artocarpus heterophyllus. Most of the crops cited as useful by households were also frequent and visible in many of the homegardens. Although homegardens still hold some valuable plants, there is also loss of important plants from the agricultural system including cowpeas, soya beans, bambara groundnuts, finger millet, cotton, aerial yams and oysternut essential for sustaining household livelihoods. This loss, precipitated by increased land-use/cover change to commercial sugarcane plantations threatens agrobiodiversity conservation and the benefits households derive from homegardens. Our findings underline the importance of homegardens in the conservation of indigenous agrobiodiversity, and indicate that with the continued expansion of commercial sugarcane cultivation this opportunity may be lost.
Edited by Christine Fürst
Introduction
Understanding the current status and forecasting the future state of tropical biodiversity requires that we understand the levels and patterns of agrobiodiversity in landscapes actively managed and modified by humans for a wide variety of traditional and commercial purposes, including commercial agriculture (Bawa et al. Citation2004; Butler et al. Citation2007; Chazdon et al. Citation2009). The drive for economic development mainly based on intensification of commercial monoculture agriculture is leading to increasing land-use/cover changes in many developing tropical countries. These land use/cover changes are a global concern as land surface processes impact on ecosystem goods and services (Lambin et al. Citation2003); and many of the drivers of biodiversity loss across all tropical ecosystems are associated with the intensification of agriculture (Hails Citation2002; Green et al. Citation2005; Donald & Evans Citation2006). In sub-Saharan Africa, land-use/cover change is driven largely by the increasing demand for more land to meet and improve food security, alleviate poverty and enhance the human and social welfare at household and community levels (Maitima & Gumbo Citation2007). These processes might affect the abundance and distribution of native biodiversity including agriculture biodiversity (i.e. agrobiodiversity) by changing the landscape structure over time and space (Wiens Citation1989).
In Uganda, like other sub-Saharan African countries, increasing efforts for poverty alleviation and wealth creation by the government have moved commercial firms and households to adopt commercial agriculture leading to expansive lands of monocultures (incl. oil palms, sugarcane, tobacco, sunflower) in historically forested and subsistence-oriented agriculture production systems. In the eastern part of Uganda, particularly the Busoga sub-region, commercial monoculture sugarcane growing that offers attractive incentives both to the Uganda government and the rural communities dominates the cultivated landscape. Consequently, there have been changes in the landscape resulting in a commercial sugarcane plantations land matrix, interspersed with small patches of homegardens where a range of plants that are important to households is maintained. In this study, a homegarden is regarded as the area around a homestead or peridomestic area where household members plant and tend useful plants including food crops, herbs, fruits, medicinal plants, fuelwood and ornamental plants (Rugalema et al. Citation1994; Howard Citation2006; Perrault-Archambault & Coomes Citation2008). Worldwide, sugarcane crop cultivation has progressively increased but sustaining this monoculture increase while ensuring minimal environmental impact is a major challenge (Lakshmanan et al. Citation2005).
Although commercial sugarcane cultivation improves household incomes and road infrastructure in areas where it is practiced in Uganda, it is taking up most of the land previously used for subsistence agriculture, leaving only a few patches of homegardens. Moreover, little is known about the implications of such changes in the landscape for agrobiodiversity sustainability, and livelihoods of rural subsistence farming households. Uganda’s economy like for many sub-Saharan African countries is predominantly agrarian, depending largely on indigenous agrobiodiversity. Agrobiodiversity includes those components of biological diversity relevant to food and agriculture as well as the components of biological diversity that constitute the agro-ecosystem (Negri & Polegri Citation2009; Frison et al. Citation2011), and the knowledge associated with them (Jackson et al. Citation2007). Conservation of biodiversity is viewed as a means of achieving adaptation strategies for human well-being in the face of climate and land use/cover changes. Its maintenance provides a broad range of essential goods and services, which support ecosystem resilience and productivity (Tilman Citation1999), thus it is a core principle of sustainable agriculture and agroecology (Altieri & Merrick Citation1987; Paoletti Citation2001; Marshall & Moonen Citation2002). Sustainable development requires the reconciliation of demands for biodiversity conservation and increased agricultural production (Eilu et al. Citation2003), aided by a clear understanding of the biodiversity within such changing landscapes. An inventory of agrobiodiversity in patches (e.g. homegardens) embedded in a landscape matrix dominated by commercial monoculture agriculture is essential for identification and promotion of appropriate management strategies for conserving biodiversity in tropical regions (e.g. Zuidema & Sayer Citation2003; Lindenmayer et al. Citation2008). In addition, understanding the geographical and social distributions of crop species, and the factors that shape patterns of species diversity is crucial to efforts aimed at promoting agrobiodiversity conservation (e.g. Zimmerer Citation1996; Brush & Meng Citation1998; Bellon Citation2004). In this study, we assessed the dominant notion in literature, which suggests that monocultures cause agrobiodiversity erosion (Masayi & Netondo Citation2012; Namb & Netondo Citation2013). We explored the following research questions; (i) What is the species diversity and composition within the homegardens in the villages that are practicing commercial sugarcane growing? (ii) Which plants within the homegardens do households consider to be the most important to their livelihoods and why? (iii) Which benefits do households derive from their homegardens? and (iv) What has been the status of indigenous important plants in the homegardens over the years? In this study, we focus on homegardens as a potential source of agrobiodiversity in a fragmented landscape dominated by commercial monoculture sugarcane plantations, which can be conserved for ecosystem services. We envisage that the findings from the current study will inform future agricultural policies and programs.
Materials and methods
Study area description
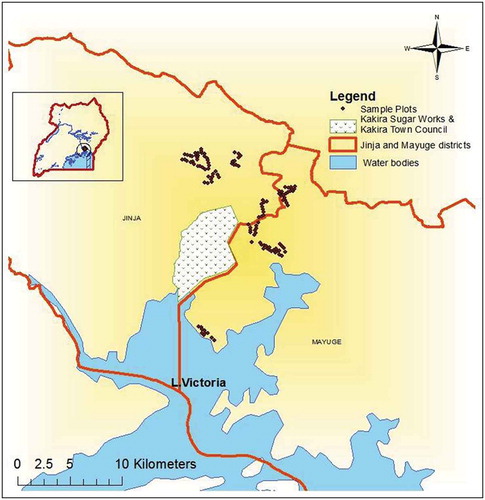
The study was conducted in homegardens within villages that are predominantly commercial sugarcane out-grower villages (i.e. areas with sugarcane farmers with contractual obligations with sugar companies) in Jinja and Mayuge districts within a radius of 21 km from Kakira Sugar Works Limited (KSWL) factory in eastern Uganda. Most sugar companies in these districts rely on contracting individual farmers to grow sugarcane for them under the out-grower scheme and therefore they are the most relevant for the current study. The 21 km radius is the area where most of the registered out-growers for KSWL are located and therefore assumed areas of large-scale commercial sugarcane growing. The study area lies at an altitude of 1180 m, and between latitude 00° 30ʹ N and longitude 33° 17ʹ E (). It has an undulating topography with an average slope of 5%. The area has a mean annual temperature of 28°C and is characterized by two rain seasons (i.e. March–June and September–December) with a mean annual precipitation of 1283 mm recorded over the last 40 years (Department of Meteorology Citation2000). The major soil types are rhodilixic ferralsols (Ssendiwanyo et al. Citation1998). Soil texture varies from clay loams/sandy clay loams to sandy loam (Isabirye et al. Citation2000). The vegetation of the area was originally a mosaic of vegetation/croplands, but it is presently monoculture sugarcane plantations dominated landscape. The 2014 Uganda National and Housing and Population Census estimated the human population of Mayuge district to be about 479,172 people of whom 48.6% were males, while Jinja with 468,256 of whom 48.6% were males (UBOS Citation2014).
Figure 1. Study area showing the 120 sampling sites (·) within a radius of 21 km from Kakira Sugar Works Limited in the commercial sugarcane cultivation villages of Jinja and Mayuge districts, eastern Uganda.
The local population in most parts of Jinja and Mayuge districts are subsistence farmers who mainly grow food crops, such as banana, maize, cassava, potatoes and beans that also serve as cash crops. Traditionally, they grew coffee and cotton as the main cash crops, but with a global slump in prices for cotton and coffee, they were replaced by commercial sugarcane cultivation in monoculture plantations by small-scale farmers under an out-growers scheme and by companies including KSWL. This has led to extensive loss of natural ecosystems and their goods and services, as well as reduction in land available for food crop cultivation. Over 15 years ago, this sub-region was predominantly a coffee–banana agro-ecological zone of Uganda.
Sampling design
Within each district, one sub-county among those adjacent to KSWL and practicing commercial sugarcane cultivation was selected purposively for the study. For Mayuge district, Baitambogwe sub-county was selected while Busedde sub-county was selected for Jinja district. Baitambogwe sub-county consists of eight parishes while Busedde sub-county has five parishes. A total of 12 villages were selected from the 13 parishes. Within each village, 10 homegardens were chosen purposively and their locations recorded using a GPS. Since homegardens have a high spatial variability and prevent the use of the standard plot sizes (Zarin et al. Citation2002), like 20 ×20 m plots, the entire homegarden was considered as a sample plot and surveyed. Sugarcane plantations were not sampled since they were monocultures devoid of plant agrobiodiversity and any agroforestry practices given their nature of management.
Data collection
Each entire homegarden was sampled for agrobiodiversity and all plants encountered enumerated, identified and recorded as present or absent in both scientific and local name. The Flora of Tropical East Africa (FTEA) (Polhill Citation1952 et seq.) was used to identify plants in the field. Voucher specimens of species that could not be identified or only be identified by local name in the field were collected, pressed and subsequently identified at Makerere University Herbarium, Botany Department (MHU), Kampala, Uganda. The trends in agrobiodiversity over the years and its contribution to local livelihoods were assessed using a face-to-face questionnaire (Appendix 1) interview of the 120 household heads (assumed to be the keeper for the sampled homegarden) comprising 10 from each of the 12 villages. The aim was to document the uses for the plants, land use type and type of ownership of the land and plants. Data collection was carried out between July 2012 and February 2013.
Ethical consideration
Respondents were allowed to exercise their right to voluntarily accept or refuse to participate in the study. They were also assured of confidentiality and anonymity.
Data analysis
Homegardens were characterized in terms of species presence/absence, species richness, diversity (i.e. α-diversity based on Shannon–Wiener Diversity Index – H’) and similarity (). For each village homegardens, species area curves were constructed using Species Diversity and Richness (SDR®) Version IV Software (Seaby & Henderson Citation2006). In this construction, a step-wise calculation of cumulative species richness as data from each replicate sample plot are added to the total village species richness approach was used. Variation in species composition among the sampling plots was calculated using the ANalysis Of SIMilarity (ANOSIM) Index technique in Community Analysis Package (CAP®) Version IV (Seaby & Henderson Citation2006). We assessed the relationship between the frequency of mention of the plant as useful and its abundance (or availability) using the Spearman rank correlation in Minitab 16 Statistical Software (MINITAB Citation2010).
Table 1. A brief description of the diversity indices and measures of similarity applied in the analysis of the plant species data from the 120 homegardens in the sugarcane cultivation villages of Jinja and Mayuge districts, eastern Uganda.
Results
Agrobiodiversity in the homegardens
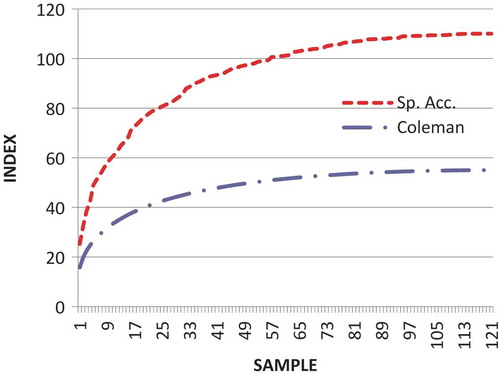
A total of 68 plant species, representing 46 genera and 27 families were recorded in the 120 homegardens selected from the 12 villages (). The species accumulation (Sp. Acc.) curve reached an asymptote, showing that species richness was not far from being completely recorded for this landscape (). The flattening of the species accumulation curve indicates that a reasonable number of samples were taken and any more intensive sampling would likely yield only a few additional species. The species accumulation curve did not, however, touch the Coleman curve suggesting high heterogeneity (patchiness) among the homegardens.
Table 2. List of plant species encountered across all the 120 homegardens in the sugarcane cultivation villages of Jinja and Mayuge districts, eastern Uganda.
Figure 2. Coleman and species accumulation (Sp. Acc.) curves of the floristic composition from the 120 homegardens of the selected 12 villages of Jinja and Mayuge districts, eastern Uganda.
The most common crop species across the homegardens were maize (Zea mays), cassava (Manihot esculenta), beans (Phaesolus spp.), coffee (Coffea spp.), bananas (Musa spp.) and jackfruits (Artocarpus heterophyllus). The most species-rich families were Moraceae (11 species), Leguminosae (4), Euphorbiaceae (3), Myrtaceae (3) and Combretaceae (3). Species richness varied among the homegardens and ranged between 6 and 19 plant species per garden. Similarly, α-diversity (H’) varied; with H’ ranging between 0.6 and 2.3, while 86.67% (i.e. 104 of 120) of the homegardens had H’ ≥1.
An ANOSIM revealed that villages differed significantly (global RANOSIM = 0.153, p = 0.001) in terms of plant species composition, corroborating the heterogeneity test results in . However, of the 66 pairwise comparisons, only 43 pairs differed significantly with RANOSIM ranging between 0.101 and 0.388, and p-values ranging between 0.001 and 0.031. Average similarities within villages ranged from 49.71 to 68.44%, with only seven of the villages having a within SIMilarity PERcentage (SIMPER) of ≥60%, and only 1 village with a SIMPER of <50% (). Hence, homegardens within the same village were not highly similar suggesting a heterogeneous nature of homegardens within the same village. The common species that contributed highly (i.e. over 60% of the cumulative %) to the average similarity among the homegardens within all the villages were: Coffea robusta L., Musa spp., Artocarpus heterophyllus Lam, Mangifera indica L. and Makharmia lutea K. Schum. Homegarden patches, although continuously under increasing threat of conversion to monoculture commercial sugar plantations, still harboured some of the major crops that originally defined the coffee–banana agro-ecological zone of Uganda (). Although villages differed in terms of species composition, the average percentage dissimilarity between them was relatively low ranging between 35.01% and 53.92% (), which corroborated the results from the ANOSIM analysis.
Table 3. A SIMilarity PERcentange (SIMPER) analysis matrix showing the average percentage dissimilarity between villages based on the homegarden agro-diversity data for each studied commercial sugarcane cultivation village in Mayuge and Jinja districts, eastern Uganda.
Figure 3. A homegarden maintained using agroforestry practices in one of the sugarcane cultivation villages of Jinja and Mayuge districts, eastern Uganda. The crops here included cassava (Manihot esculenta), maize (Zea mays L), coffee (Coffea sp.), bananas (Musa spp.) and agroforestry trees (e.g. Maesopsis eminii).

Contribution of agrobiodiversity to household livelihood strategies
Homegardens provided a variety of ecosystem goods and services, and other benefits to households as follows: food (i.e. 61.46% – Jinja; 56.13% – Mayuge district) and income from sales of crops maintained (i.e. 32.29% – Mayuge; 40.78% – Jinja). The other benefits were fuelwood, timber and shade from trees. In both districts, the most important crops cultivated in the homegardens included maize, cassava, beans, coffee, bananas and sweet potatoes. This corroborates the findings of an agrobiodiversity inventory, which showed the same plants to be the most common in the homegardens. Bananas and coffee were found in almost every homegarden studied in all the villages. In addition, fruits trees including Artocarpus heterophyllus (jackfruit), Mangifera indica (mangoes), Carica papaya (pawpaw) and Persea americana (avocado), and fuelwood species, such as Cassia siamea and Eucalyptus spp., were considered important and most cultivated in the homegardens. The area cultivated per household is small (approximately 0.4 ha/household) and continues to decline as much of the arable land is converted to monoculture sugarcane plantations. There was no significant association between the socio-economic factors (i.e. age of respondents, size of homegardens, sex of respondent and education status) and the crops grown in the homegardens (p > 0.05) for all the pairwise comparisons. On the other hand, the frequency of mention of a plant as useful was significantly correlated (Pearson correlation = 0.829, p < 0.05) with its abundance in the area. This suggests that crops that were more abundant were highly likely to be reported as useful by many respondents.
Farmers reported the following challenges in maintaining indigenous crops: limited land for crop cultivation (71%), pests and diseases (59.18%), extreme weather conditions (15.51%), low yields (10.20%) and low soil fertility (13.06%), lack of market for traditional crops, old age of farmer and labour-intensive agricultural practices. Limited land was cited as the most common reason for abandoning cultivation of some of the food crops, as most of the land, which was previously available for crop cultivation is now rented out for commercial sugarcane growing in both districts. Consequently, households had abandoned growing of key indigenous food and cash crops such as finger millet, cowpeas, groundnuts (including bambara groundnuts), soya beans, oysternut, aerial yams and cotton.
Discussion
Plant diversity and composition in homegardens
Homegardens throughout the tropics are recognized as loci for in-situ conservation of agrobiodiversity (Altieri et al. Citation1987) particularly in subsistence agricultural systems, but diversity therein varies according to ecological and socio-economic factors (Christanty et al. Citation1986). In the current study, a variety of plants were maintained in homegardens, with an alpha diversity (H’) greater than 1 for the majority of homegardens. The H’ value is within the reported range of 0.93–3.0 for tropical homegardens (Karyono Citation1990; Drescher Citation1998; Kehlenbeck & Maass Citation2004); and shows a high species diversity (Pinedo-Vasquez et al. Citation2000). However, the total plant diversity, number of useful plant species and diversity is low compared to what has been recorded in other parts of Uganda (e.g. Eilu et al. Citation2003), and elsewhere in the tropical world (e.g. Karyono Citation1990; Soemarwoto & Conway Citation1992; Abdoellah et al. Citation2001; Wezel & Bender Citation2003; Perrault-Archambault & Coomes Citation2008). Despite the small sizes of homegardens (average size 0.25–0.5 acres), a single plot contained almost all categories of crops, since it is the only available land for crop cultivation.
The homegardens differed significantly in species composition, which can be attributed to differences in household’s needs and preferences that depend on the type of foods eaten in the households and the agricultural practices. Although most households grow coffee, their choice of shade trees (i.e. agroforestry practices) varies, contributing to the overall heterogeneity of tree and crop species in this commercial sugarcane plantation matrix. Household socio-economic characteristics (i.e. age and sex of respondent, size of homegarden and level of education) did not influence variation in species composition and diversity among the homegardens. This contrasts with other studies that found species composition of homegardens to be attributed to a variety of socio-economic and demographic conditions of the farmers (e.g. Christanty et al. Citation1986; Bellon & Brush Citation1994; Quiroz et al. Citation2002; Zimmerer Citation2003; Kehlenbeck & Maass Citation2004; Perales & Brush Citation2005; Perreault Citation2005), size of homegarden (Abdoellah et al. Citation2001; Perrault-Archambault & Coomes Citation2008), distance to urban markets and ethnicity (Lamont et al. Citation1999; Kehlenbeck & Maass Citation2004; Wezel & Ohl Citation2005). The lack of association between homegarden species composition and socio-economic characteristics of households suggests other overriding factors such as sugarcane cultivation and ecological attributes such as weather and edaphic factors in the area. Garden diversity could vary according to ecological factors of gardens (Christanty et al. Citation1986), and species number and diversity are reportedly influenced by altitude of homegardens (Karyono Citation1990; Quiroz et al. Citation2002), homegarden size (Abdoellah et al. Citation2001) and level of production intensity and market access (Michon & Mary Citation1994; Peroni & Hanazaki Citation2002). The significant correlation between the frequency of mention of useful plants and their abundance suggests that such plants are highly likely to be reported as useful by a high number of respondents. This also suggests that such plants may be more available or visible to the communities (e.g. Phillips & Gentry Citation1993; Lucena et al. Citation2012; Linstädter et al. Citation2013; Ribeiro et al. Citation2014), for example, maize, bananas, coffee and cassava.
Although the homegardens differed significantly in species composition, they shared some common species, and common crops such as coffee, bananas, cassava and maize that were frequently encountered. The high contribution of Coffea robusta L., Musa spp., Artocarpus heterophyllus Lam, Mangifera indica L. and Makharmia lutea K. Schum to the average similarity among the homegardens studied suggests that households across all villages similarly valued these plants. While the maintenance of multi-purpose plants (i.e. for food, fuelwood and income) within the homegardens, is because land gets scarce and farmers invest energy in planting and maintaining plants for various home and commercial uses (Place & Otsuka Citation2000; Bolwig et al. Citation2006). Farmers prefer to grow crops that provide food and incomes (Hoogerbrugge & Fresco Citation1993; Mitchell & Hanstad Citation2004). On the other hand, bananas are usually planted with nurse trees, which account for the abundance of fuelwood and fruit trees in the homegardens.
Benefits of homegardens to households and trends in agrobiodiversity
Agrobiodiversity in the homegardens in this area forms the basis for household food production, income generation and domestic biomass energy needs thereby, enhancing rural household livelihoods. Similar findings have been reported elsewhere (Akinnifesi et al. Citation2010; Albuquerque et al. Citation2005). Homegardens wherever they occur, are characterized by a structural complexity and multi-functionality, which enables the provision of different benefits to people (Galluzzi et al. Citation2010). More notably they serve as a source of food supply ensuring food security and improved nutrition for households (Dyg & Phithayaphone Citation2004; Mitchell & Hanstad Citation2004). Furthermore, agrobiodiversity plays a pivotal role in sustaining and strengthening food, nutrition, health, domestic energy needs and livelihood security (Frei & Becker Citation2004; Toledo & Burlingame Citation2006), and is therefore essential for the survival and well-being of the human population. In the study area, bananas and coffee are the major sources of food and income. The presence of coffee plantations will contribute to biodiversity sustainability in the area compared to monoculture sugarcane plantation. Therefore, the conservation of cultivated plants in homegardens provides significant economic and nutritional benefits for the rural poor (Thrupp Citation2000; Eyzaguirre & Linares Citation2004; Kumar & Nair Citation2006).
The study area continues to experience a decrease in agrobiodiversity as the traditional food crops, such as cowpeas, soya beans, bambara groundnuts, finger millet, cotton, aerial yams and oysternut have been lost or abandoned by households. This loss of agrobiodiversity is mainly attributed to increasing commercial monoculture sugarcane cultivation that has occupied land that would be used for indigenous crops cultivation. Similarly, changes in land-use patterns leading to loss of traditional agroecosystems is one of the main causes of disappearance of traditional crop species and threatening extinction of some in western Nepal (Sunwar et al. Citation2006; Galluzzi et al. Citation2010), and south-western China (e.g. Fu et al. Citation2005, Citation2008, Citation2009). The abandonment or loss of groundnuts/peanuts (including bambara nuts) and cotton from the subsistence agricultural system in the study area has also been reported in Asia by Fu et al. (Citation2008).
Commercialization of agriculture that includes commercial monoculture sugarcane growing has been cited as the main cause of biodiversity loss including food crop diversity loss in many parts of the tropical world (Altieri et al. Citation1987; FAO Citation2004). Commercial monoculture sugarcane growing has been shown to be a major contributor to indigenous agrobiodiversity (i.e. food crops and vegetable) loss in sugar belts of western Kenya (Netondo et al. Citation2010), and has replaced crops, such as rice, cassava and maize. In the study area, sugarcane cultivation is perceived by households as the most profitable crop because of the assured market from sugar corporations operating in the area. This has also been exacerbated by the dwindling world market for other cash crops like coffee, thus leaving the local communities with no option but to concentrate on sugarcane. Commercial sugarcane cultivation and the attendant unsustainable land management practices (i.e. intensive and extensive destruction of natural ecosystems) renders the soils infertile (Clay Citation2004) and adversely affects crop production (Liwenga & Kangalawe Citation2009) and ultimately leads to loss of indigenous food crops from the subsistence agricultural system and reduced ecosystem services from agrobiodiversity.
Conclusions
The high diversity of indigenous crop species in homegardens implies that the local communities in Jinja and Mayuge districts still appreciate their value in addressing household needs. The results of this study imply that the most important plants to households are the ones that are often maintained in homegardens. Multi-purpose plant species, such as maize, cassava, beans, bananas, coffee and fruit trees dominated the homegardens. Food, income from sales of crops maintained, fuelwood, timber and shade from trees are the benefits households derive from their homegardens. However, cultivation of crops, such as finger millet, cowpeas, local land races of groundnuts (such as bambara groundnuts), soya beans, oysternut, aerial yams and cotton has declined in villages where sugarcane is commercially cultivated. Our findings underline the importance of homegardens in the conservation of indigenous agrobiodiversity, and indicate that with the continued expansion of commercial sugarcane cultivation this opportunity may be lost. There is a need to adopt agroforestry practices to support biodiversity conservation in homegardens and surrounding farmed landscapes; and judicious planning of commercial sugarcane cultivation to reduce agrobiodiversity erosion. An improved understanding of the household management decisions of homegardens may benefit from the use of various ‘Use Indices’ that take into account the general, current and potential uses in evaluating the usefulness of plants maintained therein.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Abdoellah OS, Takeuchi K, Parikesit GB, Hadikusumah HY. 2001. Structure and function of homegarden revisited. In: Proceedings of First. seminar ‘toward harmonisation between development and environmental conservation in biological production’. JSPS-DGHE Core University Program in Applied Biosciences. The University of Tokyo, Tokyo; p. 167–185.
- Akinnifesi FK, Sileshi GW, Ajayi OC, Akinnifesi AI, De Moura EG, Linhares JF, Rodrigues I. 2010. Biodiversity of the urban homegardens of São Luís city, Northeastern Brazil. Urban Ecosyst. 13:129–146.
- Albuquerque UP, Andrade LHC, Caballero J. 2005. Structure and floristics of homegardens in Northeastern Brazil. J Arid Environ. 62:491–506.
- Altieri MA, Anderson MK, Merrick LC. 1987. Peasant Agriculture and the conservation of crop and wild plant resources. Conservation Biol. 1:49–58.
- Altieri MA, Merrick LC. 1987. In situ conservation of crop genetic resources through maintenance of traditional farming systems. Econ Bot. 41:86–96.
- Bawa KS, Kress WJ, Nadkarni NM, Lele S. 2004. Beyond paradise — meeting the challenges in tropical biology in the 21st century. Biotropica. 36:437–446.
- Bellon MR. 2004. Conceptualizing interventions to support on-farm genetic resource conservation. World Developm. 32:159–172.
- Bellon MR, Brush SB. 1994. Keepers of maize in Chiapas, Mexico. Economic Bot. 48:196–209.
- Bolwig S, Pomeroy D, Tushabe H, Mushabe D. 2006. Crops, trees, and birds: biodiversity change under agricultural intensification in Uganda’s farmed landscapes. Danish J Geogr. 106:115–130.
- Brush SB, Meng E. 1998. Farmer’s valuation and conservation of crop genetic resources. Genet Resour Crop Evol. 45:139–150.
- Butler SJ, Vickery JA, Norris K. 2007. Farmland biodiversity and the footprint of agriculture. Science [Internet]. 315:381–384. Available from: www.sciencemag.org
- Chazdon RL, Harvey CA, Komar O, Griffith AM, Ferguson BG, Martínez-Ramos M, Morales H, Nigh R, Soto-Pinto L, Breugel M, Philpott SM. 2009. Beyond reserves: a research agenda for conserving biodiversity in human-modified tropical landscapes. Biotropica. 41:142–153.
- Christanty L, Abdoellah OS, Marten GG, Iskandar J. 1986. Traditional agroforestry in West Java: the pekarangan (homegardens) and kebun-talun (annual-perennial rotation) cropping systems. In: Marten GG, editor. Traditional agriculture in southeast Asia. Boulder (CO): Westview; p. 132–158.
- Clay JW. 2004. World agriculture and the environment: a commodity by-commodity guide to impacts and practices. Washington (DC), USA: Island Press.
- Department of Meteorology. 2000. Meteorological Data. Republic of Uganda: Ministry of Water and Environment.
- Donald PF, Evans AD. 2006. Habitat connectivity and matrix restoration: the wider implications of agri-environment schemes. J Appl Ecol. 43:209–218.
- Drescher AW. 1998. Hausgärten in afrikanischen Räumen: Bewirtschaftung nachhaltiger Produktionssysteme und Strategien der Ernährungssicherung in Sambia und Zimbabwe. Pfaffenweiler, Germany: Centaurus-Verlagsgesellschaft; p. 275.
- Dyg PM, Phithayaphone S 2004. Homegardenss in the Lao PDR – linkages between agricultural biodiversity and food security. Proceedings of Symposium on biodiversity and food security. Ministry of Agriculture and Forestry, Lao PDR; p. 52–59. Available from: http://s3.amazonnaws.com/zanran_storage/www.fao.org/ContentPages/81861413.pdf
- Eilu G, Obua J, Tumuhairwe JK, Nkwine C. 2003. Traditional farming and plant species diversity in agricultural landscapes of south-western Uganda. Agric Ecosyst Environ. 99:125–134.
- Eyzaguirre PB, Linares OF, editors. 2004. Homegardens and agrobiodiversity. Washington (DC): Smithsonian Books.
- FAO. 2004. What is agrobiodiversity? Food and Agricultural Organization of the UN, Rome. Available from: http://www.oecd.org/dataoecd/44/18/40713249.pdf
- Frei M, Becker K. 2004. Agro-biodiversity in subsistence-oriented farming systems in a Philippine upland region: nutritional considerations. Biodiver Conserv. 13:1591–1610.
- Frison EA, Cherfas J, Hodgkin T. 2011. Agricultural biodiversity is essential for a sustainable improvement in food and nutrition security. Sustainability. 3:238–253.
- Fu Y, Brookfield H, Guo H, Chen J, Chen A, Cui J. 2009. Smallholder rubber plantation expansion and its impact on local livelihoods, land use and agrobiodiversity, a case study from Daka, Xishuangbanna, south-western China. Int J Sustainable Dev World Ecol. 16:22–29.
- Fu Y, Chen J, Guo H, Chen A, Cui J. 2008. Utilization and conservation strategies of plant resources in tropical montane agroecosystems, a case study from Xishuangbanna, SW China. Int J Biodivers Sci Manage. 4:32–43.
- Fu Y, Guo H, Chen A, Cui J. 2005. Swidden–fallow agroecosystem change with socio-economic development of Xishuangbanna, Yunnan, China, a case study from Daka and Baka. Mt Res Dev. 25:365–371.
- Galluzzi G, Eyzaguirre P, Negri V. 2010. Homegardens: neglected hotspots of agro-biodiversity and cultural diversity. Biodivers Conserv. 19:3635–3654.
- Green RE, Cornell SJ, Scharlemann JPW, Balmford A. 2005. Farming and the fate of wild nature. Science. 307:550–555.
- Hails RS. 2002. Assessing the risks associated with new agricultural practices. Nature. 418:685–688.
- Hoogerbrugge I, Fresco LO. 1993. Homegarden systems: agricultural characteristics and challenges. London: International Institute for Environment and Development, Gatekeeper series 39; p. 21.
- Howard P. 2006. Gender and social dynamics in Swidden and homegardens in Latin America. In: Kumar BM, Nair PKR, editors. Tropical homegardens: a time-tested example of sustainable agroforestry. Dordrecht: Springer; p. 159–182.
- Isabirye M, Ronsmans E, Magunda MK, Raes D, Raju DVN, Deckers J. 2000. Irrigation of sugarcane in Mayuge district, Uganda. Available from: http://www. Uganda-Isabirye%20et%20al-Irrigation%20scheduling.pdf.
- Jackson LE, Pascual U, Hodgkin T. 2007. Utilizing and conserving agro biodiversity in agricultural landscapes. Agric Ecosys Environm. 121:196–210.
- Karyono. 1990. Homegardenss in Java. Their structure and function. In: Landauer K, Brazil M, editors. Tropical homegardenss. Tokyo, Japan: The United Nations University; p. 138–146.
- Kehlenbeck K, Maass BL. 2004. Crop diversity and classification of homegardenss in Central Sulawesi, Indonesia. Agrofor Sys. 63:53–62.
- Kumar BM, Nair PKR, editors. 2006. Tropical homegardens: a time-tested example of sustainable agroforestry. Advances in agroforestry vol. 3. Netherlands: Springer.
- Lakshmanan P, Geijskes RJ, Aitken KS, Groff CLP, Bonnett GD, Smith GR. 2005. Sugarcane biotechnology: the challenges and opportunities. Vitro Cell Dev Biology-Plant. 41:345–363.
- Lambin EF, Geist HJ, Lepers E. 2003. Dynamics of land use and land-cover change in tropical regions. Annu Rev Environ Resour. 28:205–231.
- Lamont SR, Eshbaugh WH, Greenberg AM. 1999. Species composition, diversity, and use of homegardens among three Amazonian villages. Econ Bot. 53:312–126.
- Lindenmayer DB, Hobbs RJ, Montague-Drake R, Alexandra J, Bennett A, Burgman M, Cale P, Calhoun A, Cramer V, Cullen P, et al. 2008. A checklist for ecological management of landscapes for conservation. Ecol Lett. 11:78–91.
- Linstädter A, Kemmerling B, Baumann G, Kirscht H. 2013. The importance of being reliable – Local ecological knowledge and management of forage plants in a dryland pastoral system (Morocco). J Arid Environ. 95:30–40.
- Liwenga ET, Kangalawe RYM. 2009. Climate change/variability and implications on Agricultural production and livelihoods in the Southern highlands of Tanzania. In: Maro PS, Majule AE, editors. Strengthening local agricultural innovations to adapt to climate change in Botswana, Malawi, South Africa and Tanzania; p. 124–135. Available from: http://www.sadc.int
- Lucena RFP, Medeiros PM, Araújo EL, Alves AGC, Albuquerque UP. 2012. The ecological apparency hypothesis and the importance of useful plants in rural communities from Northeastern Brazil: an assessment based on use value. J Environ Manage. 96:106–115.
- Maitima JM, Gumbo DJ 2007. Land use in Sub-Saharan Africa. Available from: http://www.ilri.org/Link/Publications/Land%20%20Use%20Chapter%20Proofs%20pd.pdf
- Marshall EJP, Moonen AC. 2002. Field margins in northern Europe: their functions and interactions with agriculture. Agric Ecosyst Environ. 89:5–21.
- Masayi N, Netondo GW. 2012. Effects of sugarcane farming on diversity of vegetable crops in Mumias Division, Western Kenya. Int J Biodivers Conserv. 4:515–524.
- Michon G, Mary F. 1994. Conversion of traditional village gardens and new economic strategies of rural households in the area of Bogor, Indonesia. Agrofor Syst. 25:31–58.
- MINITAB. 2010. Minitab 16 statistical software [Internet]. Pennsylvania, USA: Minitab Inc., State College. Available from: www.minitab.com
- Mitchell R, Hanstad T. 2004. Small homegarden plots and sustainable livelihoods for the poor. LSP Working paper No.11. Rome, Italy: Food and Agriculture Organization of the United Nations.
- Namb N, Netondo GW. 2013. Changes in agro-biodiversity as a result of sugarcane farming in Mumias Division, Western Kenya. Afr J Agric Res. 8:3735–3743.
- Negri V, Polegri L 2009. Genetic diversity in homegardenss in Umbria a cowpea case study. In: Bailey A, Eyzaguire P, Maggioni L, editors. Proceedings of a workshop on crop genetic resources in European homegardenss; Rome, Italy: Bioversity International; p 55–61
- Netondo GW, Waiswa F, Maina L, Naisiko T, Masayi N, Ngaira JK. 2010. Agrobiodiversity endangered by sugarcane farming in Mumias and Nzoia sugar belts of Western Kenya. African J Environ Sci Technol. 4:4378–4445.
- Paoletti M. 2001. Biodiversity in agroecosystems and bioindicators of environmental health. In: Shiyomi M, Koizumi H, editors. Structure and function in agroecosystems design and management advances in agroecology. Boca Raton (FL): CRC Press; p. 11–44.
- Perales HR, Brush SB. 2005. Maize diversity and ethnolinguistic diversity in Chiapas, Mexico. Proc Natl Acad Sci. 102:949–954.
- Peroni N, Hanazaki N. 2002. Current and lost diversity of cultivated varieties, especially cassava, under Swidden cultivation systems in the Brazilian Atlantic Forest. Agric. Ecosyst Environ. 92:171–183.
- Perrault-Archambault M, Coomes OT. 2008. Distribution of agrobiodiversity in homegardens along the Corrientes River, Peruvian Amazon. Econ Bot. 62:109–126.
- Perreault T. 2005. Why chacras (Swidden Gardens) persist: agrobiodiversity, food security, and cultural identity in the Ecuadorian Amazon. Hum Organizat. 64:327–339.
- Phillips O, Gentry A. 1993. The useful plants of Tambopata, Peru: II. Additional hypothesis testing in quantitative ethnobotany. Econ Bot. 47:33–43.
- Pinedo-Vasquez M, Padoch C, McGrath D, Ximenes T. 2000. Biodiversity as a product of smallholders’ strategies for overcoming changes in their natural and social landscapes: a report prepared by the Amazonia Cluster. The United Nations University project on People, Land Management and Environmental Change (PLEC) news and views no. 15. United Nations University, Australia. Availble from: http://www.unu.edu/env/plec
- Place F, Otsuka K. 2000. Population pressure, land tenure and tree resource management in Uganda. Land Econ. 76:233–251.
- Polhill RM, editor. 1952 et seq. Flora of tropical east Africa (FTEA). Kew: Royal Botanic Gardens.
- Quiroz C, Gutiérrez M, Rodríguez D, Pérez D, Ynfante J, Gámez J, Pérez De Fernandez T, Marques A, Pacheco W. 2002. Homegardens and in situ conservation of agrobiodiversity – Venezuelan component. In: Watson JW, Eyzaguirre PB, editors. Homegardens and in situ conservation of plant genetic resources in farming systems. Proceedings of the Second International Homegardens Workshop. 2001 July 1719; Rome, Italy. Witzenhausen, Germany: International Plant Genetic Resources Institute (IPGRI); p. 73–82.
- Ribeiro JES, Carvalho TKN, Ribeiro JPO, Guerra NM, Da Silva N, Pedrosa KM, Alves CAB, De Sousa Junior SP, Souto JS, Nunes AT, et al. 2014. Ecological apparency hypothesis and availability of useful plants: testing different use values. Ethnobotany Res Appl. 12:415–432.
- Rugalema GH, Okting’ati A, Johnsen FH. 1994. The homegardens agroforestry system of Bukoba district, North-Western Tanzania. 1. Farming system analysis. Agrofor Syst. 26:53–64.
- Seaby RM, Henderson PA. 2006. Species diversity & richness (SDR), Version 4. Lymington, UK: Pisces Conservation Ltd.
- Seaby RM, Henderson PA. 2006. Community Analysis Package (CAP), Version IV. Lymington, UK: Pisces Conservation Ltd.
- Soemarwoto O, Conway GR. 1992. The Javanese homegarden. J Farm Syst Res -Ext. 2:95–118.
- Ssendiwanyo EV, Isabirye M, Butegwa CN. 1998. Soils of Ikulwe district farm institute, Mayuge. Soils and soil fertility management program annual report 1998. Kampala, Uganda: National Agricultural Research Organisation.
- Sunwar S, Thornström CG, Subedi A, et al. 2006. Homegardens in western Nepal: opportunities and challenges for on-farm management of agrobiodiversity. J Biodiver Conserv. 15:4211–4238.
- Thrupp LA. 2000. Linking agricultural biodiversity and food security: the valuable role of agrobiodiversity for sustainable agriculture. Int Aff. 76:265–281.
- Tilman D. 1999. Global environmental impacts of agricultural expansion: the need for sustainable and efficient practices. Proc Natl Acad Sci. 96:1857–1861.
- Toledo A, Burlingame B. 2006. Biodiversity and nutrition: a common path toward global food security and sustainable development. J Food Composit Analys. 19:477–483.
- [UBOS] Uganda Bureau of Statistics. 2014. National Population and Housing Census 2014: Provisional Results, November 2014 Revised Edition. Republic of Uganda. Available from: http://www.ubos.org
- Wezel A, Bender S. 2003. Plant species diversity of homegardens of Cuba and its significance for household food supply. Agrofor Syst. 57:39–49.
- Wezel A, Ohl J. 2005. Does remoteness from urban centres influence plant diversity in homegardens and Swidden fields: A case study from the Matsiguenka in the Amazonian rain forest of Peru. Agroforestry Syst. 65:241–251.
- Wiens JA. 1989. Spatial scaling in ecology. Funct Ecol. 3:385–397.
- Zarin DJ, Huijun G, Enu-Kwesi L. 2002. Guidelines on the assessment of plant species in agricultural landscapes. In: Brookfield H, Padoch C, editors. Cultivating biodiversity— understanding, analysing and using agricultural diversity. London: ITDG Publishing; p. 57–69.
- Zimmerer KS. 1996. Changing fortunes: biodiversity and peasant livelihood in the Peruvian Andes. Berkeley: University of California Press.
- Zimmerer KS. 2003. Geographies of seed networks for food plants and approaches to agrobiodiversity conservation. Soc Nat Resour. 16:583–601.
- Zuidema PA, Sayer JA. 2003. Tropical forests in multi-functional landscapes: the need for new approaches to conservation and research. In: Zuidema PA, editor. Tropical forests in multi-functional landscapes. Seminar proceedings. Utrecht, The Netherlands: Prince Bernhard Centre, Utrecht University.
Appendix 1. A research questionnaire focusing on the contribution of agrobiodiversity to rural household livelihood strategies in the sugarcane cultivation villages of Jinja and Mayuge districts, eastern Uganda
MAKERERE UNIVERSITY
RESEARCH QUESTIONNAIRE
Dear respondent, this questionnaire is designed for academic purposes only and any personal information obtained will be kept confidential. The topic of the research is ‘CONTRIBUTION OF BIODIVERSITY TO RURAL LIVELIHOODS’. We request for an interview with you, but you are free not to answer any of or all the questions if you do not feel comfortable. You are also free to withdraw from the interview at any time without any worries. We look forward to your cooperation to make this study a success.
a) District……………… c) Parish ………………
b) Sub-county……………… d) Village………………
e) Household/Homegarden No………………
SECTION A: BIO DATA
a) Sex of Respondent: Male [ ] Female [ ]
b) What is your level of education attained? (i) Non-formal education [ ] (ii) Primary [ ]
(iii) Secondary [ ] (iv) Post-Secondary [ ]
c) Age …………
d) Number of children……………….
SECTION B: CROPS
1. What is the approximate size of your homegarden?
……………………………………………………………………………………………………………………………
2. What are the reasons for maintaining this homegarden?
……………………………………………………………………………………………………………………………
3. List some of the challenges you face in maintaining this homegarden.
……………………………………………………………………………………………………………………………
4. List the benefits you derive from maintaining your homegarden.
……………………………………………………………………………………………………………………………
5. Which crops do you maintain in your homegarden?
……………………………………………………………………………………………………………………………
6. Which of those crops are considered most important to local livelihoods and why?
……………………………………………………………………………………………………………………………
7. Which benefits do local households derive from the crops found within the villages?
……………………………………………………………………………………………………………………………
8. List the other crops that you used to grow on your farm but no longer cultivate.
……………………………………………………………………………………………………………………………
9. Why have you stopped growing those crops?
……………………………………………………………………………………………………………………………
10. Which benefits did you derive from those crops?
……………………………………………………………………………………………………………………………
11. Do you have alternative crops that have replaced the disappeared crops?
……………………………………………………………………………………………………………………………
12. What effect does the disappearance of these crops have on your livelihood?
……………………………………………………………………………………………………………………………
13. What measures or alternative activities do you have in place to minimize the effects in (8) above?
……………………………………………………………………………………………………………………………
