Abstract
Circular RNAs (circRNAs) are a large family of noncoding RNAs (ncRNAs) found in metazoans. Systematic studies of circRNAs have just begun. Here, we discuss circRNA biogenesis and functions with a focus on studies indicating great diversification of circRNAs. We highlight the recent identification of a special subtype of circRNAs, called EIciRNAs, and their role in transcriptional regulation. New insights on RNA-RNA interaction and other features associated with circRNA biology are also discussed.
Abbreviations
| AMO | = | antisense morpholino |
| circRNA | = | circular RNA |
| CLIP | = | cross-linked immunoprecipitation |
| dsRNA | = | double-stranded RNA |
| EIciRNA | = | exon-intron circular RNA |
| FISH | = | fluorescent in situ hybridization |
| ncRNA | = | noncoding RNA |
| QKI | = | quaking |
| MBL | = | muscleblind |
| RNAP II | = | RNA polymerase II |
| snRNP | = | small nuclear ribonucleic proteins |
| ssRNA | = | single-stranded RNA. |
Circular RNAs (circRNAs) are a recently identified large family of noncoding RNAs (ncRNAs) in metazoan.Citation1-4 Thousands of circRNAs, as alternative transcripts from exonic backsplicing of coding genes (exonic circRNAs), have been found in mammalian cells as well as in model organisms such as Drosophila melanogaster and Caenorhabditis elegans.Citation4-6 circRNAs in eukaryotic cells may include a variety of molecules, such as plant viroids, the genome of some ssRNA viruses, the loop of intronic lariats, and so on. As the majority of circRNAs identified in animal cells are composed of exonic circRNAs, in this point-of-view we will focus on exonic circRNAs; therefore, when we use the term ‘circRNAs’ we are referring to exonic circRNAs, unless otherwise specified.Citation7,8
Cotranscriptional or Posttranscriptional Biogenesis of circRNAs
In the same way as regular linear splicing, the generation of circRNAs by backsplicing takes place in the nucleus. In linear splicing, most introns in the pre-mRNA are removed before transcription is finished.Citation9 We asked whether backsplicing was also cotranscriptional? Two lines of evidence were presented to support this idea in Drosophila ().Citation10 First, a substantial number of circRNAs were found as nascent chromatin-bound RNAs. Secondly, a mutant RNA polymerase II (RNAP II) with slower elongation rate in favor of cotranscriptional splicing increased the ratio of linear to circRNAs for individual genes. These results indicated that most fly circRNAs might be generated cotranscriptionally, and linear splicing and backsplicing might compete with each other at the cotranscriptional level.Citation8 The argument for the posttranscriptional biogenesis of circRNAs was brought forward with the observation that a stable 3′ end of pre-mRNA was in favor of circRNA production in mammalian cells when tested with an overexpressing plasmid ().Citation11 Future studies are required to resolve these 2 somewhat conflicting findings, although a second possibility could also be considered.
Figure 1. circRNAs are generated co- or post-transcriptionally, and their biogenesis may be mediated by complementary sequences (purple and yellow boxes) in the flanking introns. EIciRNAs associate with U1 snRNPs to regulate gene transcription in cis. U1 snRNPs also play roles in preventing premature polyadenylation and in determining transcriptional direction. The majority of circRNAs is composed exclusively of exonic sequences and localize to the cytoplasm; 2 of them were are shown to function as microRNA sponges.
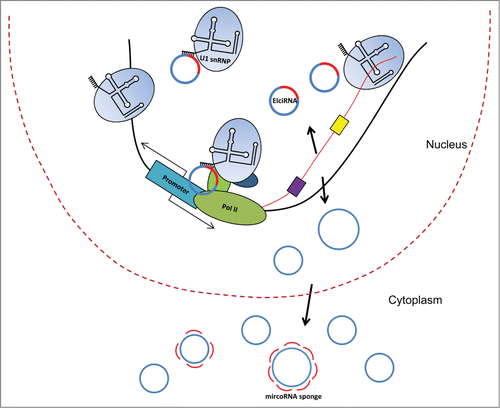
It is possible that some (maybe even the majority of) circRNAs are generated cotranscriptionally, while the biogenesis of the others may be posttranscriptional. In eukaryotic cells, most of the canonical linear splicing happens cotranscriptionally, although some introns are still removed posttranscriptionally.Citation9,12 Cotranscriptional splicing efficiency is actually higher in Drosophila than that in mice.Citation13 It is also possible that circRNAs could be divided into 2 subclasses, according to their co- or post-transcriptional biogenesis.
Sequences and Proteins Mediating the Biogenesis of circRNAs
Flanking long introns and complementary repeats such as Alu elements in flanking introns are positively associated with the biogenesis of circRNAsCitation6,11,14-16, suggesting 2 potential features of circRNA biogenesis. Long (maybe also hard-to-splice) introns could serve as marks for the regions of circularization, and then the 2 backsplicing sites might be brought together by the flanking complementary sequences (). On the other hand, not all circRNAs are associated with long flanking introns or intronic repeats, and the exonic sequences involved in backsplicing may also be crucial for the circularization of some circRNAs.Citation11,15 RNA binding proteins such as ADAR, QKI, and MBL, known to be involved in RNA editing or alternative splicing, are associated with the biogenesis of specific circRNAs.Citation6,11,17
Furthermore, circRNAs show expression profiles of cell specificity often independently from the expression patterns of their parent genes, which points to a regulated biogenesis of circRNAs that is cell- and gene-specific.Citation1,2,5,14,15,18-20 It is highly possible that circRNAs are diversified in various ways in their biogenesis.
circRNAs as microRNA Sponges
Once formed, what, if any, are the physiological roles of these non-canonical transcripts? A role in posttranscriptional regulation was demonstrated for 2 circRNAs, circCDR1as and circSry.Citation1,2 Both circRNAs function as microRNA sponges, containing multiple copies of microRNA binding sites. The canonical functional site of microRNAs is the cytoplasm, and, interestingly, most circRNAs also localize in the cytoplasm.Citation2,3 A natural question to ask following these findings is: could it be possible that many or even the majority of circRNAs function as microRNA sponges? The answer to this question may be negative when we consider that only a few out of the thousands of circRNAs in human cells harbor multiple predicted binding sites for individual microRNAs.Citation18 However, Drosophila circRNAs do harbor over a thousand computational miRNA binding sites conserved across the genus,Citation5 indicating a possible divergence in circRNA functions along different branches of the animal kingdom.
EIciRNA as a Special Subtype of circRNA
Recently, a distinct subtype of circRNAs was found in mammalian cells in our lab.Citation15 This novel subclass of circRNAs associated with RNAP II to regulate transcription in the nucleus.Citation15 We speculated that some of these ncRNAs might regulate transcription; therefore, we initially attempted to identify RNAP II-associated ncRNAs via cross-linked immunoprecipitation (CLIP) and identified >100 circRNAs.Citation15 Further analysis revealed that these circRNAs were composed of both exonic and intronic sequences of coding genes. We termed these special circRNAs exon-intron circular RNAs (EIciRNAs). The intron retention makes EIciRNAs distinct from most other circRNAs, which are composed exclusively of exonic sequences. EIciRNAs also tend to have flanking long introns as well as flanking complementary sequences, just like the other circRNAs, although other not yet identified features may lay in the flanking sequences of EIciRNAs. FISH experiments showed that EIciRNAs localized predominantly in the nucleus, consistently with their association with RNAP II. Their cellular localization, again, differentiates EIciRNAs from the other circRNAs.
EIciRNAs Regulate Transcription via RNA-RNA Interactions
Since EIciRNAs associate with RNAP II and localize almost exclusively to the nucleus, we speculated that these circRNAs might play roles in transcriptional regulation. Indeed, knockdown of EIciRNAs with either siRNAs or RNaseH-based antisense oligonucleotides (ASO) decreased the transcription of their parental genes. With multiple lines of evidence, we showed that EIciRNAs enhanced the transcription of their parental genes in cis, although the possibility of trans effects of these EIciRNAs on the expression of other genes could not be excluded.Citation15
How do EIciRNAs promote the expression of their parental genes? We found that U1 snRNPs associated with EIciRNAs at their parental gene promoter. Indeed, RNA-DNA double FISH assay showed the colocalization of EIciRNAs and U1 snRNAs. It may be even possible that the global distribution of U1 snRNAs is associated, to some degree, with the distribution of EIciRNAs in the nucleus. Further supporting the idea that U1 snRNP mediate the cis effect of EIciRNAs, we found that blocking U1 snRNA with antisense morpholino (AMO) specific for U1 snRNA abolished the effect of EIciRNA on the regulation of their parental genes at multiple levels. Therefore, the interaction between U1 snRNA and EIciRNA is indispensable for their role in transcriptional regulation. Furthermore, we suspected that EIciRNAs interacted with U1 snRNA via the 5′ splicing site of their retained introns, which is the U1 snRNA binding site in canonical splicing. Indeed, sterically blocking these sites with AMO decreased the interaction between EIciRNAs and U1 snRNP, and further interactions among RNAP II, U1 snRNP, EIciRNAs, and their parental gene promoter. This blocking eventually resulted in EIciRNA's inability to regulate parent gene transcription.Citation15
Multiple Roles of U1 snRNP
It seems that U1 snRNA plays a central role in mediating the effect of EIciRNAs. In eukaryotic cells, U1 snRNA is initially identified as a component of the spliceosome.Citation21 U1 snRNA also associates with TFIIH to mediate transcription initiation for some specific genes.Citation22 The enhancement of transcription by EIciRNAs through interaction with U1 snRNP may also be one of the regulatory mechanisms of U1 snRNA. Recent studies have demonstrated that U1 snRNAs play roles in preventing premature mRNA polyadenylation and in determining transcriptional direction of divergent promoters.Citation23-25 The number of U1 snRNA molecules is ∼100-fold higher than that of other snRNAs of the mammalian spliceosome.Citation25 Thus, it is highly possible that most or at least a large proportion of U1 snRNAs play roles in mechanisms other than splicing. U1 snRNA may even be a Swiss Army knife-like molecule involved in multiple regulatory events.
Further Insights on Transcriptional Regulation and circRNAs
Transcription is a stringently controlled process that requires many regulatory factors. Several ncRNAs participate in transcriptional regulation by using diverse mechanisms.Citation26 First, ncRNAs such as B2 RNA could directly target RNAP II to exert regulatory functions.Citation27,28 A second class of ncRNAs, such as EBER2 RNA, NEAT2 RNA, and NRSE RNA, influence the status of transcriptional activators or repressors.Citation29-31 A third class of ncRNAs includes U1 snRNA and 7SK RNA and associate with factors participating in transcriptional initiation, elongation, or termination.Citation22,32 EIciRNAs seem to utilize a novel mechanism of RNA-RNA interaction for their roles in transcriptional regulation.
A major way of regulation by RNA is via RNA-RNA interactions.Citation33,34 This is nicely exemplified by the series of RNA-RNA-mediated events between snRNAs and pre-mRNAs, which lead to the removal of eukaryotic introns.Citation9,12,21 A plethora of ncRNAs also execute their physiological roles through specific RNA-RNA recognition and binding.Citation34 RNA-RNA interaction is essential for the 2 functions so far associated with circRNAs—as microRNA sponges in the cytoplasm and as transcriptional regulators in the nucleus. It is reasonable to speculate that circRNAs may play roles by interacting with a plethora of RNAs. Also, intramolecular RNA-RNA binding is involved in circRNA biogenesis, as already discussed.
The discovery of EIciRNAs and the 2 functions so far associated with circRNAs—microRNA sponges and transcriptional regulators—as well as the already discussed disputes around circRNA biogenesis, strongly indicate that circRNAs are a family of RNAs with great diversity.Citation8 Just like linear RNAs, it is possible that the only common feature of circRNAs is that they are all circular.
For circRNAs to function as microRNA sponges the number of molecule for individual circRNAs needs to be high enough; however, most circRNAs are not abundant. Low circRNA copy numbers lead to the argument that the majority of these molecules may be piggyback products of pre-mRNA splicing and/or noise of gene expression,Citation18 although their restricted cellular localization and cell specific expression profiles argue strongly against this view. Copy numbers of EIciRNAs do not need to be high for their cis effect in the nucleus. Copy number and expression profiles are all related to their biogenesis and the completely unexplored subject of circRNA degradation. The cellular localization of individual circRNAs may also even be determined together by biogenesis, transportation, and degradation. The only thing known about circRNA degradation is that circRNAs are presumably more stable than other ncRNAs due to their inaccessibility to exonucleases such as RNase R.Citation35,36 Also, whether and how circRNAs are actively transported within eukaryotic cells are questions that require further investigation.
ncRNAs participate in numerous biological events through various mechanisms, although many ncRNAs remain poorly understood. This is particularly true about circRNAs. The identification of certain circRNAs playing microRNA sponge roles, the specific features associated with circRNA biogenesis, and the role of EIciRNAs in transcriptional regulation may provide only a glimpse of the tip of the iceberg that is circRNA biology.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Funding
The work described in Li et al., 2015Citation15 was supported by the National Basic Research Program of China (2011CBA01103 and 2015CB943000), National Natural Science Foundation of China (31471225, 81171074, and 91232702), CAS (KJZD-EW-L01-2), and the Fundamental Research Funds for the Central Universities (WK2070000034).
References
- Salzman J, Gawad C, Wang PL, Lacayo N, Brown PO. Circular RNAs are the predominant transcript isoform from hundreds of human genes in diverse cell types. PLoS One 2012; 7:e30733; PMID:22319583; http://dx.doi.org/10.1371/journal.pone.0030733
- Memczak S, Jens M, Elefsinioti A, Torti F, Krueger J, Rybak A, Maier L, Mackowiak SD, Gregersen LH, Munschauer M, et al. Circular RNAs are a large class of animal RNAs with regulatory potency. Nature 2013; 495:333-8; PMID:23446348; http://dx.doi.org/10.1038/nature11928
- Hansen TB, Jensen TI, Clausen BH, Bramsen JB, Finsen B, Damgaard CK, Kjems J. Natural RNA circles function as efficient microRNA sponges. Nature 2013; 495:384-8; PMID:23446346; http://dx.doi.org/10.1038/nature11993
- Wang PL, Bao Y, Yee MC, Barrett SP, Hogan GJ, Olsen MN, Dinneny JR, Brown PO, Salzman J. Circular RNA is expressed across the eukaryotic tree of life. PLoS One 2014; 9:e90859; PMID:24609083; http://dx.doi.org/10.1371/journal.pone.0090859
- Westholm JO, Miura P, Olson S, Shenker S, Joseph B, Sanfilippo P, Celniker SE, Graveley BR, Lai EC. Genome-wide analysis of drosophila circular RNAs reveals their structural and sequence properties and age-dependent neural accumulation. Cell Rep 2014; 9:1966-80; PMID:25544350; http://dx.doi.org/10.1016/j.celrep.2014.10.062
- Ivanov A, Memczak S, Wyler E, Torti F, Porath HT, Orejuela MR, Piechotta M, Levanon EY, Landthaler M, Dieterich C, et al. Analysis of intron sequences reveals hallmarks of circular RNA biogenesis in animals. Cell Rep 2015; 10:170-7; PMID:25558066; http://dx.doi.org/10.1016/j.celrep.2014.12.019
- Chen L, Shan G. Circular RNAs remain peculiarly unclear in biogenesis and function. Sci China Life Sci 2015; 58(6):616-8; http://dx.doi.org/10.1007/s11427-015-4855-y
- Chen L, Huang C, Wang X, Shan G. Circular RNAs in eukaryotic cells. Curr Genom 2015; 16(5): 312-8.
- Moore MJ, Proudfoot NJ. Pre-mRNA processing reaches back to transcription and ahead to translation. Cell 2009; 136:688-700; PMID:19239889; http://dx.doi.org/10.1016/j.cell.2009.02.001
- Ashwal-Fluss R, Meyer M, Pamudurti NR, Ivanov A, Bartok O, Hanan M, Evantal N, Memczak S, Rajewsky N, Kadener S. circRNA biogenesis competes with pre-mRNA splicing. Mol Cell 2014; 56:55-66; PMID:25242144; http://dx.doi.org/10.1016/j.molcel.2014.08.019
- Liang D, Wilusz JE. Short intronic repeat sequences facilitate circular RNA production. Genes Dev 2014; 28:2233-47; PMID:25281217; http://dx.doi.org/10.1101/gad.251926.114
- Bentley DL. Rules of engagement: co-transcriptional recruitment of pre-mRNA processing factors. Curr Opin Cell Biol 2005; 17:251-6; PMID:15901493; http://dx.doi.org/10.1016/j.ceb.2005.04.006
- Khodor YL, Menet JS, Tolan M, Rosbash M. Cotranscriptional splicing efficiency differs dramatically between Drosophila and mouse. RNA 2012; 18:2174-86; PMID:23097425; http://dx.doi.org/10.1261/rna.034090.112
- Jeck WR, Sorrentino JA, Wang K, Slevin MK, Burd CE, Liu J, Marzluff WF, Sharpless NE. Circular RNAs are abundant, conserved, and associated with ALU repeats. RNA 2013; 19:141-57; PMID:23249747; http://dx.doi.org/10.1261/rna.035667.112
- Li Z, Huang C, Bao C, Chen L, Lin M, Wang X, Zhong G, Yu B, Hu W, Dai L, et al. Exon-intron circular RNAs regulate transcription in the nucleus. Nat Struct Mol Biol 2015; 22:256-64; PMID:25664725; http://dx.doi.org/10.1038/nsmb.2959
- Starke S, Jost I, Rossbach O, Schneider T, Schreiner S, Hung LH, Bindereif A. Exon circularization requires canonical splice signals. Cell Rep 2015; 10:103-11; PMID:25543144; http://dx.doi.org/10.1016/j.celrep.2014.12.002
- Conn SJ, Pillman KA, Toubia J, Conn VM, Salmanidis M, Phillips CA, Roslan S, Schreiber AW, Gregory PA, Goodall GJ. The RNA Binding Protein Quaking Regulates Formation of circRNAs. Cell 2015; 160:1125-34; PMID:25768908; http://dx.doi.org/10.1016/j.cell.2015.02.014
- Guo JU, Agarwal V, Guo H, Bartel DP. Expanded identification and characterization of mammalian circular RNAs. Genome Biol 2014; 15:409; PMID:25070500; http://dx.doi.org/10.1186/s13059-014-0409-z
- You X, Vlatkovic I, Babic A, Will T, Epstein I, Tushev G, Akbalik G, Wang M, Glock C, Quedenau C, et al. Neural circular RNAs are derived from synaptic genes and regulated by development and plasticity. Nat Neurosci 2015; 18:603-10; PMID:25714049; http://dx.doi.org/10.1038/nn.3975
- Rybak-Wolf A, Stottmeister C, Glažar P, Jens M, Pino N, Giusti S, Hanan M, Behm M, Bartok O, Ashwal-Fluss R, et al.Circular RNAs in the Mammalian Brain Are Highly Abundant, Conserved, and Dynamically Expressed. Mol Cell 2015; S1097-2765: 00218-X; PMID:25921068
- Seraphin B, Rosbash M. Identification of functional U1 snRNA-pre-mRNA complexes committed to spliceosome assembly and splicing. Cell 1989; 59:349-358; PMID:2529976; http://dx.doi.org/10.1016/0092-8674(89)90296-1
- Kwek KY, Murphy S, Furger A, Thomas B, O'Gorman W, Kimura H, Proudfoot NJ, Akoulitchev A. U1 snRNA associates with TFIIH and regulates transcriptional initiation. Nat Struct Biol 2002; 9:800-5; PMID:12389039
- Almada AE, Wu X, Kriz AJ, Burge CB, Sharp PA. Promoter directionality is controlled by U1 snRNP and polyadenylation signals. Nature 2013; 499:360-3; PMID:23792564; http://dx.doi.org/10.1038/nature12349
- Berg MG, Singh LN, Younis I, Liu Q, Pinto AM, Kaida D, Zhang Z, Cho S, Sherrill-Mix S, Wan L, et al. U1 snRNP determines mRNA length and regulates isoform expression. Cell 2012; 150:53-64; PMID: 22770214; http://dx.doi.org/10.1016/j.cell.2012.05.029
- Kaida D, Berg MG, Younis I, Kasim M, Singh LN, Wan L, Dreyfuss G. U1 snRNP protects pre-mRNAs from premature cleavage and polyadenylation. Nature 2010; 468:664-8; PMID:20881964; http://dx.doi.org/10.1038/nature09479
- Goodrich JA, Kugel JF. Non-coding-RNA regulators of RNA polymerase II transcription. Nat Rev Mol Cell Biol 2006; 7:612-6; PMID:16723972; http://dx.doi.org/10.1038/nrm1946
- Espinoza CA, Allen TA, Hieb AR, Kugel JF, Goodrich JA. B2 RNA binds directly to RNA polymerase II to repress transcript synthesis. Nat Struct Mol Biol 2004; 11:822-9; PMID:15300239; http://dx.doi.org/10.1038/nsmb812
- Allen TA, Von Kaenel S, Goodrich JA, Kugel JF. The SINE-encoded mouse B2 RNA represses mRNA transcription in response to heat shock. Nat Struct Mol Biol 2004; 11:816-21; PMID:15300240; http://dx.doi.org/10.1038/nsmb813
- Lee N, Moss WN, Yario TA, Steitz JA. EBV Noncoding RNA Binds Nascent RNA to Drive Host PAX5 to Viral DNA. Cell 2015; 160: 607-18; PMID:25662012; http://dx.doi.org/10.1016/j.cell.2015.01.015
- Yang L, Lin C, Liu W, Zhang J, Ohgi KA, Grinstein JD, Dorrestein PC, Rosenfeld MG. ncRNA- and Pc2 methylation-dependent gene relocation between nuclear structures mediates gene activation programs. Cell 2011; 147: 773-88; PMID:22078878; http://dx.doi.org/10.1016/j.cell.2011.08.054
- Kuwabara T, Hsieh J, Nakashima K, Taira K, Gage FH. A small modulatory dsRNA specifies the fate of adult neural stem cells. Cell 2004; 116:779-93; PMID:15035981; http://dx.doi.org/10.1016/S0092-8674(04)00248-X
- Yang Z, Zhu Q, Luo K, Zhou Q. The 7SK small nuclear RNA inhibits the CDK9/cyclin T1 kinase to control transcription. Nature 2001; 414:317-22; PMID: 11713532; http://dx.doi.org/10.1038/35104575
- Cech TR, Steitz JA.The noncoding RNA revolution-trashing old rules to forge new ones.Cell 2014; 157:77-94; PMID:24679528; http://dx.doi.org/10.1016/j.cell.2014.03.008
- Guil S, Esteller M. RNA-RNA interactions in gene regulation: the coding and noncoding players. Trends Biochem Sci 2015; 40:248-56; PMID:25818326; http://dx.doi.org/10.1016/j.tibs.2015.03.001
- Vincent HA, Deutscher MP. Substrate recognition and catalysis by the exoribonuclease RNase R. J Biol Chem 2006; 281: 29769-75; PMID:16893880; http://dx.doi.org/10.1074/jbc.M606744200
- Suzuki H, Zuo Y, Wang J, Zhang MQ, Malhotra A, Mayeda A. Characterization of RNase R-digested cellular RNA source that consists of lariat and circular RNAs from pre-mRNA splicing. Nucleic Acids Res 2006; 34:e63; PMID:16682442; http://dx.doi.org/10.1093/nar/gkl151
