ABSTRACT
Pioneer transcription factors are defined by their ability to bind nucleosome-occupied regions. Here, we discuss the properties of nucleosomes bound by pioneers at enhancer regions. We describe how select pioneers bind nucleosome-occupied or -depleted enhancer sites. Importantly, by revisiting and expanding existing data sets, we show differential H2A.Z and p300/CBP association at bound enhancers, highlighting two possible pioneering modes.
Introduction
During development, cell fate is driven by choices in gene expression programs, which are ultimately dictated by the activation of stage-specific enhancers. These decisions are well illustrated by the Waddington epigenetic landscape metaphor in which a marble is rolling down a hill with multiple slopes, representing cell fate, with the bottom representing terminally differentiated stages.Citation1 Critical to cell fate decision is the activation or repression of regulatory elements of the genome, including enhancers. The acquisition of new, tissue-specific enhancers is largely dependent on the binding of pioneer transcription factors (TFs) able to bind nucleosome-occupied regions (NORs), ultimately turning them into nucleosome-depleted regions (NDRs) via recruitment of chromatin remodelers. Several pioneer TFs have previously been described,Citation2,3 including Forkhead as well as OSKM TFs that are able to bind nucleosomal DNA.Citation4,5 For TFs capable of binding nucleosomal DNA, the question arises as to how stable these complexes are, and whether bound nucleosomes might be epigenetically marked, if at all. A clue to answer this question was previously reported in mouse embryonic stem cells (ES), whereby distal p300- and Oct4-bound sites were highly enriched in the histone variant H2A.Z.Citation6 This histone variant has been proposed as a transitional link between closed and open chromatin conformations.Citation7 The first genome-wide studies describing H2A.Z identified positioning of this variant at nucleosomes surrounding transcriptional start sites (TSSs); however, it was typically depleted at the −1 nucleosome.Citation8,9 However, more recent developments have revealed that these early analyses might have missed potentially less stable nucleosomes associated to H2A.Z, due to technical limitations of the native ChIP of the then studies. The H2A.Z nucleosome was eventually reported to be associated with labile nucleosomes at promoters, when extracted with low salt concentrations,Citation10 although this procedure added more background to the data. Another work also suggested that H2A.Z nucleosomes protect shorter regions of DNA,Citation11 a phenomenon associated with lability for H2A.Bdb, another H2A variant.Citation12 Since then, a dedicated study using optical tweezers, measuring the number of unzipped base pairs as a function of increasing force applied to DNA/nucleosomal complexes containing canonical H2A or H2A.Z, clearly established that H2A.Z modulates the mobility of nucleosomes.Citation13 Therefore, since H2A.Z possibly hallmarks a transition between closed and open chromatin conformations, it might represent a preferential target for pioneer TFs. However, this question has remained so far essentially unexplored.
Two recent studies have shown that nucleosomes bound by pioneer TFs are differentially decorated with H2A.Z.Citation5,6 On one hand, Oct4 binding is accompanied by the presence of H2A.Z, but FoxA2 binding is not, whereby only the flanking regions were marked by H2A.Z, not the binding site proper. This suggests two modes of action for pioneer TFs. However, these studies did not explicitly distinguish NORs and NDRs. These can be identified genome-wide by exploiting high-throughput sequencing data following micrococcal nuclease treatment (MNase-Seq), subsequently ranked by increasing nucleosomal signal at distal sites.Citation14 Using this type of analysis, we also recently showed that the Ets1 TF was able to bind both NORs and NDRs at two successive developmental stages of mouse T cells, with increased association to NDRs in CD4 CD8 double positive thymocytes (DP T-cells).Citation15 These observations suggested a degree of pioneering activity for Ets1. This result was in contrast with another Ets-type TF, Pu.1, known as a pioneer and which was only found associated with NDRs at enhancers using a similar analysis.Citation14 In light of other studies detailing H2A.Z levels at TF binding sites,Citation5,6 the question remains open as to whether Ets1 binding sites harbor this histone variant. To estimate how widespread differences in H2A.Z occupancy might be among pioneer TFs, we review, revisit and expand published data that showed contrasting association of H2A.Z with bound nucleosomes at enhancers. Enticingly, recent works described that low or moderate MNase digestion allow the release of labile/unstable nucleosomesCitation5,16 at both enhancers and promoters. This allowed us to show that TFs, including pioneer such as FoxA2 previously believed to be associated with NDRs in MNase-seq experiments, were indeed bound to NORs in conditions of low digestion with the enzyme. This result opens the possibility that TF-association with NORs, such as Ets1 or Pu.1, was previously underestimated in high digestion experimental conditions, in which mono-nucleosomes represent most of the analyzed fractions.
Results and discussion
To get further insights in whether pioneer, NOR-binding TFs display different properties, we compared Ets1 in DP T-cells, Oct4 in ES cells and FoxA2 and C/EBPα in hepatocytes for H2.AZ. We also examined CBP/p300 histone acetyl transferase (HAT) recruitment, two highly related and partially redundant hallmarks of active enhancers.Citation17 As described,Citation15 Ets1 binds to both NORs and NDRs but we now found that the NOR-bound enhancers were more enriched for H2A.Z histone variant and localized at the Ets1 binding site (). Strikingly, a similar trend was observed for the Oct4 pioneer TF () in ES cells.Citation18 In none of those cases was H2A.Z found when those TFs were bound to apparent NDRs. This result suggests that H2A.Z is preferentially associated to Ets1/Oct4 at these stages, whereby enhancers are still relatively closed and in the process of being remodeled. By discriminating NORs and NDRs, we are thus extending previous findings that H2A.Z is docked on CBP/p300 and TFs at enhancer regions.
Figure 1. Two modes of association of TFs with pioneering activities with H2A.Z and p300/CBP in distal nucleosome-occupied and -depleted regions. (A) Distal, Ets1- and Oct4-bound nucleosome occupied regions are marked with H2A.Z. Average profiles of MNase-Seq and H2A.Z ChIP-Seq (left, right) in nucleosome-occupied and -depleted distal regions bound by Ets1 in DP T-cells and Oct4 in mESCs (top, bottom). (B) Nucleosomes flanking distal FoxA2- and C/EBPα-bound nucleosome occupied regions are marked with H2A.Z. Average profiles of MNase-Seq and H2A.Z ChIP-Seq (left, right) in nucleosome-occupied and -depleted distal regions bound by FoxA2 and C/EBPα in mouse hepatocytes (top, bottom). (C) Distal, p300-bound nucleosome occupied regions are marked with H2A.Z and labile. Average profiles of MNase-Seq (low and high digestion) and H2A.Z ChIP-Seq (left, right) in nucleosome-occupied and -depleted distal regions bound by p300 in mouse hepatocytes. (D) Distal, CBP-bound nucleosome occupied regions are marked with H2A.Z and labile. Average profiles of MNase-Seq and H2A.Z ChIP-Seq (left, right) in nucleosome-occupied and -depleted distal regions bound by CBP in mouse DP T-cells. (E) CBP signal is associated with Ets1 binding while p300 binding is not associated with FoxA2 binding. Average profiles of CBP and p300 signals (left, right) centered on their peak summits or Ets1 and FoxA2. (F) Model describing the association of H2A.Z, p300/CBP and nucleosome occupancy for TFs with pioneering activity.
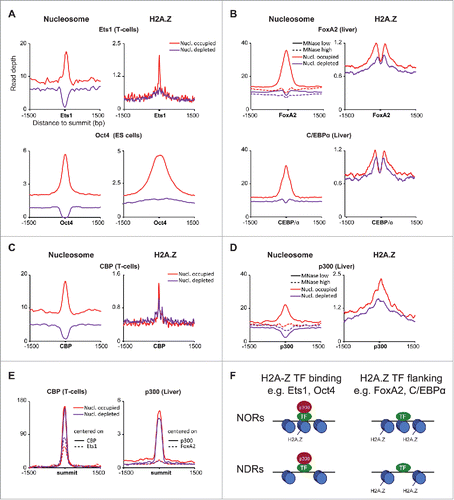
We next compared these trends to those displayed by the liver-specific FoxA2 pioneer and C/EBPα TFs in mouse liver cells. For this, we also took advantage of published MNase-Seq data performed using low concentrations of MNase (MNase low).Citation5 In the classical, higher concentration MNase-Seq assay (MNase high), 60–80% of mono-nucleosomes are typically released as compared with the whole fraction. These conditions often represent a standard for many laboratories, the rationale being that the amounts of mono-nucleosomes should be optimized in the sequenced fraction. However, this will most likely result in the loss of nucleosomes that are first digested by MNase and lost in subnucleosomal fractions. As described in Iwafuchi-Doi et al.,Citation5 high MNase-seq emphasizes FoxA2 as mainly bound to NDRs. In contrast, low MNase-seq clearly discriminates two types of FoxA2 sites bound to NORs or NDRs (). An attractive hypothesis is that NOR-bound sites represent labile/unstable nucleosomes that could hallmark enhancer binding by pioneer TFs. We performed the same analysis with the C/EBPα TF in hepatocytesCitation19 and found similar results. When examining H2A.Z levels at FoxA2 and C/EBPα sites, we also found more H2A.Z at NOR-bound sites but unlike for Ets1 and Oct4, only flanking nucleosomes were marked.
We also investigated whether NORs/NDRs behaved differently to recruit HAT activity at enhancers. At p300- or CBP-bound intergenic regions, the same NOR and NDR dichotomy could be evidenced with a prominent H2A.Z nucleosome at NORs in hepatocytes but was less pronounced in T cells (). Co-localization of CBP and Ets1 was confirmed by the presence of CBP signal at distal Ets1 sites as compared with CBP sites (). Conversely, p300 signal at distal FoxA2 sites was very low as compared with p300 sites, indicating a low level of co-localization (). We also obtained comparable results while performing this analysis on C/EBPα sites (data not shown). Taken together, our results suggest that for certain TFs with pioneering activity, e.g., Ets1 and Oct4, contacted nucleosomes in distal NORs are enriched in H2A.Z and also docked on p300/CBP, while for others, such as FoxA2 and C/EBPα, these nucleosomes are neither enriched in H2A.Z nor in p300/CBP, with flanking nucleosomes enriched in H2A.Z. Finally, according to the analysis performed on members of the co-activator complex CBP/p300 itself, H2A.Z-marked nucleosomes bound in NORs were found to be essentially labile. These findings are modeled in .
Taken together, our results suggest two classes of TFs with pioneering activity based on the presence of H2A.Z and p300/CBP with distinct modes of action for gene activation. Importantly, the presence of H2A.Z-marked nucleosomes at p300 sites, obtained via low concentration MNase-Seq, indicates that these are labile/unstable nucleosomes. Since this result was also observed with classical, “high,” MNase-Seq in mESC, this suggests that H2A.Z nucleosomes can be either labile or stable. This is consistent with H2A.Z being associated with varying lengths of nucleosomal protection.Citation13 Other activators that are not required for maintenance of open chromatin, such as FoxA2,Citation20 might probe silent chromatin and increase its accessibility via recruitment of remodelers, without necessarily behaving as co-activators. This class of TFs may thus pass on this role to another class of TFs whose function is to activate basal transcription. For another class of TFs with pioneering activity, such as Ets1 and Oct4, nucleosome binding may be associated with enhancer activity as per the presence of p300/CBP in NORs. In the case of Ets1, we had previously observed that both NORs and NDRs were associated with enhancer activity.Citation15 Thus, the presence of a labile, H2A.Z-marked nucleosome at the binding site may not be an obstacle to, but may facilitate enhancer activity. These labile nucleosomes may well represent a transient state between silent and fully open chromatin. At any rate, these two classes of TFs with pioneering activity would constitute the basis for divergent mechanisms dedicated to the opening of chromatin. The identification of such mechanisms in other TFs would also strengthen this hypothesis. On this note, we highlight the fact that while aggregate profiles of nucleosome occupancy have revealed that most of TFs studied in the literature are associated with NDRs,Citation21 this may not entirely be the case in light of the type of analysis performed in this work. Further analysis of sequence content of bound NORs and NDRs (TF motifs, GC content) may also provide additional mechanistic insights; for example it was previously shown that TF-bound distal regions exhibiting higher nucleosome occupancy had higher GC content than NDRs, which may represent more thermodynamically stable complexesCitation14 and thus require facilitation of removal via the involvement of H2A.Z in nucleosomal complexes.
Material and methods
Chromatin immunoprecipitation
ChIP-Seq was essentially performed as described previously,Citation22 using 5 × 106 cells and an Abcam Ab4174 anti-H2A.Z antibody. Sequencing was performed on a Genome Analyzer II (Illumina) according to manufacturer's instructions.
Data processing
For ChIP-Seq data produced in this work as well as for retrieved public data sets, reads were aligned to the mm9 genome via bowtie223 using –very-sensitive-local as a parameter. Peak detection and coverage track generation was performed via macs1424 using –keep-dup = auto -w -S as parameters.
Identification of distal NORs and NDRs and average profile generation
Identification of distal NORs and NDRs and average profile generation were essentially performed as described previously.Citation15 Distal TF summits were first defined as outside ± 5 kb of the transcription start site. MNase-Seq coverages were retrieved via Homer annotatePeaksCitation25 using -hist 10 -ghist -wig as parameters and subsequently ranked by decreasing read depth ± 100 bp around TF summits. NOR and NDR classes were defined as the top and bottom 25% MNase signals ± 100 bp of TF summits. Average profiles were plotted using Excel.
Data availability
ChIP-Seq data produced in this study were submitted at the Gene Expression Omnibus (GEO) under accession GSE87529.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
References
- Goldberg AD, Allis CD, Bernstein E. Epigenetics: a landscape takes shape. Cell 2007; 128:635-638; PMID:17320500; https://doi.org/10.1016/j.cell.2007.02.006
- Zaret KS, Carroll JS. Pioneer transcription factors: establishing competence for gene expression. Genes Dev 2011; 25:2227-2241; PMID:22056668; https://doi.org/10.1101/gad.176826.111
- Sherwood RI, Hashimoto T, O'Donnell CW, Lewis S, Barkal AA, van Hoff JP, Karun V, Jaakkola T, Gifford DK. Discovery of directional and nondirectional pioneer transcription factors by modeling DNase profile magnitude and shape. Nat Biotechnol 2014; 32:171-178; PMID:24441470; https://doi.org/10.1038/nbt.2798
- Soufi A, Garcia MF, Jaroszewicz A, Osman N, Pellegrini M, Zaret KS. Pioneer transcription factors target partial DNA motifs on nucleosomes to initiate reprogramming. Cell 2015; 161:555-568; PMID:25892221; https://doi.org/10.1016/j.cell.2015.03.017
- Iwafuchi-Doi M, Donahue G, Kakumanu A, Watts JA, Mahony S, Pugh BF, Lee D, Kaestner KH, Zaret KS. The pioneer transcription factor foxA maintains an accessible nucleosome configuration at enhancers for tissue-specific gene activation. Mol Cell 2016; 62:79-91; PMID:27058788; https://doi.org/10.1016/j.molcel.2016.03.001
- Hu G, Cui K, Northrup D, Liu C, Wang C, Tang Q, Ge K, Levens D, Crane-Robinson C, Zhao K. H2A.Z facilitates access of active and repressive complexes to chromatin in embryonic stem cell self-renewal and differentiation. Cell Stem Cell 2013; 12:180-192; PMID:23260488; https://doi.org/10.1016/j.stem.2012.11.003
- Fan JY, Gordon F, Luger K, Hansen JC, Tremethick DJ. The essential histone variant H2A.Z regulates the equilibrium between different chromatin conformational states. Nat Struct Biol 2002; 9:172-176; PMID:11850638; https://doi.org/10.1038/nsb0402-316b
- Raisner RM, Hartley PD, Meneghini MD, Bao MZ, Liu CL, Schreiber SL, Rando OJ, Madhani HD. Histone variant H2A.Z marks the 5′ ends of both active and inactive genes in euchromatin. Cell 2005; 123:233-248; PMID:16239142; https://doi.org/10.1016/j.cell.2005.10.002
- Schones DE, Cui K, Cuddapah S, Roh TY, Barski A, Wang Z, Wei G, Zhao K. Dynamic regulation of nucleosome positioning in the human genome. Cell 2008; 132:887-898; PMID:18329373; https://doi.org/10.1016/j.cell.2008.02.022
- Jin C, Zang C, Wei G, Cui K, Peng W, Zhao K, Felsenfeld G. H3.3/H2A.Z double variant-containing nucleosomes mark ‘nucleosome-free regions’ of active promoters and other regulatory regions. Nat Genet 2009; 41:941-945; PMID:19633671; https://doi.org/10.1038/ng.409
- Tolstorukov MY, Kharchenko PV, Goldman JA, Kingston RE, Park PJ. Comparative analysis of H2A.Z nucleosome organization in the human and yeast genomes. Genome Res 2009; 19:967-977; PMID:19246569; https://doi.org/10.1101/gr.084830.108
- Bao Y, Konesky K, Park YJ, Rosu S, Dyer PN, Rangasamy D, Tremethick DJ, Laybourn PJ, Luger K. Nucleosomes containing the histone variant H2A.Bbd organize only 118 base pairs of DNA. EMBO J 2004; 23:3314-3324; PMID:15257289; https://doi.org/10.1038/sj.emboj.7600316
- Rudnizky S, Bavly A, Malik O, Pnueli L, Melamed P, Kaplan A. H2A.Z controls the stability and mobility of nucleosomes to regulate expression of the LH genes. Nat Commun 2016; 7:12958; PMID:27653784; https://doi.org/10.1038/ncomms12958
- Barozzi I, Simonatto M, Bonifacio S, Yang L, Rohs R, Ghisletti S, Natoli G. Coregulation of transcription factor binding and nucleosome occupancy through DNA features of mammalian enhancers. Mol Cell 2014; 54:844-857; PMID:24813947; https://doi.org/10.1016/j.molcel.2014.04.006
- Cauchy P, Maqbool MA, Zacarias-Cabeza J, Vanhille L, Koch F, Fenouil R, Gut M, Gut I, Santana MA, Griffon A et al. Dynamic recruitment of Ets1 to both nucleosome-occupied and -depleted enhancer regions mediates a transcriptional program switch during early T-cell differentiation. Nucleic Acids Res 2016; 44:3567-3585; PMID:26673693; https://doi.org/10.1093/nar/gkv1475
- Mieczkowski J, Cook A, Bowman SK, Mueller B, Alver BH, Kundu S, Deaton AM, Urban JA, Larschan E, Park PJ et al. MNase titration reveals differences between nucleosome occupancy and chromatin accessibility. Nat Commun 2016; 7:11485; PMID:27151365; https://doi.org/10.1038/ncomms11485
- Holmqvist PH, Mannervik M. Genomic occupancy of the transcriptional co-activators p300 and CBP. Transcription 2013; 4:18-23; PMID:23131664; https://doi.org/10.4161/trns.22601
- Whyte WA, Orlando DA, Hnisz D, Abraham BJ, Lin CY, Kagey MH, Rahl PB, Lee TI, Young RA. Master transcription factors and mediator establish super-enhancers at key cell identity genes. Cell 2013; 153:307-319; PMID:23582322; https://doi.org/10.1016/j.cell.2013.03.035
- Sun X, Chuang JC, Kanchwala M, Wu L, Celen C, Li L, Liang H, Zhang S, Maples T, Nguyen LH et al. Suppression of the SWI/SNF component arid1a promotes mammalian regeneration. Cell Stem Cell 2016; 18:456-466; PMID:27044474; https://doi.org/10.1016/j.stem.2016.03.001
- Li XY, Thomas S, Sabo PJ, Eisen MB, Stamatoyannopoulos JA, Biggin MD. The role of chromatin accessibility in directing the widespread, overlapping patterns of Drosophila transcription factor binding. Genome Biol 2011; 12:R34; PMID:21473766; https://doi.org/10.1186/gb-2011-12-4-r34
- Wang J, Zhuang J, Iyer S, Lin X, Whitfield TW, Greven MC, Pierce BG, Dong X, Kundaje A, Cheng Y et al. Sequence features and chromatin structure around the genomic regions bound by 119 human transcription factors. Genome Res 2012; 22:1798-1812; PMID:22955990; https://doi.org/10.1101/gr.139105.112
- Koch F, Fenouil R, Gut M, Cauchy P, Albert TK, Zacarias-Cabeza J, Spicuglia S, de la Chapelle AL, Heidemann M, Hintermair C et al. Transcription initiation platforms and GTF recruitment at tissue-specific enhancers and promoters. Nat Struct Mol Biol 2011; 18:956-963; PMID:21765417; https://doi.org/10.1038/nsmb.2085
- Langmead B, Salzberg SL. Fast gapped-read alignment with Bowtie 2. Nat Methods 2012; 9:357-359; PMID:22388286; https://doi.org/10.1038/nmeth.1923
- Zhang Y, Liu T, Meyer CA, Eeckhoute J, Johnson DS, Bernstein BE, Nusbaum C, Myers RM, Brown M, Li W et al. Model-based analysis of ChIP-Seq (MACS). Genome Biol 2008; 9:R137; PMID:18798982; https://doi.org/10.1186/gb-2008-9-9-r137
- Heinz S, Benner C, Spann N, Bertolino E, Lin YC, Laslo P, Cheng JX, Murre C, Singh H, Glass CK. Simple combinations of lineage-determining transcription factors prime cis-regulatory elements required for macrophage and B cell identities. Mol Cell 2010; 38:576-589; PMID:20513432; https://doi.org/10.1016/j.molcel.2010.05.004
