ABSTRACT
Gene regulation is fundamentally important for the coordination of diverse biologic processes including homeostasis and responses to developmental and environmental stimuli. Transcription factor (TF) binding sites are one of the major functional subunits of gene regulation. They are arranged in cis-regulatory modules (CRMs) that can be more active than the sum of their individual effects. Recently, we described a mechanism of glucocorticoid (GC)-induced gene regulation in which the glucocorticoid receptor (GR) binds coordinately to multiple CRMs that are 10s of kilobases apart in the genome. In those results, the minority of GR binding sites appear to involve direct TF:DNA interactions. Meanwhile, other GR binding sites in a cluster interact with those direct binding sites to tune their gene regulatory activity. Here, we consider the implications of those and related results in the context of existing models of gene regulation. Based on our analyses, we propose that the billboard and regulatory grammar models of cis-regulatory element activity be expanded to consider the influence of long-range interactions between cis-regulatory modules.
The transcription of human genes is controlled by the compound activity of multiple gene regulatory elements acting on a promoter. Combinatorial interactions can occur when factors are bound proximally, as was observed in early studies of gene regulation by POU family TFs.Citation1,Citation2 Two models that have been proposed to explain the coordinated activity of regulatory elements are the regulatory grammar model and the billboard model. The regulatory grammar model predicates that a specific syntax of TF binding is required for co-regulation to occur (). In this model, CRMs—DNA sequences containing multiple regulatory sites—are dependent on the relative orientation, ordering, and spacing of the constituent sites to act as functional enhancers. The regulatory grammar model was first proposed in the context of microbial gene regulation and subsequently applied to eukaryotic systems.Citation3,Citation4 Recent advances in the regulatory grammar model highlight the possibility of regulatory grammar as a method of increasing the precision and specificity of transcription (reviewed, Citationref. 5). The flexible billboard model was proposed as an alternative to syntax-dependent languages of gene regulation. The billboard model of CRM organization states that the specific orientation of elements is less relevant to CRM function than the ability to recruit the appropriate complement of motif-driven and non-motif driven regulatory factors to a locus ().Citation6,Citation7
Figure 1. Models of coordinated TF function. (A) The regulatory grammar model, in which functional CRMs have a defined syntax that governs their function. (B) The flexible billboard model, in which CRM regulatory element composition rather than regulatory element ordering determines function. (C) The long-range flexible billboard model, in which CRMs with flexible ordering interact with other regulatory elements via long-range interactions to coordinate target gene expression.
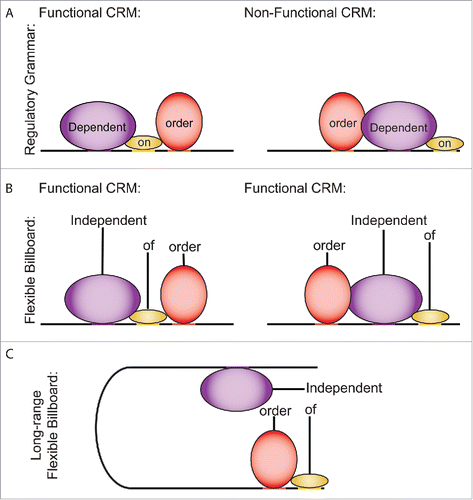
The regulatory grammar and billboard models have both been tested in several different systems. One study quantified the regulatory effects of nearly 5,000 functional element combinations in synthetic CRMs in which element composition, orientation and spacing were varied.Citation8 This study provided evidence for and improved upon the billboard model by demonstrating that orientation and spacing of regulatory elements had less of an effect on CRM function than the heterogeneity of a CRM. Heterotypic CRMs consisting of binding motifs for more than one TF had greater activity than homotypic CRMs with the same number of binding motifs for a single TF. Meanwhile, analyses of DNase hypersensitivity data support a model in which certain transcription factors open chromatin in a directional manner.Citation9 This suggests that position and orientation dependent effects may play a primary role in determining CRM activity (i.e., a regulatory grammar). Together, these studies suggest that gene regulation involves both billboard and grammar-like effects that may be specific to different TFs or classes of TFs.
Interactions between CRMs govern gene regulation
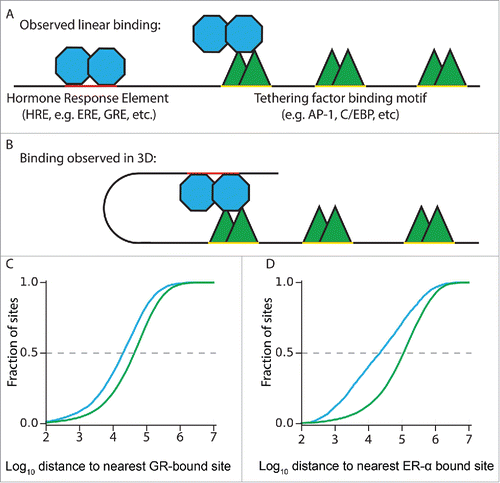
Here, we propose that the principles that govern coordinated activity between sites within a CRM extend to long-range interactions between CRMs and distal TF-binding sites following a long-range flexible billboard model (). It is well known that heterotypic interactions between TF-binding sites can occur via chromatin looping. Some of the earliest identified examples of this mode of gene regulation were characterized at the locus control region that coordinates the expression of the globin gene cluster.Citation10 These studies first characterized the importance of interactions between gene regulatory sites in the context of the position-dependent function of distal gene regulatory elements.Citation11-14 Genomic approaches to comprehensively identify long-range chromatin interactions within a cell have revealed that certain sites interact more than expected by consideration of distance alone and that those interactions often span more than 10s of kilobases.Citation15,Citation16 Meanwhile, high-throughput functional assays have revealed that sites that interact with one another can perform distinct gene regulatory functions. In the case of the glucocorticoid receptor (GR), sites can be classified into those that potentiate a response to GCs and those that modulate the activity of that response.Citation17 The GR is a type I nuclear hormone receptor that regulates transcription in response to GCs. At steady state, the GR is sequestered in the cytoplasm. However, when cells are exposed to GCs the GR translocates into the nucleus where it acts as a transcription factor. GC-mediated gene induction occurs with minutes of GC-exposure, thus it is possible to observe primary transcriptional effects of GCs without the confounding effects of the activity of secondary targets of the GR. This induced activity makes the GR an ideal subject for highly controlled gene regulation studies. GC-induced enhancers are typified by the presence of a GR response element (GRE), while non-GC-induced sites are enriched for the binding of classical GR co-regulators, such as AP-1 family member proteins, and tend to lack a GRE. Non-GC-induced tethered GR binding sites tend to act as enhancers at steady state, and are closer to direct GR binding sites than expected by chance (). The clustering of tethered GR binding sites around direct GR binding sites suggests that tethered sites and direct sites may interact via chromatin looping. Consistent with the hypothesis that direct nuclear receptor binding potentiates long-range chromatin interactions across the genome, sites bound by the estrogen receptor (ERα) are more likely to engage in long-range chromatin interactions if they have a strong match to the estrogen response element.Citation17
Figure 2. Tethering factor co-bound nuclear receptor binding sites are closer to one another than expected by chance. (A) Clusters of TF-binding sites in linear space that can be observed via ChIP-seq reflect a mixture of direct and tethered binding. Tethered binding sites tend to be closer to direct binding sites than expected by the genomic distribution of tethering factor binding. (B) Looping mechanism of TF-binding cluster formation. (C) C/EBPβ sites are closer to GR binding sites if co-bound by GR (blue) than if not co-bound (green) (19.9 kb vs. 42.9 kb; Mann–Whitney U-test p <10-100) (D) FOXA1 sites are closer to ERα binding sites if co-bound by ERα (blue) than if not co-bound (green) (21.9 kb vs. 111.9 kb; Mann–Whitney U-test p < 10-100).
Long-range nuclear receptor tethering by CEBP and FOX family TFs
The spatial relationship observed between the GR and AP-1 subunits in A549 cells is general to other types of cells, other tethering-associated factors, and other nuclear receptors. Post-hoc analysis of the co-localization between GR and ERα, and the commonly associated tethering factors, C/EBPβ and FOXA1, respectively, reveals that tethering factor-bound sites that are co-bound by the nuclear receptor are closer on average to nuclear receptor binding sites than would be expected by the genomic distribution of tethering proteins ().Citation18-20 Meanwhile, high throughput functional assays of p53 and PPARG binding sites have revealed that the direct binding motif for these factors is highly predictive of enhancer activity.Citation21,Citation22 Together, these results are consistent with a model in which the direct binding of TFs potentiates a transcriptional response, while long-range interactions with other factors modulate that response.
Chromatin looping
The role of long-range TF interactions in hormone-induced gene regulation raises the question of how chromatin looping changes in response to stimuli. More specifically, are long-distance interactions among TF-binding sites formed before stimulation, and how does new TF-binding affect such interactions? Given the functional diversity observed at GR binding sites, chromatin loops may play distinct context-dependent biologic roles. GC treatment alters the interactions of p300-bound chromatin loops. However, it is unclear if this observation is confounded by redistribution of p300 in response to GC exposure.Citation23 This concern is justified by the observation that when cells are treated with estradiol, which activates ERα binding via a mechanism similar to the way that GCs activate GR binding, there is a redistribution of p300 across the genome.Citation24 Studies focused on a small number of loci have shown that long-distance interactions exist before hormone treatment, then increase upon GC exposure.Citation25 Likewise, forced chromatin looping can induce the expression of silenced genes and broken long-range interactions can turn off gene expression.Citation26 However, TNF-α stimulation of cells does not appear to affect chromatin looping as measured by Hi-C.Citation27 It is unclear if these seemingly contradictory observations are due to differences in biologic mechanism or in experimental sensitivity. It is has been reported that long-range chromatin interactions that define topologically associated domains (TADs) are easier to observe via established methods than more short-range, sub-TAD interactions.Citation28,Citation29 Therefore, it is likely that dynamic looping interactions, which may preferentially occur within sub-TAD intervals, would be more difficult to experimentally observe. Further investigation in different biological systems is needed to determine the extent and heterogeneity of stimulus-induced changes in DNA-looping. Given limitations in resolution and scale of chromosome conformation capture approaches, innovations in DNA architecture methods will be needed to elucidate how distal TF-binding sites cooperate in the context of the 3D genome.
Integration with pioneer factor model of gene regulation
The observation that non-motif driven TF-binding sites can be detected via ChIP-seq and that those binding events can be the result of long-range TF–TF interactions suggests an alternative to the pioneer factor model of co-regulation. Substantial evidence supports a mechanism in which the binding of certain TFs, termed pioneer factors, promotes a chromatin state that potentiates the binding of other TFs.Citation30 In many cases, these secondary TF-binding events occur at sites containing a degenerate match to that TF's binding motif. In the case of the GR, AP-1 has been proposed as a pioneer factor.Citation31 Elimination of AP-1 binding by expression of a-FOS, a dominant negative version of AP-1, decreased chromatin accessibility and GR binding at sites with weak matches to the GRE that are normally co-bound by AP-1.Citation32 One interpretation of these data is that AP-1 binding and subsequent chromatin remodeling are required to allow GR to bind to weak matches to the GRE. A complementary model considers the role of long-range tethering interactions between GR and AP-1. In this model, a large proportion of GR/AP-1 co-bound sites occur as the result of long-range tethering interactions between sites bound by AP-1 that lack a GRE and distal GRE-encoded GR binding sites (). Such a model would account for the observed degeneracy of GREs at a substantial fraction of GR binding sites that are co-occupied by AP-1. Consistent with this model, direct GR binding sites potentiate an expression response to GCs in the isolated context of transient transfection based reporter assays, while tethered GR binding sites do not.Citation17 However, the presence of a motif-encoded AP-1 binding site adjacent to a GRE motif-encoded GR binding site substantially increases both steady state and GC-induced reporter gene expression.Citation17 This supports a model in which classical combinatoric interactions between the GR and AP-1 (e.g., flexible billboard model) and long-range combinatoric interactions with distal tethering proteins tune the activity of GC-induced enhancers.
Future directions
Our finding that nearby TF-binding sites potentiate clusters of GR binding raises several questions. It remains unknown if the TF-binding clusters observed with ChIP-seq results from specific physical interactions between TFs and different binding sites or from an alternate mechanism, potentially involving local increases in TF concentration or chromatin compartmentalization. Distinguishing between these possibilities will guide investigation into the specific molecular events between TF binding and changes in transcriptional output. Mapping long-range interactions with 3C-derived methods such as Hi-C can provide insights into that question. However, such assays cannot resolve whether clusters reflect multiple concurrently interacting CRMs, or instead the aggregate of mutually exclusive binary interactions aggregated over time and between cells. Recent advances in super resolution microscopy may overcome that challenge and, in our opinion, have great promise for studying CRM activity.Citation33 Depending on results from those and other studies, the long-range flexible billboard model that we propose may generalize the flexible billboard model to allow for synergistic interactions in cis between TFs separated by 10s of kilobases.
Conclusion
Several models have been proposed for the regulation of eukaryotic gene expression. TF-binding sites drive one of the most basic levels of gene regulation. CRMs containing multiple TF-binding sites have been demonstrated to act in concert allowing increased regulatory specificity. Heterotypic CRMs tend to have higher aggregate activity than CRMs containing binding motifs for a single factor. This flexible billboard model, which current data suggests explains most CRM activity, emphasizes the importance of CRM complexity rather than a specific regulatory grammar. Our recent results indicate that clusters of sites bound by the GR can be nucleated by fewer sites than expected by chance. These nucleating sites are also capable of GC-induced enhancer function. Meanwhile, the majority of GR binding sites within a cluster are tethered to the genome via other TFs. Tethering occurs as a function of long-range interactions between direct GR binding sites and nearby tethering proteins. These interactions are capable of increasing the activity of GC-induced enhancers in a manner that is independent of proximal co-activation that occurs in linear space. The observation that tethering can occur via interactions with a variety of different proteins suggests a biological role for the specific tethering protein interactions that occur within a TF-binding site cluster. It will therefore be important to test the function of the diverse proteins that can fill this role. Likewise, the observation that the fraction of PPARG and P53 binding events that confer enhancer activation by those factors is highly enriched for direct binding sites suggests a generality to the importance of motif-driven TF binding. In light of these results, we hypothesize that similar interaction clusters occur between other TFs. Such clusters could directly coordinate the amplification of enhancer effects by recruiting an abundance of transcriptional machinery to a site, could contribute to enhancer cell-type specificity, and/or could increase the stability of transcriptional complexes bound to the genome. We propose the extension of the flexible billboard model to incorporate the functional consequences of such clusters into a long-range flexible billboard mechanism.
Disclosure of potential conflicts of interest
TER is a founder of Element Genomics, a functional genomics company.
Funding
All authors were supported by National Institutes of Health under Grant U01 HG007900 (to T.E.R.). C.M.V. was also supported by National Institutes of Health under Grant F31 HL129743.
References
- LeBowitz JH, Clerc RG, Brenowitz M, Sharp PA. The Oct-2 protein binds cooperatively to adjacent octamer sites. Genes Dev 1989; 3:1625-1638; PMID:2612908; https://doi.org/10.1101/gad.3.10.1625
- Janson L, Pettersson U. Cooperative interactions between transcription factors Sp1 and OTF-1. Proc Natl Acad Sci USA 1990; 87:4732-4736; PMID:2191301; https://doi.org/10.1073/pnas.87.12.4732
- Collado-Vides J. The search for a grammatical theory of gene regulation is formally justified by showing the inadequacy of context-free grammars. Comput Appl Biosci 1991; 7:321-326; PMID:1913213; https://doi.org/10.1093/bioinformatics/7.3.321
- Collado-Vides J. Grammatical model of the regulation of gene expression. Proc Natl Acad Sci USA 1992; 89:9405-9409; PMID:1409648; https://doi.org/10.1073/pnas.89.20.9405
- Weingarten-Gabbay S, Segal E. The grammar of transcriptional regulation. Hum Genet 2014; 133:701-711; PMID:24390306; https://doi.org/10.1007/s00439-013-1413-1
- Kulkarni MM, Arnosti DN. Information display by transcriptional enhancers. Development 2003; 130:6569-6575; PMID:14660545; https://doi.org/10.1242/dev.00890
- Rastegar S, Hess I, Dickmeis T, Nicod JC, Ertzer R, Hadzhiev Y, Thies WG, Scherer G, Strähle U. The words of the regulatory code are arranged in a variable manner in highly conserved enhancers. Dev Biol 2008; 318:366-377; PMID:18455719; https://doi.org/10.1016/j.ydbio.2008.03.034
- Smith RP, Taher L, Patwardhan RP, Kim MJ, Inoue F, Shendure J, Ovcharenko I, Ahituv N. Massively parallel decoding of mammalian regulatory sequences supports a flexible organizational model. Nat Genet 2013; 45:1021-1028; PMID:23892608; https://doi.org/10.1038/ng.2713
- Sherwood RI, Hashimoto T, O'Donnell CW, Lewis S, Barkal AA, van Hoff JP, Karun V, Jaakkola T, Gifford DK. Discovery of directional and nondirectional pioneer transcription factors by modeling DNase profile magnitude and shape. Nat Biotechnol 2014; 32:171-178; PMID:24441470; https://doi.org/10.1038/nbt.2798
- Kioussis D, Vanin E, deLange T, Flavell RA, Grosveld FG. Beta-globin gene inactivation by DNA translocation in gamma beta-thalassaemia. Nature 1983; 306:662-666; PMID:6318113; https://doi.org/10.1038/306662a0
- Grosveld F, van Assendelft GB, Greaves DR, Kollias G. Position-independent, high-level expression of the human beta-globin gene in transgenic mice. Cell 1987; 51:975-985; PMID:3690667; https://doi.org/10.1016/0092-8674(87)90584-8
- Kollias G, Wrighton N, Hurst J, Grosveld F. Regulated expression of human A gamma-, beta-, and hybrid gamma beta-globin genes in transgenic mice: manipulation of the developmental expression patterns. Cell 1986; 46:89-94; PMID:3719696; https://doi.org/10.1016/0092-8674(86)90862-7
- Magram J, Chada K, Costantini F. Developmental regulation of a cloned adult beta-globin gene in transgenic mice. Nature 1985; 315:338-340; PMID:3858676; https://doi.org/10.1038/315338a0
- Townes TM, Lingrel JB, Chen HY, Brinster RL, Palmiter RD. Erythroid-specific expression of human beta-globin genes in transgenic mice. EMBO J 1985; 4:1715-1723; PMID:2992937
- Fullwood MJ, Liu MH, Pan YF, Liu J, Xu H, Mohamed YB, Orlov YL, Velkov S, Ho A, Mei PH et al. An oestrogen-receptor-alpha-bound human chromatin interactome. Nature 2009; 462:58-64; PMID:19890323; https://doi.org/10.1038/nature08497
- Lieberman-Aiden E, van Berkum NL, Williams L, Imakaev M, Ragoczy T, Telling A, Amit I, Lajoie BR, Sabo PJ, Dorschner MO et al. Comprehensive mapping of long-range interactions reveals folding principles of the human genome. Science 2009; 326:289-293; PMID:19815776; https://doi.org/10.1126/science.1181369
- Vockley CM, D'Ippolito AM, McDowell IC, Majoros WH, Safi A, Song L, Crawford GE, Reddy TE. Direct GR binding sites potentiate clusters of TF binding across the human genome. Cell 2016; 166:1269-1281.e19; PMID:27565349; https://doi.org/10.1016/j.cell.2016.07.049
- Joseph R, Orlov YL, Huss M, Sun W, Kong SL, Ukil L, Pan YF, Li G, Lim M, Thomsen JS et al. Integrative model of genomic factors for determining binding site selection by estrogen receptor-alpha. Mol Syst Biol 2010; 6:456; PMID:21179027; https://doi.org/10.1038/msb.2010.109
- Steger DJ, Grant GR, Schupp M, Tomaru T, Lefterova MI, Schug J, Manduchi E, Stoeckert CJ Jr, Lazar MA. Propagation of adipogenic signals through an epigenomic transition state. Genes Dev 2010; 24:1035-1044; PMID:20478996; https://doi.org/10.1101/gad.1907110
- Mei S, Qin Q, Wu Q, Sun H, Zheng R, Zang C, Zhu M, Wu J, Shi X, Taing L et al. Cistrome Data Browser: a data portal for ChIP-Seq and chromatin accessibility data in human and mouse. Nucleic Acids Res 2017; 45:D658-D662; PMID:27789702; https://doi.org/10.1093/nar/gkw983
- Grossman SR, Zhang X, Wang L, Engreitz J, Melnikov A, Rogov P, Tewhey R, Isakova A, Deplancke B, Bernstein BE et al. Systematic dissection of genomic features determining transcription factor binding and enhancer function. Proc Natl Acad Sci USA 2017; 114:E1291-E1300; PMID:28137873; https://doi.org/10.1073/pnas.1621150114
- Verfaillie A, Svetlichnyy D, Imrichova H, Davie K, Fiers M, Kalender Atak Z, Hulselmans G, Christiaens V, Aerts S. Multiplex enhancer-reporter assays uncover unsophisticated TP53 enhancer logic. Genome Res 2016; 26:882-895; PMID:27197205; https://doi.org/10.1101/gr.204149.116
- Kuznetsova T, Wang SY, Rao NA, Mandoli A, Martens JH, Rother N, Aartse A, Groh L, Janssen-Megens EM, Li G et al. Glucocorticoid receptor and nuclear factor kappa-b affect three-dimensional chromatin organization. Genome Biol 2015; 16:264; PMID:26619937; https://doi.org/10.1186/s13059-015-0832-9
- Guertin MJ, Zhang X, Coonrod SA, Hager GL. Transient estrogen receptor binding and p300 redistribution support a squelching mechanism for estradiol-repressed genes. Mol Endocrinol 2014; 28:1522-1533; PMID:25051172; https://doi.org/10.1210/me.2014-1130
- Hakim O, John S, Ling JQ, Biddie SC, Hoffman AR, Hager GL. Glucocorticoid receptor activation of the Ciz1-Lcn2 locus by long range interactions. J Biol Chem 2009; 284:6048-52; PMID:19124469; https://doi.org/10.1074/jbc.C800212200
- Deng W, Rupon JW, Krivega I, Breda L, Motta I, Jahn KS, Reik A, Gregory PD, Rivella S, Dean A et al. Reactivation of developmentally silenced globin genes by forced chromatin looping. Cell 2014; 158:849-860; PMID:25126789; https://doi.org/10.1016/j.cell.2014.05.050
- Jin F, Li Y, Dixon JR, Selvaraj S, Ye Z, Lee AY, Yen CA, Schmitt AD, Espinoza CA, Ren B. A high-resolution map of the three-dimensional chromatin interactome in human cells. Nature 2013; 503:290-294; PMID:24141950; https://doi.org/10.1038/nature12644
- Dixon JR, Gorkin DU, Ren B. Chromatin domains: the unit of chromosome organization. Mol Cell 2016; 62:668-680; PMID:27259200; https://doi.org/10.1016/j.molcel.2016.05.018
- Rao SS, Huntley MH, Durand NC, Stamenova EK, Bochkov ID, Robinson JT, Sanborn AL, Machol I, Omer AD, Lander ES et al. A 3D map of the human genome at kilobase resolution reveals principles of chromatin looping. Cell 2014; 159:1665-1680; PMID:25497547; https://doi.org/10.1016/j.cell.2014.11.021
- Cirillo LA, Lin FR, Cuesta I, Friedman D, Jarnik M, Zaret KS. Opening of compacted chromatin by early developmental transcription factors HNF3 (FoxA) and GATA-4. Mol Cell 2002; 9:279-289; PMID:11864602; https://doi.org/10.1016/S1097-2765(02)00459-8
- John S, Sabo PJ, Thurman RE, Sung MH, Biddie SC, Johnson TA, Hager GL, Stamatoyannopoulos JA. Chromatin accessibility pre-determines glucocorticoid receptor binding patterns. Nat Genet 2011; 43:264-268; PMID:21258342; https://doi.org/10.1038/ng.759
- Biddie SC, John S, Sabo PJ, Thurman RE, Johnson TA, Schiltz RL, Miranda TB, Sung MH, Trump S, Lightman SL et al. Transcription factor AP1 potentiates chromatin accessibility and glucocorticoid receptor binding. Mol Cell 2011; 43:145-155; PMID:21726817; https://doi.org/10.1016/j.molcel.2011.06.016
- Wang S, Su JH, Beliveau BJ, Bintu B, Moffitt JR, Wu CT, Zhuang X. Spatial organization of chromatin domains and compartments in single chromosomes. Science 2016; 353:598-602; PMID:27445307; https://doi.org/10.1126/science.aaf8084
