ABSTRACT
There are hundreds of copies of rDNA repeats in mammalian chromosomes and the ratio of active, poised, or inactive rDNA is regulated in epigenetic manners. Recent studies demonstrated that a post-DNA replication repair enzyme, SHPRH affects rRNA transcription by recognizing epigenetic markers on rDNA promoters and unveiled potential links between DNA repair and ribosome biogenesis. This study suggests that SHPRH could be a link between mTOR-mediated epigenetic regulations and rRNA transcription, while concomitantly affecting genomic integrity.
Ribosomal DNA (rDNA) and ribosomal RNA (rRNA) transcription
The ribosome is a macromolecular machine that builds proteins necessary for cellular metabolism. The ribosome is composed of multiple proteins and rRNAs. Since ribosome biogenesis determines many metabolic processes, including cell proliferation, apoptosis, and autophagy, syntheses of rRNA and proteins are tightly regulated Citation[1]. Failure of such regulation causes diverse diseases such as anemia and cancers, as well as the aging of cells.
Eukaryotic ribosomes consist of a 60S subunit, which is composed of 49 proteins and 5.8S, 5S, and 28S rRNAs, and a 40S subunit, which is made up of about 33 proteins and 18S rRNA. The 5.8S, 18S, and 28S rRNA components of the ribosome are transcribed together as a 47S precursor rRNA (pre-rRNA) and processed to generate each of these rRNAs Citation[2]. 47S pre-rRNAs are transcribed from multiple tandem repeats of 42.9 kb rDNA that are organized in transcribed and intergenic regions (). In humans, only half of the hundreds of rDNA repeats are actively transcribed and others remain silent. rDNAs are localized in the nucleoli. Thus, transcription of the 47S pre-rRNA and processing to 5.8S, 18S, and 28S rRNAs also occur in the nucleoli. 47S pre-rRNA is transcribed by the RNA polymerase I complex, whose activity is controlled by cellular responses to nutritional states, cellular stresses, growth, differentiation, and cell cycle.
Figure 1. Human ribosomal DNA (rDNA) structure. rDNA transcription and processing occur in the nucleoli to maintain appropriate number or ribosomes. Each rDNA unit consists of multiple tandem repeats of rRNA genes and intergenic spaces. During transcription, pre-rRNA is transcribed from an individual active rDNA unit and processed into 18S, 5.8S, and 28S rRNAs. Fibrillarin and DAPI indicate nucleoli and nucleus, respectively. In ChIP experiment, SHPRH is bound to H42.9, a rRNA gene promoter, but not to other rDNA regions including H1, H4 and etc. Citation[1].
![Figure 1. Human ribosomal DNA (rDNA) structure. rDNA transcription and processing occur in the nucleoli to maintain appropriate number or ribosomes. Each rDNA unit consists of multiple tandem repeats of rRNA genes and intergenic spaces. During transcription, pre-rRNA is transcribed from an individual active rDNA unit and processed into 18S, 5.8S, and 28S rRNAs. Fibrillarin and DAPI indicate nucleoli and nucleus, respectively. In ChIP experiment, SHPRH is bound to H42.9, a rRNA gene promoter, but not to other rDNA regions including H1, H4 and etc. Citation[1].](/cms/asset/39a0d024-9b45-42fc-a716-c95452ab6ab4/ktrn_a_1381795_f0001_oc.jpg)
Recent advances in sequencing technology started to reveal that human populations have extensive polymorphisms in the number of copies of DNA segment in certain regions of their chromosomes Citation[3]. Such changes in copy numbers, known as copy number variation (CNV) are estimated to affect up to 12% of human genomes and appear to be a major driving force in evolution and diseases development. Due to high similarity of hundreds of rDNA repeats, they can easily become subjects for recombination and result in CNV in rDNA repeats. Whole-genome short-read DNA sequencing of human rDNA copy numbers showed correlations with the expression of chromatin components targeting the nucleoli, such as CCCTC-binding factor (CTCF) and heterochromatin protein 1β (HP1β) Citation[4]. Recent observations of lower rDNA copy numbers in mTOR-activated cancers shed light on the potential link between rDNA copy numbers and tumorigenesis Citation[5]. Similarly in yeast, susceptibility of tandem repeats of rDNAs for deletions and insertions was observed. Multiple factors including cohesion, helicases, and homologous recombination affect the CNVs of rDNA repeats in yeast Citation[6,7].
Epigenetic regulation of ribosomal RNA transcription
Transcription of genes, including rRNA, is tightly controlled by several epigenetic mechanisms Citation[8]. In addition to methylation of DNA that silences rRNA transcription, acetylation and methylation of histone proteins are also associated with transcriptional regulation of rDNA (). In rDNA, TTF-I and CSB (Cockayne syndrome protein B)-dependent nucleosome remodeling establishes the open chromatin structure and recruits RNA polymerase I to transcribe the rRNAs Citation[9]. rRNA transcription is associated with acetylation of rDNA histones, which is stimulated by overexpression of transacetylase p300, CBP, and PCAF and suppressed by an inhibitor of HDAC, trichostatin A Citation[10]. For rDNA silencing, NoRC (nucleolar remodeling complex) and eNoSC (energy-dependent nucleolar silencing complex) are involved in epigenetic modifications Citation[11,12]. NoRC, which consists of SNF2h (SMARCA5) and Tip5, recruits DNA methyltransferase and histone deacetylase and establishes the heterochromatin structure to silence the rDNA promoter Citation[12,13]. eNoSC senses cellular energy status and represses rRNA transcription in complex with SIRT1 and SUV39H1 Citation[11]. Nucleomethylin in eNoSC binds to histone H3K9me2, which is an epigenetic inactivation mark.
Table 1. Abbreviations of epigenetic regulators in rDNA transcription.
Recently, the poised state of rDNA was unveiled. Poised chromatin shows bivalent epigenetic features containing both an active histone modification, H3K4me3, and an inactive one, H3K27me3 Citation[14]. The quantity of rDNA with this poised structure is shown to be higher in differentiated cells Citation[15]. The NuRD (nucleosome remodeling and deacetylation) complex is composed of HDAC 1,2 (histone deacetylase 1/2), CHD4 (chromodomain helicase DNA binding protein 4), RBBP/4 (retinoblastoma binding protein 4), RBBP7 (retinoblastoma binding protein 7), MBD3 (methyl CpG binding domain protein 3), and MTAs (metastasis-associated proteins) Citation[16]. The NuRD complex is responsible for establishing the poised rRNA promoter. However, disruption of the NuRD complex impairs rRNA transcription. NuRD also suppresses the function of NoRC to maintain the rDNA promoter in an unmethylated state Citation[17]. The poised rRNA promoter is occupied by the pre-initiation complex without RNA Polymerase I Citation[15]. Therefore, once the rDNA promoter is epigenetically activated, RNA Polymerase I is delivered to the rDNA promoter to initiate rRNA transcription.
The mTOR complex and ribosomal control
Ribosome biosynthesis is regulated by nutrient concentrations, growth factors, stresses, and environmental changes. Several studies suggest that TOR (target of rapamycin) regulates ribosome biosynthesis by sensing the concentration of nutrients in yeast and mammals Citation[18]. mTOR interacts with other proteins and makes two different complexes (mTORC1 and mTORC2) and each complex has unique functions (). mTORC1 is composed of mTOR, Raptor, mLST8, PRAS40, and Deptor. mTORC2 consists of mTOR, Rictor, mSin1, Protor, mLst8, and Deptor Citation[19]. Many growth factors and nutrients activate mTOR pathways, which induce protein synthesis and suppress protein degradation. In contrast, when the mTOR pathway is inactivated by nutrient deficiency, protein synthesis is down-regulated. mTORC1 appears to sense nutrients and controls cellular metabolic processes. Growth factors stimulate PI3K (Phosphoinositide 3-kinase) and AKT (Protein kinase B). In turn, these kinases activate mTORC1 and inhibit TSC1/2, a negative regulator of mTORC1 Citation[20,21]. The other major negative regulator is PTEN (phosphatase and tensin homolog), which can block AKT activation by dephosphorylating PIP3 (plasma membrane intrinsic protein 3) Citation[22].
Table 2. Components of mTORC1 and mTORC2 complexes.
MAT2 in the NuRD complex recruits EZH2 (enhancer of zeste 2 polycomb repressive complex 2 subunit), which silences target genes such as TSC2 and results in activation of the mTORC1 pathway.
Structural and functional differentiation of SHPRH with HLTF and Rad5
SHPRH (SNF2 histone linker PHD RING helicase) is a functional ortholog of S. cerevisiae Rad5, a SWI2/SNF2-family ATP dependent chromatin remodeling protein that promotes an error-free DNA damage avoidance in eukaryotes by polyubiquitylating the DNA-sliding clamp PCNA (proliferating cell nuclear antigen) Citation[23]. The human Rad5 family includes SHPRH and HLTF (helicase like transcription factor). These proteins share a unique structural feature whereby a RING (really interesting new gene) domain is embedded between the conserved motifs of SWI2/SNF2 (A). The SWI/SNF helicase domain and the RING domain are responsible for translocating along dsDNA and ubiquitylating PCNA, respectively. Rad5 and HLTF have a HIRAN (HIP116, Rad5p N-terminal domain) domain that recognizes DNA features at stalled replication forks, but it is not present in SHPRH Citation[24].
Figure 2. A. Protein domain structures of SHPRH and HLTF (Rad5 ortholog). Locations of the Nuclear Localization Signal sequence (NLS), Linker Histone domain H1/H5 domain (LH), Plant Homeodomain (PHD), SWI2/SNF2 helicase domain (I, Ia, II, III, IV, V, VI), and RING domain are presented. B. Models for the role of SHPRH in the rRNA transcription. During poised rDNA status, histone H3 lysine 4 is trimethylated with only pre-initiation complexes, which lacks SHPRH and RNA polymerase I. NuRD inhibits transition of the poised rDNA status to the inactive rDNA status. When the rDNA promoter status shifts to the active one for rRNA transcription, SHPRH recruits RNA polymerase I. When nutrients become scarce, SHPRH makes foci structures in the nucleoli. The exact role of the SHPRH foci is unclear at this moment.
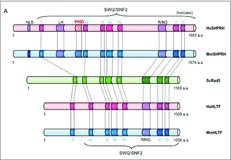
SHPRH contains a histone H1 and H5 linker sequence and a PHD (plant homeodomain), which are not present in Rad5 or HLTF (A). PHD is found in many chromatin-remodeling proteins and functions as an epigenetic reader that binds specific epigenetic marks on histone tails and recruits transcription factors and nucleosome-associated complexes to chromatin Citation[25]. A PHD can read the N-terminal tail of histone H3, mainly the methylation state of H3K4 (K4me0 vs. K4me3/2). The binding preference of H3K4 methylation occurs through interaction with an aromatic cage on PHD, and the recognition of non-modified H3K4me0 does not require an aromatic cage but occurs via interaction with hydrophobic and acidic amino acids. PHDs are versatile components of the epigenetic machineries that act in a multifaceted manner to alter chromatin structure and control fundamental DNA processes, such as transcriptional activation and repression Citation[26].
The PHD of SHPRH adopts a canonical PHD fold with a central two-stranded anti-parallel beta sheet flanked on both sides by the two zinc binding sites. Sequence alignment of the SHPRH PHD with other PHDs shows that SHPRH contains the conserved tryptophan residue that binds the tri- and dimethylated H3K4 as well as the two aromatic residues that form the aromatic cage Citation[1,27]. More importantly, the SHPRH PHD has a glutamate (E660), that is a negatively charged acidic amino acid that is conserved amongst other PHDs such as CHD4 and PHF21A. This residue is important to interact with unmodified H3K4me0 or H3K4me2. A mutation in this residue of SHPRH decreases the H3K4me2 binding affinity Citation[1]. Interestingly, a recent NMR study shows that the SHPRH PHD does not specifically interact with the H3-derived peptides, irrespective of K4 methylation. This suggests that the SHPRH PHD might have evolved a different function Citation[27]. This may indicate that the H3K4 binding selectivity occurs in a context-dependent manner or requires other adjacent effectors.
Regulation of ribosomal RNA transcription by SHPRH
The PHD in SHPRH suggests SHPRH is an epigenetic reader Citation[1]. In vitro and in vivo interaction studies demonstrated that the PHD of SHPRH could not interact with histone H3K4me3, which is the histone H3 modification at a poised promoter (B). When cells reduce their metabolic processes upon starvation or differentiation, a number of inactive or poised rDNA copies are increased. In contrast, when cells enhance metabolic processes, the proportion of active rDNA increases to accelerate the ribosome biosynthesis and cellular metabolism. To initiate the transcription of rRNA from the poised promoter, RNA polymerase I needs to be recruited to the pre-initiation complex on the rDNA promoter.
Recently, we showed that SHPRH interacts with the RNA polymerase I complex and that the loading of RNA polymerase I onto the rDNA promoter was impaired by silencing the expression of SHPRH Citation[1]. NuRD complex is required for rDNA activation by blocking the change of the rDNA promoters from poised to inactive epigenetic modifications and by keeping the poised rDNA unmethylated Citation[15]. Interestingly, the targeting of SHPRH to the rDNA promoter is dependent on CHD4 Citation[1]. Consistently, recruitment of RNA polymerase I to the rDNA promoter depends on the presence of SHPRH at the rDNA promoter in response to histone H3 methylation, suggesting that the PHD of SHPRH is important for the recruitment of RNA polymerase I to the rDNA promoter H42.9 (Fig. and B). Thus, SHPRH could act as an epigenetic reader for the transition from poised-to-active rDNA status.
Although nucleolar location of SHPRH is detected as foci during cell starvation, the distribution of SHPRH was observed throughout entire chromosomes, not just restricted to rDNA Citation[1]. Bivalent poised promoters are frequently found and controlled in many biological processes including development and differentiation Citation[28],Citation[29]. Therefore, it is possible that the function of SHPRH on chromatin is involved in broad epigenetic transitions in addition to rRNA transcription.
Perspectives and future direction
SHPRH was originally identified as an E3 ubiquitin ligase targeting PCNA as a substrate similar to the yeast Rad5 protein. Subsequent studies found HLTF as an ortholog closer to yeast Rad5. A recent observation demonstrating SHPRH is a regulator of rDNA transcription by recognizing the histone codes of rDNA in an mTOR-dependent manner shed light on a new direction to study SHPRH function Citation[1].
PCNA polyubiquitylation directs an uncharacterized template switching pathway for post-replication repair to avoid DNA damage and the blocking of proper DNA replication. Enzymatic activity of SHPRH for PCNA polyubiquitylation and its function promoting transcription of pre-rRNA could have a close link to stability of rDNA. Yeast rDNA expansion or deletion has a close relation with transcription within rDNA Citation[6]. In addition, a high level of rDNA transcription, which disrupts cohesion locally, makes sister chromatids loosely bound and DNA more vulnerable to damage Citation[30]. SHPRH stimulates rRNA transcription in high nutrition states in an mTOR dependent manner Citation[1]. When nutrition sources become scarce, SHPRH moves to another location on rDNA to reduce rRNA transcription. It might block rDNA copy number changes by recombination between rDNA repeats, since low transcription of rDNA enhances interaction between sister chromatids. Ubiquitylation of PCNA or other substrates by SHPRH in rDNA repeats might be important for suppressing recombination between rDNA repeats.
Abnormalities in ribosome biogenesis are linked with many genetic diseases including Diamond-Blackfan anemia and 5q- syndrome Citation[31]. However, the causative mutations are unknown and still under investigation in large portions of patients. In addition, strong correlation between mTOR-enhanced cancers and reduced rDNA CNV was observed Citation[5]. Anemic condition and tumorigenesis caused by ribosome biogenesis and rDNA CNV could be directly linked to rRNA transcription either by transcriptional regulation or rDNA CNVs. Recent observations of SHPRH regulating rRNA transcription in an mTOR-dependent manner and the function of its yeast ortholog, Rad5 for template switching raises several new questions: Can SHPRH control copy number changes in rDNA? If so, is PCNA polyubiquitylation also important to copy number changes in rDNA? Can active transcription of rDNA by SHPRH affect rDNA stability through loosening sister chromatid cohesion similar to yeast? Can mTOR-enhanced tumorigenesis be controlled by SHPRH? By answering these questions, we could eventually link tumorigenesis as well as potentially the aging process to rDNA stability and rRNA transcription.
Disclosure of potential conflicts of interest
No potential conflict of interest was reported by the authors
Additional information
Funding
References
- Lee D, An J, Park YU, et al. SHPRH regulates rRNA transcription by recognizing the histone code in an mTOR-dependent manner. Proc Natl Acad Sci U S A. 2017;114(17):E3424–E3433. doi:10.1073/pnas.1701978114. PMID:28400511
- Boisvert FM, van Koningsbruggen S, Navascues J, et al. The multifunctional nucleolus. Nat Rev Mol Cell Biol. 2007;8(7):574–585. doi:10.1038/nrm2184. PMID:17519961
- Hastings PJ, Lupski JR, Rosenberg SM, et al. Mechanisms of change in gene copy number. Nat Rev Genet. 2009;10(8):551–564. doi:10.1038/nrg2593. PMID:19597530
- Gibbons JG, Branco AT, Yu S, et al. Ribosomal DNA copy number is coupled with gene expression variation and mitochondrial abundance in humans. Nat Commun. 2014;5:4850. doi:10.1038/ncomms5850. PMID:25209200
- Xu B, Li H, Perry JM, et al. Ribosomal DNA copy number loss and sequence variation in cancer. PLoS Genet. 2017;13(6):e1006771. doi:10.1371/journal.pgen.1006771. PMID:28640831
- Kobayashi T, Ganley AR. Recombination regulation by transcription-induced cohesin dissociation in rDNA repeats. Science. 2005;309(5740):1581–1584. doi:10.1126/science.1116102. PMID:16141077
- Kobayashi T, Horiuchi T, Tongaonkar P, et al. SIR2 regulates recombination between different rDNA repeats, but not recombination within individual rRNA genes in yeast. Cell. 2004;117(4):441–453. doi:10.1016/S0092-8674(04)00414-3. PMID:15137938
- McStay B, Grummt I. The epigenetics of rRNA genes: from molecular to chromosome biology. Annu Rev Cell Dev Biol. 2008;24:131–157. doi:10.1146/annurev.cellbio.24.110707.175259. PMID:18616426
- Bradsher J, Auriol J, Proietti de Santis L, et al. CSB is a component of RNA pol I transcription. Mol Cell. 2002;10(4):819–829. doi:10.1016/S1097-2765(02)00678-0. PMID:12419226
- Hirschler-Laszkiewicz I, Cavanaugh A, Hu Q, et al. The role of acetylation in rDNA transcription. Nucleic Acids Res. 2001;29(20):4114–4124. doi:10.1093/nar/29.20.4114. PMID:11600700
- Murayama A, Ohmori K, Fujimura A, et al. Epigenetic control of rDNA loci in response to intracellular energy status. Cell. 2008;133(4):627–639. doi:10.1016/j.cell.2008.03.030. PMID:18485871
- Santoro R, Li J, Grummt I. The nucleolar remodeling complex NoRC mediates heterochromatin formation and silencing of ribosomal gene transcription. Nat Genet. 2002;32(3):393–396. doi:10.1038/ng1010. PMID:12368916
- Strohner R, Nemeth A, Nightingale KP, et al. Recruitment of the nucleolar remodeling complex NoRC establishes ribosomal DNA silencing in chromatin. Mol Cell Biol. 2004;24(4):1791–1798. doi:10.1128/MCB.24.4.1791-1798.2004. PMID:14749393
- Lesch BJ, Page DC. Poised chromatin in the mammalian germ line. Development. 2014;141(19):3619–3626. doi:10.1242/dev.113027. PMID:25249456
- Xie W, Ling T, Zhou Y, et al. The chromatin remodeling complex NuRD establishes the poised state of rRNA genes characterized by bivalent histone modifications and altered nucleosome positions. Proc Natl Acad Sci U S A. 2012;109(21):8161–8166. doi:10.1073/pnas.1201262109. PMID:22570494
- Zhang Y, Ng HH, Erdjument-Bromage H, et al. Analysis of the NuRD subunits reveals a histone deacetylase core complex and a connection with DNA methylation. Genes Dev. 1999;13(15):1924–1935. doi:10.1101/gad.13.15.1924. PMID:10444591
- Ling T, Xie W, Luo M, et al. CHD4/NuRD maintains demethylation state of rDNA promoters through inhibiting the expression of the rDNA methyltransferase recruiter TIP5. Biochem Biophys Res Commun. 2013;437(1):101–107. doi:10.1016/j.bbrc.2013.06.045. PMID:23796711
- Mayer C, Grummt I. Ribosome biogenesis and cell growth: mTOR coordinates transcription by all three classes of nuclear RNA polymerases. Oncogene. 2006;25(48):6384–6391. doi:10.1038/sj.onc.1209883 PMID:17041624
- Zoncu R, Efeyan A, Sabatini DM. mTOR: from growth signal integration to cancer, diabetes and ageing. Nat Rev Mol Cell Biol. 2011;12(1):21–35. doi:10.1038/nrm3025. PMID:21157483
- Inoki K, Li Y, Xu T, et al. Rheb GTPase is a direct target of TSC2 GAP activity and regulates mTOR signaling. Genes Dev. 2003;17(15):1829–1834. doi:10.1101/gad.1110003. PMID:12869586
- Sarbassov DD, Guertin DA, Ali SM, et al. Phosphorylation and regulation of Akt/PKB by the rictor-mTOR complex. Science. 2005;307(5712):1098–1101. doi:10.1126/science.1106148. PMID:15718470
- Song MS, Salmena L, Pandolfi PP. The functions and regulation of the PTEN tumour suppressor. Nat Rev Mol Cell Biol. 2012;13(5):283–296. PMID:22473468
- Motegi A, Sood R, Moinova H, et al. Human SHPRH suppresses genomic instability through proliferating cell nuclear antigen polyubiquitination. J Cell Biol. 2006;175(5):703–708. doi:10.1083/jcb.200606145. PMID:17130289
- Unk I, Hajdu I, Blastyak A, et al. Role of yeast Rad5 and its human orthologs, HLTF and SHPRH in DNA damage tolerance. DNA repair. 2010;9(3):257–267. doi:10.1016/j.dnarep.2009.12.013. PMID:20096653
- Sanchez R, Zhou MM. The PHD finger: a versatile epigenome reader. Trends Biochem Sci. 2011;36(7):364–372.
- Musselman CA, Kutateladze TG. PHD fingers: epigenetic effectors and potential drug targets. Mol Interv. 2009;9(6):314–323. doi:10.1124/mi.9.6.7. PMID:20048137
- Machado LE, Pustovalova Y, Kile AC, et al. PHD domain from human SHPRH. J Biomol NMR. 2013;56(4):393–399. doi:10.1007/s10858-013-9758-2. PMID:23907177
- Harikumar A, Meshorer E. Chromatin remodeling and bivalent histone modifications in embryonic stem cells. EMBO Rep. 2015;16(12):1609–1619. doi:10.15252/embr.201541011. PMID:26553936
- Matsumura Y, Nakaki R, Inagaki T, et al. H3K4/H3K9me3 Bivalent Chromatin Domains Targeted by Lineage-Specific DNA Methylation Pauses Adipocyte Differentiation. Mol Cell. 2015;60(4):584–596. doi:10.1016/j.molcel.2015.10.025. PMID:26590716
- Ide S, Miyazaki T, Maki H, et al. Abundance of ribosomal RNA gene copies maintains genome integrity. Science. 2010;327(5966):693–696. doi:10.1126/science.1179044. PMID:20133573
- Karbstein K. Quality control mechanisms during ribosome maturation. Trends Cell Biol. 2013;23(5):242–250. doi:10.1016/j.tcb.2013.01.004. PMID:23375955
