ABSTRACT
The identification of FACT as a histone chaperone enabling transcription through chromatin in vitro has strongly shaped how its roles are envisioned. However, FACT has been implicated in essentially all aspects of chromatin biology, from transcription to DNA replication, DNA repair, and chromosome segregation. In this review, we focus on recent literature describing the role and mechanisms of FACT during transcription. We highlight the prime importance of FACT in preserving chromatin integrity during transcription and challenge its role as an elongation factor. We also review evidence for FACT’s role as a cell-type/gene-specific regulator of gene expression and briefly summarize current efforts at using FACT inhibition as an anti-cancer strategy.
Introduction
More than 20 years ago, FACT was originally identified as a biochemical entity from HeLa cells that stimulates transcription elongation by RNA polymerase II (RNAPII) on a chromatinized DNA template in vitro [Citation1] and was therefore named FAcilitates Chromatin Transcription. Simultaneously, FACT was also purified from yeast, notably as a factor interacting with DNA polymerase I [Citation2–4], and from Xenopus eggs as a DNA unwinding factor [Citation5], supporting a role in DNA replication. Later, FACT was shown to be involved in transcription by RNAPI and III, DNA repair, and even in chromosome segregation [Citation6–8]. For all these reasons, FACT is sometimes called Facilitates Chromatin Transactions, a name that appropriately describes its wider role.
While its ability to facilitate transcription through nucleosomes in vitro has led the field to view FACT as a factor that disassembles nucleosomes in front of RNAPII, early experiments in yeast rather pointed to a role in assembling nucleosomes in the wake of the elongation complex. Over the years, a growing amount of data revealed that FACT indeed plays a role in preserving chromatin structure during transcription elongation [Citation9–23], while a role in stimulating transcription elongation per se remains to be demonstrated in vivo.
FACT is an unusual histone chaperone in that it can bind all components of the nucleosome (the H3-H4 tetramer, the H2A-H2B dimer, the histone tails, and even nucleosomal DNA) [Citation24], while other histone chaperones typically have more dedicated specificities [Citation25]. In biochemical assays, FACT can promote either chromatin assembly or disassembly depending on the context [Citation24]. Conceivably, one can therefore envision FACT promoting transcription by virtue of its nucleosome disassembly activity ‒either by promoting chromatin opening at promoters or facilitating histone/nucleosome eviction during elongation‒, or promoting transcription repression via its chromatin assembly activity. Alternatively, it may assist chromatin reassembly during transcription elongation without necessarily contributing to transcription per se. This last scenario is supported by a large amount of recent data [Citation6] so FACT is increasingly recognized as a guardian of chromatin structure during transcription. In this review, we focus on recent literature about the role of FACT in the context of transcription, with a focus on recent structural and genomic studies. Emerging data on FACT as a potential drug target in cancer will also be discussed. We encourage readers interested in the roles of FACT in other contexts such as DNA replication and repair to consult excellent recent reviews [Citation6–8].
FACT composition
FACT is conserved from yeast to humans and is made of two subunits named SUPT16H (Suppressor of Ty 16 Homolog) and SSRP1 (Structure-Specific Recognition Protein-1) in humans [Citation26], though SUPT16H is generally referred to as SPT16 after the yeast homolog. Both proteins are composed of several domains independently capable of binding different subnucleosomal particles with different affinities (). SPT16 harbors a highly conserved N-terminal domain (NTD), a dimerization domain (DD), a middle domain (MD), and a C-terminal domain (CTD). The SPT16-NTD resembles bacterial aminopeptidases but lacks the catalytic residues [Citation27,Citation28]. Instead, the SPT16-NTD was shown to interact (somewhat weakly) with the H3 and H4 N-terminal tails [Citation27]. Although highly conserved, the SPT16-NTD is not essential for viability in yeast [Citation29] and its role in FACT’s function remains largely unknown. The SPT16-DD and MD respectively contain one and two pleckstrin homology (PH) motifs. The SPT16-DD allows the dimerization with SSRP1 [Citation30] but also plays a key role in nucleosome binding as will be discussed below. The SPT16-MD interacts with the (H3-H4)2 tetramer [Citation31] while the SPT16-CTD is acidic, structurally disordered, and binds to the H2A-H2B dimer [Citation32]. SSRP1 interacts with SPT16 using its N-terminal domain (NTD). The SSRP1-NTD is therefore sometimes called SSRP1-DD. The SSRP1-NTD/DD is followed by a middle domain (MD) that, like the SPT16-MD, is made of two PH motifs and interacts with the (H3-H4)2 tetramer [Citation30,Citation33]. C-terminal to the SSRP1-MD lies an intrinsically disordered domain (IDD) followed by a High mobility group (HMG) box domain and a CTD that is also intrinsically disordered. These domains remain relatively poorly characterized. The HMG box domain of SSRP1, which is closely related to the HMGB family of HMG proteins, can bind DNA [Citation34] but its role in FACT’s function remains enigmatic.
Figure 1. FACT domains and structure. (a) Domain organization of human FACT subunits. The gray double arrows indicate known physical interactions of FACT domains with histones. NTD, N-terminal domain; DD, dimerization domain; MD, middle domain; CTD, C-terminal domain; IDD, intrinsically disordered domain; HMG, High mobility group domain; PH, Pleckstrin homology. (b) Structure of human FACT bound to a nucleosome (PDB ID: 6UPL) [Citation50]. The structure of the SPT16-NTD (PDB ID: 5E5B) [Citation55] and SSRP1-HMG (PDB ID: 6L34) [Citation56] were added (boxed structures) to contextualize their relative size. (c) A view of the FACT-nucleosome structure (PDB ID: 6UPL; containing 79 bp of DNA, dark gray) that also displays the DNA of a fully-wrapped (146 bp) nucleosome from PDB ID: 2CV5 (old pink) [Citation210]. Notice the clash between the path of the DNA and the Spt16-MD, as well as the position of the SPT16-CTD, mimicking the path of the DNA in the full nucleosome. Molecular graphics and analyses were performed with UCSF ChimeraX [Citation211]. The docking in panel C was done on the histone proteins from PDB ID: 6UPL and PDB ID: 2CV5 using the MatchMaker tool in ChimeraX. The histones from PDB ID: 2CV5 were hidden.
![Figure 1. FACT domains and structure. (a) Domain organization of human FACT subunits. The gray double arrows indicate known physical interactions of FACT domains with histones. NTD, N-terminal domain; DD, dimerization domain; MD, middle domain; CTD, C-terminal domain; IDD, intrinsically disordered domain; HMG, High mobility group domain; PH, Pleckstrin homology. (b) Structure of human FACT bound to a nucleosome (PDB ID: 6UPL) [Citation50]. The structure of the SPT16-NTD (PDB ID: 5E5B) [Citation55] and SSRP1-HMG (PDB ID: 6L34) [Citation56] were added (boxed structures) to contextualize their relative size. (c) A view of the FACT-nucleosome structure (PDB ID: 6UPL; containing 79 bp of DNA, dark gray) that also displays the DNA of a fully-wrapped (146 bp) nucleosome from PDB ID: 2CV5 (old pink) [Citation210]. Notice the clash between the path of the DNA and the Spt16-MD, as well as the position of the SPT16-CTD, mimicking the path of the DNA in the full nucleosome. Molecular graphics and analyses were performed with UCSF ChimeraX [Citation211]. The docking in panel C was done on the histone proteins from PDB ID: 6UPL and PDB ID: 2CV5 using the MatchMaker tool in ChimeraX. The histones from PDB ID: 2CV5 were hidden.](/cms/asset/72f36d2f-d71e-492e-8cdc-32bc754b1406/ktrn_a_2069995_f0001_oc.jpg)
In yeast, where FACT has been the most deeply studied, FACT has a similar composition but the SSRP1 ortholog (named Pob3; Polymerase One Binding protein 3), is lacking the HMG box domain. It has been proposed that Nhp6, a highly abundant HMG protein, covers the function of the SSRP1 HMG box domain in yeast [Citation35,Citation36]. Although yeast FACT does not stably associate with Nhp6 [Citation35,Citation36], supplementing FACT with Nhp6 allows for the histone chaperone to bind nucleosomes in vitro [Citation37] and also stimulates its chaperoning activity in various assays [Citation38,Citation39]. However, at least a six-fold molar excess of Nhp6 (relative to nucleosomes) is necessary to stimulate the binding of yeast FACT to the nucleosome [Citation37]. While the high abundance of Nhp6 in yeast cells is compatible with such a scenario [Citation40,Citation41], recent evidence has raised doubts on the in vivo relevance of Nhp6 to FACT function. First, if Nhp6 was the functional equivalent of the SSRP1 HMG box domain, one may expect that fusing Nhp6 to Pob3 would alleviate the need for additional Nhp6. Yet, a minimum of three Nhp6s have to be fused to Pob3 to render FACT independent of external Nhp6 in vitro 41. Second, human FACT, despite containing an HMG box domain, likewise requires exogenous Nhp6 to bind nucleosomes in vitro [Citation41]. Finally, Nhp6 is also required for another histone chaperone, Spt6, to bind nucleosomes in vitro [Citation42]. Collectively, these data suggest that while Nhp6 can “stimulate” FACT and other histone chaperones in a test tube, it may not be physiologically equivalent to the HMG box found in SSRP1 in some species. Whether Nhp6 in yeast and perhaps some HMGB proteins in some other species contribute to FACT function in cells remains to be determined.
Nevertheless, in vitro experiments performed in the presence of Nhp6 contributed to revealing a key aspect of FACT: its dependence on partial nucleosome unfolding for binding. FACT does not bind nucleosomes in vitro, most likely because the different domains of FACT recognize histone surfaces that are covered by DNA within the nucleosome [Citation43–45]. Nhp6 (and HMGB proteins in general) is known to bind and bend DNA [Citation46,Citation47] so that adding an excess of Nhp6 likely contributes to uncoiling DNA contacts at the entry/exit sites of the nucleosome, hence exposing histone surfaces crucial for FACT binding. Accordingly, other means of reducing histone-DNA contacts also stimulate FACT-nucleosome interactions and alleviate the need for Nhp6. These include the use of small molecules [Citation48], the presence of a double-stranded break in the nucleosomal DNA [Citation43,Citation49], and nucleosomal particles made of sub-nucleosomal size DNA [Citation45,Citation50]. Structural data presented in the next section now provide explanations for how this happens. We and others have proposed that, in cells, FACT senses the presence of partially unwrapped nucleosomes, produced during transcription or other chromatin-related processes [Citation6,Citation51,Citation52], a phenomenon that can be somewhat mimicked in vitro by the addition of an excess of Nhp6 or other DNA-histone perturbing agents.
FACT structure
Work from several laboratories over the years has provided structural information on the different domains of FACT, either alone or in interaction with histones [Citation27,Citation28,Citation30–32,Citation43,Citation53–56]. Collectively, these studies show how the different FACT domains can shield the DNA binding surfaces of histones. More recently, the group of Karolin Luger reported a high-resolution structure of human FACT bound to a nucleosome [Citation50]. This structure illustrates why FACT requires partial DNA unwrapping for binding. Indeed, to obtain a stable FACT-nucleosome complex, Liu, Zhou et al. had to use a much shorter piece of DNA (79 bp) relative to a fully wrapped nucleosome (146 bp). These subnucleosomal particles were stably bound by FACT and successfully analyzed by cryo-EM (). The resulting structure resembles a unicycle where the (H3-H4)2 tetramer forms the wheel and the two H2A-H2B dimers act as the pedals. The stacked SPT16 and SSRP1 DDs sit on the dyad, making contact with the DNA, forming the saddle/seat. This was unexpected since no prior work had suggested a role for either DD in DNA binding. SPT16 and SSRP1 MDs reach down on both sides, interacting with the DNA at the dyad, the tetramer underneath, and reaching down to the dimer “pedals”, hence forming both forks of the unicycle. The positions of both MDs with H3 and H4 histones clash with the trajectory of the DNA in a fully wrapped nucleosome, hence explaining why destabilizing the histone-DNA interaction stimulates FACT binding, as suggested by a wealth of biochemical data (see above). Despite being disordered, the SPT16-CTD is visible in the structure and adopts an interesting location. It essentially follows the path of the missing DNA, hence working as a DNA mimic, most likely preventing DNA-rebinding in more natural (full-length nucleosomal DNA) contexts and further explaining why fully wrapped nucleosomes are not stably bound by FACT (, right panel, and ). The FACT-nucleosome structure, therefore, revealed extensive FACT-DNA interactions, in addition to the expected FACT-histone interactions [Citation50]. Interestingly, FACT does not bind naked DNA yet a truncation of the SPT16 and SSRP1 CTDs led to FACT-DNA interaction in vitro [Citation50]. This led the Luger group to propose that the CTDs of both proteins fold on their MDs, keeping FACT in closed conformation to prevent DNA binding. Once an H2A-H2B dimer is exposed via nucleosomal DNA unwrapping, SPT16-CTD would bind to the dimer and hence free the SPT16-MD, allowing FACT to “sit” on the dyad via contacts between the SPT16-MD and DD with the DNA and the tetramer. Similar mechanisms may take place on the other side of the nucleosome with the SSRP1-CTD and MD. A recent study from the Studitsky lab showed that Nhp6 can bind the SPT16 and SSRP1 CTDs, leading them to suggest that Nhp6 may also promote FACT binding (in vitro) by contributing to the opening of the compact MD/CTD fold [Citation57].
Some parts of the FACT complex are not visible in the cryo-EM structure, notably the SPT16-NTD and the SSRP1-IDD, -HMG, and -CTD [Citation50]. The structure of individual SPT16-NTD [Citation55] and SSRP1-HMG [Citation56] domains were included in (boxed models in the left panel) to provide a sense of their size relative to the core of FACT. FACT was recently shown to act on tetranucleosome substrates, helping in the decondensation of packed nucleosomes [Citation58]. An intriguing possibility is that some of the domains not visible in the FACT-nucleosome structure are involved in oligo-nucleosome contexts. Alternatively, these domains may become engaged with the nucleosome in subsequent steps of the remodeling process. Indeed, one has to keep in mind that the cryo-EM structure of the FACT-nucleosome complex [Citation50] represents only one snapshot of the very complex and dynamic process of nucleosome (dis)assembly. Finally, these domains may simply be inherently mobile and hence not compatible with structure determination.
FACT recruitment to chromatin
Shortly after Liu, Zhou et al. published their cryo-EM structure of the human FACT-nucleosome complex [Citation50], the Cramer lab published the cryo-EM structure of the yeast FACT complex with an interesting twist: their structure contains full-length nucleosomal DNA and an RNAPII engaging the FACT-bound nucleosome [Citation59]. shows a model of the yeast FACT-nucleosome-RNAPII-Spt4/5 structure [Citation59] docked onto the structure of a human RNAPII elongation complex containing the Paf1 complex (PAF1C), and the elongation factors DSIF and SPT6 [Citation60]. Topologically, the human and yeast FACT-nucleosome complexes are similar except that the interaction of FACT with the DNA is slightly downstream (by about 10 bp) in the yeast complex relative to the human complex. This may reflect differences between both species but perhaps most likely reflects differences in the actual complexes being examined (presence of full nucleosomal DNA versus partial nucleosomal DNA, and presence or absence of RNAPII). Importantly, the yeast cryo-EM structure shows how the presence of an engaging polymerase allows stable binding of FACT despite the presence of full-length DNA. In this structure, RNAPII peels off about 40 bp of DNA, hence exposing one surface of the tetramer and the first dimer, which are bound by SPT16-MD and SPT16-CTD in a similar topology as observed in the human complex [Citation50,Citation59]. Importantly, in the yeast FACT complex, the second half of the nucleosomal DNA (downstream of the dyad) is fully wrapped, demonstrating that asymmetrical unwrapping of the nucleosomal DNA from the upstream side (the Spt16 side) is sufficient to accommodate FACT binding. Recent in vivo mapping experiments showed that FACT associates with such asymmetrically unwrapped nucleosomes in the body of transcribed yeast genes [Citation51]. These MNase footprinting experiments, combined with chromatin immunoprecipitation (ChIP), also revealed the presence of RNAPII-FACT-nucleosome particles, with partially unwrapped DNA, on active yeast genes [Citation51,Citation52]. A picture hence emerges from recent biochemical, structural, and genomics data where FACT is recruited directly to partially unwrapped nucleosomes as they are being engaged by RNAPII. This is different from other chromatin regulators, which often rely on interactions with the polymerase or one of its associated elongation factors for their recruitment. For example, the histone chaperone Spt6 contacts the RNAPII CTD and its linker, as well as the RNAPII stalk, and these interactions contribute to Spt6 recruitment to genes [Citation61–63](see ). Similarly, the Set2 histone methyltransferase [Citation64,Citation65] and the Rpd3S histone deacetylase complex [Citation66,Citation67] are well known to use their affinity with the phosphorylated RNAPII CTD for their recruitment. While FACT was shown to make interactions with several components of the RNAPII elongation complex as well as with elongation-associated histone modifications [Citation68–74], a systematic survey of their contribution to FACT chromatin occupancy in yeast failed to identify an input for these interactions in FACT occupancy on genes [Citation51]. Consistently, FACT and RNAPII do not interact in the cryo-EM structure of the RNAPII-nucleosome-FACT complex [Citation59] (see ). In addition, some mutations in histones H3 and H4 reduce FACT occupancy, and in some cases, this was shown to occur without affecting RNAPII occupancy on genes [Citation75–79], further supporting direct recruitment of FACT on the nucleosome. The most parsimonious model to explain how FACT-chromatin interactions occur on transcribed regions (and depend on transcription [Citation51]) is therefore that FACT directly recognizes nucleosomes that are in the process of being transcribed (). This may be possible given the very high abundance of FACT in yeast cells. Indeed, FACT is estimated to be present at 15,000 to 42,000 molecules per cell [Citation6]. Given there are 70,000 nucleosomes in a yeast genome, there could be as many as one FACT molecule per two nucleosomes. When further considering that a significant fraction of the genome is not being transcribed at any given time (most transcription is concentrated on a few highly transcribed genes), this leaves many available “free-FACT” molecules ready to intervene whenever a nucleosome begins to unfold.
Figure 2. A model of the RNAPII elongation complex engaging a nucleosome bound by FACT. The structure of the human RNAPII elongation complex (RNAPII, PAF1C, DSIF, SPT6; PDB ID: 6TED) [Citation60] was docked on the structure of yeast FACT bound to a nucleosome engaged by RNAPII-Spt4/5 (PDB ID: 7NKY) [Citation59]. The RNA, RNAPII, and DSIF are from PDB ID: 6TED (the ones from PDB ID: 7NKY are hidden). The docking was done on the RNAPII molecules using the MatchMaker tool in ChimeraX [Citation211].
![Figure 2. A model of the RNAPII elongation complex engaging a nucleosome bound by FACT. The structure of the human RNAPII elongation complex (RNAPII, PAF1C, DSIF, SPT6; PDB ID: 6TED) [Citation60] was docked on the structure of yeast FACT bound to a nucleosome engaged by RNAPII-Spt4/5 (PDB ID: 7NKY) [Citation59]. The RNA, RNAPII, and DSIF are from PDB ID: 6TED (the ones from PDB ID: 7NKY are hidden). The docking was done on the RNAPII molecules using the MatchMaker tool in ChimeraX [Citation211].](/cms/asset/86a509ff-827a-4ba8-bfb4-7c5455cde5c1/ktrn_a_2069995_f0002_oc.jpg)
Figure 3. A proposed model of the dynamic interactions between FACT and a nucleosome transcribed by RNAPII. The nucleosome is represented linearly for simplification and the regions contacted by the various histones along the DNA are represented as white rectangles. Superhelical locations (SHL −7 to +7) and the dyad axis are indicated. Yeast FACT is represented with the same color code as human FACT in and proposed dynamic interactions with histones and DNA are depicted at key steps of RNAPII transcribing through the nucleosome. This model is based on available biochemical, structural, and genomic data cited in the main text but remains highly speculative. Note that the Pob3-CTD and Spt16-NTD are depicted in step 2 despite not being visible in the structure from Farnung et al. [Citation59].
![Figure 3. A proposed model of the dynamic interactions between FACT and a nucleosome transcribed by RNAPII. The nucleosome is represented linearly for simplification and the regions contacted by the various histones along the DNA are represented as white rectangles. Superhelical locations (SHL −7 to +7) and the dyad axis are indicated. Yeast FACT is represented with the same color code as human FACT in Figure 1a and proposed dynamic interactions with histones and DNA are depicted at key steps of RNAPII transcribing through the nucleosome. This model is based on available biochemical, structural, and genomic data cited in the main text but remains highly speculative. Note that the Pob3-CTD and Spt16-NTD are depicted in step 2 despite not being visible in the structure from Farnung et al. [Citation59].](/cms/asset/a141b0b3-228f-4179-b215-14a7724377d0/ktrn_a_2069995_f0003_oc.jpg)
Single-molecule tracking (SMT) experiments revealed that only about 3% of all FACT molecules are stably engaged on chromatin in yeast cells [Citation51]. This represents about 450–1,500 FACT molecules, a number that matches the number of elongating RNAPII molecules, as estimated by a combination of ChIP and global run-on experiments [Citation80]. These findings further support a model where a large number of available FACT molecules work as a supply for chromatin binding with little signal other than the affinity of FACT for exposed histone surfaces on partially unwrapped nucleosomes. Satisfyingly, this model provides a universal mechanism for FACT recruitment in the context of any of the chromatin activity in which it has been implicated. Indeed, nucleosome unwrapping inevitably occurs during transcription by all three RNA polymerases, as well as during DNA replication and DNA repair. As recently proposed by Formosa and Winston [Citation6], one can therefore see FACT as a chromatin repair factor, recognizing, maintaining, and “fixing” nucleosomes that become incompletely folded.
Transcription through a gene implies that RNAPII will engage several nucleosomes in a row (5 or 6 for a typical yeast gene and many more for a mammalian gene). The simple chromatin repair model described above would predict that a different FACT molecule may engage with each nucleosome along a transcribed gene. Surprisingly, however, while transcription through a nucleosome takes about 6 seconds on average, transcription-dependent FACT-chromatin interactions are much longer, rather matching the time it takes to transcribe through an entire yeast gene (20–25 seconds) [Citation51]. Combined with the fact that the number of chromatin-bound FACT molecules matches that of elongating RNAPII, these SMT experiments are (strikingly) consistent with a processive mechanism for FACT during transcription. While FACT may directly target partially unfolded nucleosomes, the data implies that additional mechanisms allow the maintenance of FACT on a gene during its entire transcription. A single FACT molecule would then be reused from one nucleosome to the next, tracking along with RNAPII. Recent work from our laboratory suggests that the Chd1 chromatin remodeler plays a role in this process. Indeed, the deletion of CHD1 leads to the decrease of FACT occupancy in 3’ ends, coupled with an increase in 5’ ends, suggesting that Chd1 affects the distribution of FACT on genes without affecting its recruitment [Citation51]. Interestingly, despite Chd1 and FACT being known to physically interact [Citation59,Citation71,Citation81–84], the described interaction surface appears to be dispensable for this activity. Instead, the ATPase activity of Chd1 appears critical for determining FACT distribution [Citation51]. The current data, therefore, suggest that chromatin remodeling by Chd1 favors the recycling of FACT molecules from one nucleosome to the next as RNAPII elongates (), perhaps relating to the genetic interactions between FACT and CHD1 mutants [Citation85].
The role of Chd1 in FACT distribution appears to be conserved in Drosophila [Citation51] suggesting that recognition of partial nucleosome unwrapping followed by Chd1-dependent spreading, as dissected in yeast, may explain how FACT is recruited and maintained on transcribed regions in other species as well. But how is FACT recruited to chromatin in other contexts? FACT has been shown to be recruited to the yeast HO promoter where it contributes to chromatin opening over the promoter and therefore activation of HO transcription [Citation86,Citation87]. FACT also contributes to the activation of other yeast genes via less well-characterized mechanisms [Citation11,Citation88]. In higher eukaryotes, FACT contributes to the function of several promoters (see below). How FACT is recruited to promoters and how it promotes chromatin opening is far less understood. One proposed model is that initial chromatin remodeling by transcription factors (TFs) or chromatin remodelers provides the trigger for FACT recruitment, similar to how it is recruited during elongation. Accordingly, the transient association of FACT with the HO promoter is dependent on the Swi5 TF and the SWI/SNF chromatin remodeling complex [Citation86]. Similarly, in mammalian cells, FACT is transiently recruited to pluripotency genes regulated by OCT4 in embryonic stem cells (ESCs) undergoing retinoic acid-mediated differentiation through a mechanism that is not understood but that depends on initial chromatin opening by histone demethylation [Citation89]. Alternatively (or in addition), FACT may be more directly recruited by TFs via protein-protein interactions. This model is supported by a few reports describing TFs [Citation90–92] and transcription co-factors [Citation93,Citation94] interacting with FACT. How FACT is recruited to chromatin during DNA replication is also ill-defined and may involve some of the previously identified interactions with replication factors [Citation3,Citation4,Citation53,Citation95–98] as well as direct recruitment to partially unwrapped nucleosomes. During DNA repair, FACT may be recruited by the repair machinery [Citation99–104] and/or unwrapped nucleosomes but some evidence suggests that the HMG domain of SSRP1 may contribute to recognizing the DNA at the damaged site [Citation105,Citation106].
Role of FACT during elongation: elongation factor or chromatin safe keeper?
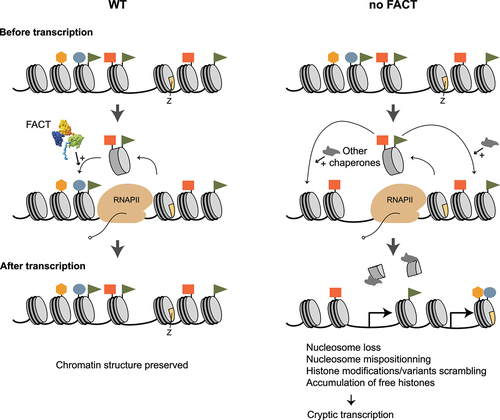
In cell-free systems, FACT stimulates transcription through nucleosomes [Citation1,Citation45,Citation107,Citation108]. This has been proposed to involve the removal of an H2A-H2B dimer [Citation107] and argued otherwise by others [Citation39,Citation108]. Coupled with the fact that FACT can both assemble and disassemble nucleosomes in vitro [Citation45,Citation50,Citation107–109], it has been proposed FACT promotes RNAPII transcription through nucleosomes while preserving nucleosome integrity [Citation107,Citation108]. The mechanism has been carefully examined by Hsieh et al [Citation108] who showed that, by competing with DNA for binding to the proximal dimer, FACT helps uncoil DNA from the nucleosome, which helps with the progression of RNAPII. At the same time, by binding to the dimer, FACT protects from dimer loss. This model fits very well the recent structural data described above. Experiments performed in cells support a role for FACT in promoting nucleosome assembly during elongation, but so far failed to validate a role for FACT in promoting elongation. Indeed, inactivating FACT for only a few minutes leads to massive chromatin defects (). Very rapidly, nucleosomes are lost and those remaining become mislocalized [Citation9–15]. In addition, the distribution of histone variants [Citation13,Citation16–18] and histone modifications [Citation15] becomes blurred, as the cell attempts to fill the nucleosome gaps via alternative chromatin assembly pathways, as evidenced by the increased histone turnover [Citation9], increased H3K56 acetylation [Citation15], and recruitment of other histone chaperones (Asf1 and HIR) [Citation15] over transcribed regions, as well as the accumulation of soluble histones associated with Asf1 [Citation19]. Importantly, this chromatin chaos can be minimized by inhibiting transcription [Citation15,Citation20]. Hence, one prominent role of FACT is to prevent chromatin defects otherwise promoted by transcription. More recently, some of these chromatin defects were shown to occur as a consequence of FACT inactivation in higher eukaryotes as well with a magnitude that varies between studies [Citation21–23]. FACT can therefore be considered as a chromatin safe keeper, as it uses its ability to recognize unwrapped nucleosomes produced by RNAPII and preserve their integrity as the polymerase traverses them (). This is to say that FACT’s chromatin assembly activity prevails during transcription elongation. These data contrast with the initial in vitro experiments that rather suggested FACT would promote disassembly (minimally by ejecting an H2A-H2B dimer) in order to promote RNAPII passage [Citation107]. But does FACT inactivation affect transcription in cells?
Figure 4. Roles of FACT during transcription elongation (chromatin safe keeper model). A nucleosome array is represented before (top), during (middle) and after (bottom) the passage of RNAPII, in WT cells (left) and in cells where FACT function is impaired (right). The distribution of some histones modifications (colored rectangles, ovals, triangles, and hexagons) and H2A.Z histone variant (z) are shown.
The most apparent transcription defect observed in FACT mutants is the appearance of transcription initiated from cryptic sites inside genes, a phenomenon well-characterized in yeast [Citation10,Citation13,Citation73,Citation110,Citation111] and recently observed in plants [Citation112] and mouse ESCs [Citation113]. Such cryptic transcription, which is also observed in mutants for other chromatin regulators [Citation10,Citation110], inevitably occurs as a consequence of the chromatin defects arising upon FACT’s loss. So, the exposure of cryptic promoters ‒otherwise covered by nucleosomes‒ in FACT mutants, leads to fortuitous initiation, but does FACT play a role in elongation?
Elongation properties can be altered in at least three different ways. First, the elongation rate (speed) may be reduced so that the time required to fully transcribe a gene increases. Second, RNAPII may be less processive; that is, its probability to transcribe until the end of the gene is reduced, for example by premature termination. Finally, more frequent or longer pauses at a subset of positions along the gene may also affect the apparent elongation rate. All these defects would lead to reduced transcription output but work from several groups repeatedly reported either no/modest decrease or increase in RNA output upon tampering with FACT [Citation12,Citation21,Citation22,Citation113–118]. Some groups have reported massive gene expression changes in both directions, but these were after long (>24 hours) FACT inactivation [Citation91,Citation119]. Also, in those experiments, the expression changes did not correlate with FACT occupancy, suggesting that these were indirect effects of FACT inactivation. RNA-seq experiments from mouse skin fibroblasts revealed that increased transcription upon SSRP1 knockout is characterized by reads in introns and intergenic regions, as well as by read density biases in some exons [Citation21]. Collectively, these data suggest that the increased RNA-seq signal measured upon loss of FACT mainly stems from the emergence of cryptic initiation. This, however, will require confirmation by methods allowing mapping of transcription initiation sites in FACT mutants, as shown for FACT in plant cells [Citation112] and another histone chaperone, Spt6, in yeast [Citation120].
Demonstrating a role for FACT in stimulating elongation through nucleosomes in vivo has therefore been a challenge. While FACT may simply not play any role in stimulating elongation in vivo, it remains possible that such a role is not easily detected for technical or biological reasons. One possibility is that the effect of FACT on elongation is obscured by the presence of redundant factors in cells. Candidates for that include other histone chaperones such as Spt6 but also other factors including LEDGF, HDGF2, Brd2, Brd3, and NDF [Citation121–123] that have been shown to allow transcription through nucleosomes in vitro using similar assays as originally used to isolate FACT [Citation1]. Another possibility is that the chromatin reassembly role of FACT obscures its elongation function. Indeed, the massive chromatin loss quickly occurring upon FACT inactivation implies that subsequent rounds of transcription take place on nucleosome-(partially) depleted chromatin, hence obviating the need for FACT in elongation. To circumvent this limitation, one would have to look specifically at the first round of transcription after FACT inactivation or use a system where FACT has a limited effect on nucleosome occupancy. Another possible explanation for the apparent absence of elongation defect in FACT mutants is that cryptic transcription, which is so prominent in these mutants, makes it very difficult to address whether the chaperone affects elongation. Indeed, processivity defects are typically measured by looking for 5’ bias in RNAPII occupancy or activity [Citation124], but the fact that the presence of cryptic transcription also leads to similar biases makes this approach inapplicable [Citation124]. Elongation rate can be measured by looking at RNAPII occupancy or activity over time in cells where transcription has been synchronized [Citation124–127]. Here again, the presence of signals emanating from cryptic transcripts can compromise those measurements. Nevertheless, except for one notable exception [Citation128], attempts at detecting elongation defects in FACT mutants/inhibition using these approaches revealed no appreciable phenotype [Citation115,Citation129]. In a very recent report, FACT was proposed to affect the elongation of hypoxia-induced genes [Citation130] but because only one region per gene was analyzed, one can not conclude on an effect on elongation. Finally, intragenic pausing can be detected in cells using native elongating transcript sequencing (NET-seq) [Citation131,Citation132] but this was never tested for FACT mutants and would inevitably be complicated by the presence of cryptic initiation. Highlighting a role for FACT in promoting elongation through nucleosomes in vivo will therefore require additional efforts. Combinations of genomic methods measuring RNAPII occupancy and activity, combined with acute protein depletion, were recently used to highlight the contribution of Spt6 to transcription elongation in mammalian cells [Citation133,Citation134]. Such an approach may allow determining whether FACT promotes elongation in cells as it does in vitro but for the time being, direct evidence for a role for FACT in promoting elongation in cells remains scarce.
Cooperation with other histone chaperones
The phenotypes associated with FACT mutants are not unique to FACT. Indeed, mutations in SPT6, another histone chaperone, also lead to severe chromatin defects and massive cryptic transcription [Citation10,Citation13,Citation15,Citation120,Citation135–140]. Similar phenotypes, although milder, are also observed in mutants for other histone chaperones such as Spn1/Iws1 [Citation141], CAF-1 [Citation142], HIR [Citation143], Asf1 [Citation143], Rtt106 [Citation143,Citation144], and Spt2 [Citation145,Citation146], as well as in mutants for other (non-histone chaperone) chromatin regulators [Citation65,Citation110,Citation142,Citation147]. Oppositely, mutations in other histone chaperones such as Nap1 and Vsp75 can suppress cryptic transcription defects [Citation148]. The maintenance of nucleosomes during transcription elongation, therefore, involves several players, engaged in a complex choreography where FACT and Spt6 play the main parts. While many of these factors may play redundant roles (explaining their mild phenotypes), some, notably FACT and Spt6, appear to have essential and complementary functions. This complementarity may be explained (at least in part) by their different genomic localization. Indeed, in yeast, while FACT occupies active genes relatively evenly from start to finish [Citation51,Citation52,Citation149,Citation150], Spt6 occupancy is lower at 5’ ends and increases toward the end of genes [Citation149,Citation151]. The difference in localization is even more pronounced in higher eukaryotes where FACT occupancy was sometimes, (but not always [Citation21]), reported being biased toward the 5’ end of transcription units [Citation22,Citation119,Citation152]. Other data, however, suggest that FACT, Spt6, and other chaperones may truly collaborate, rather than splitting the duty between different nucleosomes. First, many of the other histone chaperones interact physically with one another (e.g. Spt6-Spn1/Iws1 [Citation42,Citation153], Spt6-Spt2 [Citation154], Rtt106-CAF-1 [Citation144]). Interestingly, this is not the case for FACT since, to our knowledge, no direct interactions between FACT and another histone chaperone have been reported so far. As shown in , this may not be so surprising given that FACT and Spt6 are quite far from each other in current structural models of elongation complexes. This is not to say that FACT does not collaborate with other histone chaperones. On the contrary, recent evidence suggests a complex interplay between FACT, Spt6, and Spn1 [Citation155]. In this work, Viktorovskaya et al. show that the chromatin defects observed upon the deletion of SPN1 (or abrogating the interaction between Spn1 and Spt6) can be suppressed by mutations in FACT that lead to reduced FACT-chromatin interactions. Because abrogating the Spt6-Spn1 interaction leads to reduced Spt6 occupancy on genes, the authors proposed that the balance between FACT and Spt6 (rather than their absolute levels) is a key determinant for chromatin integrity during transcription. While the molecular details remain to be elucidated, the current literature strongly suggests that FACT, Spt6, and many other histone chaperones work together to safeguard chromatin during transcription elongation.
The role of FACT in transcription initiation: What dictates whether FACT preserves or dissociates nucleosomes?
In addition to its role during elongation, FACT is also documented to regulate promoter activity. As mentioned above, in yeast, inactivation of FACT leads to transcription initiation defects at selected genes including HO, GAL1/10, PHO5, and SER3 [Citation11,Citation86–88,Citation156]. Some of the effects on initiation may be consequent to the role of FACT in repressing cryptic transcription, as cryptic transcription -running over promoter region- was shown to alter initiation at a few specific genes (GAL1 and SER3) in FACT mutants [Citation156–158]. At HO, however, FACT was shown to bind the promoter so it may act more directly. At this promoter, FACT promotes nucleosome disassembly, hence promoting the recruitment of TFs and, ultimately, transcription initiation [Citation86,Citation87]. Whether FACT directly regulates other promoters in yeast remains to be demonstrated. The association of FACT with the HO promoter is very transient and could only be detected in a careful time course in synchronized cells [Citation86,Citation87]. Such a transient interaction, if a common feature of FACT at promoters, may explain why a wider role of FACT in promoter regulation remains unexplored.
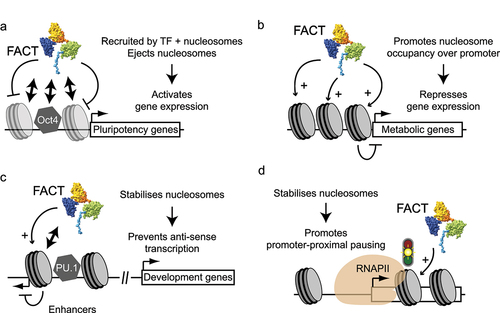
In mammals, FACT was also shown to affect initiation at some genes (). FACT is transiently recruited to pluripotency genes regulated by OCT4 in ESCs undergoing retinoic acid-mediated differentiation [Citation89] (). In a recent preprint, the Hainer lab showed that acute depletion of FACT in ESCs leads to downregulation of master TFs (including OCT4) and loss of pluripotency (bioRxiv 2021.07.30.454509; doi: https://doi.org/10.1101/2021.07.30.454509). FACT could regulate these cell-identity genes by controlling chromatin structure over the promoters and enhancers of their target genes. Another group rather suggested that FACT helps maintain the pluripotency state of mouse ESCs via the repression of promoters of metabolic genes [Citation119] (). There is therefore evidence for FACT either “opening” or “closing” chromatin structure at promoters. In macrophages, FACT binds positioned nucleosomes flanking sites occupied by the TF PU.1 in enhancers near genes essential for macrophage development and function, where it would maintain a stable chromatin structure and prevent non-coding transcription [Citation94] (), a phenomenon also observed at some genes in mouse ESCs [Citation119]. FACT is also recruited to muscle-specific genes at the onset of C2C12 differentiation by the TF myogenin [Citation92]. In Arabidopsis, FACT was shown to rhythmically bind to the promoter of genes involved in the circadian rhythms [Citation159,Citation160]. In addition, FACT mutants show defects in the expression of genes involved in seed dormancy [Citation161] and the response to light stress [Citation118]. Hence, regardless of the organism, in addition to its role at transcribed chromatin, FACT appears to have gene-specific roles by transiently targeting some promoters or enhancers. One outstanding question regarding this less-well characterized function of FACT is what dictates whether FACT promotes chromatin accessibility via nucleosome disassembly or a more repressive structure via nucleosome assembly. Perhaps, the local context (presence of other factors, histone modifications, DNA methylation, etc) influences the outcome of FACT binding to promoters.
Figure 5. Role of FACT during transcription initiation at specific genes in mammalian cells. (a-d) Four different previously described roles played by FACT at specific promoters or enhancers are depicted (see main text for details). Physical interactions are represented as double arrows. Stabilizing (arrows with “+” signs) and destabilizing (inhibitory arcs) effects on nucleosomes are depicted.
Could FACT play a more general role in initiation? In yeast, the occupancy of RNAPII [Citation15,Citation162] and the pre-initiation component TFIIB [Citation162] decreases dramatically genome-wide upon FACT inactivation. This may suggest a global role for FACT in initiation but this conclusion is uncertain since it remains possible that the initiation machinery is titrated away from bona fide promoters by the emergence of massive cryptic initiation in FACT mutants [Citation15]. This mechanism has been proposed to explain a similar initiation defect in spt6 mutants, another histone chaperone [Citation120]. Such a mechanism is plausible in light of the demonstration that the transcription machinery is limiting in yeast cells [Citation163].
In Drosophila S2 cells, the knockdown of FACT leads to decreased RNAPII promoter-proximal pausing, suggesting the FACT helps maintain RNAPII at promoter-proximal pause sites [Citation22] (). Increased RNAPII over gene bodies ‒indicative of more frequent release into elongation‒ was only observed at a few genes, however. Hence, at many genes, the knockdown of FACT may lead to early termination of the paused polymerases, rather than its release into elongation. Nevertheless, this work suggests that FACT promotes the paused state. How this is achieved remains unclear. FACT may promote pausing by maintaining nucleosomes (including the +1 nucleosome) over genes. This may be related to the recently discovered “second pause site” that occurs at (and presumably due to) the +1 nucleosome [Citation164,Citation165]. Alternatively, FACT may regulate pausing in a histone chaperone-independent manner. In support of this possibility, FACT was shown to affect promoter-proximal pausing on naked DNA in vitro, although ‒in such a system‒ FACT promotes escape rather than pausing [Citation166].
FACT is a context-dependent essential factor
FACT was long assumed to be a ubiquitous and essential factor, akin to other SPT factors SPT6 and SPT5/DSIF, but work from several labs in the last decade clearly showed that while some cells rely on FACT for their viability, others can grow well without it. Coincidently, FACT expression levels vary enormously between cell types, and those expressing high levels of FACT are also those that depend on it [Citation23,Citation117,Citation167–171] (). These observations raised the question, what makes some cells dependent on FACT? A hint to this question comes from looking at which cells express high levels of FACT. Analysis of different cell lines and tissues showed high FACT levels in highly proliferating/less differentiated cells, while more differentiated cells showed low to undetectable FACT levels [Citation23,Citation117,Citation167–171]. FACT levels are notably very high in cancer cells, especially those with poorly differentiated phenotypes [Citation167,Citation171,Citation172].
Figure 6. Different aspects of FACT in mammalian cells. (a) A summary of key characteristics of stem, differentiated, and cancer cells. (b) Previously proposed mechanisms that may explain why FACT is only essential in some cell types. See main text for details.
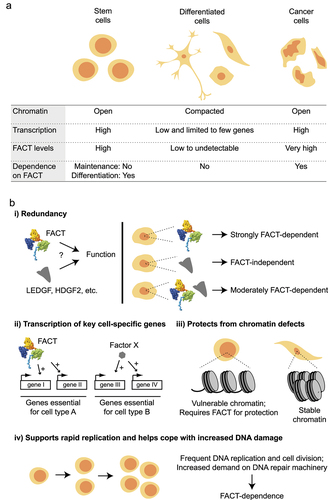
Several non-mutually exclusive reasons may explain why FACT is essential in some but not all cellular contexts (). First, cells not expressing FACT may express other factors, playing similar roles. LEDGF and HDGF2, like FACT, were identified as factors that allow transcription from a chromatin template in vitro and have nucleosome disassembly activity [Citation121]. These two proteins were therefore proposed to be FACT-like factors and to cover for FACT’s role in cells not expressing FACT. Mouse ESCs, despite expressing high levels of LEDGF and HDGF2, did not survive a FACT knockdown, however, suggesting they cannot replace all of FACT activities [Citation121]. Hence, while LEDGF and HDGF2 may have activities related to FACT, the current data do not support a simple redundancy model. Other proteins such as NDF, Brd2, and Brd3 [Citation122,Citation123], like LEDGF and HDGF2, have been shown to enable transcription through nucleosomes in vitro but their possible redundancy with FACT in vivo has not yet been investigated. Second, FACT may be important for the transcription of key proliferation genes. For example, inhibition or knockdown of FACT in different cancer cell lines led to gene-specific transcription defects, affecting genes important for cancer cell proliferation such as H-RAS, p53, NFκB, NOTCH1, MYC, and many others [Citation117,Citation173–176]. Interestingly, different sets of genes are affected by FACT inhibition in different cell types, suggesting that the effect of FACT on transcription is cell type-specific. A third possibility is that highly proliferating cells such as stem and cancer cells have an increased need for FACT because of their high level of transcription. While FACT may not be needed for global transcription per se (see above), the high level of transcription in these cells may render FACT essential in order to prevent lethal chromatin chaos. Because their chromatin is more compact and less frequently transcribed, differentiated cells may be able to cope with fewer FACT molecules than stem and cancer cells that are more transcriptionally active and have more open (and hence more vulnerable) chromatin [Citation177,Citation178]. In addition to defects in chromatin per se, the absence of FACT in proliferating cells may lead to increased levels of free histones, which are known to be toxic [Citation179]. Hence, FACT may be essential in contexts where chromatin is more vulnerable and high transcription activity may be one of the main determinants of this vulnerability, together with the differentiation stage. Consistent with this model, Goswami et al. showed recently that, similarly to what was extensively shown in yeast (see above), mesenchymal stem cells suffer from transcription-mediated chromatin defects upon the loss of FACT, while more differentiated cells appear insensitive to FACT loss [Citation23]. A counter-argument for this model was provided by Mylonas and Tessarz [Citation119] who showed that FACT knockdown led to increased proliferation of mouse ESCs [Citation119]. Finally, the dependence on FACT may be linked to other roles of FACT not discussed in this review. For example, highly proliferative cells with more open chromatin may be more susceptible to DNA damage and hence may depend on the role of FACT in DNA repair (reviewed in [Citation7]). This may be exacerbated in cancer cells that are known to have reduced DNA damage checkpoints [Citation180]. FACT is also involved in DNA replication (reviewed in [Citation6,Citation7]) and chromosome segregation [Citation181,Citation182], two processes more active in proliferating cells.
FACT in cancer: a novel drug target
For a long time, general cellular processes such as transcription were assumed not to be druggable given their general and ubiquitous roles. Sequence-specific TFs, notably those that are also hormone receptors, have been successfully drugged some long time ago (think of Tamoxifen, targeting the estrogen receptor in breast cancer for example [Citation183]) but it is only recently that general transcription factors or coregulators have been considered as potential therapeutic targets. Most likely because of their fast proliferation, cancer cells are addicted to transcription [Citation184]. This creates a therapeutic window to treat cancer cells with drugs targeting rather global transcription regulators such as CDK7, BRD4, and HDACs [Citation185–188]. Regardless of the mechanism, cancer cells tend to rely more on FACT than “normal cells” (see above), and high FACT levels are associated with high-grade tumors and poor cancer prognosis [Citation117,Citation171,Citation174,Citation189,Citation190]. Cancer cells can therefore be considered addicted to FACT. About ten years ago, the Gurova lab identified small molecules named Curaxins that appear to trap FACT on chromatin, hence titrating the histone chaperone away from active genes [Citation152,Citation173] (reviewed in [Citation191]). Curaxins were shown to have an anti-tumor effect on many cancer models including breast cancer [Citation117], neuroblastoma [Citation174], medulloblastomas [Citation192–194], glioblastoma [Citation190], diffuse intrinsic pontine glioma [Citation195], liver cancer [Citation128], MLL-rearranged leukemia [Citation196], and small-cell lung cancer [Citation175]. More recently, combining the curaxin CBL0137 with either HDAC [Citation195] or CDK7 [Citation194] inhibitors showed promising results, demonstrating that simultaneously targeting several cancer cells’ addictions might be a potential therapeutic avenue. Curaxins were also successfully used in combination with other cancer drugs such as cisplatin [Citation175,Citation192], sorafenib [Citation130,Citation197], and anti-angiogenic inhibitors [Citation130] as well as with radiotherapy [Citation192].
Regulation of FACT
The high variability of FACT levels between cell types (see above), and the fact that its expression dramatically drops upon differentiation (and raises upon transformation) in different systems [Citation168], suggests the presence of mechanisms regulating FACT abundance. Accordingly, both SSRP1 and SPT16 are regulated by several miRNAs and these pathways are often misregulated in cancers, contributing to the high FACT expression level in cancer cells [Citation198–201]. In addition, FACT protein levels are regulated by the proteasome [Citation130,Citation202,Citation203]. In yeast, the San1 E3 ligase regulates the abundance of FACT [Citation202], while in a human hepatocellular carcinoma-derived cell line, FACT was recently shown to be ubiquitylated by the von Hippel-Lindau (VHL) and KEAP1 E3 ligases following proline hydroxylation [Citation128,Citation130]. Interestingly, in the hypoxic environment of tumor cells, the VHL-dependent degradation of FACT is dampened, contributing to increased FACT levels, which in turn allows the tumor cells to survive [Citation130].
Remarkably, both FACT subunits rapidly drop upon knockdown of the other subunit, suggesting that individual subunits are unstable when not part of the complex [Citation119,Citation130,Citation171,Citation203]. Surprisingly, the SSRP1 and SPT16 mRNAs were shown to be physically associated with FACT, and contribute to the stability of the complex as well as that of the mRNAs themselves [Citation203]. This mRNA-protein stability reinforcing loop may explain why both FACT subunits are so quickly degraded as one of the two mRNAs is downregulated and may provide a mechanism for a tight synchronous expression during cellular differentiation and transformation.
The activity of FACT is also regulated in some conditions. For example, the polyADP-ribosylation of SPT16 by PARP-1 in response to DNA damage leads to both reduced chromatin binding and reduced histone exchange activity, which may contribute to the accumulation of H2A.X at damages sites [Citation204–206]. Similarly, in yeast, the association of FACT with replication origins is regulated by Rtt101-mediated ubiquitylation of SPT16 [Citation207]. Finally, SSRP1 is phosphorylated by CK2 [Citation105,Citation208,Citation209]. Interestingly, FACT phosphorylated on SSRP1 has reduced affinity for DNA [Citation208,Citation209] but stimulates binding to UV-damaged DNA [Citation105]. FACT phosphorylation may therefore shape its chromatin binding preference, perhaps contributing to the redistribution of FACT from transcribed genes to damaged DNA [Citation101].
Concluding remarks
Initially identified as an elongation factor, FACT is now better characterized as an elongation-associated histone chaperone whose role is to preserve chromatin structure integrity during transcription elongation. Whether FACT regulates elongation in cells remains a possibility but its demonstration will necessitate alleviating some technical limitations. In addition to its role as a chromatin safe keeper, FACT also contributes to the expression of specific genes and its knockdown or pharmacological inhibition highlighted its paramount importance in the expression of genes key for the maintenance of pluripotency in stem cells and the growth and survival of cancer cells. Although not extensively covered in this review, FACT is also important for DNA replication, DNA repair, and mitosis. Somewhat surprisingly, FACT is not expressed in “normal” differentiated tissues. Its expression is however high in stem and cancer cells, where it is required for viability, most likely because these cells have vulnerable chromatin that relies on FACT for its integrity. Taking advantage of cancer cells’ “addiction” to FACT, various groups are currently investigating the potential of FACT inhibitors, alone or in combination with other drugs/treatments, as a promising anti-cancer therapy.
Acknowledgments
We are grateful to Craig Kaplan for his critical reading of the manuscript. We would like to apologize to those whose work could not be cited owing to space restrictions. The Robert lab is currently funded by grants from the Canadian Institutes of Health Research (PJT-156383 and PJT-162334) and the Natural Sciences and Engineering Research Council of Canada (2018-04519).
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Orphanides G, LeRoy G, Chang CH, et al. FACT, a factor that facilitates transcript elongation through nucleosomes. Cell. 1998;92(1):105–116.
- Brewster NK, Johnston GC, Singer RA. Characterization of the CP complex, an abundant dimer of Cdc68 and Pob3 proteins that regulates yeast transcriptional activation and chromatin repression. J Biol Chem. 1998;273(34):21972–21979.
- Wittmeyer J, Formosa T. The Saccharomyces cerevisiae DNA polymerase alpha catalytic subunit interacts with Cdc68/Spt16 and with Pob3, a protein similar to an HMG1-like protein. Mol Cell Biol. 1997;17(7):4178–4190.
- Wittmeyer J, Joss L, Formosa T. Spt16 and Pob3 of Saccharomyces cerevisiae form an essential, abundant heterodimer that is nuclear, chromatin-associated, and copurifies with DNA Polymerase α. Biochemistry. 1999;38(28):8961–8971.
- Okuhara K, Ohta K, Seo H, et al. A DNA unwinding factor involved in DNA replication in cell-free extracts of Xenopus eggs. Curr Biol. 1999;9(7):341–350.
- Formosa T, Winston F. The role of FACT in managing chromatin: disruption, assembly, or repair? Nucleic Acids Res. 2020;48(21):11929–11941.
- Gurova K, Chang HW, Valieva ME, et al. Structure and function of the histone chaperone FACT - resolving fACTual issues. Biochim Biophys Acta Gene Regul Mech. 2018;1861(9):892–904.
- Bhakat KK, Ray S. The FAcilitates Chromatin Transcription (FACT) complex: its roles in DNA repair and implications for cancer therapy. DNA Repair (Amst). 2022;109:103246.
- Jamai A, Puglisi A, Strubin M. Histone chaperone spt16 promotes redeposition of the original h3-h4 histones evicted by elongating RNA polymerase. Mol Cell. 2009;35(3):377–383.
- Kaplan CD, Laprade L, Winston F. Transcription elongation factors repress transcription initiation from cryptic sites. Science. 2003;301(5636):1096–1099.
- Schwabish MA, Struhl K. Evidence for eviction and rapid deposition of histones upon transcriptional elongation by RNA polymerase II. Mol Cell Biol. 2004;24(23):10111–10117.
- Voth WP, Takahata S, Nishikawa JL, et al. A role for FACT in repopulation of nucleosomes at inducible genes. PloS one. 2014;9(1):e84092.
- Jeronimo C, Watanabe S, Kaplan CD, et al. The Histone Chaperones FACT and Spt6 Restrict H2A.Z from Intragenic Locations. Mol Cell. 2015;58(6):1113–1123.
- Jeronimo C, Robert F. Histone chaperones FACT and Spt6 prevent histone variants from turning into histone deviants. BioEssays. 2016;38(5):420–426.
- Jeronimo C, Poitras C, Robert F. Histone recycling by fact and spt6 during transcription prevents the scrambling of histone modifications. Cell Rep. 2019;28(5):1206–1218 e1208.
- Chen CC, Bowers S, Lipinszki Z, et al. Establishment of Centromeric Chromatin by the CENP-A Assembly Factor CAL1 Requires FACT-Mediated Transcription. Dev Cell. 2015;34(1):73–84.
- Deyter GM, Biggins S. The FACT complex interacts with the E3 ubiquitin ligase Psh1 to prevent ectopic localization of CENP-A. Genes Dev. 2014;28(16):1815–1826.
- Okada M, Okawa K, Isobe T, et al. CENP-H-containing complex facilitates centromere deposition of CENP-A in cooperation with FACT and CHD1. Mol Biol Cell. 2009;20(18):3986–3995.
- Morillo-Huesca M, Maya D, Muñoz-Centeno MC, et al. FACT prevents the accumulation of free histones evicted from transcribed chromatin and a subsequent cell cycle delay in G1. PLoS Genet. 2010;6(5):e1000964.
- Feng J, Gan H, Eaton ML, et al. Noncoding transcription is a driving force for nucleosome instability in spt16 mutant cells. Mol Cell Biol. 2016;36(13):1856–1867.
- Sandlesh P, Safina A, Goswami I, et al. Prevention of Chromatin Destabilization by FACT Is Crucial for Malignant Transformation. iScience. 2020;23(6):101177.
- Tettey TT, Gao X, Shao W, et al. A Role for FACT in RNA Polymerase II Promoter-Proximal Pausing. Cell Rep. 2019;27(13):3770–3779 e3777.
- Goswami I, Sandlesh P, Stablewski A, et al. FACT maintains nucleosomes during transcription and stem cell viability in adult mice. EMBO Rep. 2022;53684: 10.15252/embr.202153684.
- Zhou K, Liu Y, Luger K. Histone chaperone FACT FAcilitates Chromatin Transcription: mechanistic and structural insights. Curr Opin Struct Biol. 2020;65:26–32.
- Hammond CM, Stromme CB, Huang H, et al. Histone chaperone networks shaping chromatin function. Nat Rev Mol Cell Biol. 2017;18(3):141–158.
- Orphanides G, Wu WH, Lane WS, et al. The chromatin-specific transcription elongation factor FACT comprises human SPT16 and SSRP1 proteins. Nature. 1999;400(6741):284–288.
- Stuwe T, Hothorn M, Lejeune E. The FACT Spt16 “peptidase” domain is a histone H3-H4 binding module. Proc Natl Acad Sci USA. 2008;105(26):8884–8889.
- VanDemark AP, Xin H, McCullough L, et al. Structural and functional analysis of the Spt16p N-terminal domain reveals overlapping roles of yFACT subunits. J Biol Chem. 2008;283(8):5058–5068.
- O’Donnell AF, Brewster NK, Kurniawan J, et al. Domain organization of the yeast histone chaperone FACT: the conserved N-terminal domain of FACT subunit Spt16 mediates recovery from replication stress. Nucleic Acids Res. 2004;32(19):5894–5906.
- Hondele M, Stuwe T, Hassler M, et al. Structural basis of histone H2A-H2B recognition by the essential chaperone FACT. Nature. 2013;499(7456):111–114.
- Kemble DJ, Whitby FG, Robinson H, et al. Structure of the Spt16 middle domain reveals functional features of the histone chaperone FACT. J Biol Chem. 2013;288(15):10188–10194.
- Kemble DJ, McCullough LL, Whitby FG, et al. FACT disrupts nucleosome structure by binding H2A-H2B with Conserved Peptide Motifs. Mol Cell. 2015;60(2):294–306.
- Zunder RM, Antczak AJ, Berger JM, et al. Two surfaces on the histone chaperone Rtt106 mediate histone binding, replication, and silencing. Proc Natl Acad Sci USA. 2012;109(3):E144–153.
- Rottgers K, Krohn NM, Lichota J, et al. DNA-interactions and nuclear localisation of the chromosomal HMG domain protein SSRP1 from maize. Plant J. 2000;23(3):395–405.
- Formosa T, Eriksson P, Wittmeyer J, et al. Spt16-Pob3 and the HMG protein Nhp6 combine to form the nucleosome-binding factor SPN. EMBO J. 2001;20(13):3506–3517.
- Brewster NK, Johnston GC, Singer RA. A bipartite yeast SSRP1 analog comprised of Pob3 and Nhp6 proteins modulates transcription. Mol Cell Biol. 2001;21(10):3491–3502.
- Ruone S, Rhoades AR, Formosa T. Multiple Nhp6 molecules are required to recruit Spt16-Pob3 to form yFACT complexes and to reorganize nucleosomes. J Biol Chem. 2003;278(46):45288–45295.
- Valieva ME, Armeev GA, Kudryashova KS, et al. Large-scale ATP-independent nucleosome unfolding by a histone chaperone. Nat Struct Mol Biol. 2016;23(12):1111–1116.
- Xin H, Takahata S, Blanksma M, et al. yFACT induces global accessibility of nucleosomal DNA without H2A-H2B displacement. Mol Cell. 2009;35(3):365–376.
- Ho B, Baryshnikova A, Brown GW. Unification of Protein Abundance Datasets Yields a Quantitative Saccharomyces cerevisiae Proteome. Cell Syst. 2018;6(2):192–205 e193.
- McCullough LL, Connell Z, Xin H, et al. Functional roles of the DNA-binding HMGB domain in the histone chaperone FACT in nucleosome reorganization. J Biol Chem. 2018;293(16):6121–6133.
- McDonald SM, Close D, Xin H, et al. Structure and biological importance of the Spn1-Spt6 interaction, and its regulatory role in nucleosome binding. Mol Cell. 2010;40(5):725–735.
- Tsunaka Y, Fujiwara Y, Oyama T, et al. Integrated molecular mechanism directing nucleosome reorganization by human FACT. Genes Dev. 2016;30(6):673–686.
- Tsunaka Y, Ohtomo H, Morikawa K, et al. Partial Replacement of Nucleosomal DNA with Human FACT Induces Dynamic Exposure and Acetylation of Histone H3 N-Terminal Tails. iScience. 2020;23(10):101641.
- Wang T, Liu Y, Edwards G, et al. The histone chaperone FACT modulates nucleosome structure by tethering its components. Life Sci Alliance. 2018;1(4):e201800107.
- Allain FH, Yen YM, Masse JE, et al. Solution structure of the HMG protein NHP6A and its interaction with DNA reveals the structural determinants for non-sequence-specific binding. EMBO J. 1999;18(9):2563–2579.
- Sarangi MK, Zvoda V, Holte MN, et al. Evidence for a bind-then-Bend mechanism for architectural DNA binding protein yNhp6A. Nucleic Acids Res. 2019;47(6):2871–2883.
- Nesher E, Safina A, Aljahdali I, et al. Role of chromatin damage and chromatin trapping of fact in mediating the anticancer cytotoxicity of DNA-Binding Small-molecule drugs. Cancer Res. 2018;78(6):1431–1443.
- Mayanagi K, Saikusa K, Miyazaki N, et al. Structural visualization of key steps in nucleosome reorganization by human FACT. Sci Rep. 2019;9(1):10183.
- Liu Y, Zhou K, Zhang N, et al. FACT caught in the act of manipulating the nucleosome. Nature. 2020;577(7790):426–431.
- Jeronimo C, Angel A, Nguyen VQ, et al. FACT is recruited to the +1 nucleosome of transcribed genes and spreads in a Chd1-dependent manner. Mol Cell. 2021;81(17):3542–3559 e3511.
- Martin BJE, Chruscicki AT, Howe LJ. Transcription promotes the interaction of the facilitates chromatin transactions (fact) complex with nucleosomes in Saccharomyces cerevisiae. Genetics. 2018;210(3):869–881.
- VanDemark AP, Blanksma M, Ferris E, et al. The structure of the yFACT Pob3-M domain, its interaction with the DNA replication factor RPA, and a potential role in nucleosome deposition. Mol Cell. 2006;22(3):363–374.
- Zhang W, Zeng F, Liu Y, et al. crystal structure of human ssrp1 middle domain reveals a role in DNA binding. Sci Rep. 2015;5(1):18688.
- Marciano G, Huang DT. Structure of the human histone chaperone FACT Spt16 N-terminal domain. Acta Crystallogr F Struct Biol Commun. 2016;72(2):121–128.
- Li X, Li H, Jing Q, et al. Structural insights into multifunctionality of human FACT complex subunit hSSRP1. J Biol Chem. 2021;297(6):101360.
- Sivkina AL, Karlova MG, Valieva ME, et al. Electron microscopy analysis of ATP-independent nucleosome unfolding by FACT. Commun Biol. 2022;5(1):2.
- Li W, Chen P, Yu J, et al. FACT Remodels the tetranucleosomal unit of chromatin fibers for gene transcription. Mol Cell. 2016;64(1):120–133.
- Farnung L, Ochmann M, Engeholm M, et al. Structural basis of nucleosome transcription mediated by Chd1 and FACT. Nat Struct Mol Biol. 2021;28(4):382–387.
- Vos SM, Farnung L, Linden A, et al. Structure of complete Pol II-DSIF-PAF-SPT6 transcription complex reveals RTF1 allosteric activation. Nat Struct Mol Biol. 2020;27(7):668–677.
- Burugula BB, Jeronimo C, Pathak R, et al. Histone deacetylases and phosphorylated polymerase II C-terminal domain recruit Spt6 for cotranscriptional histone reassembly. Mol Cell Biol. 2014;34(22):4115–4129.
- Sdano MA, Fulcher JM, Palani S, et al. A novel SH2 recognition mechanism recruits Spt6 to the doubly phosphorylated RNA polymerase II linker at sites of transcription. eLife. 2017;6:e28723.
- Vos SM, Farnung L, Boehning M, et al. Structure of activated transcription complex Pol II-DSIF-PAF-SPT6. Nature. 2018;560(7720):607–612.
- Krogan NJ, Kim M, Tong A, et al. Methylation of Histone H3 by Set2 in Saccharomyces cerevisiae Is Linked to Transcriptional Elongation by RNA Polymerase II. Mol Cell Biol. 2003;23(12):4207–4218.
- Carrozza MJ, Li B, Florens L, et al. Histone H3 methylation by Set2 directs deacetylation of coding regions by Rpd3S to suppress spurious intragenic transcription. Cell. 2005;123(4):581–592.
- Drouin S, Laramée L, Jacques P-É, et al. DSIF and RNA polymerase II CTD phosphorylation coordinate the recruitment of Rpd3S to actively transcribed genes. PLoS Genet. 2010;6(10):e1001173.
- Govind CK, Qiu H, Ginsburg DS, et al. Phosphorylated Pol II CTD recruits multiple HDACs, including Rpd3C(S), for methylation-dependent deacetylation of ORF nucleosomes. Mol Cell. 2010;39(2):234–246.
- Adelman K, Wei W, Ardehali MB, et al. Drosophila Paf1 Modulates Chromatin Structure at Actively Transcribed Genes. Mol Cell Biol. 2006;26(1):250–260.
- Carvalho S, Raposo AC, Martins FB, et al. Histone methyltransferase SETD2 coordinates FACT recruitment with nucleosome dynamics during transcription. Nucleic Acids Res. 2013;41(5):2881–2893.
- John S, Howe L, Tafrov ST, et al. The Something About Silencing protein, Sas3, is the catalytic subunit of NuA3, a yTAF II 30-containing HAT complex that interacts with the Spt16 subunit of the yeast CP (Cdc68/Pob3)–FACT complex. Genes Dev. 2000;14(10):1196–1208.
- Krogan NJ, Kim M, Ahn SH, et al. RNA polymerase II elongation factors of Saccharomyces cerevisiae: a targeted proteomics approach. Mol Cell Biol. 2002;22(20):6979–6992.
- Kwon SH, Florens L, Swanson SK, et al. Heterochromatin protein 1 (HP1) connects the FACT histone chaperone complex to the phosphorylated CTD of RNA polymerase II. Genes Dev. 2010;24(19):2133–2145.
- Mason PB, Struhl K. The FACT complex travels with elongating RNA polymerase II and is important for the fidelity of transcriptional initiation in vivo. Mol Cell Biol. 2003;23(22):8323–8333.
- Sen R, Kaja A, Ferdoush J, et al. An mRNA Capping Enzyme Targets FACT to the Active Gene To Enhance the Engagement of RNA Polymerase II into Transcriptional Elongation. Mol Cell Biol. 2017;37(13):e00029–00017.
- Duina AA, Rufiange A, Bracey J, et al. Evidence that the localization of the elongation factor spt16 across transcribed genes is dependent upon histone H3 Integrity in Saccharomyces cerevisiae. Genetics. 2007;177(1):101–112.
- Nguyen HT, Wharton W 2nd, Harper JA, et al. A nucleosomal region important for ensuring proper interactions between the transcription elongation factor Spt16 and transcribed genes in saccharomyces cerevisiae. G3 (Bethesda). 2013;3(6):929–940.
- Johnson P, Mitchell V, McClure K, et al. A systematic mutational analysis of a histone H3 residue in budding yeast provides insights into chromatin dynamics . G3 (Bethesda). 2015;5(7):741–749.
- Hainer SJ, Martens JA. Regulation of chaperone binding and nucleosome dynamics by key residues within the globular domain of histone H3. Epigenetics Chromatin. 2016;9(1):17.
- Nyamugenda E, Cox AB, Pierce JB, et al. Charged residues on the side of the nucleosome contribute to normal Spt16-gene interactions in budding yeast. Epigenetics. 2018;13(1):1–7.
- Pelechano V, Chavez S, Perez-Ortin JE. A complete set of nascent transcription rates for yeast genes. PloS one. 2010;5(11):e15442.
- Krogan NJ, Cagney G, Yu H, et al. Global landscape of protein complexes in the yeast Saccharomyces cerevisiae. Nature. 2006;440(7084):637–643.
- Krogan NJ, Peng W-T, Cagney G, et al. High-definition macromolecular composition of yeast RNA-processing complexes. Mol Cell. 2004;13(2):225–239.
- Simic R, Lindstrom DL, Tran HG, et al. Chromatin remodeling protein Chd1 interacts with transcription elongation factors and localizes to transcribed genes. EMBO J. 2003;22(8):1846–1856.
- Farnung L, Vos SM, Wigge C, et al. Nucleosome-Chd1 structure and implications for chromatin remodelling. Nature. 2017;550(7677):539–542.
- Biswas D, Dutta-Biswas R, Stillman DJ. Chd1 and yFACT act in opposition in regulating transcription. Mol Cell Biol. 2007;27(18):6279–6287.
- Takahata S, Yu Y, Stillman DJ. FACT and Asf1 regulate nucleosome dynamics and coactivator binding at the HO promoter. Mol Cell. 2009;34(4):405–415.
- Yu Y, Yarrington RM, Stillman DJ. FACT and Ash1 promote long-range and bidirectional nucleosome eviction at the HO promoter. Nucleic Acids Res. 2020;48(19):10877–10889.
- Ransom M, Williams SK, Dechassa ML, et al. FACT and the proteasome promote promoter chromatin disassembly and transcriptional initiation. J Biol Chem. 2009;284(35):23461–23471.
- Shakya A, Callister C, Goren A, et al. Pluripotency transcription factor Oct4 mediates stepwise nucleosome demethylation and depletion. Mol Cell Biol. 2015;35(6):1014–1025.
- Ding J, Xu H, Faiola F, et al. Oct4 links multiple epigenetic pathways to the pluripotency network. Cell Res. 2012;22(1):155–167.
- Hossan T, Nagarajan S, Baumgart SJ, et al. Histone Chaperone SSRP1 is essential for wnt signaling pathway activity during osteoblast differentiation. Stem Cells. 2016;34(5): 1369–1376.
- Lolis AA, Londhe P, Beggs BC, et al. Myogenin recruits the histone chaperone facilitates chromatin transcription (FACT) to promote nucleosome disassembly at muscle-specific genes. J Biol Chem. 2013;288(11):7676–7687.
- Zhou D, Park JG, Wu Z, et al. FACT subunit SUPT16H associates with BRD4 and contributes to silencing of antiviral interferon signaling. bioRxiv. 2021; DOI:10.1101/2021.04.21.440833.
- Ferri F, Petit V, Barroca V, et al. Interplay between FACT subunit SPT16 and TRIM33 can remodel chromatin at macrophage distal regulatory elements. Epigenetics Chromatin. 2019;12(1):46.
- Tan BC-M, Chien C-T, Hirose S, et al. Functional cooperation between FACT and MCM helicase facilitates initiation of chromatin DNA replication. EMBO J. 2006;25(17):3975–3985.
- Miles J, Formosa T. Protein affinity chromatography with purified yeast DNA polymerase alpha detects proteins that bind to DNA polymerase. Proc Natl Acad Sci USA. 1992;89(4):1276–1280.
- Foltman M, Evrin C, De Piccoli G, et al. Eukaryotic replisome components cooperate to process histones during chromosome replication. Cell Rep. 2013;3(3):892–904.
- Safaric B, Chacin E, Scherr MJ, et al. The fork protection complex recruits FACT to reorganize nucleosomes during replication. Nucleic Acids Res. 2022;50(3):1317–1334.
- de Vivo A, Sanchez A, Yegres J, et al. The OTUD5-UBR5 complex regulates FACT-mediated transcription at damaged chromatin. Nucleic Acids Res. 2019;47(2):729–746.
- Gao Y, Li C, Wei L, et al. SSRP1 Cooperates with PARP and XRCC1 to facilitate single-strand dna break repair by chromatin priming. Cancer Res. 2017;77(10):2674–2685.
- Charles Richard JL, Shukla MS, Menoni H, et al. FACT Assists Base Excision Repair by Boosting the Remodeling Activity of RSC. PLoS Genet. 2016;12(7):e1006221.
- Wienholz F, Zhou D, Turkyilmaz Y, et al. FACT subunit Spt16 controls UVSSA recruitment to lesion-stalled RNA Pol II and stimulates TC-NER. Nucleic Acids Res. 2019;47(8):4011–4025.
- Kumari A, Mazina OM, Shinde U, et al. A role for SSRP1 in recombination-mediated DNA damage response. J Cell Biochem. 2009;108(2):508–518.
- Oliveira DV, Kato A, Nakamura K, et al. Histone chaperone FACT regulates homologous recombination by chromatin remodeling through interaction with RNF20. J Cell Sci. 2014;127(Pt 4):763–772.
- Krohn NM, Stemmer C, Fojan P, et al. Protein kinase CK2 phosphorylates the high mobility group domain protein SSRP1, inducing the recognition of UV-damaged DNA. J Biol Chem. 2003;278(15):12710–12715.
- Yarnell AT, Oh S, Reinberg D, et al. Interaction of FACT, SSRP1, and the high mobility group (HMG) domain of SSRP1 with DNA damaged by the anticancer drug cisplatin. J Biol Chem. 2001;276(28):25736–25741.
- Belotserkovskaya R, Oh S, Bondarenko VA, et al. FACT facilitates transcription-dependent nucleosome alteration. Science. 2003;301(5636):1090–1093.
- Hsieh FK, Kulaeva OI, Patel SS et al. Histone chaperone FACT action during transcription through chromatin by RNA polymerase II. Proc Natl Acad Sci USA. 2013;110(19):7654–7659.
- Chen P, Dong L, Hu M, et al. Functions of FACT in Breaking the Nucleosome and Maintaining Its Integrity at the Single-Nucleosome Level. Mol Cell. 2018;71(2):284–293 e284.
- Cheung V, Chua G, Batada NN, et al. Chromatin- and transcription-related factors repress transcription from within coding regions throughout the Saccharomyces cerevisiae genome. PLoS Biol. 2008;6(11):e277.
- Myers CN, Berner GB, Holthoff JH, et al. Mutant versions of the S. cerevisiae transcription elongation factor Spt16 define regions of Spt16 that functionally interact with histone H3. PloS one. 2011;6(6):e20847.
- Nielsen M, Ard R, Leng X, et al. Transcription-driven chromatin repression of Intragenic transcription start sites. PLoS Genet. 2019;15(2):e1007969.
- Chen F, Zhang W, Xie D, et al. Histone chaperone FACT represses retrotransposon MERVL and MERVL-derived cryptic promoters. Nucleic Acids Res. 2020;48(18):10211–10225.
- True JD, Muldoon JJ, Carver MN, et al. The modifier of transcription 1 (Mot1) atpase and spt16 histone chaperone co-regulate transcription through preinitiation complex assembly and nucleosome organization. J Biol Chem. 2016;291(29):15307–15319.
- Biswas D, Dutta-Biswas R, Mitra D, et al. Opposing roles for Set2 and yFACT in regulating TBP binding at promoters. EMBO J. 2006;25(19):4479–4489.
- Li Y, Zeng SX, Landais I, et al. Human SSRP1 has Spt16-dependent and -independent roles in gene transcription. J Biol Chem. 2007;282(10):6936–6945.
- Fleyshman D, Prendergast L, Safina A, et al. Level of FACT defines the transcriptional landscape and aggressive phenotype of breast cancer cells. Oncotarget. 2017;8(13):20525–20542.
- Pfab A, Breindl M, Grasser KD. The Arabidopsis histone chaperone FACT is required for stress-induced expression of anthocyanin biosynthetic genes. Plant Mol Biol. 2018;96(4–5):367–374.
- Mylonas C, Tessarz P. Transcriptional repression by FACT is linked to regulation of chromatin accessibility at the promoter of ES cells. Life Sci Alliance. 2018;1(3):e201800085.
- Doris SM, Chuang J, Viktorovskaya O, et al. Spt6 Is required for the fidelity of promoter selection. Mol Cell. 2018;72(4):687–699 e686.
- LeRoy G, Oksuz O, Descostes N, et al. LEDGF and HDGF2 relieve the nucleosome-induced barrier to transcription in differentiated cells. Sci Adv. 2019;5(10):eaay3068.
- LeRoy G, Rickards B, Flint SJ. The double bromodomain proteins Brd2 and Brd3 couple histone acetylation to transcription. Mol Cell. 2008;30(1):51–60.
- Fei J, Ishii H, Hoeksema MA, et al. NDF, a nucleosome-destabilizing factor that facilitates transcription through nucleosomes. Genes Dev. 2018;32(9–10):682–694.
- Mason PB, Struhl K. Distinction and relationship between elongation rate and processivity of RNA polymerase II in vivo. Mol Cell. 2005;17(6):831–840.
- Fuchs G, Voichek Y, Rabani M, et al. Simultaneous measurement of genome-wide transcription elongation speeds and rates of RNA polymerase II transition into active elongation with 4sUDRB-seq. Nat Protoc. 2015;10(4):605–618.
- Jonkers I, Kwak H, Lis JT. Genome-wide dynamics of Pol II elongation and its interplay with promoter proximal pausing, chromatin, and exons. eLife. 2014;3:e02407.
- Jonkers I, Lis JT. Getting up to speed with transcription elongation by RNA polymerase II. Nature Reviews Molecular Cell Biology. 2015;16(3):167–177.
- Shen J, Chen M, Lee D, et al. Histone chaperone FACT complex mediates oxidative stress response to promote liver cancer progression. Gut. 2020;69(2):329–342.
- Fleming AB, Kao CF, Hillyer C, et al. H2B ubiquitylation plays a role in nucleosome dynamics during transcription elongation. Mol Cell. 2008;31(1):57–66.
- Shen J, Yang C, Zhang MS, et al. Histone chaperone FACT complex coordinates with HIF to mediate an expeditious transcription program to adapt to poorly oxygenated cancers. Cell Rep. 2022;38(5):110304.
- Gajos M, Jasnovidova O, Van bömmel A, et al. Conserved DNA sequence features underlie pervasive RNA polymerase pausing. Nucleic Acids Res. 2021;49(8):4402–4420.
- Feng P, Xiao A, Fang M et al. A machine learning-based framework for modeling transcription elongation Proc Natl Acad Sci USA. 2021;118(6), doi:10.1073/pnas.2007450118.
- Zumer K, Maier KC, Farnung L, et al. Two distinct mechanisms of RNA polymerase II elongation stimulation in vivo. Mol Cell. 2021;81(15):3096–3109 e3098.
- Narain A, Bhandare P, Adhikari B, et al. Targeted protein degradation reveals a direct role of SPT6 in RNAPII elongation and termination. Mol Cell. 2021;81(15):3110–3127 e3114.
- Bortvin A, Winston F. Evidence that Spt6p controls chromatin structure by a direct interaction with histones. Science. 1996;272(5267):1473–1476.
- Ivanovska I, Jacques P-É, Rando OJ, et al. Control of chromatin structure by Spt6: different consequences in coding and regulatory regions. Mol Cell Biol. 2010;31(3):531–541.
- Perales R, Erickson B, Zhang L, et al. Gene promoters dictate histone occupancy within genes. EMBO J. 2013;32(19):2645–2656.
- DeGennaro CM, Alver BH, Marguerat S, et al. Spt6 regulates intragenic and antisense transcription, nucleosome positioning, and histone modifications genome-wide in fission yeast. Mol Cell Biol. 2013;33(24):4779–4792.
- Uwimana N, Collin P, Jeronimo C, et al. Bidirectional terminators in Saccharomyces cerevisiae prevent cryptic transcription from invading neighboring genes. Nucleic Acids Res. 2017;45(11):6417–6426.
- Kato H, Okazaki K, Iida T, et al. Spt6 prevents transcription-coupled loss of posttranslationally modified histone H3. Sci Rep. 2013;3(1):2186.
- Reim NI, Chuang J, Jain D, et al. The conserved elongation factor Spn1 is required for normal transcription, histone modifications, and splicing in Saccharomyces cerevisiae. Nucleic Acids Res. 2020;48(18):10241–10258.
- van Bakel H, Tsui K, Gebbia M, et al. A compendium of nucleosome and transcript profiles reveals determinants of chromatin architecture and transcription. PLoS Genet. 2013;9(5):e1003479.
- Silva AC, Xu X, Kim H-S, et al. The replication-independent histone H3-H4 chaperones HIR, ASF1, and RTT106 co-operate to maintain promoter fidelity. J Biol Chem. 2012;287(3):1709–1718.
- Imbeault D, Gamar L, Rufiange A, et al. The Rtt106 Histone Chaperone Is Functionally Linked to Transcription Elongation and Is Involved in the Regulation of Spurious Transcription from Cryptic Promoters in Yeast. J Biol Chem. 2008;283(41):27350–27354.
- Chen S, Rufiange A, Huang H, et al. Structure-function studies of histone H3/H4 tetramer maintenance during transcription by chaperone Spt2. Genes Dev. 2015;29(12):1326–1340.
- Nourani A, Robert F, Winston F. Evidence that Spt2/Sin1, an HMG-like factor, plays roles in transcription elongation, chromatin structure, and genome stability in Saccharomyces cerevisiae. Mol Cell Biol. 2006;26(4):1496–1509.
- Venkatesh S, Smolle M, Li H, et al. Set2 methylation of histone H3 lysine 36 suppresses histone exchange on transcribed genes. Nature. 2012;489(7416):452–455.
- Xue YM, Kowalska AK, Grabowska K, et al. Histone chaperones Nap1 and Vps75 regulate histone acetylation during transcription elongation. Mol Cell Biol. 2013;33(8):1645–1656.
- Mayer A, Lidschreiber M, Siebert M, et al. Uniform transitions of the general RNA polymerase II transcription complex. Nat Struct Mol Biol. 2010;17(10):1272–1278.
- Vinayachandran V, Reja R, Rossi MJ, et al. Widespread and precise reprogramming of yeast protein-genome interactions in response to heat shock. Genome Res. 2018;28(3):357–366.
- Connell Z, Parnell TJ, McCullough LL, et al. The interaction between the Spt6-tSH2 domain and Rpb1 affects multiple functions of RNA Polymerase II. Nucleic Acids Res. 2022;50(2):784–802.
- Chang HW, Valieva ME, Safina A, et al. Mechanism of FACT removal from transcribed genes by anticancer drugs curaxins. Sci Adv. 2018;4(11):eaav2131.
- Diebold ML, Koch M, Loeliger E, et al. The structure of an Iws1/Spt6 complex reveals an interaction domain conserved in TFIIS, Elongin A and Med26. EMBO J. 2010;29(23):3979–3991.
- Bhat W, Boutin G, Rufiange A, et al. Casein kinase 2 associates with the yeast chromatin reassembly factor Spt2/Sin1 to regulate its function in the repression of spurious transcription. Mol Cell Biol. 2013;33(21):4198–4211.
- Viktorovskaya O, Chuang J, Jain D, et al. Essential histone chaperones collaborate to regulate transcription and chromatin integrity. Genes Dev. 2021;35(9–10):698–712.
- Hainer SJ, Charsar BA, Cohen SB, et al. Identification of mutant versions of the spt16 histone chaperone that are defective for transcription-coupled nucleosome occupancy in Saccharomyces cerevisiae. G3 (Bethesda). 2012;2(5):555–567.
- Hainer SJ, Pruneski JA, Mitchell RD, et al. Intergenic transcription causes repression by directing nucleosome assembly. Genes Dev. 2011;25(1):29–40.
- Leng H, Liu S, Lei Y, et al. FACT interacts with Set3 HDAC and fine-tunes GAL1 transcription in response to environmental stimulation. Nucleic Acids Res. 2021;49(10):5502–5519.
- Perales M, Mas P. A functional link between rhythmic changes in chromatin structure and the arabidopsis biological clock. Plant Cell. 2007;19(7):2111–2123.
- Ma Y, Gil S, Grasser KD, et al. Targeted Recruitment of the Basal Transcriptional Machinery by LNK Clock Components Controls the Circadian Rhythms of Nascent RNAs in Arabidopsis. Plant Cell. 2018;30(4):907–924.
- Michl-Holzinger P, Mortensen SA, Grasser KD. The SSRP1 subunit of the histone chaperone FACT is required for seed dormancy in Arabidopsis. J Plant Physiol. 2019;236:105–108.
- Petrenko N, Jin Y, Dong L, et al. Requirements for RNA polymerase II preinitiation complex formation in vivo. eLife. 2019;8(e43654). 10.7554/eLife.43654
- Metzl-Raz E, Kafri M, Yaakov G, et al. Gene Transcription as a Limiting Factor in Protein Production and Cell Growth. G3 (Bethesda). 2020;10(9):3229–3242.
- Aoi Y, Smith ER, Shah AP, et al. NELF regulates a promoter-proximal step distinct from RNA Pol II pause-release. Mol Cell. 2020;78(2):261–274 e265.
- Cheon Y, Han S, Kim T, et al. The chromatin remodeler Ino80 mediates RNAPII pausing site determination. Genome Biol. 2021;22(1):294.
- Wada T, Orphanides G, Hasegawa J, et al. FACT relieves DSIF/NELF-mediated inhibition of transcriptional elongation and reveals functional differences between P-TEFb and TFIIH. Mol Cell. 2000;5(6):1067–1072.
- Garcia H, Fleyshman D, Kolesnikova K, et al. Expression of FACT in mammalian tissues suggests its role in maintaining of undifferentiated state of cells. Oncotarget. 2011;2(10):783–796.
- Hertel L, De Andrea M, Bellomo G, et al. The HMG protein T160 colocalizes with DNA replication foci and is down-regulated during cell differentiation. Exp Cell Res. 1999;250(2):313–328.
- Xiang YY, Wang D-Y, Tanaka M, et al. Expression of structure-specific recognition protein mRNA in fetal kidney and Fe-nitrilotriacetate-induced renal carcinoma in the rat. Cancer Lett. 1996;106(2):271–278.
- Duroux M, Houben A, Ruzicka K, et al. The chromatin remodelling complex FACT associates with actively transcribed regions of the Arabidopsis genome. Plant J. 2004;40(5):660–671.
- Garcia H, Miecznikowski J, Safina A, et al. Facilitates chromatin transcription complex is an “accelerator” of tumor transformation and potential marker and target of aggressive cancers. Cell Rep. 2013;4(1):159–173.
- Hudson ME, Pozdnyakova I, Haines K, et al. Identification of differentially expressed proteins in ovarian cancer using high-density protein microarrays. Proc Natl Acad Sci USA. 2007;104(44.):17494–17499.
- Gasparian AV, Burkhart CA, Purmal AA, et al. Curaxins: anticancer compounds that simultaneously suppress NF-kappaB and activate p53 by targeting FACT. Sci Transl Med. 2011;3(95). 10.1126/scitranslmed.3002530
- Carter DR, Murra J, Cheung BB, et al. Therapeutic targeting of the MYC signal by inhibition of histone chaperone FACT in neuroblastoma. Sci Transl Med. 2015;7(312):312ra176.
- De S. The FACT inhibitor cbl0137 synergizes with cisplatin in small-cell lung cancer by increasing notch1 expression and targeting tumor-initiating cells. Cancer Res. 2018;78(9):2396–2406.
- Bi L, Xie C, Yao M, et al. The histone chaperone complex FACT promotes proliferative switch of G 0 cancer cells. Int J Cancer. 2019;145(1):164–178.
- Schlesinger S, Meshorer E. Open chromatin, epigenetic plasticity, and nuclear organization in pluripotency. Dev Cell. 2019;48(2):135–150.
- Morgan MA, Shilatifard A. Chromatin signatures of cancer. Genes Dev. 2015;29(3):238–249.
- Singh RK, Liang D, Gajjalaiahvari UR, et al. Excess histone levels mediate cytotoxicity via multiple mechanisms. Cell Cycle. 2010;9(20):4236–4244.
- Visconti R, Della Monica R, Grieco D. Cell cycle checkpoint in cancer: a therapeutically targetable double-edged sword. J Exp Clin Cancer Res. 2016;35(1):153.
- Garcia-Luis J, Lazar-Stefanita L, Gutierrez-Escribano P, et al. FACT mediates cohesin function on chromatin. Nat Struct Mol Biol. 2019;26(10):970–979.
- Shintomi K, Takahashi TS, Hirano T. Reconstitution of mitotic chromatids with a minimum set of purified factors. Nat Cell Biol. 2015;17(8):1014–1023.
- Legare S, Basik MM. Minireview: The link between eralpha corepressors and histone deacetylases in tamoxifen resistance in breast cancer. Mol Endocrinol. 2016;30(9):965–976.
- Bradner JE, Hnisz D, Young RA. Transcriptional Addiction in Cancer. Cell. 2017;168(4):629–643.
- Martin RD, Hebert TE, Tanny JC. Therapeutic Targeting of the General RNA Polymerase II Transcription Machinery. Int J Mol Sci. 2020;21(9):3354.
- Sava GP, Fan H, Coombes RC, et al. CDK7 inhibitors as anticancer drugs. Cancer Metastasis Rev. 2020;39(3):805–823.
- Cai SF, Chen CW, Armstrong SA. Drugging Chromatin in Cancer: recent Advances and Novel Approaches. Mol Cell. 2015;60(4):561–570.
- White ME, Fenger JM, Carson WE 3rd. Emerging roles of and therapeutic strategies targeting BRD4 in cancer. Cell Immunol. 2019;337:48–53.
- Dermawan JK, Gurova K, Pink J, et al. Quinacrine overcomes resistance to erlotinib by inhibiting FACT, NF-kappaB, and cell-cycle progression in non-small cell lung cancer. Mol Cancer Ther. 2014;13(9):2203–2214.
- Dermawan JK, Hitomi M, Silver DJ, et al. Pharmacological targeting of the histone chaperone complex fact preferentially eliminates glioblastoma stem cells and prolongs survival in preclinical models. Cancer Res. 2016;76(8):2432–2442.
- Chang HW, Nizovtseva EV, Razin SV, et al. Histone Chaperone FACT and Curaxins: effects on Genome Structure and Function. Journal of Cancer Metastasis and Treatment. 2019;5: DOI:10.20517/2394-4722.2019.31.
- Song H, Xi S, Chen Y, et al. Histone chaperone FACT complex inhibitor CBL0137 interferes with DNA damage repair and enhances sensitivity of medulloblastoma to chemotherapy and radiation. Cancer Lett. 2021;520:201–212.
- Wang J, Sui Y, Li Q, et al. Effective inhibition of MYC-amplified group 3 medulloblastoma by FACT-targeted curaxin drug CBL0137. Cell Death Dis. 2020;11(12):1029.
- Mo J, Liu F, Sun X, et al. Inhibition of the FACT complex targets aberrant hedgehog signaling and overcomes resistance to smoothened antagonists. Cancer Res. 2021;81(11):3105–3120.
- Ehteda A, Simon S, Franshaw L, et al. Dual targeting of the epigenome via FACT complex and histone deacetylase is a potent treatment strategy for DIPG. Cell Rep. 2021;35(2):108994.
- Somers K, Kosciolek A, Bongers A, et al. Potent antileukemic activity of curaxin CBL0137 against MLL-rearranged leukemia. Int J Cancer. 2020;146(7):1902–1916.
- Safina A, Cheney P, Pal M, et al. FACT is a sensor of DNA torsional stress in eukaryotic cells. Nucleic Acids Res. 2017;45(4):1925–1945.
- Gao L, Xiong X. MiR-223 inhibits the proliferation, invasion and EMT of nasopharyngeal carcinoma cells by targeting SSRP1. Int J Clin Exp Pathol. 2018;11(9):4374–4384.
- Huang H, Cai L, Li R, et al. A novel lncRNA LOC101927746 accelerates progression of colorectal cancer via inhibiting miR-584-3p and activating SSRP1. Biochem Biophys Res Commun. 2019;509(3):734–738.
- Wu W, He K, Guo Q, et al. SSRP1 promotes colorectal cancer progression and is negatively regulated by miR-28-5p. J Cell Mol Med. 2019;23(5):3118–3129.
- Yang L, Wang X, Jiao X, et al. Suppressor of Ty 16 promotes lung cancer malignancy and is negatively regulated by miR-1227-5p. Cancer Sci. 2020;111(11):4075–4087.
- Sen R, Ferdoush J, Kaja A, et al. Fine-Tuning of FACT by the Ubiquitin Proteasome System in Regulation of Transcriptional Elongation. Mol Cell Biol. 2016;36(11):1691–1703.
- Safina A, Garcia H, Commane M, et al. Complex mutual regulation of facilitates chromatin transcription (FACT) subunits on both mRNA and protein levels in human cells. Cell Cycle. 2013;12(15):2423–2434. .
- Heo K, Kim H, Choi SH, et al. FACT-mediated exchange of histone variant H2AX regulated by phosphorylation of H2AX and ADP-ribosylation of Spt16. Mol Cell. 2008;30(1):86–97.
- Huang JY, Chen WH, Chang YL, et al. Modulation of nucleosome-binding activity of FACT by poly(ADP-ribosyl)ation. Nucleic Acids Res. 2006;34(8):2398–2407.
- Piquet S, Le Parc F, Bai S-K, et al. The Histone Chaperone FACT Coordinates H2A.X-Dependent Signaling and Repair of DNA Damage. Mol Cell. 2018;72(5):888–901 e887.
- Han J, Li Q, McCullough L, et al. Ubiquitylation of FACT by the cullin-E3 ligase Rtt101 connects FACT to DNA replication. Genes Dev. 2010;24(14):1485–1490.
- Tsunaka Y, Toga J, Yamaguchi H, et al. Phosphorylated intrinsically disordered region of FACT masks its nucleosomal DNA binding elements. J Biol Chem. 2009;284(36):24610–24621.
- Li Y, Keller DM, Scott JD, et al. CK2 phosphorylates SSRP1 and inhibits its DNA-binding activity. J Biol Chem. 2005;280(12):11869–11875.
- Tsunaka Y. Alteration of the nucleosomal DNA path in the crystal structure of a human nucleosome core particle. Nucleic Acids Res. 2005;33(10):3424–3434.
- Pettersen EF, Goddard TD, Huang CC, et al. UCSF ChimeraX: structure visualization for researchers, educators, and developers. Protein Sci. 2021;30:70–82.
