Abstract
Background. Compared to warfarin, the non-vitamin K antagonist oral anticoagulant rivaroxaban may have advantages in treating patients with venous thromboembolism, because injectable bridging therapy and routine laboratory monitoring are not required. The objective of this study was to compare the rate of hospitalization in patients treated with rivaroxaban after its introduction with what it would have been before the introduction of rivaroxaban. Methods. A retrospective claims analysis was conducted using the MarketScan Hospital Drug Database from January 2011 to December 2013. Adult patients with a primary diagnosis of deep vein thrombosis (DVT) treated with rivaroxaban or low-molecular-weight heparin (LMWH) bridged to warfarin during the first day of an evaluation at a hospital were identified. Based on propensity-score methods, historical LMWH/warfarin patients (i.e., patients who received LMWH/warfarin before the approval of rivaroxaban) were matched 4:1 to rivaroxaban patients, and the rates of hospitalization were compared. Results. All rivaroxaban-treated patients (n = 134) in the database were well matched with four historical LMWH/warfarin-treated patients (n = 536). Among the rivaroxaban cohort, 60% of the patients were admitted to the hospital, compared to 82% of the historical patients treated with LMWH/warfarin in the matched cohort. The difference was statistically significant and corresponded to a 27% reduction in hospital admissions (rate ratio [95% confidence interval]: 0.73 [0.62–0.84]). Hospital admission rates adjusted for time-trend analyses also led to similar results. Conclusion. The availability of rivaroxaban significantly reduced the hospitalization rate in patients with DVT treated with rivaroxaban compared to what it would have been if only LMWH/warfarin were available.
Introduction
Low-molecular-weight heparin (LMWH) followed by an oral vitamin K antagonist (VKA) therapy has been, for some 20 years, the standard of care for patients diagnosed with venous thromboembolism (VTE, which includes deep vein thrombosis [DVT] and pulmonary embolism [PE]) [Citation1]. In the United States, the prevalence of patients with VTE was estimated at 950,000 in 2006 and is projected to double to 1.82 million by 2050 [Citation2]. The most common form of VTE is DVT, which constitutes approximately two-thirds of all diagnoses [Citation3,4].
Recently, large international trials of non-VKA oral anticoagulant (NOAC) agents, including rivaroxaban, dabigatran, apixaban, and edoxaban, have shown comparable or superior efficacy and safety to the combination of LMWH/warfarin for the treatment of DVT [Citation1,5-8]. These treatments have been approved by the US Food and Drug Administration (FDA) to prevent or treat VTE patients: rivaroxaban in November 2012, dabigatran in April 2014, apixaban in August 2014, and edoxaban in January 2015 [Citation9,10,11,12]. These new agents were also found to have a more predictable bioavailability, with no need for hematological monitoring, compared to the standard of care for which serial assays of the international normalized ratio (INR) and directed dose adjustments are required [Citation1,13].
Use of the first of these agents to be approved for treatment of VTE without bridging or lead-in therapy with a parenteral agent (i.e., rivaroxaban) may offer the advantage of convenient initiation of therapy and discharge home from the site of diagnosis (frequently an emergency department [ED]), thereby averting or at least shortening hospital length of stay (LOS). The EINSTEIN trials demonstrated this shorter LOS in rivaroxaban-treated patients [Citation5,14], but no studies have evaluated the impact of the availability of this new agent on the likelihood of being admitted to a hospital in a real-world setting. The objective of this analysis was to compare the rate of hospitalization in patients treated with rivaroxaban after its introduction with what the rate would have been before the introduction of rivaroxaban.
Methods
Study design
A retrospective matched-cohort design adjusting for change in practice patterns over time was used to evaluate the impact of the availability of rivaroxaban on hospitalization rates among a population of patients who presented at a hospital with DVT and were treated with rivaroxaban.
Before the introduction of rivaroxaban, there were predominantly two possible interventions for patients who presented with DVT in the hospital setting: treat with LMWH/warfarin and not hospitalize; or treat with LMWH/warfarin and hospitalize. After the introduction of rivaroxaban, two additional interventions became available for such patients in this setting: treat with rivaroxaban and not hospitalize; or treat with rivaroxaban and hospitalize. The objective of this study is to compare the rate of hospitalization among rivaroxaban-treated patients to what it would have been before rivaroxaban became available – this is why we compare the hospitalization rate in a current rivaroxaban cohort to that in a matched historical LMWH/warfarin cohort.
Data source/patients
Health-insurance claims from the Truven MarketScan Hospital Drug Database (HDD) for care between January 2011 and December 2013 were used to conduct the study. MarketScan HDD is a large US hospital-based, service-level database that represents >550 teaching and nonteaching hospitals. The database contains hospitalization episodes and the information is collected from the hospital’s point of view, as opposed to traditional claims data, in which the information collected is from the insurer’s point of view. The patient information collected by MarketScan HDD includes demographics (e.g. age, gender, primary payer type, regions), primary and secondary diagnoses, primary and secondary procedures, length of hospital stay, drug administration on a daily basis, department charge and cost details, day-of-stay, and physician specialty. The MarketScan HDD data are de-identified and fully compliant with all Health Insurance Portability and Accountability Act privacy and security requirements to protect patient anonymity and confidentiality. Institutional review board approval was not required for this study.
Adult (aged ≥18 years) patients included in the study had a primary diagnosis of DVT and were administered rivaroxaban or LMWH/warfarin during the first day of a hospital-based evaluation (the index hospital visit). Patients were excluded if they were diagnosed with a PE during an associated inpatient stay.
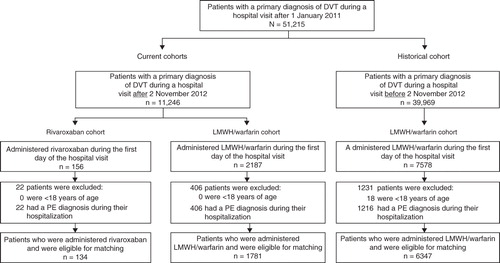
Three mutually exclusive cohorts were established: 1) rivaroxaban cohort, 2) current LMWH/warfarin cohort, and 3) historical LMWH/warfarin cohort. Patients using LMWH/warfarin were considered part of the historical LMWH/warfarin cohort if the date of their index hospital visit was before 2 November 2012; otherwise, they were part of the current LMWH/warfarin cohort. As described in the “Statistical Analysis” section, historical and current LMWH/warfarin groups were defined to enable both the assessment of the effect of the availability of rivaroxaban and the assessment of any time trend in the hospitalization rate.
Patients were observed until discharge (i.e., only the first day if they were discharged within 24 hours of their hospital visit or until the hospitalization discharge if they had been admitted in the hospital). Records up to 12 months before and on the index hospital visit were used to evaluate baseline characteristics.
LMWH/warfarin and rivaroxaban interventions
Prior to the approval of rivaroxaban for the indication of treatment of DVT and PE in November 2012, only two interventions were available to physicians when they treated patients at the hospital: treat DVT patients with LMWH/warfarin agents and then 1) admit them to the hospital or 2) discharge them the same day (i.e. no hospitalization). Patients using unfractionated heparin were not considered in the study as they would all have been admitted. However, since the FDA’s approval of rivaroxaban for the treatment of VTE, clinicians now have four possible interventions: 1) treat patients with rivaroxaban and admit them to hospital; 2) treat patients with rivaroxaban and discharge them the same day; 3) treat patients with LMWH/warfarin and admit them to hospital; or 4) treat patients with LMWH/warfarin and discharge them the same day.
Study end points
The main end point of this study was whether or not a patient was admitted to the hospital on the day of the index hospital visit with admission being defined as not being discharged within 24 hours of their hospital visit. In addition, the rates of subsequent hospital visits (i.e., hospital visits after the index hospital visit) within 1 month, 2 months, 3 months, and 6 months after the index hospital visit were determined.
Statistical analysis
To assess the impact of the availability of rivaroxaban on hospitalization rates, a three-step methodology adjusting for the practice pattern change over time (analogous to a difference-in-difference model) was used. First, the ratio of the rate of hospital admission in patients treated with rivaroxaban to that of matched patients with similar characteristics treated with LMWH/warfarin before the approval of rivaroxaban was evaluated. The likelihood of hospitalization for each treatment in the matched cohort was reported using absolute-rate measures. Rate ratios (RRs) were calculated as the ratio of the rate (rivaroxaban rate divided by historical LMWH/warfarin rate), and the 95% confidence intervals (CIs) were calculated using nonparametric bootstraps with 999 replications.
Second, the time trend in the likelihood of hospital admission over time was evaluated. The time trend was estimated by the RR of hospital admission between different populations of LMWH/warfarin users for the time period before (i.e. historical LMWH/warfarin users) versus after the approval of rivaroxaban (i.e. current LMWH/warfarin users). Finally, the association between the use of rivaroxaban and likelihood of hospital admission estimated in the first step was adjusted to account for the time trend estimated in the second step and thus estimate that part of the relative rate estimated in the first step that could be attributed to the availability of rivaroxaban.
Step 1: Rates of hospital admission among patients treated with rivaroxaban and matched historical LMWH/warfarin users
All patients in the rivaroxaban cohort were matched exactly with four patients of the historical LMWH/warfarin cohort using propensity-score calipers of 5% to form the rivaroxaban and historical LMWH/warfarin matched cohort. Propensity scores were calculated using a multivariate logistic regression model that incorporated the following baseline characteristics: age, gender, primary payer type, region of the hospital, hospital characteristics (i.e. urban, large [≥500 beds], teaching), admission source (i.e. physician referral, clinical referral, and transfer from hospital), and VTE risk factors (e.g. age ≥60 years, hypertension, hyperlipidemia, heart failure, and active malignancy). Since only two interventions (i.e. hospital admission or discharge) were available for the historical LMWH/warfarin cohort, the difference in the rate of hospital admission observed between the patients treated with rivaroxaban and the matched patients treated with LMWH/warfarin was attributable potentially to 1) the availability of rivaroxaban and/or 2) the trend in hospital admissions over time.
Step 2: Change in the likelihood of hospital admission over time
To assess the time trend in hospital admissions, three alternative scenarios were used among different populations of LMWH/warfarin users (see footnotes of for descriptions of the populations). The same variables used in the calculation of the propensity score of the rivaroxaban and historical LMWH/warfarin matched cohort were used in the calculation of the propensity scores for all three time-trend matched scenarios.
Step 3: Rates of hospital admission adjusted for the time trend
In the last step, the observed ratio of the rate of hospital admission between rivaroxaban and historical LMWH/warfarin (step 1) was adjusted to account for the estimated ratio in hospital admissions over time between current and historical LMWH/warfarin cohorts (step 2), which isolates the potential impact of the availability of rivaroxaban on hospitalizations. The RR of hospital admission calculated for the rivaroxaban and historical LMWH/warfarin matched cohort was divided by the corresponding RRs of the time-trend matched cohorts. CIs and P values were calculated using nonparametric bootstraps with 999 replications [Citation15].
The rate of subsequent hospital visits was also reported for patients treated with rivaroxaban and their matched patients treated with LMWH/warfarin before the approval of rivaroxaban among subgroups of patients whose follow up (time to end of data) was at least 1 month, 2 months, 3 months, and 6 months. Patients in the rivaroxaban cohort were matched exactly with four patients of the historical LMWH/warfarin cohort using propensity-score calipers of 5% to form the rivaroxaban and historical LMWH/warfarin matched cohorts among patients with sufficient follow up for each of the time periods evaluated (i.e. 1 month, 2 months, 3 months, and 6 months). Comparisons between cohorts were assessed using RRs, and 95 CIs were calculated using nonparametric bootstraps with 999 replications.
Descriptive statistics were generated to summarize the baseline characteristics of both the unmatched and matched cohorts, as well as the mean hospital LOS of the matched cohorts. Means, medians, and standard deviations were used to describe continuous variables, while frequencies and percentages were used to describe categorical variables. Standardized differences were used to compare the baseline characteristics and the mean hospital LOS between cohorts, and standardized differences <10% were considered well balanced. All statistical analyses were conducted using SAS 9.3 (SAS Institute, Inc., Cary, NC, USA).
Results
The rivaroxaban, current LMWH/warfarin, and historical LMWH/warfarin cohorts included 134, 1781, and 6347 patients, respectively (). The 134 rivaroxaban patients were matched with 536 historical LMWH/warfarin patients to form the rivaroxaban and historical LMWH/warfarin matched cohort. After matching, the mean age of both cohorts was 62 years and ∼50% of patients were female (). The most prevalent VTE risk factors were hypertension, age ≥60 years, hyperlipidemia, and tobacco in both cohorts. The matched cohorts, constructed to assess the time trend in hospital admissions among different populations, were also well balanced (Appendices 1–3).
Table 1. Patients’ demographics and clinical characteristics – unmatched and matched rivaroxaban and historical LMWH/warfarin cohorts.
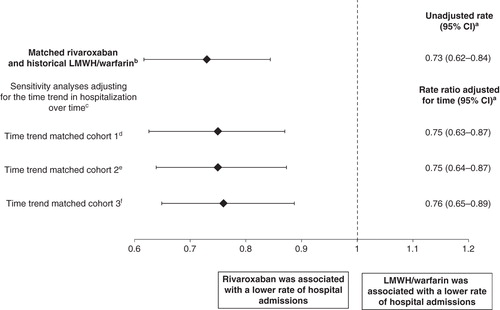
presents the number of events and the absolute rate of hospitalization for the matched rivaroxaban and historical LMWH/warfarin cohort, as well as the comparisons to assess the time trend in hospital admissions among different populations. In the rivaroxaban and historical LMWH/warfarin matched cohort, the hospitalization rate for rivaroxaban was 60%, while that for LMWH/warfarin was 82%, suggesting that the new availability of rivaroxaban after regulatory approval was associated with a significant reduction in hospitalization rate. In addition, the difference between cohorts was statistically significant with a corresponding RR (95% CI) of 0.73 (0.62–0.84); compared to standard of care, rivaroxaban showed a 27% reduction in the rate of hospitalization (P < 0.001, ).
Table 2. Comparison of the rate of hospital admission among rivaroxaban and LMWH/warfarin users.
The analyses to assess the time trend in hospital admissions among different populations estimated a 3% to 5% reduction in the rate of hospital admission before versus after November 2012. After adjustment for the change in practice patterns over time, new availability of rivaroxaban following its approval was also associated with significantly fewer hospital admissions compared to the standard of care. The RR (95% CI) of hospital admission between rivaroxaban and historical LMWH/warfarin users, adjusted for the time-trend scenarios, was still significantly associated with a lower likelihood of hospital admission for rivaroxaban users ().
Figure 2. Effect of rivaroxaban versus LMWH/warfarin on hospitalization avoided in DVT patients using rivaroxaban.
The mean (median) hospital LOS (counting 1 day for patients who were discharged within 24 hours) for the 134 rivaroxaban users and the matched 536 LMWH/warfarin historical users was 2.6 (2) and 3.8 (3) days, respectively (standardized difference = 46.2%). For the matched populations used for evaluating the time trend, the mean LOS ranged from 3.5 to 3.7 days for the current cohorts and from 3.7 to 3.8 days for the historical cohorts (standardized differences <10% for all).
presents the rate of subsequent hospital visits evaluated within 1 month, 2 months, 3 months, and 6 months after the index hospital visit for the current rivaroxaban and historical LMWH/warfarin cohorts. The rate of hospital visits was lower for the rivaroxaban patients compared to the historical LMWH/warfarin patients during the first 6 months (within 1 month: 1.5% vs. 2.2%; within 2 months: 3.0% vs. 4.7%; within 3 months: 4.8% vs. 6.2%; within 6 months: 4.4% vs. 7.2%; all P values >0.05). In addition, similar results were also found after adjusting for the time trend as described in the hospital admission analysis (data not shown).
Table 3. Comparison of the rate of subsequent hospital visits among matched rivaroxaban and historical LMWH/warfarin users.a
Discussion
This retrospective matched-cohort study compared the likelihood of provider-directed hospitalization during a hospital visit between DVT patients treated with rivaroxaban and a matched sample of DVT patients treated with the standard of care, LMWH/warfarin, before rivaroxaban became available. With the regulatory approval and subsequent US availability of rivaroxaban, there was a 27% reduction in the hospital admission rate in patients treated with rivaroxaban compared to what the rate would have been before the availability of rivaroxaban when the treatment would have been LMWH/warfarin.
Recently, van Bellen et al. used data from the EINSTEIN DVT trial to compare the rate of hospitalization between patients treated with rivaroxaban and patients treated with the standard therapy, enoxaparin/VKA. The authors reported a rate of hospitalization of 50.6% for DVT patients treated with rivaroxaban, compared to 53.1% for patients treated with enoxaparin/VKA agents (P = 0.144) [Citation14]. While the likelihood of hospitalization among rivaroxaban users found in the current study (60%) is similar in range to the likelihood using the data from the clinical trial, the likelihood of hospitalization among LMWH/warfarin users in the current study (81%) was higher. The difference could partly be explained by the differences between randomized clinical trials and real-world analyses, such as different patient populations. More specifically, the data used by van Bellen et al. came from an international trial, while the data used in this study were US claims data. As mentioned by van Bellen et al., the highly monitored environment of clinical trials could lead to reducing the perceived need for hospital admissions [Citation16].
The LOS of the initial hospitalization between patients treated with rivaroxaban and patients treated with the standard therapy, enoxaparin/VKA, was also compared in the study by van Bellen et al. among a population of DVT patients. The authors found that 54% of rivaroxaban patients had a LOS <5 days, compared to only 31% for patients treated with enoxaparin/VKA [Citation14]. In addition, even though NOAC agents were used to treat a different condition, several studies have evaluated their impact in stroke prevention compared to that of warfarin among populations of patients with nonvalvular atrial fibrillation and have found that new agents such as rivaroxaban, dabigatran, and apixaban were associated with shorter hospital LOS [Citation17-19]. These results could be explained by the more predictable bioavailability of the NOAC and the less cumbersome concern for food and drug interactions [Citation1]. More specifically in the case of DVT patients, rivaroxaban has the advantage of not requiring a parenteral anticoagulant agent to obtain a rapid onset of action, which could also decrease the LOS of rivaroxaban patients compared to that of LMWH/VKA patients [Citation5]. These advantages may potentially explain our findings that patients treated with rivaroxaban had a significantly lower likelihood of hospitalization compared to patients treated with LMWH/warfarin, as rivaroxaban provides more flexibility and makes it easier for physicians to send home patients who would otherwise have been hospitalized, resulting in avoided or shortened hospitalization.
The proportion of patients with VTE who were treated entirely in the outpatient setting appears to be on the rise as patterns in clinical practice change over time and physicians may be encouraged to send DVT patients home sooner. Clinical trials and recent studies have documented the safety and efficacy of this approach in a specific subset of patients with careful monitoring [Citation20-24]. The ongoing effort since the early 2000s to manage outpatients with uncomplicated DVT that was diagnosed in the ED involved self-administration of enoxaparin, which meant training patients/caregivers, overcoming needle phobia, finding a pharmacy with drug availability (often after-hours), and managing INR monitoring during the bridging period. Monotherapy with an oral drug not requiring monitoring advanced the goal of supporting (or accelerating) outpatient care. Rivaroxaban patients are more likely to be discharged from the ED simply because they can be. Obviously, discharge of patients on unfractionated heparin is not possible, and most EDs do not have the resources to both acquire and teach patients how to use LMWH, thus preventing same-day discharge. The ability to begin rivaroxaban or another oral anticoagulant is easier as patients can receive the first dose in the ED and be given a prescription to obtain the medication the next day.
Moreover, in the current study, we found a lower rate of subsequent hospital visits for rivaroxaban and their matched historical LMWH/warfarin users in the first months after their index hospital visit. These findings suggest that rivaroxaban users, despite a high proportion of them being quickly discharged after their index hospital visit, received sufficient initial care (i.e., at least as good as patients treated with warfarin who had longer hospital stays) since they did not have more hospital visits than their matched historical LMWH/warfarin users within 1 month, 2 months, 3 months, and 6 months of the index hospital visit.
In computerized printouts of metropolitan Worcester (MA, USA) residents with healthcare system encounters during calendar years 1999, 2001, and 2003, the proportion of patients diagnosed with VTE and treated as outpatients rose from 22.4% to 28.5% [Citation25]. In the current study, the trend in hospital admissions over time was assessed among different populations pre- and post-November 2012, and the proportion of patients admitted to the hospital was found to decline from 3% to 5%. Although not directly comparable because of a different study design and population, the current study concurs with the literature that a trend toward fewer hospital admissions was observed for the treatment of patients with VTE. In order to estimate the impact of the availability of rivaroxaban on hospitalization rate, the current study adjusted for this time trend and thus isolated the impact of the availability of rivaroxaban. The sensitivities for the time-trend adjustments (i.e. using different populations) produced very similar results, which reduced the uncertainty about the populations chosen to evaluate the change in practice patterns over time.
This study has several limitations. First, the study is subject to limitations inherent to administrative claims data, including possible inaccuracies in billing and missing data. Second, patients were matched only on information collected during hospital visits and hospitalizations occurring at the same hospital up to 12 months before and on the index hospital visit. This particular limitation could affect the comprehensiveness of the baseline characteristics, as well as the analysis of subsequent hospital visits. Third, a general limitation of observational studies is that adjustment can be made only for observable factors and cannot be made for unmeasured confounders. Fourth, the observational design was susceptible to additional potential biases such as those of information or classification (e.g. identification of false-positive or false-negative DVT events). Last, the sample size of the rivaroxaban cohort was relatively small, which may limit generalizability. Despite these limitations, the current study has several advantages, including reliance on the real-world utilization of rivaroxaban and LMWH/warfarin anticoagulant agents among DVT patients.
Conclusion
In this real-world study of patients presenting at a hospital with DVT and treated with rivaroxaban, the hospitalization rate after the availability of rivaroxaban for treatment was significantly reduced compared to what it would have been if only LMWH/warfarin were available.
IHOP_A_1021659_SM8925.doc
Download MS Word (329 KB)Acknowledgments
The authors would like to acknowledge Brahim Bookhart and Jeff R. Schein, of Janssen Scientific Affairs, LLC, Raritan, NJ, USA, who provided assistance during the study, as well as Guillaume Germain, of Groupe d’analyse, Ltée, Montréal, QC, Canada, who contributed to the analysis and writing phases with funding from Janssen Scientific Affairs. Editorial assistance was provided by Shannon O’Sullivan, of MedErgy (Yardley, PA), and was funded by Janssen Scientific Affairs.
Declaration of interest
This research was funded by Janssen Scientific Affairs, LLC, Raritan, NJ, USA. GJ Merli and JE Hollander have received research grants from Janssen Scientific Affairs. MK Raut and WH Olson are employees of Janssen Scientific Affairs. P Lefebvre and F Laliberté are employees of Analysis Group, Inc., a consulting company that has received research grants from Janssen Scientific Affairs. CV Pollack reports receiving consulting fees for scientific activities from Janssen, Boehringer Ingelheim, Pfizer, and Daiichi-Sankyo. CV Pollack has engaged in no promotional activities. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
Notes
References
- Agnelli G, Becattini C, Franco L. New oral anticoagulants for the treatment of venous thromboembolism. Best Pract Res Clin Haematol 2013;26:151–61
- Deitelzweig SB, Johnson BH, Lin J, Schulman KL. Prevalence of clinical venous thromboembolism in the USA: current trends and future projections. Am J Hematol 2011;86:217–20
- Beckman MG, Hooper WC, Critchley SE, Ortel TL. Venous thromboembolism: a public health concern. Am J Prev Med 2010;38:S495–501
- Dobesh PP. Economic implications of inadequate treatment of venous thromboembolism and potential solutions. J Pharm Pract 2014;27:178–86
- Landman GW, Gans RO. Oral rivaroxaban for symptomatic venous thromboembolism. N Engl J Med 2011;364:1178
- Schulman S, Kearon C, Kakkar AK, et al. Dabigatran versus warfarin in the treatment of acute venous thromboembolism. N Engl J Med 2009;361:2342–52
- Agnelli G, Buller HR, Cohen A, et al. Oral apixaban for the treatment of acute venous thromboembolism. N Engl J Med 2013;369:799–808
- Büller HR, Décousus H, Grosso MA, et al. Edoxaban versus warfarin for the treatment of symptomatic venous thromboembolism. N Engl J Med 2013;369:1406–15
- U.S. Food and Drug Administration. FDA expands use of Xarelto to treat, reduce recurrence of blood clots. Available from http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm326654.htm. [Last accessed February 2015]
- Boehringer Ingelheim. FDA approves Pradaxa® (dabigatran etexilate mesylate) for treatment and reduction in the risk of recurrence of deep venous thrombosis and pulmonary embolism. Available from http://us.boehringer-ingelheim.com/news_events/press_releases/press_release_archive/2014/04-07-14-fda-approves-pradaxa-dabigatran-etexilate-mesylate-treatment-reduction-risk-of-recurrence-deep-venous-thrombosis-pulmonary-embolism.html. [Last accessed February 2015]
- U.S. FDA approves Eliquis (apixaban) for the treatment of deep vein thrombosis (DVT) and pulmonary embolism (PE), and for the reduction in the risk of recurrent DVT and PE following initial therapy. Available from http://www.pfizer.com/news/press-release/press-release-detail/u_s_fda_approves_eliquis_apixaban_for_the_treatment_of_deep_vein_thrombosis_dvt_and_pulmonary_embolism_pe_and_for_the_reduction_in_the_risk_of_recurrent_dvt_and_pe_following_initial_therapy. [Last accessed February 2015]
- FDA approves anti-clotting drug Savaysa. Available from http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm429523.htm. [Last accessed February 2015]
- Becattini C, Vedovati MC, Agnelli G. Old and new oral anticoagulants for venous thromboembolism and atrial fibrillation: a review of the literature. Thromb Res 2012;129:392–400
- van Bellen B, Bamber L, Correa de Carvalho F, et al. Reduction in the length of stay with rivaroxaban as a single-drug regimen for the treatment of deep vein thrombosis and pulmonary embolism. Curr Med Res Opin 2014;24432872
- Efron B, Tibshirani RJ. An introduction to the bootstrap. 1st edition. New York: Chapman & Hall; 1993
- van Bellen B, Bamber L, Correa de Carvalho F, et al. Reduction in the length of stay with rivaroxaban as a single-drug regimen for the treatment of deep vein thrombosis and pulmonary embolism. Curr Med Res Opin 2014;30:829–37
- Laliberté F, Pilon D, Raut MK, et al. Hospital length of stay: is rivaroxaban associated with shorter inpatient stay compared to warfarin among patients with non-valvular atrial fibrillation? Curr Med Res Opin 2014;30:645–53
- Fonseca E, Walker DR, Hill J, Hess GP. Dabigatran etexilate is associated with shorter hospital length of stay compared to warfarin in patients with nonvalvular atrial fibrillation. Circulation 2012;5:A282
- Cowper PA, Pan W, Anstrom K, et al. Apixaban reduces hospitalization in patients with atrial fibrillation: an analysis of the effect of apixaban therapy on resource use in the apixaban for reduction in stroke and other thromboembolic events in atrial fibrillation trial. J Am Coll Cardiol 2013;61:E1576
- Hull RD, Raskob GE, Pineo GF, et al. Subcutaneous low-molecular-weight heparin compared with continuous intravenous heparin in the treatment of proximal-vein thrombosis. N Engl J Med 1992;326:975–82
- Prandoni P, Lensing AW, Büller HR, et al. Comparison of subcutaneous low-molecular-weight heparin with intravenous standard heparin in proximal deep-vein thrombosis. Lancet 1992;339:441–5
- Levine M, Gent M, Hirsh J, et al. A comparison of low-molecular-weight heparin administered primarily at home with unfractionated heparin administered in the hospital for proximal deep-vein thrombosis. N Engl J Med 1996;334:677–81
- Lozano F, Trujillo-Santos J, Barrón M, et al. Home versus in-hospital treatment of outpatients with acute deep venous thrombosis of the lower limbs. J Vasc Surg 2014;59:1362–7.e1
- Othieno R, Abu Affan M, Okpo E. Home versus in-patient treatment for deep vein thrombosis. Cochrane Database Syst Rev 2007:CD003076
- Spencer FA, Emery C, Joffe SW, Pacifico L, et al. Incidence rates, clinical profile, and outcomes of patients with venous thromboembolism. The Worcester VTE study. J Thromb Thrombolysis 2009;28:401–9
Supplementary material available online
Appendices 1–3