ABSTRACT
Objective: Flowable agents such as Floseal® (F) are often reserved as adjuncts to non-flowable agents (i.e. gelatin (G) sponges and thrombin (T)) when bleeding is not sufficiently controlled. Based on their perceived positive impact, it is postulated that flowable agents alone may result in better clinical and resource utilization outcomes. Clinical and health-care utilization outcomes were compared in this retrospective analysis of spine surgery cases with charges for Floseal only (FO) and F + G/T.
Methods: The United States Premier Hospital Database was searched for adult spine surgeries performed between October 2010 and September 2015 with FO or F and G/T charges. To obtain an unbiased treatment estimate, 1:1 propensity-score matching was used to identify FO and F + G/T cohorts. The cohorts were compared for rates of intraoperative, perioperative, postoperative and transfusion; blood loss-related, serious and other complications; hospital length-of-stay (LOS), surgical time, and volume of hemostat charged.
Results: Among 40,335 spine surgeries, 15,105 FO and F + G/T matched pairs were compared. Significantly (p < 0.0001) lower percentages of FO than F + G/T cases received intraoperative (1.4% vs. 2.5%), perioperative (1.6% vs. 2.8%), postoperative (1.6% vs 3.0%), and any transfusion (2.3% vs. 4.3%). FO cases had significantly less blood loss complications than F + G/T cases (0.5% vs. 0.8%, p = 0.0022) and significantly (p < 0.0001) shorter hospital LOS (−0.45 days), surgical time (−39.0 min), and used less hemostat (−12.5 mL).
Conclusions: Results from this observational hospital database analyses indicate that FO use in spine surgery is associated with lower blood transfusion use and blood loss complications compared to its use with adjunct non-flowable hemostatic agents. The shorter hospital stay, reduced surgical time, and less hemostat volume health-care utilization outcomes that favored FO versus combination use may translate to health system cost savings. Further validation of these findings using controlled clinical trials and cost-consequence studies is warranted.
Clinical relevance: The use of flowable hemostatic agents alone may result in better clinical and possibly economic outcomes in spine surgery.
Introduction
Bleeding is an anticipated consequence of virtually all types of surgical procedures. Ideally, each and every surgical procedure concludes with hemostasis of the surgical field and an optimal patient outcome. However, a retrospective database analysis of over 1.6 million inpatient surgeries conducted in the US between 1 January 2006 and 31 December 2007 revealed that the percentages of patients who experience bleeding and the rates of bleeding-related consequences (e.g. intervention(s) to control bleeding including a return to the operating room, blood product transfusion) are substantial.[Citation1] The rates of these bleeding and bleeding-related complications ranged from 7.5% with reproductive organ procedures to 47.4% with cardiac procedures [Citation1]. The percentage of patients reported in Stokes, E. et al. (), with any type of bleeding-related complication varied according to subgroup (general: 27.5%; cardiac: 47.4%; solid organ: 28.5%; non-cardiac thoracic: 34.3%; vascular: 31.5%; knee/hip replacement: 29.8%; reproductive organ: 7.5%; spine: 15.0%; and overall: 29.9%) [Citation1].
Table 1. Percentages of patients with specific complication events, by surgical subgroup.
In the same Stokes et al retrospective database analyses of 107,187 spine surgeries, bleeding and bleeding-related complications occurred in 15.0% of cases [Citation1]. The most common type of event in the spine surgery cases was blood product transfusion (14.3%) [Citation1]. Spine surgery cases with a bleeding/bleeding-related complication exhibited longer hospital and intensive care unit (ICU) length-of-stay (LOS) as compared to those that did not experience these events (overall: 7.8 vs. 3.3 days; ICU: 1.7 vs. 0.3 days) [Citation1]. In addition, these events were associated with increased mean total hospital costs (+$17,279) [Citation1]. The findings of this large database analysis in addition to other reports support that the failure to manage hemostasis during any surgical procedure can result in excess bleeding and bleeding-related complications and an increase in patient morbidity and mortality, greater health-care resource utilization, and overall costs [Citation1–Citation3]. In the case of spine surgery procedures, excessive and/or uncontrolled bleeding also imparts challenges related to the adequate visualization of the neural elements.
The control of bleeding during surgical procedures is often managed using a variety of hemostatic agents and tissue sealants, especially when conventional surgical techniques such as suturing, cautery, or manual compression fail or are impractical [Citation4–Citation13]. Hemostatic agents are generally categorized as either passive or active, which refers to their mechanism of action and interaction with or activation of the clotting cascade [Citation6,Citation8,Citation13]. Specifically, passive agents such as gelatins, polysaccharide spheres, collagens, and cellulose act via bleeding site contact activation and promotion of platelet aggregation. The surgical practicality of these agents may be limited in certain situations such as difficult-to-reach sites, patients who are receiving anticoagulants, or cases of active bleeding [Citation14–Citation16]. On the other hand, active agents, such as thrombin, act biologically on the clotting cascade to potentiate the hemostatic effect.
The gelatin-thrombin hemostatic matrix, Floseal® (F, Baxter BioSurgery, Vienna, Austria), is an active flowable topical hemostat that combines two independent hemostatic agents: patented bovine-derived gelatin granules and human thrombin that work in combination to form a stable clot at the bleeding site [Citation16–Citation18]. An advantage to the use of flowable hemostats is that they exhibit both passive and active mechanisms of action on the blood clotting cascade. The gelatin granules passively swell to produce a tamponade effect and the high concentrations of human thrombin component actively convert fibrinogen into fibrin monomers, accelerating clot formation [Citation14,Citation17,Citation18]. F is currently approved by the US Food and Drug Administration for use in surgical procedures other than ophthalmologic, as an adjunct to hemostasis when control of bleeding by ligature or conventional procedures is ineffective or impractical.
Randomized clinical trials and observational studies across different surgical procedures, including spine surgery, have shown that the use of flowable gelatin–thrombin hemostatic matrix products provides significant clinical outcome benefits. These include reduction of time to hemostasis, less blood loss and economic/health-care resource utilization benefits such as shorter surgery times, lower transfusion needs and reduced hospital LOS as compared to the use of non-flowable hemostatic agents including topical hemostatic patches or sponges (e.g. oxidized cellulose, absorbable hemostatic gelatin sponges plus thrombin) or other comparators [Citation5,Citation11,Citation14–Citation16,Citation19–Citation22].
There is a wide variation in the surgical utilization of flowable hemostatic agents by spine surgeons. Some spine surgeons will use a single flowable agent such as F as the only hemostatic agent while a majority of others adopt a combination use approach where F is reserved as a ‘last line of defense’ for the most challenging bleeding circumstances after other traditional non-flowable hemostatic agents (e.g. gelatin (G), thrombin (T)) have failed. To our knowledge, there is limited clinical evidence in the literature and no evidence-based practice guideline(s) or algorithm available to spine surgeons to make a clinically informed choice regarding the benefit of one strategy over the other (e.g. single use F versus stepwise combination use of F, G and T). Without such evidence and guidance, it is difficult to determine whether the single use of a flowable hemostatic agent alone provides more effective clinical and resource utilization outcomes and waste reduction as compared to its use in a combination fashion when a non-flowable hemostatic agent hasfailed. By comparing the single-use strategy of Floseal only (FO) versus a combination strategy of F + G/T, the aim of this retrospective health-care database analysis is to identify the most positive strategy to treat challenging and uncontrolled bleeding in spine surgery. To achieve this, tangible surgical outcomes (e.g. clinical and healthcare resource utilization) were compared in surgical cases that had charges for FO with those where F was charged in combination with a non-flowable absorbable G and T (F + G/T). In the surgical setting, it is not uncommon for the staff to bring in, and possibly open (although likely an uncommon practice due to economic reasons), both types of hemostatic agents. The choice of one or both agents is selected for use during the procedure, with opened items, whether used or not, being charged to the case and therefore identified in this database retrieval. In this type of analysis, it is also not possible to determine the order of hemostat use if both were utilized, and/or the amount and type of opened hemostat that may have been abandoned/not used. Other factors that are difficult to assess and also limit the interpretation of these analyses are clinical items such as the degree of bleeding severity and the expertise/skill level of the surgeon, though this effect should be normalized by the large multicenter sample.
The authors understand that there is selection bias associated with this retrospective observational data. The limitation of the study is associated with retrospective observational evaluation of registry database. It is less robust than prospective randomized trials. Several important clinical variables were not available; certain data points could not be captured or could not be clearly identified. However, the strengths of the analysis included the relatively recent and national hospital representation covered broad geographic and demographic setting; the large sample size of the spine surgery population analyzed and propensity scoring methodology reduced the selection bias of large observational study to maximum. After propensity score matching, the differences of baseline characteristics between two cohorts were reduced to minimum.
The authors are currently performing simulation model-based analyses to evaluate the overall differences in cost associated with the FO versus F + G/T hemostasis strategy. Evidence of approximately 1000 iterations from our different ‘economic Floseal databases’ utilized in a previous cost-consequence analysis of a large retrospective analysis of Floseal and Surgiflo kit with thrombin in major spine surgery cases (Price et al. [Citation15]; Makhija et al. [Citation23]) found that the use of F was associated with less transfusion use, less operative time and less product volume utilization with these factors resulting in a cost savings of $151 per major and $574 per severe spine surgery.
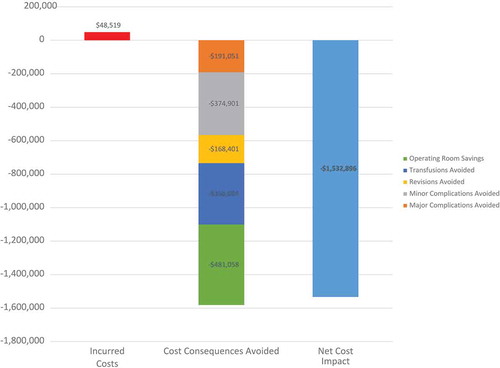
Based on Makhija et al. [Citation23] (), the objective was to estimate the cost-consequence of two flowable hemostatic matrices (F or Surgiflo) for US hospitals and the main conclusion was as follows: even if F acquisition cost is higher ($48,519 for 245 surgeries), F is expected to generate substantial annual net cost savings ($1,532,896 for 245 surgeries) compared to Surgiflo. This cost consequence analysis suggests that cost savings at the hospital level may be generated by using F rather than Surgiflo.
Figure 1. Estimated net cost impact of using Floseal versus Surgiflo in an average US hospital performing 245 cardiac surgical procedures per year.
Source: Own elaboration with data obtained from Makhija D, Rock M, Xiong Y, et al. Cost-consequence analysis of different active flowable hemostatic matrices in cardiac surgical procedures, Journal of Medical Economics. 2017;20(6):565–573, DOI: 10.1080/13,696,998.2017.1284079 homepage: http://www.tandfonline.com/loi/ijme20.
Materials and methods
Analysis design
This retrospective observational analysis assessed clinical and health-care resource utilization outcomes between FO or in combination with G and T (F + G/T) in spine surgery cases identified through procedural and billing charges in the Premier’s US Perspective Hospital Database.
Data source
Data for this retrospective database analysis was obtained from Premier’s US Perspective Hospital Database, a repository of clinical, economic and resource use data developed for quality and utilization benchmarking purposes. The database includes nationally representative hospital data based on bed size, geographic region and teaching hospital status. The database comprises data from more than 600 hospital facilities throughout the US, capturing approximately 25% of hospital discharges and containing over 490 million hospital encounters with approximately 6 million added each month [Citation24]. To protect confidentiality, patient-specific data are de-identified according to US Health Insurance Portability and Accountability Act regulations. Therefore, this analysis using the registry database does not constitute human subject research and is not subject to International Review Board approval.
Database case selection criteria
The records of spine surgery cases included in this retrospective database analysis were of patients who underwent elective, emergent or urgent spine surgeries with hospitalizations and discharges that occurred between 1 October 2010 and 30 September 2015; had presence of a charge on the day of surgery for F alone or F with G/T and were at least 18 years old on the day of hospital admission. lists the ICD-9 codes used in the identification of the cases and in characterizing their primary or secondary surgical type and severity (i.e. minor, major/severe). In cases of multiple hospitalizations within the same hospital during the study period, only the first hospitalization for one of the target procedures was identified and considered. Database records indicating the use of a sealant or hemostatic agent other than F with or without G/T and those that lacked complete demographic/baseline values and/or had unevaluable outcome measures were excluded from the analysis. If only the volume of hemostatic agent was missing, the case was included for all other outcomes.
Table 2. Group assignment by primary or secondary ICD-9 procedural codes.
Data extracted for analyses
Data regarding patient characteristics (i.e. age at the time of admission, gender, race, Charlson Comorbidity Index [Citation25], antiplatelet/anticoagulant use and admission type [i.e. elective, emergent, urgent]) were extracted. Hospital characteristics data were also extracted (i.e. teaching hospital status, urban or rural setting, US geographic region and bed size [0–199, 200–299, 300–499, 500+]).
Outcome variables extracted from the records included blood product administration, complication outcomes and health-care resource utilization outcomes. Blood product administration outcomes were intraoperative transfusion (defined as any blood product transfusions during the day of surgery), perioperative transfusion (defined as any blood product transfusions 4 days before or after surgery during the hospitalization period), postoperative transfusions (defined as any blood product transfusions 7 days after surgery during the hospitalization period) and transfusion (defined as any packed red blood cell (pRBC) transfusions during the hospitalization period).
Complication outcomes extracted were blood loss complications (e.g. hemorrhage, anemia, thrombocytopenia, hematoma complicating a procedure, visual complication such as dural puncture during the operation, embolic events or venous thrombosis), severe complications (e.g. encephalopathy, sepsis, postoperative infection or acute respiratory failure) and other complications (e.g. seroma complicating a procedure; urinary, cardiac or respiratory complications; or volume depletion/dehydration). Complication-related information that was not present at the time of admission was derived from the primary or secondary discharge diagnosis records.
Health-care resource utilization outcomes extracted were hospital LOS (in days), surgery time (in minutes and derived from charges where the clinical summary description was ‘surgery time’) and amount of hemostatic matrix volume (i.e. F in mL and T in IU/mL) used during surgery.
Statistical analyses
All patient and hospital characteristics were summarized descriptively by hemostat charge cohort (i.e. FO or F + G/T). Continuous variables were summarized using descriptive statistics (number, mean, standard deviation, median, minimum and maximum) and categorical variables were presented as counts and percentages. To compare the equivalency of the cohorts, t-tests and chi-square tests were performed on continuous and categorical variables, respectively.
Baseline and demographic variables were used in the propensity score model to obtain a propensity score for every subject in the FO and F + G/T charge cohorts. The propensity score for each subject was calculated from a logistic regression model that included all baseline and demographic data as covariates and the dependent variable of study treatment (i.e. FO or F + G/T). A 1:1 matching without replacement utilizing the nearest available Mahalanobis metric within calipers defined by the propensity score technique was utilized to match each FO subject with a F + G/T subject [Citation26–Citation28]. All FO charged cases were then randomly ordered and a F + G/T charged case with a propensity score closest to the first treatment case was selected.
Group comparisons of the propensity matched pairs were made using McNemar’s test for categorical variables (clinical outcomes) and a paired t-test for continuous variables (health-care utilization outcomes). All analyses were performed using SAS/GRAPH® 9.4 software and Base SAS® 9.4. Copyright© 2002–2012, SAS Institute Inc. SAS and all other SAS Institute INC. Treatment effects were evaluated on the basis of a two-sided significance level of 0.05. Cases with missing values for any variable(s), with the exception of hemostat volume, were excluded from analysis.
Results
A total of 40,335 spinal surgery cases were identified with data extracted from 18,844 cases with a FO charge and 21,491 cases with F + G/T charges. Propensity score analysis resulted in a total of 15,105 FO charged cases matched with 15,105 F + G/T charged cases. After propensity matching, a small percentage but statistically significant difference was observed between the F and F + G/T cohorts for race, anticoagulant/antiplatelet use, admission type and hospital characteristics of teaching facility, location and number of hospital beds ().
Table 3. Patient and provider characteristics after propensity score matching.
In the 15,105 matched pairs, blood product administration and blood loss complication outcomes were significantly different between the groups (). Significantly lower (p < 0.0001 for all comparisons) percentages of the FO than the F + G/T cohort received intraoperative transfusions (1.4% vs. 2.5%), perioperative transfusions (1.6% vs. 2.8%), postoperative transfusions (1.6% vs. 3.0%) and transfusions (2.3% vs. 4.3%). The percentage of patients who experienced blood loss complications was significantly lower in the FO versus the F + G/T cohort (0.5% vs. 0.8%, p = 0.0022), with no difference in the rates of severe or other complications.
Table 4. Results of clinical outcomes on propensity matched pairs (Floseal only vs. Floseal + gelatin/thrombin).
Definition of terms: Intraoperative transfusions were any blood product transfusions during the day of surgery; perioperative transfusions were any blood product transfusions 4 days before or after surgery during the hospitalization period; postoperative transfusions were any blood product transfusions 7 days after surgery during the hospitalization period. Pure-blood/pRBC transfusions were any packed RBC transfusions during the hospitalization period. Blood loss complications were those of hemorrhage, anemia, thrombocytopenia, hematoma complicating a procedure, visual complications such as dural puncture during the operation, embolic events or venous thrombosis. Severe complications were those of encephalopathy, sepsis, postoperative infection or acute respiratory failure. Other complications included seroma complicating a procedure; urinary, cardiac or respiratory complications or volume depletion/dehydration.
Health-care resource utilization outcomes were significant and favored the FO over the F + G/T strategy. As summarized in , FO as compared to F + G/T was associated with a shorter hospital LOS (mean difference of 0.446 days, p < 0.0001), less surgical time (mean difference of 39.0 min, p < 0.0001) and a lower volume of hemostat (mean difference ‒12.514 mL, p < 0.001). Further analyses of hemostat volume (i.e. F in mL and T in IU/mL) revealed that the mean volume of F was slightly lower in the FO than the F + G/T group (11.385 and 11.393 mL, respectively). The mean volume of T was 0 and 15.340 IU/mL in the FO and F + G/T cohort, respectively [Citation29].
Table 5. Results of healthcare resource utilization outcomes on propensity matched pairs (Floseal only vs. Floseal + gelatin/thrombin).
Discussion
This 5-year period, retrospective analysis of a large US database registry identified over 40,000 spine surgery cases in which the active, flowable gelatin–thrombin hemostatic matrix, F, was charged and used alone (FO) in 18,844 cases or charged with gelatin/thrombin (F + G/T) in 21,491 cases. In the matched pairs, the use of intraoperative, perioperative and postoperative transfusions and the rate of transfusions and blood loss complications (only) was significantly less in the FO compared to the F + G/T group. Significant differences favoring FO over the combination approach (F + G/T) were also observed in health-care utilization parameters of hospital LOS, surgery time and hemostat volume. Further analysis of hemostat volume revealed that the mean volume of F used was similar (between 11.365 and 11.767 mL) in the FO and F + G/T cohorts. In the F + G/T cohort, a mean thrombin volume of 15.340–16.118 mL was utilized, which in addition to the higher utilization of blood products in this group represents a substantial cost component [Citation29].
These clinical and health-care utilization benefits observed in the FO as compared with F + G/T cohort in spine surgery cases suggest that the use of F alone is effective and potentially cost-effective given the reductions in transfusion utilization, complications, hospital LOS, surgical time and hemostat use. Other research studies in spine surgery have also shown that F when used alone results in greater clinical and health-care utilization outcomes as compared to other flowable and non-flowable hemostatic agents [Citation15,Citation16,Citation30,Citation31].
Spine surgery presents unique challenges in attaining hemostasis due to the difficulty in visualizing and controlling epidural and bony bleeding. The use of flowable hemostatic agents may offer a unique advantage due to their ability to conform to irregular surfaces that are ubiquitous in spine surgery cases. Some spine surgeons may utilize F in combination with non-flowable G/T for different practical and clinical reasons. To our knowledge, there are no clinical practice guidelines that support combination use of F and non-flowable G/T nor any randomized controlled trials that have been conducted to evaluate the clinical and health-care utilization outcomes between the use of a flowable hemostatic matrix alone and its use in combination with a non-flowable hemostat agent in patients undergoing spine surgery. Furthermore, this is the first and largest retrospective observational study known to compare the use of flowable FO with F + non-flowable G/T. Our findings of the clinical and healthcare resource utilization advantages with the use of FO alone are relevant to the continued understanding of the role of FO as compared with F + G/T in spine surgery cases. It remains to be determined as to why the combined use of F with G/T was associated with less favorable surgical outcomes than FO. A possible explanation for this observation may be a staged approach towards hemostasis control, with the surgeon using G/T as an ‘economical’ first-line strategy and then utilizing F in those cases where hemostasis is not achieved. Data from this analysis, and as we can attest from our clinical practice, supports that this strategy may result in longer surgical time, use of greater amounts of blood transfusions and hospital LOS, all of which incur greater health-care costs and patient morbidity. This observation underscores the need for well-controlled randomized comparative trials in order to confirm these differences and provide surgeons with additional guidance as to the selection and use of hemostatic agents.
The strengths of the analysis included the relatively recent and national hospital representation covering a broad geographic and demographic setting; large number of treating physicians from teaching and non-teaching hospitals; the large sample size of the spine surgery population analyzed and propensity scoring methodology to minimize potential selection bias. Limitations of the analysis include its retrospective observational evaluation of a registry database design, which is less robust than the conduct of prospective randomized trials. Our interpretation of the findings is limited by the fact that certain data points could not be captured or could not be clearly identified. For example, while one can fairly comfortably assume that those in the FO charged cohort received F, it is less clear as to what hemostatic agent or agents and the amount of each that were actually received by cohort for which both F + G/T were charged. In the surgical setting, it is not uncommon for the staff to bring in, and possibly open (although likely an uncommon practice due to economic reasons), both types of hemostatic agents.
The choice of one or both agents is selected for use during the procedure, with opened items, whether used or not, being charged to the case and therefore identified in this database retrieval. In this type of analysis, it is also not possible to determine the order of hemostat use if both were utilized and/or the amount and type of opened hemostat that may have been abandoned/not used. Other factors that are difficult to assess and also limit the interpretation of these analyses are clinical items such as the degree of bleeding severity and the expertise/skill level of the surgeon, though this effect should be normalized by the large multicenter sample. Unfortunately, there is heterogeneity among bleeding definitions and severity ratings among clinicians [Citation32]. Lewis et al [Citation33] recently described the validated intraoperative bleeding scale, which is an intra-operative bleeding severity scale that standardizes the definition of bleeding and may enhance the effective utilization of hemostatic agents for the appropriate bleed severity and improve the evaluation of hemostatic agents and their effects. Limitations such as these clearly underscore the need for randomized controlled trials to accurately assess the impact of FO as compared to F + G/T in spine surgery cases.
There is an increased focus on cost and waste across the spectrum of health care. Spine surgery is a high-cost intervention and there have been many studies looking at ways to decrease costs. Many spine surgeries are moving to the outpatient setting with patients only requiring same day or overnight stays. Hemostasis is a key component in being able to decrease length of stay and morbidity for these patients. The cumulative effect of minimally invasive surgery, multimodal anesthesia, effective hemostasis and blood management utilization has significant potential to bend the cost curve historically associated with spine surgery.
Conclusion
The findings of the observational propensity score matched analysis of patients undergoing spine surgery indicate that the use of F alone and likely first line resulted in low rates of intraoperative perioperative postoperative and transfusion. Its use was also associated with shorter surgical times, less hemostat volume and shorter hospital length of stay as compared to cases in which F and a non-flowable absorbable gelatin compressed sponge and thrombin were charged. Further, well-controlled clinical trials and cost-consequence studies are needed to confirm and further elucidate these findings and to quantify the cost-savings that may be realized with the utilization of a single flowable hemostatic matrix versus combination management of bleeding with flowable and non-flowable hemostatic agents in patients undergoing spine surgery.
Limitations
Limitations of the analysis include its retrospective observational evaluation of a registry database design, which is less robust than the conduct of prospective randomized trials and inherently associated with selection biases. As mentioned earlier, the utilization of propensity score matching was done to reduce the differences between the two cohorts. Specifically, after propensity score matching methodology was applied, the two cohorts were closely matched with the exceptions of race, admission type, anticoagulant/antiplatelet use and several hospital-related characteristics. With the exception of anticoagulant use, it is highly unlikely that the other variable mismatches contributed significantly to the outcome findings. Furthermore, with respect to the 1.6% mismatch between the cohorts () in anticoagulant/antiplatelet use (FO 14.9% and F + G/T 16.5%), this reflects how closely these two cohorts were matched using this methodology, with this small difference unlikely to fully explain the significant differences that were observed in bleeding rates, transfusion use and health-care resource utilization. Our interpretation of the findings is limited by the fact that certain data points could not be captured or could not be clearly identified. For example, while one can fairly comfortably assume that those in the FO charged cohort received F, it is less clear as to what hemostatic agent or agents and the amount of each that were actually received by cohort for which both F + G/T were charged. We did not think through the concept that G and T alone may have been opened in many cases and F only opened later during the procedure when severe bleeding was encountered. In the surgical setting, it is not uncommon for the staff to bring in, and possibly one open (although likely an uncommon practice due to economic reasons), both types of hemostatic agents. The choice of one or both agents is selected for use during the procedure, with opened items, whether used or not, being charged to the case and therefore identified in this database retrieval. In this type of analysis, it is also not possible to determine the order of hemostat use if both were utilized, and/or the amount and type of opened hemostat that may have been abandoned/not used. Further, well-controlled clinical trials and cost-consequence studies are needed to confirm and further elucidate these findings and to quantify the cost-savings that may be realized with the utilization of a single flowable hemostatic matrix versus combination management of bleeding with flowable and non-flowable hemostatic agents in patients undergoing spine surgery.
Declaration of interest
MGR, DY, DC and EK are employees of Baxter Healthcare, which is involved in the manufacture of a product examined in this analysis. HD has served as a consultant for Baxter and receives royalties from RTI Surgical. NK serves as a consultant for Nuvasive, Globus, Edge Surgical, Alpheon, Baxter, and Casetabs.
Acknowledgment
Editorial support provided by Susan M. Garabedian-Ruffalo, PharmD., MedWrite, Inc. Newport Coast, CA.
Additional information
Funding
References
- Stokes E, Ye X, Shah M, et al. Impact of bleeding-related complications and/or blood product transfusions on hospital costs in inpatient surgical patients. BMC Health Serv Res. 2011;11:135.
- Boucher BA, Traub O. Achieving hemostasis in the surgical field. Pharmacotherapy. 2009;29:2S–7S.
- Levi M, Cromheecke ME, de Jonge E, et al. Pharmacological strategies to decrease excessive blood loss in cardiac surgery: a meta-analysis of clinically relevant endpoints. Lancet. 1999;354(9194):1940–1947.
- De la Torre RA, Bachman SL, Wheeler AA, et al. Hemostasis and hemostatic agents in minimally invasive surgery. Surgery. 2007;142:S39–S45.
- Esposito P, Cappabianca P, Angileri FF, et al. Gelatin-thrombin hemostatic matrix in neurosurgical procedures: hemostasis effectiveness and economic value of clinical and surgical procedure-related benefits. J Neurosurg Sci. 2016 July 26. [Epub ahead of print]. PMID 27456032.
- Fiss I, Danne M, Stendel R. Use of gelatin-thrombin matrix hemostatic sealant in cranial neurosurgery. Neurol Med Chir (Tokyo). 2007;47:462–467.
- Gazzeri R, Galarza M, Neroni M, et al. Hemostatic matrix sealant in neurosurgery: a clinical and imaging study. Acta Neurochir (Wien). 2011;153(1):148–154.
- Gerald AG. Update on hemostasis: neurosurgery. Surgery. 2007;142:S55–S60.
- Lewis KM, Atlee HD, Mannone AJ, et al. Comparison of two gelatin and thrombin combination hemostasis in a porcine liver abrasion model. J Invest Surg. 2013;26(3):141–148.
- Mozet C, Prettin C, Dietze M, et al. Use of Floseal and effects on wound healing and pain in adults undergoing tonsillectomy: randomised comparison versus electrocautery. Eur Arch Otorhinolaryngol. 2012;269(10):2247–2254.
- Nasso G, Piancone F, Bonifazi R, et al. Prospective, randomized clinical trial of the FloSeal matrix sealant in cardiac surgery. Ann Thorac Surg. 2009;88(5):1520–1526.
- Raga F, Sanz-Cortes M, Bonilla F, et al. Reducing blood loss at myomectomy with use of a gelatin-thrombin matrix hemostatic sealant. Fertil Steril. 2009;92(1):356–360.
- Spotnitz WD. Active and mechanical hemostatic agents. Surgery. 2007;142:S34–S38.
- Oz MC, Cosgrove DM 3rd, Badduke BR, et al. Controlled clinical trial of a novel hemostatic agent in cardiac surgery. Ann Thorac Surg. 2000;69:1376–1382.
- Price JS, Tackett S, Patel V. Observational evaluation of outcomes and resource utilization from hemostatic matrices in spine surgery. J Med Econ. 2015;18:777–786.
- Renkens KL Jr., Payner TD, Leipzig TJ, et al. A multicenter, prospective, randomized trial evaluating a new hemostatic agent for spinal surgery. Spine (Phila PA 1976). 2001;26(15):1645–1650.
- Oz MC, Rondinone JF, Shargill NS. FloSeal matrix: new generation topical hemostatic sealant. J Card Surg. 2003;18:486–493.
- FLOSEAL. Hemostatic Matrix Instructions for Use. Hayward, CA: Baxter Healthcare Corporation; 2017.
- Albala DM, Lawson JH. Recent clinical and investigational applications of fibrin sealant in selected surgical specialties. J Am Coll Surg. 2005;202:685–697.
- Carless PA, Henry DA, Anthony DM. Fibrin sealant use for minimizing peri-operative allogeneic blood transfusion. Cochrane Database Syst Rev. 2003;1:CD004171.
- Tomizawa Y. Clinical benefits and risk analysis of topical hemostats: a review. J Artif Organs. 2005;8:137–142.
- Weaver FA, Hood DB, Zatina M, et al. Gelatin-thrombin-based hemostatic sealant for intraoperative bleeding in vascular surgery. Ann Vasc Surg. 2002;16(3):286–293.
- Makhija D, Rock M, Xiong Y, et al. Cost-consequence analysis of different active flowable hemostatic matrices in cardiac surgical procedures. J Med Econ. 2017;20(6):565–573.
- Finn T. Premier perspectives database: comparative effectiveness research (CER) – validated 2012. Available from: https://hcmatters.com. Accessed on 01/10/18.
- Charlson ME, Pompei P, Ales KL, et al. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40(5):373–383.
- D’agostino R. Propensity score methods for bias reduction in the comparison of a treatment to a nn-randomized control group. Stat Med. 1998;17(19):2265–2281.
- Rosenbaum PR, Rubin DB. Reducing bias in observational studies using classification on the propensity score. J Amer Stat Assoc. 1984;79:516–524.
- Rosenbaum PR, Rubin DB. The central role of the propensity score in observational studies for causal effects. Biometrika. 1983;70:41–55.
- Ikeme S, et al. Clinical and healthcare resource utilization outcomes in spine surgery: Research Poster presented at the North (NASS) Meeting, Oct 25–28, 2017, Orlando, FL, USA.
- David G, Lim S, Gunnarsson C, et al. Similar patient outcomes yet different hospital costs between flowable hemostatic agents. J Med Econ. 2015;18:735–745.
- Landi A, Gregori F, Marotta N, et al. Efficacy, security, and manageability of gelified hemostatic matrix in bleeding control during thoracic and lumbar spine surgery: floSeal versus Surgiflo. J Neurol Surg A Cent Eur Neurosurg. 2016;77:139–143.
- Mehran R, Rao SV, Bhatt DL, et al. Standardized bleeding definitions for cardiovascular clinical trials. Circulation. 2011;123:2736–2747.
- Lewis KM, Li Q, Jones DS, et al. Development and validation of an intraoperative bleeding severity scale for use in clinical studies of hemostatic agents. Surgery. 2017;161:771–781.
