Abstract
This exploratory study aimed to assess associations of baseline nutritional status and in-hospital step count with muscle quantity, quality, and function. Seventy-nine participants aged ≥70 years (mean age 79.1 years, 44.3% female) were recruited (elective colorectal surgery, emergency abdominal surgery, and general medical patients with infections). Baseline nutrition (Mini Nutritional Assessment) and in-hospital step count (Fitbit Inspire devices) were assessed. Ultrasound quadriceps, bioelectrical impedance analysis, and physical function were assessed at baseline and 7 (±2) days and 13 (±1) weeks post-admission/post-operatively. Baseline nutritional status was associated with baseline rectus femoris ultrasound echogenicity (normal: 58.5, at risk: 68.5, malnourished: 81.2; p = 0.025), bilateral anterior thigh thickness (normal: 5.07 cm, at risk: 4.03 cm, malnourished: 3.05 cm; p = 0.021), and skeletal muscle mass (Sergi equation) (normal: 21.6 kg, at risk: 18.2 kg, malnourished: 12.0 kg; p = 0.007). Step count was associated with baseline patient-reported physical function (<900 37.1, ≥900 44.5; p = 0.010). There was a significant interaction between nutrition, step count, and time for skeletal muscle mass (Janssen equation) (p = 0.022).
Keywords:
Background
Acute sarcopenia (acute muscle insufficiency) is defined by the development of incident sarcopenia (low muscle strength with low muscle quantity and/or quality) within six months, normally following a stressor event.Citation1–3 It is increasingly recognized as a target for therapeutic trials.Citation4 Acute sarcopenia may lead on to the development of chronic sarcopenia over time,Citation1 which is known to impact upon quality of life.Citation5 Trials that demonstrate benefit in ameliorating negative changes in muscle quantity and function during hospitalization, therefore, have potential to improve quality of life of older adults following hospitalization in the longer-term. Interventions that have been previously trialed include physical activity, nutritional, neuromuscular electrical stimulation, and pharmaceutical (testosterone, nandrolone, erythropoietin).Citation4 A previous multi-center cohort study showed that patients at greatest risk of acute sarcopenia were those who were already vulnerable, but not yet fully dependent (i.e. living at home and mobile but with some dependency on others for help with daily tasks).Citation6 Mean step count over up to three consecutive days during hospitalization has previously been shown to be associated with functional decline from premorbid function (two weeks prior to hospitalization) to discharge, controlling for baseline functional status.Citation7 Physical activity measured by accelerometry has also been shown to be associated with severity of acute illness during hospitalization.Citation8 However, the benefits of physical activity upon skeletal muscle can be blunted when nutrition, particularly protein intake, is inadequate.Citation9 Interventional strategies where exercise has been combined with inadequate protein intake have proven ineffective in preventing muscle quantity loss in critical illness.Citation10 The interactions of the effects of nutrition and step count with dynamic changes in skeletal muscle in hospitalized older adults have not previously been characterized. This study aimed to assess the associations between in-hospital step count and baseline nutritional status with muscle quantity, quality, and function measurements.
Methods
Study design and setting
The protocol for the main study has been published previouslyCitation11 and the study was prospectively registered (NCT03858192). This exploratory study represents a post hoc analysis of the main study. Results from the main study have been published elsewhere.Citation12,Citation13 Participants were recruited from a single center, the Queen Elizabeth Hospital Birmingham. Three groups of participants aged 70 years and older were recruited: patients expected to undergo elective colorectal surgery, patients who had undergone emergency abdominal surgery, and general medical patients with acute infectious diseases. Participants were excluded if they were considered to be imminently approaching the end of life, or if they were unable to understand verbal or written English. Ethical approval was obtained from Wales Research Ethics Committee 4. Participants provided written informed consent or consultee declaration was obtained if they were deemed to lack capacity to consent for themselves. Baseline assessments were performed in preoperative assessment clinic in the elective cohort, within 48 hours of surgery in the emergency surgery cohort, and within 48 hours of admission in the medical cohort. Further assessments were performed at 7 (±2) days post-operatively/post-admission, and 13 (±1) weeks post-operatively/post-admission. demonstrates the timing of the assessments relevant to this analysis that were performed at each timepoint. If participants were discharged home prior to assessment at 7 (±2) days, then the investigator contacted the participant to arrange to review them in their own home within the same timeframe. Recruitment and assessments were performed by a clinician with training and expertise in geriatric medicine.
Muscle quantity and quality assessment
Muscle quantity and quality were assessed at each timepoint using quadriceps ultrasound and bioelectrical impedance analysis (BIA).Citation11 Bilateral anterior thigh thickness (BATT) was calculated as the total thickness of the right and left rectus femoris and vastus intermedius muscles, measured at the midpoint between the greater trochanter and lateral joint line in the transverse plane using a linear probe (Venue 50, GE Healthcare).Citation14 Rectus femoris echogenicity was measured using longitudinal images taken at the same location by grey scale analysis using Image J software.Citation14 Skeletal muscle mass (SMM) was calculated from BIA measurements (Bodystat Quadscan 4000) using two previously validated equations: SMMJanssenCitation15 and SMMSergiCitation16 (Table S1, Supplement). Both equations were used in wider analysis and it was unknown which would demonstrate the greatest sensitivity to change and associations with outcomes in this exploratory study.
Physical function assessment
Handgrip strength was measured at each timepoint using a Jamar dynamometer. Participants were advised to squeeze as hard as they were able to, and the best result from two measures on each side was used in analysis.Citation17 Usual gait speed was measured at each timepoint across a four meter course, by advising participants to walk at a “comfortable pace,”Citation18 except for emergency surgery patients at baseline assessment, where this was not possible. Patient-reported physical function was assessed using the Patient-Reported Outcomes Measurements Information System (PROMIS®) item bank V2.0 Physical Function Short Form 10b questionnaire at each timepoint.Citation19 Raw scores were entered into the HealthMeasures Scoring Service, powered by Assessment CenterSM to derive T-scores.
Nutritional assessment
Baseline nutritional assessment was performed using the Mini Nutritional Assessment (Full Form) (MNA).Citation20 Participants were categorized as normal (24–30 points), at risk of malnutrition (17–23.5 points), or malnourished (less than 17 points).
Step count
Step count was measured during the inpatient stay from initial post-operative/post-admission assessment to 7 (±2) days post-operatively using Fitbit Inspire devices worn on the non-dominant wrist. This was included as an optional aspect of the study. Mean daily step count was calculated from days where whole day data was available (i.e. excluding the days that the device was applied or removed for data extraction). Participants were advised to wear the device all the time but were able to remove the device when washing or sleeping for comfort. Participants were categorized as having <900 or ≥900 steps/day as per previously validated cutoffs for functional decline.Citation7
Frailty assessment
Frailty was assessed at each timepoint using both a frailty indexCitation21 and Clinical Frailty Scale (CFS).Citation22 The variables included within the frailty index were adapted from those previously validated within the electronic Frailty Index (eFI), which was developed from a UK community population.Citation23 The CFS was assessed by a clinician with expertise in geriatric medicine, based on function two weeks prior to admission. All information from a full comprehensive assessment including KatzCitation24 and LawtonCitation25 Activities of Daily Living (ADLs) were used when assessing the CFS.
Statistical analysis
All statistical analyses were performed using IBM SPSS Statistics Version 26 (IBM Corp). This study represents a substudy of the main study. The study was initially powered to enable detection of clinically significant differences in muscle and physical function measurements within groups (45 to follow-up in each group). In light of recruitment to the study being paused during the Coronavirus 2019 (COVID-19) pandemic, the recruitment target was revised to enable detection of differences across groups (45 to follow-up across groups). Descriptive statistics were summarized at baseline according to categories of nutrition and step count. Statistical significance of differences were analyzed using analysis of variance (nutrition) or independent samples t-tests (step count) for parametric continuous variables, Kruskal–Wallis (nutrition) or Mann–Whitney U tests (step count) for non-parametric continuous or ordinal variables, and chi-squared tests for proportions. Statistical significance of differences in muscle quantity, quality, and physical function measurements between nutrition and step count groups, and between timepoints were analyzed using linear mixed models (parametric variables) or generalized linear mixed models (non-parametric variables). Interaction terms for nutrition × timepoint, step count × timepoint, and nutrition × step count × timepoint were included within the models. Estimated marginal means across timepoints and groups were calculated from these models. Statistical significance was set at p < 0.050.
Results
Eighty-one participants were recruited to this study. Two participants were excluded from this analysis (one elective admission in the emergency surgery cohort and one emergency surgery participant did not undergo surgery). Recruitment and drop-out rates are shown in Figure S1 (Supplement). Full feasibility analysis and screening and recruitment rates have been published previously.Citation26 Fitbit data were collected for 51.5% (35/68) of participants across all groups, with no significant difference between groups. Baseline nutritional status was assessed in all participants. Baseline characteristics are shown in . The mean age of participants was 79.1 (6.6), and 44.3% were female. There were no significant differences between nutrition and step count groups in terms of age, sex, or ethnicity of participants. However, a greater proportion of at risk or malnourished participants were medical patients and had greater frailty indices and CFS compared to those with normal nutrition. Participants with lower step counts also had higher CFS at baseline.
Table 1. Baseline characteristics of participants separated by nutritional status and step count.
Muscle quantity and quality
demonstrates results of mixed models with estimated marginal means derived from interaction terms with timepoint. These results are shown graphically for muscle quantity and quality in . Participants with greater risk of malnutrition had significantly lower BATT, SMMSergi, and SMMJanssen, and higher echogenicity across timepoints. Differences were statistically significant for SMMJanssen with a model including interaction terms for nutrition, step count, and timepoint together. Non-statistically significant lower BATT, SMMSergi, and SMMJanssen, and higher echogenicity were demonstrated for participants with lower step counts.
Figure 2. Estimated marginal means of muscle quantity and quality, and physical function measurements according to nutritional status and step count. BATT: bilateral anterior thigh thickness; PROMIS: Patient-Reported Outcomes Measurements Information System, T-score for physical function; SMMSergi: skeletal muscle mass (Sergi equation).
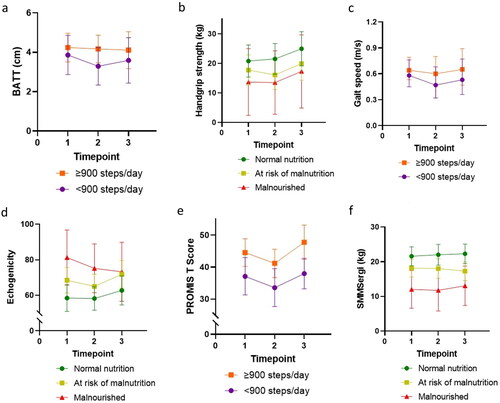
Table 2. Estimated marginal means with 95% confidence intervals for muscle quantity and quality measures derived from mixed models for nutritional status and step count across timepoints.
Muscle and physical function
No associations were demonstrated between nutrition and PROMIS T-score or gait speed. Handgrip strength was lower in participants with greater risk of malnutrition (), although these differences were not statistically significant. Participants with lower step count had significantly lower PROMIS T-scores across all timepoints, but step count was not predictive of differences across timepoints. Lower handgrip strength and slower gait speeds were demonstrated in participants with lower step counts, but these differences were not statistically significant ().
Table 3. Estimated marginal means with 95% confidence intervals for physical function measures derived from mixed models for nutritional status and step count across timepoints.
Discussion
In this study, baseline nutritional status was associated with baseline muscle quantity (BATT, SMMSergi, SMMJanssen) and quality (low rectus femoris echogenicity). This suggests that malnutrition is associated with the development of chronic sarcopenia, but the relationship with acute sarcopenia is unclear. This study was exploratory in nature, and further evaluation of the trends demonstrated in this study may assist in planning of future higher powered observational and targeted interventional studies. We have demonstrated that it is feasible to conduct a study of this design on a complex heterogeneous population, and that it is possible to perform a comprehensive nutritional assessment on all participants. The use of Fitbit devices was shown to be feasible in half of participants; further studies may be valuable to assess how uptake and compliance with devices can be improved. It should be noted that the use of the Fitbit devices was an optional aspect of the study design. However, nutritional status was not associated with physical function or performance, indicating that additional factors are involved in these pathways. Although not statistically significant, results suggested that the most malnourished participants were the least likely to recover acute losses in muscle quantity. Malnourished hospital patients represent a specific group to target further research in larger powered studies.
Conversely, step count was significantly associated with patient-reported physical function, although this does not necessarily indicate causation. Step count itself could be considered a marker of physical function, rather than low step count being causative of declines in physical function. Although not statistically significant, muscle quantity, quality, and strength were consistently reduced with reduced step count, and there was suggestion of greater acute decline in BATT. This implies that a low step count in-hospital may be acutely detrimental regardless of baseline muscle size, and that step count may not simply be a marker of baseline function. There was a statistically significant interaction term between nutrition, step count, and timepoint for SMMJanssen, although there were no statistically significant differences for interaction terms with timepoint and either nutrition or step count alone. Low step count was associated with declines in muscle quantity with normal nutrition, whereas high step count was associated with recovery of muscle quantity with malnutrition.
Results in the context of other literature
These results are consistent with previous studies, which have demonstrated low fat-free mass measured by BIA in malnourished (defined by Subjective Global Assessment) hospitalized patients admitted with exacerbations of chronic obstructive pulmonary disease,Citation27 and older hospitalized patients at high risk of malnutrition (defined by the Short Nutritional Assessment Questionnaire).Citation28 The latter study included serial measurements and no change in muscle mass during hospitalization was demonstrated regardless of nutritional status.Citation28
The cutoff of 900 steps/day was used as this has previously been demonstrated to predict decline in functional independence, as measured by the Barthel index and instrumental ADLs, during hospitalization.Citation7 A unit-tailored mobility program with a specific goal of at least 900 steps/day was shown to be associated with a lesser decline in ADLs in a quasi-experimental study.Citation29 However, a subsequent study suggested that this cutoff may have high specificity but low sensitivity for hospital associated disability; lower step count was demonstrated in patients experiencing declines in ADLs.Citation30 The results of this study suggest that 900 steps/day may be a functionally significant cutoff, but analysis of linear trends in a larger powered study would be valuable.
Considering underlying mechanisms of the associations encountered, 14 days of reduced steps in healthy older adults have been shown to be associated with declines in leg fat-free mass measured using dual energy X-ray absorptiometry (DXA). These declines were associated with reduced postprandial rates of muscle protein synthesis.Citation31 This suggests that reduced step count is associated with anabolic resistance; thus, negative effects may be exacerbated if protein intake is especially low. This is consistent with the results of our study, demonstrating an interaction between nutrition and step count in terms of change in SMMJanssen over time, and suggests that interventions may be most effective when targeted toward physical activity and nutrition together.
Limitations
We acknowledge that there are a number of important limitations related to this study. Firstly, the MNA provides an assessment of nutrition over the preceding three monthsCitation20 and is less sensitive to acute illness related effects. We did not record nutritional intake during hospitalization in this study, and it is unclear how this may have impacted upon dynamic changes. Secondly, we recognize that there are limitations in the use of Fitbit devices as opposed to raw accelerometry data. In older adults with very low step count, this may have led to a floor effect, and it was not possible to assess for the effects of differences in position.Citation32 Additionally, although step count provides a quantitative estimate of physical activity, it does not differentiate between resistance exercise, aerobic exercise, and simple mobilization. Thirdly, no gold standard measure of muscle quantity (DXA, magnetic resonance imaging, or computer tomographyCitation2) was used in this study. Fluid balance affects estimation of SMM with BIACitation33 and may affect ultrasound measurements in the presence of significant edema.Citation34 Research by our group also previously demonstrated that ultrasound in particular can be affected by changes in position. However, these changes were not significant with only minor changes in body position, and variability with position was less pronounced with BIA.Citation35 Fourthly, due to recruitment challenges during the COVID-19 pandemic, it was not possible to recruit sufficient numbers of participants to meet the original sample size target. The study was not powered toward this exploratory study, and particularly recruitment technique was not modified to increase the number of malnourished participants included within the study. The number of participants who met criteria for being malnourished at baseline was particularly small, and confidence intervals were wide. This may explain why nutritional status was not associated with physical function/performance, as similar trends to other measures were demonstrated in those with normal and at risk nutrition. Fifthly, there were higher rates of frailty and medical patients amongst participants who met criteria for malnutrition, and higher rates of frailty at baseline in participants with low step counts. The direction of causality is unclear for these associations. Finally, we analyzed our results assessing changes across all three groups. This is particularly relevant when considering the applicability of baseline assessments, which were performed pre-insult in the elective group but during the acute phase of illness in the emergency surgery and medical groups. As the MNA assesses nutritional status over the previous three months, this should not have affected the overall assessment of nutritional status. However, we acknowledge that there are important differences in the clinical characteristics of these three populations.
Recommendations for clinical practice and future research
Further research is warranted to establish the effectiveness of combined nutritional and physical activity interventions in hospitalized older adults. In-hospital supervised exercised interventions have been shown to be safe and effective for improving declines in functional independence in hospitalized older adults,Citation36 although few studies inform nutritional management of inpatients with or at risk of sarcopenia.Citation37 Large community studies are currently underway to establish the effectiveness of protein supplementation and resistance exercise to prevent and treat chronic sarcopenia.Citation38 This exploratory study provides important proof-of-concept results, but further larger powered observational studies are warranted to establish causality and underlying mechanisms of associations. This should in turn drive forward interventional studies and translation into clinical practice. Importantly, our study has shown that it is feasible to perform a comprehensive nutritional assessment in all three patient groups, and feasible to measure step count in at least half of participants. The identification of patients who are malnourished or at risk of malnutrition in hospital is important, and nutritional assessment should be incorporated into any Comprehensive Geriatric Assessment that includes assessment of frailty and sarcopenia status. Step count is not currently measured within routine clinical practice, but our study is confirmatory of previous studies, which have demonstrated that assessment of step count within a clinical environment is feasible. Routine use of physical activity monitors in hospital could allow real-time monitoring with trends over time, which could be viewed remotely in a similar manner to vital signs.
Conclusion
Baseline nutritional status is associated with baseline muscle quantity and quality in hospitalized older adults. Baseline physical function is associated with reduced step count during hospitalization. There is some suggestion that there may be interactions between the effects of nutritional status and physical activity in predicting dynamic changes in muscle quantity. Further research should aim to stratify and examine underlying mechanisms, to enable the development of targeted combined nutritional and physical activity interventions.
Take away points
Our exploratory study shows that baseline nutritional status is associated with reduced muscle quantity and quality, and strength in older adults admitted to hospital, reaffirming the relationship between nutritional status and sarcopenia.
Participants with lower patient-reported physical function as measured by the PROMIS T-score had lower in-hospital step count, which suggests that the PROMIS T-score is very predictive of objectively measured physical activity.
In hospital, a combination of suboptimal baseline nutritional status and reduced step count leads to increased risk of loss of muscle quantity as measured by SMMJanssen.
Interventions to target acute sarcopenia should therefore aim to target nutrition and physical activity together.
Ethical approval
This research has been sponsored by and reviewed by the University of Birmingham research governance team. Ethical approval has been obtained from Wales Research Ethics Committee 4 (19/WA/0036), the Health Research Authority, and the University Hospitals Birmingham NHS Trust Research and Development department. Written informed consent was obtained from all participants who were considered to have capacity to consent for themselves. Written personal or professional consultee declaration was obtained if the participant was considered to lack capacity to consent to participation. The use of both informed consent and consultee declaration was approved by the ethics committee. All methods were performed in accordance with the relevant guidelines and regulations.
Author contributions
CW designed the research question and study protocol. TJ, CG, TM, and TP all contributed toward design of the study protocol. CW, ZM, and HM all significantly contributed toward recruitment and follow-up assessments of participants in the study. DL and BS contributed toward data collection. CW analyzed and interpreted the results and was responsible for manuscript preparation. All authors significantly contributed toward the writing of the manuscript and approved the final submitted version.
Supplemental Material
Download MS Word (165 KB)Acknowledgments
No further acknowledgments.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
The anonymized dataset is available from the corresponding author upon reasonable request.
Additional information
Funding
References
- Welch C, Hassan-Smith ZK, Greig CA, Lord JM, Jackson TA. Acute sarcopenia secondary to hospitalisation—an emerging condition affecting older adults. Aging Dis. 2018;9(1):151–164. doi:10.14336/AD.2017.0315.
- Cruz-Jentoft AJ, Bahat G, Bauer J, et al. Sarcopenia: revised European consensus on definition and diagnosis. Age Ageing. 2019;48(1):16–31. doi:10.1093/ageing/afy169.
- Montero-Errasquín B, Cruz-Jentoft AJ. Acute sarcopenia. Gerontology. 2023;69(5):519–525. doi:10.1159/000529052.
- Welch C, Majid Z, Greig C, Gladman J, Masud T, Jackson T. Interventions to ameliorate reductions in muscle quantity and function in hospitalised older adults: a systematic review towards acute sarcopenia treatment. Age Ageing. 2021;50(2):394–404. doi:10.1093/ageing/afaa209.
- Beaudart C, Reginster JY, Petermans J, et al. Quality of life and physical components linked to sarcopenia: The SarcoPhAge study. Exp Gerontol. 2015;69:103–110. doi:10.1016/j.exger.2015.05.003.
- De Spiegeleer A, Kahya H, Sanchez-Rodriguez D, et al. Acute sarcopenia changes following hospitalization: influence of pre-admission care dependency level. Age Ageing. 2021;50(6):2140–2146. doi:10.1093/ageing/afab163.
- Agmon M, Zisberg A, Gil E, Rand D, Gur-Yaish N, Azriel M. Association between 900 steps a day and functional decline in older hospitalized patients. JAMA Intern Med. 2017;177(2):272–274. doi:10.1001/jamainternmed.2016.7266.
- Hartley P, DeWitt AL, Forsyth F, Romero-Ortuno R, Deaton C. Predictors of physical activity in older adults early in an emergency hospital admission: a prospective cohort study. BMC Geriatr. 2020;20(1):177. doi:10.1186/s12877-020-01562-3.
- Shad BJ, Thompson JL, Breen L. Does the muscle protein synthetic response to exercise and amino acid-based nutrition diminish with advancing age? A systematic review. Am J Physiol Endocrinol Metab. 2016;311(5):E803–E817. doi:10.1152/ajpendo.00213.2016.
- Nickels MR, Blythe R, White N, et al. Predictors of acute muscle loss in the intensive care unit: a secondary analysis of an in-bed cycling trial for critically ill patients. Aust Crit Care. 2023. doi:10.1016/j.aucc.2022.12.015.
- Welch C, Greig CA, Masud T, Pinkney T, Jackson TA. Protocol for understanding acute sarcopenia: a cohort study to characterise changes in muscle quantity and physical function in older adults following hospitalisation. BMC Geriatr. 2020;20(1):239. doi:10.1186/s12877-020-01626-4.
- Welch C, Greig C, Majid Z, et al. Induced frailty and acute sarcopenia are overlapping consequences of hospitalisation in older adults. J Frailty Sarcopenia Falls. 2022;7(3):103–116. doi:10.22540/jfsf-07-103.
- Welch C, Greig C, Lewis D, et al. Trajectories of muscle quantity, quality and function measurements in hospitalized older adults. Geriatr Gerontol Int. 2022;22(4):311–318. doi:10.1111/ggi.14366.
- Wilson DV, Moorey H, Stringer H, et al. Bilateral anterior thigh thickness: a new diagnostic tool for the identification of low muscle mass? J Am Med Dir Assoc. 2019;20(10):1247–1253.e2. doi:10.1016/j.jamda.2019.04.005.
- Janssen I, Heymsfield SB, Baumgartner RN, Ross R. Estimation of skeletal muscle mass by bioelectrical impedance analysis. J Appl Physiol. 2000;89(2):465–471. doi:10.1152/jappl.2000.89.2.465.
- Sergi G, De Rui M, Veronese N, et al. Assessing appendicular skeletal muscle mass with bioelectrical impedance analysis in free-living Caucasian older adults. Clin Nutr. 2015;34(4):667–673. doi:10.1016/j.clnu.2014.07.010.
- Roberts HC, Denison HJ, Martin HJ, et al. A review of the measurement of grip strength in clinical and epidemiological studies: towards a standardised approach. Age Ageing. 2011;40(4):423–429. doi:10.1093/ageing/afr051.
- Rydwik E, Bergland A, Forsén L, Frändin K. Investigation into the reliability and validity of the measurement of elderly people’s clinical walking speed: a systematic review. Physiother Theory Pract. 2012;28(3):238–256. doi:10.3109/09593985.2011.601804.
- Tatsuoka C, DeMarco L, Smyth KA, et al. Evaluating PROMIS physical function measures in older adults at risk for Alzheimer’s disease. Gerontol Geriatr Med. 2016;2:2333721416665502. doi:10.1177/2333721416665502.
- Vellas B, Guigoz Y, Garry PJ, et al. The Mini Nutritional Assessment (MNA) and its use in grading the nutritional state of elderly patients. Nutrition. 1999;15(2):116–122. doi:10.1016/s0899-9007(98)00171-3.
- Rockwood K, Mitnitski A. Frailty in relation to the accumulation of deficits. J Gerontol A Biol Sci Med Sci. 2007;62(7):722–727. doi:10.1093/gerona/62.7.722.
- Pulok MH, Theou O, van der Valk AM, Rockwood K. The role of illness acuity on the association between frailty and mortality in emergency department patients referred to internal medicine. Age Ageing. 2020;49(6):1071–1079. doi:10.1093/ageing/afaa089.
- Clegg A, Bates C, Young J, et al. Development and validation of an electronic frailty index using routine primary care electronic health record data. Age Ageing. 2016;45(3):353–360. doi:10.1093/ageing/afw039.
- Katz S, Ford AB, Moskowitz RW, Jackson BA, Jaffe MW. Studies of illness in the aged. The index of ADL: A standardized measure of biological and psychosocial function. Jama. 1963;185(12):914–919. doi:10.1001/jama.1963.03060120024016.
- Lawton MP, Brody EM. Assessment of older people: self-maintaining and instrumental activities of daily living. Gerontologist. 1969;9(3 Part 1):179–186. doi:10.1093/geront/9.3_Part_1.179.
- Welch C, Greig C, Majid Z, et al. The feasibility of conducting acute sarcopenia research in hospitalised older patients: a prospective cohort study. Eur Geriatr Med. 2022;13(2):463–473. doi:10.1007/s41999-021-00565-6.
- Teixeira PP, Kowalski VH, Valduga K, de Araújo BE, Silva FM. Low muscle mass is a predictor of malnutrition and prolonged hospital stay in patients with acute exacerbation of chronic obstructive pulmonary disease: a longitudinal study. J Parenter Enteral Nutr. 2021;45(6):1221–1230. doi:10.1002/jpen.1998.
- Pierik VD, Meskers CGM, Van Ancum JM, et al. High risk of malnutrition is associated with low muscle mass in older hospitalized patients—a prospective cohort study. BMC Geriatr. 2017;17(1):118. doi:10.1186/s12877-017-0505-5.
- Cohen Y, Zisberg A, Chayat Y, et al. Walking for better outcomes and recovery: the effect of WALK-FOR in preventing hospital-associated functional decline among older adults. J Gerontol A Biol Sci Med Sci. 2019;74(10):1664–1670. doi:10.1093/gerona/glz025.
- Pavon JM, Sloane RJ, Pieper CF, et al. Accelerometer-measured hospital physical activity and hospital-acquired disability in older adults. J Am Geriatr Soc. 2020;68(2):261–265. doi:10.1111/jgs.16231.
- Breen L, Stokes KA, Churchward-Venne TA, et al. Two weeks of reduced activity decreases leg lean mass and induces “anabolic resistance” of myofibrillar protein synthesis in healthy elderly. J Clin Endocrinol Metab. 2013;98(6):2604–2612. doi:10.1210/jc.2013-1502.
- Heesch KC, Hill RL, Aguilar-Farias N, van Uffelen JGZ, Pavey T. Validity of objective methods for measuring sedentary behaviour in older adults: a systematic review. Int J Behav Nutr Phys Act. 2018;15(1):119. doi:10.1186/s12966-018-0749-2.
- Nakanishi N, Tsutsumi R, Okayama Y, et al Monitoring of muscle mass in critically ill patients: comparison of ultrasound and two bioelectrical impedance analysis devices. J Intensive Care. 2019;7(1):61. doi:10.1186/s40560-019-0416-y.
- Fischer A, Spiegl M, Altmann K, et al. Muscle mass, strength and functional outcomes in critically ill patients after cardiothoracic surgery: does neuromuscular electrical stimulation help? The Catastim 2 randomized controlled trial. Crit Care. 2016;20(1):30. doi:10.1186/s13054-016-1199-3.
- Welch C, Majid Z, Andrews I, et al. Effect of position and exercise on measurement of muscle quantity and quality: towards a standardised pragmatic protocol for clinical practice. BMC Sports Sci Med Rehabil. 2021;13(1):3. doi:10.1186/s13102-020-00227-3.
- Valenzuela PL, Morales JS, Castillo-García A, et al. Effects of exercise interventions on the functional status of acutely hospitalised older adults: a systematic review and meta-analysis. Ageing Res Rev. 2020;61:101076. doi:10.1016/j.arr.2020.101076.
- Rus GE, Porter J, Brunton A, et al. Nutrition interventions implemented in hospital to lower risk of sarcopenia in older adults: A systematic review of randomised controlled trials. Nutr Diet. 2020;77(1):90–102. doi:10.1111/1747-0080.12608.
- Landi F, Cesari M, Calvani R, et al. The "Sarcopenia and Physical fRailty IN older people: multi-componenT Treatment strategies" (SPRINTT) randomized controlled trial: design and methods. Aging Clin Exp Res. 2017;29(1):89–100. doi:10.1007/s40520-016-0715-2.