Abstract
The objective of this article was to review the biochemical basis of the total antioxidant capacity (TAC) test and its applications to exercise science and promotion of healthy aging. Measurement of TAC allows evaluation of the antioxidant content of muscle, heart and other exercise-target organs. Acute non-regular physical exercise and body exposition to higher altitude have been associated with a considerable decrease of blood TAC. However, regular practice of physical activity and exercise is associated with improved antioxidant response increasing TAC levels. TAC methodologies did not address lipophylic antioxidants and have some other limitations. However, TAC is useful as a complementary test for the evaluation of antiperoxidation assays and blood, erythrocyte and cytosolic antioxidant enzyme assays (glutathion reductase, glutathion peroxidase, superoxide dismutase and catalase). Exercise induces both molecular antioxidant and hormetic responses which have been suggested to be linked with a healthy aging.
Introduction
The molecular participation of free radicals from oxygen, nitrogen and chlorine sources in cell pathogenesis has been strongly consolidated. At least 100 diseases are closely associated with oxidative and nitrosative stresses and its biochemical consequences – lipid peroxidation (measured by biomarkers such as malonaldehyde, MDA, and 4-hydroxynonenal, HNE and acrolein), protein peroxidation (measured by protein carbonyls), nucleic acid peroxidation (quantification of oxidized DNA bases), and carbohydrate oxidation products (measured by glycosilation products) (Ferrari Citation2000; Halliwell Citation2011; Niki Citation2011).
This molecular pathogenesis is based on an imbalance in favor of oxygen free radicals (or nitrogen and chlorine radicals) in relation to levels of the antioxidant molecules, resulting in oxidative stress (Halliwell Citation2011; Niki Citation2011), which is correlated to cell membrane damage, DNA damage and gene mutation, and triggers cell death by necrosis or apoptosis (Ferrari Citation2000; Gross et al. Citation2011).
Over many decades, a great number of research groups have been studying the biomarkers of oxidative stress and antioxidant activity. Antioxidant activity has been estimated by measuring antioxidant cell enzymes including superoxide dismutase (SOD), catalase (CAT), glutathion reductase (GSH), glutathion peroxidase (GPx), ceruloplasmin and metallothioneins (Ferrari Citation2000; Gross et al. Citation2011; Halliwell Citation2011).
Miller et al. Citation(1993) developed the first well-replicated antioxidant capacity test: total antioxidant capacity (TAC). The major advantage of this test is that it can measure the antioxidant capacity of virtually all components of a biological sample (blood, urine, feces), vegetable extracts, medicinal plants, beverages and food.
This article summarizes the antioxidant capacity tests and their applications for exercise sciences and healthy aging.
TAC tests
There are three major TAC tests (Rice-Evans Citation2000):
(1) Ferric reducing antioxidant power (FRAP), developed by Benzie and Strain Citation(1996), which has been widely used in food and nutritional studies – the essay is based on the reduction of the ferric to ferrous ion by adding a sample (plasma, urine, vegetable/fruit extract or food) with presumptive reducing activity. The samples are measured by spectrophotometry at 593 nm (Benzie and Strain Citation1996). | |||||
(2) Oxygen radical absorbance capacity (ORAC) created by Cao and Alessio Citation(1993) – this test is based on the capacity of the plasma constituents in scavenging peroxyl radicals formed by thermal decomposition of azo initiators, such as 2,2′-diazobis[2-diaminepropane hydrochloride (ABAP) and measurement of fluorescence decay 540 nm wavelength (excitation) and 565 nm (emission); | |||||
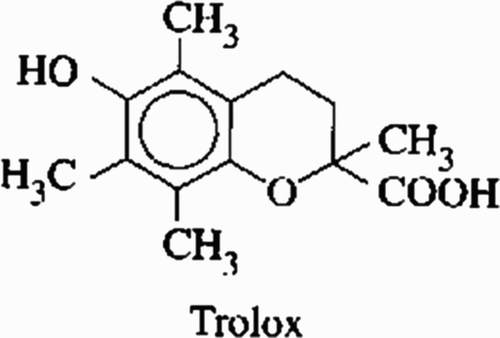
(3) Trolox-equivalent antioxidant capacity (TEAC), created by Miller et al. (2005), and transformed in diagnostic kit by Randox Laboratories (Crumlin, UK) – the test is based on ABTS+ radical formation [2,2′-azino-di-(3-ethylbenzothiazoline sulfonate) , with a green-bluish color, and its removal by sample compounds, e.g. the serum, pharmaceutical or foods, measured by spectrophotometry. | |||||
If the sample has antioxidants, its addition produces proportional inhibition of the ABTS+ radical decreasing absorbance at 600 nm. This absorbance is inversely associated with the antioxidant concentration in the sample which can be compared to the Trolox antioxidant (the standard), a hydrophilic vitamin E analog (). Results are expressed in mmol of trolox equivalents (TEq)/l (blood, liquids or fluids) or in μ mol TEq/100 g of sample (food or solid samples).
TAC can also be estimated by chemiluminescence methods. These methods are based on the time of induction of oxidation of a lipid dispersion exposed to the 2,2′-azo-bis (2-amidinepropane) (ABAP) in environmental temperature and the measurement of chemiluminescence intensity after sample incubation with luminol or luciferol (Whitehead et al. Citation1992; Said et al. Citation2003; Bastos et al. Citation2006).
Reference values for TAC
Although presents reference values for TAC, a very important recommendation is that each laboratory should maintain their own reference data, as gender, age, lifestyle variables, ethnic differences, genome diversity and other factors can change the antioxidant responses.
Table 1. Total antioxidant capacity of plasma or serum.
There are three important limitations to TAC tests: they did not measure the role of the important lipophylic antioxidants (vitamin E, fatty acids, carnosine) nor the activity of antioxidant enzyme systems (SOD, CAT, GSH), and they are very much influenced by uric acid levels. Because of its hydrophilic nature and reducing activity, uric acid is considered an important interfering factor in TAC evaluation (Benzie and Strain Citation1996; Rice-Evans Citation2000).
Total antioxidant capacity in exercise physiology studies
It is well established that acute or exhausting exercise induces oxidative and nitrosative stresses and subsequent damage to cell membranes, genome and organeles (Möller et al. Citation1996). Liu et al. Citation(1999) reported that marathon athletes presented increased susceptibility of low density lipoprotein (LDL) cholesterol to peroxidation. They also observed increased levels of both plasma total antioxidant capacity and uric acid, although a significant decrement in potent antioxidant blood thiols also occurred. It was demonstrated that after running a marathon there was a reduction in TAC as well as increased DNA oxidation from human lymphocytes (Briviba et al. Citation2005). Another interesting study pointed out improved TAC levels as well as DNA peroxidation in blood of athletes after an ultramarathon competition (Skenderi et al. Citation2008). In this regard, overtraining hampers tissue and organ adaptations to exercise, resulting in rising of oxidative stress levels and reduction of the TAC, a fact that was confirmed later in study of protocols of military acute physical training (Taskanen et al. Citation2010).
Beyond acute effects of exercise, chronic physical training has been also associated with TAC. Chronic swimming training induced blood TAC and GSH decay with augmentation of blood SOD levels (Aguilar-Silva et al. Citation2002). Reduction of TAC and increasing lipid peroxidation were also confirmed in athletes after an acute endurance testing (Caimi Citation2009). In rowing athletes, including kayaking, there was reduction of TAC concomitant with increasing lipid peroxidation and creatine kinase (Teixeira et al. Citation2009).
After an intensive chronic 18-day physical training, skiing athletes from French Federation submitted to higher altitudes could not recover their plasma TAC levels after two weeks of resting compared to the basal pre-training levels (Pialoux et al. Citation2010). Training at higher altitudes also decreased TAC of elite swimmers (Subudhi et al. Citation2004), confirming previous research which demonstrated increased oxidative stress at higher altitudes after nutritional supplementation with antioxidants (Heinicke et al. Citation2009; Castell et al. Citation2010). However, in high-fitness trained military marine men, the total antioxidant capacity remained elevated despite training at higher altitudes during the winter (Ji et al. Citation2009).
Regular physical exercise: adaptation to oxidative stress and induction of total antioxidant capacity
Regular practice of physical activity and exercise can improve TAC by modulating the synthesis of both enzymatic (SOD, CAT, GPX) and non-enzymatic (uric acid, albumin, ceruloplasmin, metallothioneins) cell antioxidants in skeletal muscles, liver, heart, brain and other organs, and can reduce lipid peroxidation, postprandial oxidative and nitrosative stresses and LDL cholesterol oxidation (Heitkamp et al. Citation2008; Neubauer et al. Citation2008; Bloomer et al. Citation2009; Heinicke et al. Citation2009; Ji et al. Citation2009; Castell et al. Citation2010). DNA damage can be reduced with regular physical exercise by enhancing expression of genome repairing enzymes on striatal skeletal muscles (Radák et al. Citation2003; Kim et al. Citation2010). The influence of physical exercise regularity on TAC concentrations was tested. After consecutive days of exercise training a progressive decreasing on oxidative stress was observed, whereas the TAC remained at higher levels (Sahlin et al. Citation2010). Although ultraendurance exercise had increased ROS production in isolated mitochondria this effect was abolished after 28 h and no mitochondrial protein damage was observed (Shing et al. Citation2007). Mitochondria of untrained muscles released sustained higher levels of reactive oxygen species, whereas progressive adaptation to exercise lowered the production of free radicals through abrogation of NFkB and activator protein-1 (AP-1) pathway (Brookes et al. Citation2008). Concurrently with the modulation of the antioxidant factors there is induction of other cytoprotective factors such as chaperones or heat shock proteins (HSPs), as well as the regulation of the nuclear kappa beta (NFkB) proliferating factor, increasing synthesis of the anti-inflammatory cytokine IL-10, and decreasing production of pro-inflammatory cytokines such as tumor necrosis factor-α (TNF-α), IL-1, IL-6, and further reduction of inflammatory lineages of monocytes (CD14+/CD16+) (Timmerman et al. Citation2008; Czarkowska-Paczek et al. Citation2009; Giraldo et al. Citation2010; Rahman et al. Citation2010; Ropelle et al. Citation2010) in response to physical exertion. In this sense, it was observed that skeletal muscle activation of nitric oxide synthesis is dependent on heat shock protein-90 (HSP90) expression (Harris et al. Citation2008). In fact, acute exercise increases inflammation, but chronic adaptation to physical exercise also leads to a positive anti-inflammatory response (Cunniffe et al. Citation2010). Physical training also induces antioxidant protection against radiation. A study pointed out that exercised rats exposed to gamma radiation produced more SOD (41%), Mn-SOD (51%), and the mitochondrial enzymes such as cytochrome c oxidase (38%), and citrate synthase (42%) when compared with sedentary radiation-exposed rats (De Lisio et al. Citation2011).
The exposure of healthy sedentary subjects to strenuous physical exercise has been associated with inhibition of macrophage migration due to TGF-β activation, and this inhibitory effect on migratory activities of macrophages was associated with increased total antioxidant capacity (Czepluch et al. Citation2011).
In another study, during 12 initial minutes of treadmill running plasma TAC increased by 36%, and the decayed by 59% after 26 minutes of exertion in comparison to the basal levels (de Souza et al. Citation2005). Although intense physical exercise increases free radical release, regular sports training can increase plasma total antioxidant capacity, as seen in two independent studies with soccer athletes (Brites et al. Citation1999; Mukherjee and Chia Citation2009). Regular practice of physical activities leads to tissue adaptation against free radicals. Even anaerobic exercise promotes adaptation to oxidative stress, increasing brain, heart and muscle TAC (Qiao et al. Citation2006). Thus, exercise induces enhancement of vascular TAC and decrement of endothelial oxidative stress (Suvorava and Kojda Citation2007). Regular physical activity and exercise increased by 78% to 83% the TAC of adolescent athletes (Carlsohn et al. Citation2008). After motocross running, increased lipid peroxidation was reported as well as rising of TAC levels, which were associated with increased uric acid levels (Ascensão et al. Citation2007). Similar results were obtained after mountain bike competitions in two independent research reports (Tauler et al. Citation2006; Martarelli et al. Citation2009). In a study with wrestler combat athletes, their physical training provoked an adaptation characterized by increased oxidative stress, but also increased TAC (Kürkçu et al. Citation2010).
It should be emphasized that TAC levels do not always change; depending on training intensity, frequency and timing, as well as other endogenous (genomic and phenotypic) and exogenous factors. A study with untrained subjects submitted to an aerobic training over eight weeks did not reporte increased TAC, but observed positive association between TAC and VO2max (Afzalpour et al. Citation2008). In the same manner, although there were studies demonstrating positive correlations between TAC and oxidative stress biomarkers, there were also many studies demonstrating inverse associations between these two parameters (Falone et al. Citation2009).
After the above-mentioned questions, it should be noted there are three important limitations to those TAC tests: they did not measure the role of the important lipophylic antioxidants (vitamin E, fatty acids, carnosine) nor the activity of antioxidant enzyme systems (SOD, CAT, GSH), and they are very much influenced by uric acid levels. Because of its hydrophilic nature and reducing activity, uric acid is considered an important interfering factor in TAC evaluation (Benzie and Strain Citation1996; Liu et al. Citation1999; Rice-Evans Citation2000; Neubauer et al. Citation2008).
Antioxidant capacity and healthy aging: does exercise matter?
Aging per se not always risk oxidative and nitrosative stresses. In fact, oxidative/nitrosative stresses in aging depend on age, gender, smoking, alcohol drinking, body composition, genome, and other intrinsic and extrinsic factors (Lesgards et al. Citation2002; Gutteridge and Halliwell Citation2010). However, since the 1950s a great number of studies have been reported increased oxidative/nitrosative stresses and decreased cell antioxidant defenses in aging animals and humans (Ferrari Citation2004; Barja Citation2005; Pamplona et al. Citation2005; da Silva and Ferrari Citation2011).
The increased oxidative/nitrosative stresses found in aging animals and humans have been linked to peroxidative damage to lipids, DNA, proteins and carbohydrates as well increased levels of SOD and CAT, and impairment of the total antioxidant capacity (İnal et al. Citation2001; Lasheras et al. Citation2002; Mutlu-Türkoǧlu et al. Citation2003; Rizvi and Maurya Citation2007; Dayhoff-Brannigan et al. Citation2008; Alexandrova and Bochev Citation2009). In fact, excessive production of hydrogen peroxide is very harmful to aging muscles. It has been suggested excessive release of H2O2 is cytotoxic to muscle cells and markedly increases mitochondrial disruption (Fan et al. Citation2010), and hydrogen peroxide overload triggers the expression of both autophagy and atrophy genes (McClung et al. Citation2010). In addition, until the age of 45 years, GSH redox system levels are maintained which do not occur in later life (Jones et al. Citation2002).
Further, for many elderly patients the presence of multiple pathologies is a common feature, especially overweight/obesity, hypertension, atherosclerosis, type 2 diabetes, chronic kidney disease, neurodegenerative disorders and the metabolic syndrome (Jones et al. Citation2002; Ferrari Citation2007; Qureshi and Parvez Citation2007; Morais et al. Citation2008; Tsirpanlis Citation2008; Thorin and Thorin-Trescases Citation2009). These diseases usually shorten the life span (Fontaine et al. Citation2003; Qureshi and Parvez Citation2007). It is important to note that those chronic non-transmissible diseases (atherosclerosis, metabolic syndrome, type 2 diabetes, Alzheimer's disease and Parkinson's disease) are frequently associated with increased production of oxygen and nitrogen free radicals and its reactive species, also including sarcopenia and physical fitness decay (Leiter and Lewanczuk Citation2005; Qureshi and Parvez Citation2007; Dayhoff-Brannigan et al. Citation2008; Schiffrin Citation2010; Noirez and Butler-Browne Citation2006). For example, among adult patients with metabolic syndrome higher levels of systemic inflammation and oxidative stress and lower TAC were demonstrated (On et al. Citation2005). A practice of regular exercise in the elderly leads to adaptation to oxidative and nitrosative stresses, decreasing mitochondrial release of free radicals and their reactive species, increasing TAC and cellular antioxidants such as GSH and nitric oxide, which in turn improve endothelial health and reduce systemic inflammation contributing to decreased risk of hypertension, type 2 diabetes, atherosclerosis and metabolic syndrome (Fatouros et al. Citation2004; Maeda et al. Citation2004; Suvorava and Kojda Citation2007; Seals et al. Citation2009; Gando et al. Citation2010; Newsholme et al. Citation2010; Teixeira-Lemos et al. Citation2011). Thus, regular exercise can afford an excellent maintenance of lower levels of oxidative stress with adequate levels of total antioxidants, promoting healthy aging. The molecular pathways of exercise regulation of TAC and its health benefits are discussed below.
TAC in aging and detraining: is the lack of exercise the origin of chronic diseases?
Aging is characterized by molecular and cellular changes in tissues and organs. In this sense, aging is associated with accumulation of lipid peroxidation products, and reduction of TAC into the heart, brain, liver and other target organs (Siqueira et al. Citation2005; Kaplan et al. Citation2007; Aydin et al. Citation2010; Terryn et al. Citation2011). Although aging has been associated with decreasing antioxidant capacity of the organism, dietary supplementation with higher antioxidant capacity plant foods did not enhance lifespan in Caenorhabditis elegans worms (Pun Citation2010). Cell aging is characterized by mitochondrial failure with ATP synthesis decay and increment of oxygen free radical production (Passarino et al. Citation2010; Osiewacz Citation2011). It is well established that the massive free radical production in aging cells and organisms has been closely associated with higher rates of both DNA and RNA mutation (Robert et al. Citation2010), confirming previous reports of an inverse association between DNA oxidation rates and longevity (Barja and Herrero et al. Citation2000). It also has been demonstrated that mtDNA mutation impaired oxidative phosphorylation, without further free radical enhancement, leading to apoptosis of skeletal muscle cells and sarcopenia (Hiona et al. Citation2010). By the same manner, detraining and sedentary behavior reduce mitochondrial biogenesis. Thus, the aging subject frequently has lower muscle mitochondrial density and many senescent mitochondria which are related to reduced vital capacity, decreased physical fitness and to an increased risk of chronic and metabolic diseases (Ferrari Citation2008; Figueiredo et al. Citation2008; Hiona et al. Citation2010; Jackson et al. Citation2010). Therein, positive associations have been observed between oxygen free radicals and both systolic and diastolic blood pressure values, whereas the association between ROS and arterial stiffness was only observed among hypertensive men (Kruger et al. Citation2012).
Beyond sedentary behavior, aging per se induces over-expression of NADPH oxidase (phagocyte oxidase) contributing to overreaching release of free radicals which, in turn, degrades nitric oxide cytosolic pools, causing impaired endothelium-dependent arterial dilation in old rats but not among young animals (Trott et al. Citation2011).
A very interesting experimental study announced that denervation-induced atrophy was associated with a 30-fold increasing on mitochondrial free radical production (Muller et al. Citation2007). In animal models of sarcopenia it has been suggested that both defective mitochondria and SOD deficiency in senescent skeletal muscle cells resulted in massive release of superoxide anion which decisively contributes to aging-related sarcopenia (Jackson Citation2009). In spite of this mitochondrial decay of aging with increased free radical production and decreased total antioxidant capacity, mitochondrial biogenesis is not impaired since exercised elderly subjects produce more skeletal muscle mitochondria than elderly sedentary individuals (Figueiredo et al. Citation2008).
Although the heat shock response was preserved, elderly people had decreased TAC which was associated with lower heat-shock protein synthesis compared with young subjects (Rao et al. Citation2003). One year of swimming training improved antioxidant enzymes of heart, liver, and lung of older rats compared to the older control (Günduz et al. Citation2004).
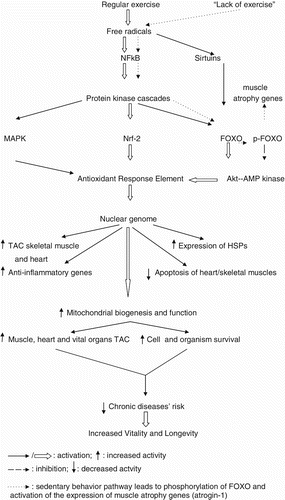
As discussed above, cells and tissues can react against exercise-induced free radicals by molecular pathways involving triggering NFkB, which in turn stimulates protein kinases that can activate other protein mitogen-activated kinases (MAPK), or the zinc-containing nuclear respiratory transcription factor-2 (Nrf-2) (George et al. Citation2008) or even activating the forkhead box proteins (FOXO) (Rattan and Ali Citation2007; Rattan and Demirovic Citation2010; Yin et al. Citation2010). In the absence of insulin and insulin-growth factor-1 (IGF-1), FOXO can trigger the kinase protein pathway, akt, which in turn activates the adenosine monophosphate activated protein kinase (AMP-kinase or AMPK) resulting in cell proliferation, survival, suppressing of tumors, and longevity (Greer and Brunet Citation2005; Greer et al. Citation2007). Chronic AMPK activation due to exercise improves muscle oxidative metabolism, increasing myogenic responses (Llubicic et al. Citation2011). The FOXO3A gene which encodes information for the synthesis of FOXO proteins has been considered a longevity gene in very different cell types (Anselmi et al. Citation2009; Rattan and Demirovic Citation2010). It should be noted that on contrast to other tissues and organs, in skeletal muscles akt is a survival pathway and FOXO proteins mediate myostatin-induced sarcopenia (Greer and Brunet Citation2005; Favier et al. Citation2008). Regular exercise activates akt which in turn downregulates FOXO phosphorylation, causing inhibition of the expression of muscle atrophy genes (Anselmi et al. Citation2009). Another cell longevity group factor is represented by sirtuins – the NAD+-dependent deacetylases (Blander and Guarente Citation2004; Yang et al. Citation2007). In response to oxidative stress insults, sirtuins activate FOXO transcription factors leading to decrease of free radical production (Wang et al. Citation2007). Endurance exercise has been associated with increased sirtuin levels and sirtuin activation in skeletal muscle cells of young and old mammals (Suwa et al. Citation2008; Koltai et al. Citation2010).
These transcription factors (MAPK, Nrf-2, akt, AMPK) stimulate the antioxidant response element (ARE) and other target genes in the cell nucleus, resulting in increased synthesis and release of antioxidants, anti-inflammatory factors, and other cytoprotective responses with massive production of HSPs, DNA-repair enzymes, nitric oxide, anti-inflammatory cytokines, and heme-oxygenase. In fact, MAPK signal transduction regulates expression of Nrf-2, and it has been suggested that aging cells have lower expression of Nfr-2 experiencing higher degree of oxidative stress (Shih and Yen Citation2007). As discussed above, exercise stimulates Nfr-2 and NFkB, improving antioxidant defenses in decreasing free radicals. It has been suggested aging-related sedentary lifestyle is associated with impaired function of the Nfr-2 signaling pathway and increased oxidative stress (Safdar et al. Citation2010). In fact, polymorphisms of the Nrf-2 encoding gene have been associated with endurance performance of athletes (He et al. Citation2007; Eynon et al. Citation2010). These adaptive responses to exercise and toxic insults are known as hormesis, a concept that increases cytoprotection and promotes longevity (Motta et al. Citation2004; Rattan Citation2008a). In this regard, any natural or synthetic compound which activates a stress response resulting in positive effects is known as hormetin (Rattan Citation2008b). So, exercise could also be considered a hormetin factor (Rattan et al. Citation2009). These molecular pathways of exercise-induced longevity are represented in . Regular practice of exercise or physical activity triggers activation of the antioxidant response element and other cytoprotective pathways (antioxidant, anti-inflammatory and anti-apoptotic pathways), whereas a lack of exercise induces NFkB-induction of FOXO phosphorylation and expression of skeletal muscle atrophy genes ().
Other total antioxidant capacity modulators
Many different physiological or pathological states result in oxidative stress response. Psychological stress, arterial hypertension, acute massive physical exercise and aging are intimately related to increased release of free radicals, as well as to decreasing on antioxidant defenses, and in a consequently reduction of the TAC (Liu et al. Citation1999; Briviba et al. Citation2005; Rattan Citation2008c; Czepluch et al. Citation2011; Gross et al. Citation2011; Halliwell Citation2011). Aging and detraining in rats were associated with reduction of muscle strength as well as TAC, which was partially recovered by resveratrol administration (Kashyap et al. Citation2005). Acute stress causes reduction of TAC but chronic exposure to stress can result in an adaptation characterized by improvement in plasma TAC (Torres et al. Citation2004). Psychological stress reduced plasma levels of TAC, an effect that was partially reverted by an eight week physical exercise program in rats (Rosety-Rodriguez et al. Citation2006).
Conclusion
Studies of exercise physiology can incorporate TAC evaluation before, during and after physical exercise; this strategy could be very useful to determine the real needs of antioxidant supplementation for athletes.
It should be emphasized that regular engagement in physical activities enhances TAC, reducing the oxidative stress (Shing et al. Citation2007; Brookes et al. Citation2008; Ji et al. Citation2009), being recommended for promotion of a healthy aging of the skeletal muscle and cardiovascular system (Seals et al. Citation2009).
References
- Afzalpour , M E , Gharakhanlou , R , Gaeini , A A , Mohebbi , H , Hedayati , M and Khazaei , M . 2008 . The effects of aerobic exercises on the serum oxidized LDL and total antioxidant capacity in non-active men . CVD Prev Contr. , 3 ( 2 ) : 77 – 82 .
- Aguilar-Silva , R H , Cintra , B B , Milani , S , Moraes , T P and Tsuji , H . 2002 . Estado antioxidante total do sangue como indicador da eficiência do treinamento em nadadores . Rev Brasil Ciênc Movim. , 10 : 7 – 11 .
- Alexandrova , M L and Bochev , P G . 2009 . Reduced extracellular phagocyte oxidase activity, antioxidant levels changes and increased oxidative damage in healthy human blood as a function of age . AGE. , 31 : 99 – 107 .
- Anselmi , C V , Malovini , A , Roncarati , R , Novelli , V , Villa , F , Condorelli , G , Bellazzi , R and Pucca , A A . 2009 . Association of the FOXO3A locus with extreme longevity in a southern Italian Centenarian Study . Rejuv Res. , 12 : 95 – 103 .
- Ascensão , A , Ferreira , R , Marques , F , Oliveira , E , Azevedo , V , Soares , J and Magalhães , J . 2007 . Effect of off-road competitive MotoCross race on plasma oxidative stress and damage markers . Brit J Sport Med. , 41 : 101 – 105 .
- Aydin , S , Atukeren , P , Çakatay , U , Uzun , H and Altuǧ , T . 2010 . Gender-dependent oxidative variations in liver of aged rats . Biogerontology. , 11 : 335 – 346 .
- Barja , G . 2005 . Radicales libres de origen mitocondrial y longevidad . An R Acad Nac Farm. , 71 : 783 – 798 .
- Barja , G and Herrero , A . 2000 . Oxidative damage to mitochondrial DNA is inversely related to maximum life span in the heart and brain of mammals . Faseb J. , 14 : 312 – 318 .
- Bastos , E L , Ciscato , LFM L , Bartoloni , F H , Catalani , L H , Romoff , P and Baader , W J . 2006 . Studies on PVP hydrogel-supported luminol chemiluminescence: 2. luminometer calibration and potential analytical applications . Luminescence. , 22 : 126 – 133 .
- Benzie , IF F and Strain , J J. 1996 . The reducing ability of plasma as a measure of antioxidant power – the FRAP assay . Anal Biochem , 239 : 70 – 76 .
- Blander , G and Guarente , L . 2004 . The Sir2 family of protein deacetylases . Annu Rev Biochem. , 73 : 417 – 435 .
- Bloomer , R J , Ferebee , D E , Fischer-Wellman , K H , Quindry , J C and Schilling , B K . 2009 . Postprandial oxidative stress: influence of sex and exercise training status . Med Sci Sports Exerc. , 41 ( 12 ) : 2111 – 2119 .
- Brites , F D , Evelson , P A , Christiansen , M G , Nicol , M F , Basílico , M J , Wikinski , R W and Llesuy , S F . 1999 . Soccer players under regular training show oxidative stress but an improved plasma antioxidant status . Clin Sci. , 96 : 381 – 385 .
- Briviba , K , Watzl , B , Nickel , K , Kulling , S , Bös , K , Haertel , S , Rechkemmer , G and Bub , A . 2005 . A half-marathon and a marathon run induce oxidative DNA damage, reduce antioxidant capacity to protect DNA against damage and modify immune function in hobby runners . Redox Rep. , 10 : 325 – 331 .
- Brookes , S V , Vasilaki , A , Larkin , L M , McArdle , A and Jackson , M . 2008 . Repeated bouts of aerobic exercise lead to reductions in skeletal muscle free radical generation and nuclear factor kB activation . J Physiol. , 586 : 3979 – 3990 .
- Caimi , G , Canino , B , Amodeo , G , Montana , M and Lo Presti , R . 2009 . Lipid peroxidation and total antioxidant status in unprofessional athletes before and after a cardiopulmonary test . Clinical Hemorheology and Microcircul. , 43 ( 3 ) : 233 – 239 .
- Cao , G and Alessio , H M . 1993 . Oxygen-radical absorbance capacity assay for antioxidants . Free Rad Biol Med. , 14 : 303 – 311 .
- Carlsohn , A , Rohn , S , Bittmann , F , Raila , J , Mayer , F and Schweigert , F J . 2008 . Exercise increases the plasma antioxidant capacity of adolescent athletes . Ann Nutr Metab. , 53 ( 2 ) : 96 – 103 .
- Castell , L M , Douglas Thake , C and Ensign , W . 2010 . Biochemical markers of possible immunodepression in military training in harsh environments . Mil Med. , 175 ( 3 ) : 158 – 165 .
- Child , R , Brown , S , Day , S , Donnelly , A , Roper , H and Saxton , J . 1999 . Changes in indices of antioxidant status, lipid peroxidation and inflammation in human skeletal muscle after eccentric muscle actions . Clin Sci. , 96 : 105 – 115 .
- Cunniffe , B , Hore , A J , Whitcombe , D M , Jones , K P , Baker , J S and Davies , B . 2010 . Time course of changes in immuneoendocrine markers following an international rugby game . Eur J Appl Physiol. , 108 ( 1 ) : 113 – 122 .
- Czarkowska-Paczek , B , Zendzian-Piotrowska , M , Bartlomiejczyk , I , Przybylski , J and Gorski , J . 2009 . The effect of acute and prolonged endurance exercise on transforming growth factor-beta1 generation in rat skeletal and heart muscle . J Physiol Pharmacol. , 60 ( 4 ) : 157 – 162 .
- Czepluch , F S , Barrès , R , Caidahl , K , Olieslagers , S , Krook , A , Rickenlund , A , Zierath , J R and Waltenberger , J . 2011 . Strenous physical exercise adversely affects monocyte chemotaxis . Thromb Haemost. , 105 : 122 – 130 .
- da Silva , WJ M and Ferrari , CK B . 2011 . Mitochondrial metabolism, free radicals and aging . Rev Bras Geriatr Gerontol. , 14 : 441 – 451 .
- Dayhoff-Brannigan , M , Ferrucci , L , Sun , K , Fried , L P , Walston , J , Varadhan , R , Guralnik , J M and Semba , R D . 2008 . Oxidative protein damage is associated with elevated serum interleukin-6 levels among older moderately to severely disabled women living in the community . J Gerontol A Biol Sci Med Sci. , 63 : 179 – 183 .
- De Lisio , M , Kaczor , J J , Phan , N , Tarnopolsky , M A , Boreham , D R and Parise , G. 2011 . Exercise training enhances the skeletal muscle response to radiation-induced oxidative stress . Muscle Nerve. , 43 ( 1 ) : 58 – 64 .
- de Souza , T P Jr , de Oliveira , P R and Pereira , B . 2005 . Efeitos do exercício físico intenso sobre a quimioluminescência urinária e malondialdeído plasmático . Rev Bras Med Esport. , 11 : 91 – 96 .
- Eynon , N , Alves , A J , Sagiv , M , Yamin , C , Sagiv , M and Meckel , Y . 2010 . Interaction between SNPs in the NRF2 gene and elite endurance performance . Physiol Gen. , 41 : 78 – 81 .
- Falone , S , Mirabilio , A , Passerini , A , Izzicupo , P , Cacchio , M , Baldassarre , A D and Amicarelli , F . 2009 . Aerobic performance and antioxidant protection in runners . Int J Sports Med. , 30 ( 11 ) : 782 – 788 .
- Fan , X , Hussien , R and Brooks , G A . 2010 . H2O2-induced mitochondrial fragmentation in C2C12 myocytes . Free Rad Biol Med. , 49 : 1646 – 1654 .
- Fatouros , I G , Jamurtas , A Z , Villiotou , V , Poulipoulou , S , Fotinakis , P , Taxildaris , K and Deliconstantinos , G. 2004 . Oxidative stress responses in older men during endurance training detraining . Med Sci Sport Exerc. , 36 : 2065 – 2072 .
- Favier , F B , Benoit , H and Freyssenet , D . 2008 . Cellular and molecular events controlling skeletal muscle mass in response to altered use . Pfluger Arch Eur J Physiol. , 456 : 587 – 600 .
- Ferrari , CK B . 2000 . Free radicals, lipid peroxidation and antioxidants in apoptosis: implications in cancer, cardiovascular and neurological diseases . Biologia. , 55 : 581 – 590 .
- Ferrari , C K . 2004 . Functional foods, herbs and nutraceuticals: towards biochemical mechanisms of healthy aging . Biogerontology. , 5 : 275 – 289 .
- Ferrari , CK B . 2007 . Functional foods and physical activities in health promotion of aging people . Maturitas. , 58 : 327 – 339 .
- Ferrari , CK B . 2008 . Metabolic syndrome and obesity: Epidemiology and prevention by physical activity and exercise . J Exerc Sci Fit. , 6 : 87 – 96 .
- Figueiredo , P A , Mota , M P , Appell , H J and Duarte , J A . 2008 . The role of mitochondria in aging of skeletal muscle . Biogerontology. , 9 : 67 – 84 .
- Fontaine , K R , Redden , D T , Wang , C , Westfall , A O and Allison , D B . 2003 . Years of life lost due to obesity . JAMA. , 289 : 187 – 193 .
- Gando , Y , Yamamoto , K , Murakami , H , Ohmori , Y , Kawakami , R , Sanada , K , Higuchi , M , Tabata , I and Miyachi , M . 2010 . Long time spent in light physical activity is associated with reduced arterial stiffness in older adults . Hypertension. , 56 : 540 – 546 .
- George , L , Asghar , M and Lokhandwala , M F . 2008 . Exercise stimulates transcription factors (Nrf2 & NFkB), increases antioxidant defenses, decreases oxidative stress, and restores renal dopamine D1 receptor function in aging . Faseb J. , 22 ( Suppl. ) : 1159.6
- Giraldo , E , Martin-Cordero , L , Garcia , J J , Gerhmann , M , Multhoff , G and Ortega , E . 2010 . Exercise-induced extracellular 72kDa heat shock protein (Hsp72) stimulates neutrophil phagocytic and fungicidal capacities via TLR-2 . Eur J Appl Physiol. , 108 ( 2 ) : 217 – 225 .
- Greer , E L and Brunet , A . 2005 . FOXO transcription factors at the interface between longevity and tumor suppression . Oncogene. , 24 : 7410 – 7425 .
- Greer , E L , Dowlatshahi , D , Banko , M R , Villen , J , Hoang , K , Blanchard , D , Gygi , S P and Brunet , A . 2007 . An AMPK-FOXO pathway mediates longevity induced by a novel method of dietary restriction in C. elegans . Curr Biol. , 17 ( 19 ) : 1646 – 1656 .
- Gross , M , Baum , O and Hoppeler , H . 2011 . Antioxidant supplementation and endurance training: win or loss? . Eu J Sport Sci. , 11 ( 1 ) : 27 – 32 .
- Günduz , F , Ṣentürk , U K , Kuru , O , Aktekin , B and Aktekin , M R . 2004 . The effect of one year's swimming exercise on oxidant stress and antioxidant capacity in aged rats . Physiol Res. , 53 : 171 – 176 .
- Gutteridge , J M and Halliwell , B . 2010 . Antioxidants: molecules, medicines, and myths . Biochem Biophys Res Commun. , 393 : 561 – 564 .
- Halliwell , B . 2011 . Free radicals and antioxidants – quo vadis . Trend Pharmacol Sci. , 32 ( 3 ) : 125 – 130 .
- Harris , M B , Mitchell , B M , Sood , S G , Webb , R C and Venema , R C . 2008 . Increased nitric oxide synthase activity and Hsp90 association in skeletal muscle following chronic exercise . Eur J Appl Physiol. , 104 : 795 – 802 .
- He , Z , Hu , Y , Feng , L , Lu , Y , Liu , G , Xi , Y , Wen , L and McNaughton , L R. 2007 . NRF2 genotype improves endurance capacity in response to training . Int J Sport Med. , 28 : 717 – 721 .
- Heinicke , I , Boehler , A , Rechsteiner , T , Bogdanova , A , Jelkmann , W , Hofer , M , Rawlings , P , Araneda , O F , Behn , C , Gassmann , M and Heinicke , K . 2009 . Moderate altitude but not additional endurance training increases markers of oxidative stress in exhaled breath condensate . Eur J Appl Physiol. , 106 ( 4 ) : 599 – 604 .
- Heitkamp , H C , Wegler , S , Brehme , U and Heinle , H . 2008 . Effect of an 8-week endurance training program on markers of antioxidant capacity in women . J Sports Med Phys Fitness. , 48 ( 1 ) : 113 – 119 .
- Hiona , A , Sanz , A , Kujoth , G C , Pamplona , R , Seo , A Y , Hofer , T , Someya , S , Miyakawa , T , Nakayama , C , Samhan-Arias , A K , Servais , S , Barger , J L , Portero-Otín , M , Tanokura , M , Prolla , T A and Leeuwenburgh , C . 2010 . Mitochondrial DNA mutations induce mitochondrial dysfunction, apoptosis, and sacopenia in skeletal muscle of mitochondrial DNA mutator mice . PLoS One. , 5 ( 7 ) : e11468 doi: 10.1371/Journal.pone.0011468
- İnal , M E , Kanbak , G and Sunal , E. 2001 . Antioxidant enzyme activities and malondialdehyde levels related to aging . Clin Chim Acta. , 305 : 75 – 80 .
- Jackson , J R , Ryan , M J , Hao , Y and Alway , S E . 2010 . Mediation of endogenous antioxidant enzymes and apoptotic signaling by resveratrol following muscle disuse in the gastrocnemius muscles of young and old rats . Am J Physiol Regul Integr Comp Physiol. , 299 ( 6 ) : R1572 – R1581 .
- Jackson , M J . 2009 . Skeletal muscle aging: role of reactive oxygen species . Crit Care Med. , 37 ( 10 Suppl. ) : S368 – S371 .
- Ji , L L , Gomez-Cabrera , M C and Viña , J. 2009 . Role of free radicals and antioxidant signaling in skeletal muscle health and pathology . Infect Disord Drug Targets. , 9 ( 4 ) : 428 – 444 .
- Jones , D P , Mody Jr , V C , Carlson , J L , Lynn , M J and Sternberg Jr , P . 2002 . Redox analysis of human plasma allows separation of pro-oxidant events of aging from decline in antioxidant defense . Free Rad Biol Med. , 33 : 1290 – 1300 .
- Kaplan , P , Sivonova , M , Tatarkova , Z , Babusikova , E , Lehotsky , J and Dobrota , D . 2007 . Total antioxidant capacity and oxidative damage to proteins and lipids in aged rat heart . J Mol Cel Cardiol. , 42 ( 6 Suppl.1 ) : S117 – S118 .
- Kashyap , M K , Yadav , V , Sherawat , B S , Jain , S , Kumari , S , Khullar , M , Sharma , P C and Nath , R . 2005 . Different antioxidant status, total antioxidant power and free radicals in essential hypertension . Mol Cell Biochem. , 277 : 89 – 99 .
- Kim , K S , Paik , I Y , Woo , J H and Kang , B Y . 2010 . The effect of training type on oxidative DNA damage and antioxidant capacity during three-dimensional space exercise . Med Princ Pract. , 19 ( 2 ) : 133 – 141 .
- Koltai , E , Szabo , Z , Atalay , M , Boldogh , I , Naito , H , Goto , S , Nyakas , C and Radak , Z . 2010 . Exercise alters SIRT1, SIRT6, NAD and NAMPT levels in skeletal muscle of aged rats . Mech Aging Develop. , 131 : 21 – 28 .
- Kruger , R , Schutte , R , Huisman , H W , Van Rooyen , J M , Malan , N T , Fourie , CM T , van der Westhuizen , F H , van Deventer , C A , Malan , L and Schutte , A E . 2012 . Associations between reactive oxygen species, blood pressure and arterial stiffness in Black South Africans: the SABPA study . J Hum Hypertens. , 26 : 91 – 97 .
- Kürkçu , R , Tekin , A , Özdag , S and Akçakoyun , F . 2010 . The effects of regular exercise on oxidative and antioxidative parameters in young wrestlers . Afr J Pharmac Pharmacol. , 4 : 244 – 251 .
- Lasheras , C , Huerta , J M , Gonzalez , S , Braña , A F , Patterson , A M and Fernandez , S . 2002 . Independent and interactive association of blood antioxidants and oxidative damage in elderly people . Free Rad Res. , 36 : 875 – 882 .
- Leiter , L A and Lewanczuk , R Z . 2005 . Of the renin-angiotensin system and reactive oxygen species type 2 diabetes and angiotensin II inhibition . Am J Hypertens. , 18 : 121 – 128 .
- Lesgards , J-F , Durand , P , Lassarre , M , Stocker , P , Lesgards , G , Lanteaume , A , Prost , M and Lehucher-Michel , M-P . 2002 . Assessment of lifestyle effects on the overall antioxidant capacity of healthy subjects . Environm Health Perspect. , 110 : 479 – 486 .
- Liu , M-L , Bergholm , R , Mäkimattila , S , Lahdenperä , S , Valkonen , M , Hilden , H , Yki-Järvinen , H and Taskinen , M-R . 1999 . A marathon run increases the susceptibility of LDL to oxidation in vitro and modifies plasma antioxidants . Am J Physiol. , 276 : E1083 – E1091 .
- Llubicic , V , Miura , P , Burt , M , Boudreault , L , Khogali , S , Lunde , J A , Renaud , J M and Jasmin , B J . 2011 . Chronic AMPK activation evokes the slow, oxidative myogenic program and triggers beneficial adaptations in mdx mouse skeletal muscle . Hum Mol Genet. , 20 : 3478 – 3493 .
- Maeda , S , Tanabe , T , Otsuki , T , Sugawara , J , Iemitsu , M , Miyauchi , T , Kuno , S , Ajisaka , R and Matsuda , M . 2004 . Moderate regular exercise increases basal production of nitric oxide in elderly women . Hypertens Res. , 27 : 947 – 953 .
- Martarelli , D and Pompei , P . 2009 . Oxidative stress and antioxidant changes during a 24-hours mountain bike endurance exercise in master elite athletes . J Sport Med Phys Fitness. , 49 ( 1 ) : 122 – 127 .
- McClung , J M , Judge , A R , Powers , S K and Yan , Z . 2010 . p38 MAPK links oxidative stress to autophagy-related gene expression in cachetic muscle wasting . Am J Physiol Cell Physiol. , 298 : C542 – C549 .
- Miller , N J , Rice-Evans , C , Davies , M J , Gopinathan , V and Milner , A . 1993 . A novel method for measuring antioxidant capacity and its application to monitoring the antioxidant status in premature neonates . Clin Sci. , 84 : 407 – 412 .
- Möller , P , Wallin , H and Knudsen , L E . 1996 . Oxidative stress associated with exercise, psychological stress and life-style factors . Chem-Biol Inter. , 102 : 17 – 36 .
- Morais , T C , Fujimori , M , Toledo , O R , Batalini , C , França , E L , Ferrari , CK B and Honorio-França , A C . 2008 . Pharmacoepidemiology and health in a Brazilian older population . Int J Gerontol. , 2 : 103 – 108 .
- Motta , M C , Divecha , N , Lemieux , M , Kamel , C , Chen , D , Gu , W , Bultsma , Y , McBurney , M and Guarante , L . 2004 . Mammalian SIRT1 represses Forkhead transcription factors . Cell. , 116 : 551 – 563 .
- Mukherjee , S and Chia , M . 2009 . Urinary total antioxidant capacity in soccer players . Acta Kinesiologica. , 3 ( 1 ) : 26 – 33 .
- Muller , F L , Song , W , Jang , Y C , Liu , Y , Sabia , M , Richardson , A and Van Remmen , H . 2007 . Denervation-induced skeletal muscle atrophy is associated with increased mitochondrial ROS production . Am J Physiol Regu Physiol. , 293 ( 3 ) : R1159 – R1168 .
- Mutlu-Türkoǧlu , Ü , İlhan , E , Öztecan , S , Kuru , A , Aykaç-Toker , G and Uysal , M . 2003 . Age-related increases in plasma malondialdehyde and protein carbonyl levels and lymphocyte DNA damage in elderly subjects . Clin Biochem. , 36 : 397 – 400 .
- Neubauer , O , König , D , Kern , N , Nics , L and Wagner , K H . 2008 . No indications of persistent oxidative stress in response to an ironman triathlon . Med Sci Sports Exerc. , 40 ( 12 ) : 2119 – 2128 .
- Newsholme , P , Homem de Bittencourt Jr , P I , Hagan , C O , De Vito , G , Murphy , C and Krause , M S . 2010 . Exercise and possible molecular mechanisms of protection from vascular disease and diabetes: the central role of ROS and nitric oxide . Clin Sci. , 118 : 341 – 349 .
- Niki , E . 2011 . Do free radicals play causal role in atherosclerosis? Low density lipoprotein and vitamin E revisited . J Clin Biochem Nutr. , 48 ( 1 ) : 3 – 7 .
- Noirez , P and Butler-Browne , G . 2006 . “ Slowing down age-related muscle loss and sarcopenia ” . In Prevention and treatment of age-related diseases , 1 , Edited by: Rattan , SI S and Kassem , M . 71 – 85 . Dordrecht : Springer .
- On , Y K , Park , R , Hyon , M S , Kim , S K and Kwon , Y J. 2005 . Are low total antioxidant status and elevated levels of C-reactive protein and monocyte chemotactic protein-1 associated with cardiac syndrome X? . Circ J. , 69 : 1212 – 1217 .
- Osiewacz , H D . 2011 . Regulation of the mitochondrial transition pore: impact on mammalian aging . Aging. , 3 ( 1 ) : 10 – 11 .
- Pamplona , R , Portero-Otín , M , Sanz , A , Ayala , V , Vasileva , E and Barja , G . 2005 . Protein and lipid oxidative damage and complex I content are lower in the brain of budgerigar and canaries than in mice. Relation to aging rate . AGE. , 27 : 267 – 280 .
- Passarino , G , Rose , G and Bellizzi , D . 2010 . Mitochondrial function, mitochondrial DNA and ageing: a reappraisal . Biogerontology. , 11 : 575 – 588 .
- Pialoux , V , Brugniaux , J V , Rock , E , Mazur , A , Schmitt , L , Richalet , J- P , Robach , P , Clottes , E , Coudert , J , Fellmann , N and Mounier , R . 2010 . Antioxidant status of elite athletes remains impaired 2 weeks after a simulated altitude training camp . Eur J Nutr. , 49 : 285 – 292 .
- Pun , PB L , Gruber , J , Tang , S Y , Schaffer , S , Ong , RL S , Fong , S , Ng , L F , Cheah , I and Halliwell , B . 2010 . Ageing in nematodes: do antioxidant extend lifespan in Caenorhabditis elegans? . Biogerontology. , 11 : 17 – 30 .
- Qiao , D , Hou , L and Liu , X . 2006 . Influence of intermittent anaerobic exercise on muose physical endurance and antioxidant components . Brit J Sport Med. , 40 ( 3 ) : 214 – 218 .
- Qureshi , G A and Parvez , S H . 2007 . Oxidative damage and neurodegenerative disorders , 1 , Amsterdam : Elsevier .
- Radák , Z , Apor , P , Pucsok , J , Berkes , I , Ogonovszky , H , Pavlik , G , Nakamoto , H and Goto , S . 2003 . Marathon running alters the DNA base excision repair in human skeletal muscle . Life Sci. , 72 : 1627 – 1633 .
- Rahman , Z A , Abdullah , N , Singh , R and Sosroseno , W . 2010 . Effect of acute exercise on the levels of salivary cortisol, tumor necrosis factor-alpha and nitric oxide . J Oral Sci. , 52 ( 1 ) : 133 – 136 .
- Rao , D V , Boyle , G M , Parsons , P G , Watson , K and Jones , G L . 2003 . Influence of aging, heat shock treatment and in vivo total antioxidant status on gene-expression profile and protein synthesis in human peripheral lymphocytes . Mech Ageing Develop. , 124 : 55 – 69 .
- Rattan , SI S . 2008a . Hormesis in aging . Ageing Res Rev. , 7 : 63 – 78 .
- Rattan , SI S . 2008b . Hormetic interventions in aging . Am J Pharmacol Toxicol. , 3 : 30 – 43 .
- Rattan , SI S . 2008c . Principles and practice of hormetic treatment of aging and age-related diseases . Human Exp Toxicol. , 27 : 151 – 154 .
- Rattan , SI S and Ali , R E . 2007 . Hormetic prevention of molecular damage during cellular aging of human skin fibroblasts and keratinocytes . Ann NY Acad Sci. , 1100 : 424 – 430 .
- Rattan , SI S and Demirovic , D . 2010 . Hormesis can and does work in humans . Dose-Response. , 8 ( 1 ) : 58 – 63 .
- Rattan , SI S , Fernandes , R A , Demirovic , D , Dymek , B and Lima , C F . 2009 . Heat stress and hormetin-induced hormesis in human cells: effects on aging, wound healing, angiogenesis, and differentiation . Dose Response. , 7 : 90 – 103 .
- Rice-Evans , C . 2000 . Measurement of Total Antioxidant Activity as a marker of antioxidant status in vivo: procedures and limitations . Free Rad Res. , 33 : S59 – S66 .
- Rizvi , S I and Maurya , P K . 2007 . Alterations in antioxidant enzymes during aging in humans . Mol Biotechnol. , 37 : 58 – 61 .
- Robert , L , Labat-Robert , J and Robert , A M . 2010 . Genetic, epigenetic and posttranslational mechanisms of aging . Biogerontology. , 11 : 387 – 399 .
- Ropelle , E R , Flores , M B , Cintra , D E , Rocha , G Z , Pauli , J R , Morari , J , de Souza , C T , Moraes , J C , Prada , P O , Guadagnini , D , Marin , R M , Oliveira , A G , Augusto , T M , Carvalho , H F , Velloso , L A , Saad , M J and Carvalheira , J B . 2010 . IL-6 and IL-10 anti-inflammatory activity links exercise to hypothalamic insulin and leptin sensitivity through IKKbeta and ER stress inhibition . PLoS Biol , 8 ( 8 ) : e1000465 doi:10.1371/Journal.pbio.1000465
- Rosety-Rodriguez , M , Ordonez , F J and Rosety , M . 2006 . Eight week moderate exercise training program increases plasmatic total antioxidant status in rats stressed by immobilization . Haema. , 9 : 124 – 126 .
- Safdar , A , deBeer , J and Tarnopolsky , M A . 2010 . Dysfunctional Nrf-2-keap1 redox signaling in skeletal muscle of the sedentary old . Free Rad Biol Med. , 49 : 1487 – 1493 .
- Sahlin , K , Shabalina , I G , Mattson , C M , Bakkman , L , Fernström , M , Rozhdestvenskaya , Z , Enqvist , J K , Nedergaard , J , Ekblom , B and Tonkonogi , M . 2010 . Ultraendurance exercise increases the production of reactive oxygen species in isolated mitochondria from human skeletal muscle . J Appl Physiol. , 108 ( 4 ) : 780 – 787 .
- Said , T M , Kattal , N , Sharma , R K , Sikka , S C , Thomas Jr , A J , Mascha , E and Agarwal , A . 2003 . Enhanced chemiluminescence assay vs colorimetric assay for measurement of the total antioxidant capacity of human seminal plasma . J Androl. , 24 : 676 – 680 .
- Schiffrin , E L . 2010 . Antioxidants in hypertension and cardiovascular disease . Mol Interv. , 10 : 354 – 362 .
- Seals , D R , Walker , A E , Pierce , G L and Lesniewski , L A . 2009 . Habitual exercise and vascular ageing . J Physiol. , 587 ( 23 ) : 5541 – 5549 .
- Shih , P-H and Yen , G-C . 2007 . Differential expressions of antioxidant status in aging rats: the role of transcription factor Nrf2 and MAPK signaling pathway . Biogerontology. , 8 : 71 – 80 .
- Shing , C M , Peake , J M , Ahern , S M , Strobel , N A , Wilson , G , Jenkins , D G and Coombes , J S . 2007 . The effect of consecutive days of exercise on markers of oxidative stress . Appl Physiol Metab. , 32 ( 4 ) : 677 – 685 .
- Siqueira , I R , Fochesatto , C , Andrade , A de , Santos , M , Hagen , M , Bello-Klein , A and Netto , C A . 2005 . Total antioxidant capacity is impaired in different structures from aged rat brain . Int J Develop Neurosci. , 23 ( 8 ) : 663 – 671 .
- Skenderi , K P , Tsironi , M , Lazaropoulou , C , Anastasiou , C A , Matalas , C- A , Kanavaki , I , Thalmann , M , Goussetis , E , Papassotiriou , I and Chrousos , G P . 2008 . Changes in free radical generation and antioxidant capacity during ultramarathon foot race . Eur J Clin Invest. , 38 ( 3 ) : 159 – 165 .
- Subudhi , A W , Jacobs , K A , Hagobian , T A , Fattor , J A , Fulco , C S , Muza , S R , Rock , P B , Hoffman , A R , Cymerman , A and Friedlander , A L . 2004 . Antioxidant supplementation does not attenuate oxidative stress at high altitude . Aviat Space Environm Med. , 75 ( 10 ) : 881 – 888 .
- Suvorava , T and Kojda , G . 2007 . Prevention of transient endothelial dysfunction in acute exercise: a friendly fire? . Thromb Haemost. , 97 : 331 – 333 .
- Suwa , M , Nakano , H , Radak , Z and Kumagai , S . 2008 . Endurance exercise increases the SIRT1 and peroxisome proliferator-activated receptor γ coativator-1α protein expressions in rat skeletal muscle . Metabol Clin Exp. , 57 : 986 – 998 .
- Taskanen , M , Atalay , M and Uusitalo , A . 2010 . Altered oxidative stress in overtrained athletes . J Sport Sci. , 12 ( 1 ) : 1 – 9 .
- Tauler , P , Sureda , A , Cases , N , Aguiló , A , Rodríguez-Marroyo , J A , Villa , G , Tur , J A and Pons , A . 2006 . Increased lymphocyte antioxidant defenses in response to exhaustive exercise do not prevent oxidative damage . J Nutr Biochem. , 17 ( 10 ) : 665 – 671 .
- Teixeira , V , Valente , H , Casal , S , Marques , F and Moreira , P . 2009 . Antioxidant status, oxidative stress, and damage in elite trained kayakers and canoeists and sedentary controls . Int J Sport Nutr Exerc Metab. , 19 ( 5 ) : 443 – 456 .
- Teixeira-Lemos , E , Nunes , S , Teixeira , F and Reis , F . 2011 . Regular physical exercise training assists in preventing type 2 diabetes development: focus on its antioxidant and anti-inflammatory properties . Cardiovasc Diabetol. , 10 : 12
- Terryn , S and Devuyst , O . 2011 . “ Oxidative stress in the kidney: proximal tubule disorders ” . In Studies on renal disorders , Edited by: Miyata , T , Eckardt , K-U and Nangaku , M . 179 – 203 . Dordrecht : Springer .
- Thorin , E and Thorin-Trescases , N . 2009 . Vascular endothelial ageing, heartbeat after heartbeat . Cardiovasc Res. , 84 : 24 – 32 .
- Timmerman , K L , Flynn , M G , Coen , P M , Markofski , M M and Pence , B D . 2008 . Exercise training-induced lowering of inflammatory (CD14+CD16+) monocytes: a role in the anti-inflammatory influence of exercise? . J Leukoc Biol. , 84 ( 5 ) : 1271 – 1278 .
- Torres , R L , Torres , IL S , Gamaro , G D , Fontella , F U , Silveira , P P , Moreira , JS R , Lacerda , M , Amoretti , J R , Rech , D , Dalmaz , C and Belló , A A . 2004 . Lipid peroxidation and total radical-trapping potential of the lungs of rats submitted to chronic and sub-chronic stress . Braz J Med Biol Res. , 37 : 185 – 192 .
- Trott , D W , Seawright , J W , Luttrell , M J and Woodman , C R . 2011 . NAD(P)H oxidase-derived reactive oxygen species contribute to age-related impairments of endothelium-dependent dilation in rat soleus feed arteries . J Appl Physiol. , 110 : 1171 – 1180 .
- Tsirpanlis , G . 2008 . Cellular senescence, cardiovascular risk, and CKD: a review of the established and hypothetical interconnections . Am J Kidney Dis. , 51 : 131 – 144 .
- Wang , F , Nguyen , M , Qin , FX-F and Tong , Q . 2007 . SIRT2 deacetylates FOXO3a in response to oxidative stress and caloric restriction . Aging Cell. , 6 : 505 – 514 .
- Whitehead , T P , Thorpe , GH G and Maxwell , SR J . 1992 . Enhanced chemiluminescent assay for antioxidant capacity in biological fluids . Anal Chim Acta. , 266 : 265 – 277 .
- Yang , H , Baur , J A , Chen , A , Miller , C and Sinclair , D A . 2007 . Design and synthesis of compounds that extend yeast replicative lifespan . Aging Cell. , 6 : 35 – 43 .
- Yin , F , Liu , J , Zheng , X , Guo , L and Xiao , H . 2010 . Geniposide induces the expression of heme oxygenase-1 via PI3K/Nrf-2-signaling to enhance the antioxidant capacity in primary hippocampal neurons . Biol Pharm Bull. , 33 : 1841 – 1846 .