Abstract
The antioxidant activity, phenolic contents and phytochemical profile of acetone, methanol, water and ethyl acetate extracts of the stem bark of B. monosperma were investigated. Extracts showed highest antioxidant activity at 100 μg ml−1 with application of different assays: 2′-2′ diphenyl–2′ picrylhydrazyl (DPPH) scavenging (83.5% and 78.1%), reducing power (1.85 and 1.78), deoxyribose degradation site-specific (65.5% and 61.5%) and non-site-specific (67.1% and 66.5%) and chelating power (63.4% and 58.9%) in decreasing and increasing polarity of acetone extracts, respectively. However, nitroblue tetrazolium (NBT) showed scavenging of O2• radicals by 56.4% and 53.1% in decreasing and increasing polarity methanol extracts, respectively. Similarly, electron paramagnetic resonance (EPR)/spin-trapping exhibited highest radical scavenging activity with acetone extracts (12.6 mg g−1 Trolox). The results pointed to the significant antioxidant activities in acetone and methanol extracts. In most cases, the extracts obtained through decreasing polarity showed higher antioxidant activity. The phenolic content exhibited a strong association (r 2>0.9) with antioxidant activity. These results suggest that bark of B. monosperma can be a potential source of natural antioxidants.
Introduction
Drugs from plant origin are relied upon by 80% of the world's population (World Health Organization 2008). In India, the use of herbal drugs is an important component of the traditional system of medicine. In the past decade, considerable attention has been paid to the improvement of human health by consumption of functional food and herbal products. Many plants synthesize substances including phenolic compounds that are useful in the maintenance of good health in humans and animals. There is much interest in the study of phenolic compounds because of their antioxidant, anti-inflammatory, antimicrobial and anti cancerous activity (Jiménez et al. Citation2010; Pandey and Mishra Citation2010).
Free radicals are known be the product of normal metabolism. However, when oxygen is supplied in excess or its reduction is insufficient, reactive oxygen species (ROS) such as superoxide anions, hydroxyl radicals, singlet oxygen and hydrogen peroxide are generated (Aruoma Citation1999) that can cause cellular injuries and initiate peroxidation of polyunsaturated fatty acids in biological membranes. ROS may also cause tissue injury leading to DNA strand breakage, protein damage and oxidation of important enzymes in the human body. These events could consequently lead to free-radical related several chronic and degenerative diseases such as carcinogenesis, malaria, heart diseases, arteriosclerosis, diabetes and many other ailments related to ageing (Halliwell and Gutteridge Citation1984; Honda et al. Citation2004).
It is well known that the majority of plants are an excellent source of phytochemicals such as phenolic and polyphenolic compounds (e.g. phenolic acids, flavonoids, tannins, etc.), many of which have potent antioxidant activities which can be exploited in the preparation of food and pharmaceutical products (Liu et al. Citation2008; Li et al. Citation2009). Phenolic compounds exert their protective effect mainly because of their redox properties, which allow them to act as reducing agents, hydrogen donators and singlet oxygen quenchers. Earlier studies have shown that phenolic compounds can be beneficial as prophylactic agents owing to their antibacterial, anticancer, anti-inflammatory, anti-viral, anti-allergic and immune-stimulating activities (Liu et al. Citation2008; Viuda-Martos et al. Citation2008). Nevertheless, all aerobic organisms, including humans, have antioxidant defences that protect against oxidative harm and repair damaged molecules. However, as natural antioxidant mechanisms can be inadequate, the supply of antioxidants through dietary ingredients is of great interest for a healthy life. A number of plants have been documented for their health promoting benefits (Scalbert and Williamson 2000). The use of these plant materials as a source of natural antioxidants and for other applications is important not only for food safety reasons, but also because they are natural and are more readily acceptable to consumers.
Butea monosperma (Lam.) Kuntze, also known as flame of the forest, is found in greater parts of India, Burma and Sri Lanka and belongs to the family Fabaceae. It is a sacred deciduous tree, attaining a height of 12–15 m. It is reported to have excellent medicinal properties. B. monosperma flowers are reported to contain butrin, isobutrin, butein, butin, flavonoids, glycosides and steroids. It has been dubbed a wonder drug and is reported to possess chemopreventive and anticancer (Choedon et al. Citation2010), anti-hepatotoxic (Sharma and Shukla Citation2011), and antidiabetic (Somani et al. Citation2006) properties. The bark exudes a red juice that dries to form Butea gum. Roots of B. monosperma are useful in the treatment of filariasis, night blindness, helminthiasis, piles, ulcers and tumours. Pippali rasayana, an Indian ayurvedic drug, employs B. monosperma and is used in the management of giardiasis (Aggarwal et al. Citation1997). Its gum contains leucocyanidin tetramers, procyanidin and gallic acid which are reported to possess anti-implantation, anti-ovulatory activity and is useful in elephantiasis and in curing night blindness and other eyesight defects. The bark is reported to possess astringent, pungent, alliterative, aphrodisiac and antihelminthic properties. It is also useful in tumours, bleeding piles, gonorrhoea and ulcers (Burli and Khade Citation2007). Thus, most of these studies reported a good pharmaceutical potential of B. monosperma. However, to the best of our knowledge, antioxidant studies on the bark of this plant and their correlation with phenolics are scarce. Therefore, the objectives of the present investigation were to evaluate the antioxidant activity of various extracts/fractions of the bark of B. monosperma by employing a number of antioxidant assays and solvent systems in addition to provide data on total phenolics and phytochemical profile of the extracts.
Materials and methods
Chemicals and plant material
2′-2′ Diphenyl–2′ picrylhydrazyl (DPPH), 2–thiobarbituric acid (2–TBA), deoxyribose, ferrozine, potassium ferricyanide, ferric chloride, EDTA, hydrogen peroxide, L-ascorbic acid, sodium hydroxide, trichloroacetic acid, gallic acid, sodium carbonate, butylated hydroxyanisole (BHA) butylated hydroxytoluene (BHT) and chemicals for phytochemical screening were of analytical grade and obtained from Hi-media Pvt. Ltd., Mumbai, India. Other chemicals, namely Folin Ciocalteu reagent and organic solvents were obtained from Fischer Scientific, Mumbai.
Stem bark of Butea monosperma (Lam.) Kuntze. was obtained from a tree growing in a locality in Sirsa town, Haryana, India in the month of February. Its bark is hard, fissured and ash coloured. It was washed thoroughly initially with tap water and then with distilled water to remove any debris or dust particles and allowed to dry in an oven below 40°C for 24 h. The dried bark was ground to powder and stored in airtight containers until used for further studies.
Preparation of extracts
For preparation of extracts two methods were followed based on solvent polarity. In increasing order of solvent polarity, extraction was initiated with a least polar solvent, i.e. hexane, which was followed by chloroform → ethyl acetate → acetone → methanol → water. In decreasing order of solvent polarity, the extraction was started with most polar solvent (water), followed by methanol → acetone → ethyl acetate → chloroform → hexane. In both methods, 500 g of bark powder was soaked with 1500 ml of each solvent for 24 h at room temperature. After recovering the supernatant, the respective solvents were added twice to the residue. All the three supernatants recovered from each solvent were combined and the solvents were eliminated using a rotary vacuum evaporator (Buchi type) to obtain a dry extract. The ethyl acetate extract was further partitioned into water fraction (WF) and ethyl acetate fraction (EAF). All extracts were stored at 4°C until use. Extractive value was calculated as follows:
Determination of total phenolic content
The total phenolic content (TPC) of the extracts was determined using Folin–Ciocalteu reagent following Kujala et al. Citation(2000). In this method, briefly, to 100 μl of extract (100 μg dry extract per ml solvent) were added 500 μl of appropriately diluted (50%) Folin–Ciocalteu reagent followed by 1 ml of 20% Na2CO3 aqueous solution. The mixture was incubated at room temperature for 20 minutes before recording the absorbance at 730 nm. The results were expressed as gallic acid equivalents (GAE) in mg g−1 of dry plant material and correlated with different antioxidant assays.
Antioxidant activity
DPPH (2′-2′ diphenyl–2′ picrylhydrazyl) radical scavenging assay
The free radical scavenging capacity of different samples was measured by the DPPH radical scavenging method of Yen and Chen Citation(1995) with slight modifications. The method involves the reaction of the antioxidants present in the extracts with the stable DPPH in 0.1 mM methanol solution. Briefly, the reaction mixture contained 300 μl of extract of varying concentrations (10–100 μg ml−1) and 2 ml of DPPH solution. After 10 minutes, the change in absorbance was recorded at 517 nm in a spectrophotometer against a blank, which did not contain the extract. L-ascorbic acid was used as a positive control. The DPPH radical scavenging capacities were expressed as Vitamin-C Equivalent Antioxidant Capacity (VCEAC) in mmol g−1 of extract.
The % DPPH• scavenging activity was calculated by the equation:
Reducing power assay
Reducing power of the plant extracts was determined following the method of Oyaizu Citation(1986). Briefly, to different concentrations of extracts (1 ml) were added 2.5 ml of phosphate buffer (0.2 M, pH 6.6) and 2.5 ml of potassium ferricyanide (1%). The reaction mixture was allowed to incubate at 50°C for 20 min. Then 2.5 ml of trichloroacetic acid (10%) was added to the reaction mixture, which was then centrifuged at 9500 rpm for 10 min. The upper layer of solution (2.5 ml) was recovered and mixed with 2.5 ml distilled water and 2.5 ml FeCl3 (0.1%). The absorbance was recorded at 700 nm in a spectrophotometer. An increase in the absorbance of reaction mixture indicated the increased reducing power.
Deoxyribose degradation assay
The non-site and site-specific deoxyribose assays were performed respectively following the method of Halliwell et al. Citation(1987) and Arouma et al. (1987) with some modifications. In non-site specific deoxyribose assay, the extracts (10–100 μg ml−1) were mixed with a Haber–Weiss reaction buffer [10 mM FeCl3, 1 mM EDTA (pH 7.4), 10 mM H2O2, 10 mM deoxyribose, and 1 mM L-ascorbic acid] and the final volume of all mixtures was made to 1.0 ml. The mixture was then incubated at 37°C for 1 h following heating at 80°C for 30 min with 1 ml of 2-TBA (0.5% 2-TBA in 0.025 M NaOH, 0.02% BHA) and 1 ml of 10% trichloroacetic acid (TCA) in water bath for 45 min. After cooling, the absorbance of mixture was measured at 532 nm. A site-specific assay was performed following slight modifications where the EDTA was replaced with a same volume of phosphate buffer. The percentage inhibition was calculated employing the formula as given for DPPH scavenging assay and was correlated with total phenolic content. The IC50 value was also calculated.
Chelating effects on ferrous ions
The chelating effect on ferrous ions was determined as per Dinis et al. Citation(1994) with certain modifications. Briefly, the extracts (250 μl) were mixed with 1.75 ml of methanol and 250 μl of FeCl2 (250 mM) followed by the addition of 250 μl of 2 mM ferrozine, and allowed to react for 10 min at room temperature before measuring the absorbance of mixture at 562 nm in a spectrophotometer. The chelating effect (%) was also calculated from the formula as given for DPPH radical scavenging assay.
NBT superoxide radical scavenging assay
The scavenging activity of the plant extracts towards superoxide anion radicals was measured by following the method of Liu et al. Citation(1997). The superoxide anion was generated in 3 ml of Tris-HCL buffer (100 mM, pH 7.4) containing 750 μl of NADH (936 μM) solution and 300 μl of different concentrations (10–100 μg ml−1) of extracts. L-ascorbic acid was used as positive control. The reaction was initiated by adding 750 μl of PMS (120 μM) to the mixture. After 5 min of incubation at the room temperature, the absorbance was measured at 560 nm. The per cent NBT decolourization of the sample was calculated by the equation:
EPR (electron paramagnetic resonance) spin trapping technique
The thermal decomposition of potassium persulfate (K2S2O8) in dimethyl sulphoxide (DMSO) at 333 K was used as a source of reactive radicals. To measure the radical scavenging capacity of acetone and methanol extracts of B. monosperma, the EPR spin trapping technique (Rapta et al. Citation2005) was used, employing 5,5-dimethylpyrroline N-oxide (DMPO, Sigma-Aldrich, St. Louis, MO, USA) as a spin trap. Sulfate radical anions generated upon thermal decomposition of K2S2O8 represent reactive species with high reduction potential, capable of reacting with a variety of organic compounds. In DMSO solvent these paramagnetic species are added to the double bond of DMPO spin trapping agent producing the corresponding spin adducts (Zalibera et al. Citation2009). All EPR measurements were carried out in a single 4 mm flat quartz cell in a Bruker TE102 (ER 4102 ST) cavity using the EMX EPR spectrometer (Bruker, Rheinstetten, Germany) working in the X-band. The ER 4111 VT temperature unit (Bruker, Germany) was used for temperature regulation. The reaction mixture consisted of 25 μl of respective extracts dissolved in DMSO (pure DMSO in reference), 175 μl DMSO, 25 μl of 0.2 M DMPO dissolved in DMSO and 25 μl of 0.01 M K2S2O8 (DMSO). A time course of EPR spectra of the DMPO spin adducts was recorded in 66 s intervals for 22 minutes at 333 K (each spectrum was an accumulation of three scans). The integral EPR intensity (double integral) found after 22 minutes of thermal treatment for the sample solution was compared with the reference measurement. The difference between the integral EPR intensities of the reference and the samples in the 22nd minute characterizes the amount of radicals scavenged by the various components present in the sample acting as radical scavengers. The radical scavenging capacity (RSC) values were calculated as a percentage of scavenged radicals relative to the reference sample (DMSO). These values were recalculated to Trolox equivalent antioxidant capacity (TEAC) using a calibration curve measured analogously for Trolox solutions in K2S2O8/DMPO/DMSO systems, and so obtained radical scavenging characteristics of investigated samples were evaluated in mmol of Trolox per 1 g of extract.
Phytochemical screening
Phytochemical screening of various extracts/fractions was carried out according to the standard methods as described by Trease and Evans Citation(1996).
Statistical analysis and correlations
The mean values and the standard deviations were calculated from the data obtained from three independent experiments. Statistical differences at p<0.05 were considered to be significant coefficient of determination (r 2) to determine the relationship between two variables were calculated using Microcal Origin 5.0 and Microsoft Excel.
Results and discussion
Phytochemicals and total phenolic contents
Results of phytochemical analysis showed that both increasing and decreasing solvent polarity extracts exhibited the presence of various phytochemicals (). Qualitatively, acetone and methanol extracts of decreasing solvent polarity exhibited the presence of most phytochemicals investigated. The highest extractive value in decreasing solvent polarity was obtained in water extract (4.03%) indicating the presence of a high amount of water soluble phytoconstituents. Conversely, increasing polarity extracts exhibited maximum extractive value (2.09%) in acetone extracts.
Table 1. Phytochemical analysis and extractive value of different solvent extracts of B. monosperma bark in increasing and decreasing order of solvent polarity.
Phenolic compounds have been widely investigated in many medicinal plants, fruits and vegetables (Djeridane et al. Citation2006). Phenolic compounds or polyphenols are the principal antioxidant constituents with more than 8000 phenolic structures currently known. The total phenolic content of different extracts of stem bark of B. monosperma was estimated by the Folin–Ciocalteu method. From the results, it was observed that total phenolics ranged from 45 to 141 mg GAE/g of extract (). Among the extracts investigated, the amount of phenolic compounds in acetone extracts of both solvent polarities were highest, and lowest in ethyl acetate extract (p<0.05). Tawaha et al. Citation(2007) estimated that a total phenolic content higher than 20 mg GAE/g dry weight could be considered as very high. This suggests that, the methanol, acetone, ethyl acetate and water extracts of B. monosperma bark must be considered as good source of phenolic compounds. However, a very high amount of phenolic content (>775 mg GAE/g extracts) was reported in Acacia auriculiformis (Singh et al. Citation2007). This tremendous increase in phenolic content could be explained due to further fractionation of the crude extracts used in their study.
Table 2. Total phenolic content (as mg gallic acid equivalents (GAE) per g of extract) in different extracts of B. monosperma.
Antioxidant activity
Antioxidant activity may possibly result by the synergy between a number of mechanisms. Owing to complex nature of the phytochemicals present (Hall and Cuppett Citation1997), the antioxidant activity of plant extracts cannot be evaluated by a single method. In order to explore these additional mechanisms, several antioxidant assays, namely DPPH, reducing power, deoxyribose degradation, chelating power, NBT and EPR, were employed in the present investigation to evaluate antioxidant activity of B. monosperma bark extracts and were correlated with their total phenolic contents.
DPPH radical scavenging activity
The stable DPPH free radical has been widely used to test the antiradical power of various natural products (Brand-Williams et al. Citation1995) and has been accepted as a model compound for free radicals originating in lipids (Yasuda et al. Citation2000). In the present study, various extracts of B. monosperma were effective in reducing the stable DPPH radical to yellow coloured diphenylpicrylhydrazine, indicating that these extracts are active in DPPH radical scavenging (). Strikingly, acetone and methanol extracts of B. monosperma tested had exceptionally high scavenging activity when compared with the water and ethyl acetate extracts. In this study, we expressed DPPH antioxidant capacity results by considering kinetic parameters by testing different initial concentrations (20–100 μg ml−1) of the test samples. In the present investigation, we also established the IC50, which was the amount of sample needed to scavenge 50% of the initial concentration of the free radical (DPPH). Results of the IC50 values revealed that acetone extracts of increasing polarity were more powerful and depleted the initial concentration of DPPH by 50% with 44.77 μ g ml−1 extract concentration in 10 minutes. Meanwhile this concentration was highest (139.88 μ g ml−1) in water extract. Similarly, in case of decreasing polarity extracts, the IC50 value was also found to be minimum (34.96 μg ml−1) in acetone extract and maximum (71.37 μg ml−1) in ethyl acetate fraction. The lower the IC50 value the higher the free radical scavenging activity of a sample.
Table 3. Percentage inhibition of DPPH radical in the presence of different concentrations of increasing and decreasing polarity extracts of B. monosperma.
The DPPH scavenging activity of B. monosperma extracts in increasing order of solvent polarity ranged between 36.68% and 78.10% at 100 μ g ml−1 extract concentration. For decreasing polarity extracts it ranged between 59.26% and 83.02% at 100 μ g ml−1 extract concentration (). A linear correlation between DPPH scavenging activity and total phenolic content showed a statistically significant correlation with r 2 of 0.809 to 0.980 (not shown) with different extracts. These data are in accordance with earlier research (Liu et al. Citation2008; Amensour et al. Citation2010; Kuate et al. Citation2010), suggesting that a high total phenolic content increases antioxidant activity.
Reducing power assay
Reducing power is based on the reduction of Fe3+ to Fe2+ in the presence of reductants present in the extracts. shows the relative reducing power of various extracts of B. monosperma prepared by increasing and decreasing solvent polarities. It is clear from the results that regardless of extraction procedure, acetone extracts exhibited maximum (1.779, 1.852) reducing potential, whereas water extracts depicted minimum (0.985, 0.838) reducing potential in increasing and decreasing order, respectively, of solvent polarities (p<0.05), at 100 μg ml−1 extract concentration. There was no significant difference of activities as shown by increasing and decreasing solvent polarity extracts. Reducing power assay is an important parameter used in evaluating antioxidant activities of natural extracts. It inhibits lipid peroxidation (LPO) by donating a hydrogen atom, resulting in termination of free radical chain reaction (Yen and Chen Citation1995). In this study, reducing potential increased in a dose dependent manner which is concomitant with the study of Yen and Duh Citation(1993), who reported that the reducing power of peanut hull extract increased with increase in concentration and correlated (r 2=0.979) well with the extent of antioxidant activity.
Table 4. Relative reducing power of different concentrations of increasing and decreasing polarity extracts of B. monosperma.
Deoxyribose degradation assay
Hydroxyl radical is the most reactive ROS and attacks almost every molecule in the body resulting in peroxidation of cell membrane lipids and in formation of malondialdehyde, which is mutagenic and carcinogenic. The results obtained in deoxyribose degradation assay (site-specific and non-site-specific) to prevent 2-deoxy-D-ribose oxidation mediated by OH• radicals are presented in and , respectively. This assay was carried out under two different experimental conditions: in the presence of EDTA (non-site-specific) which allows the study of OH radical scavenging activity; and in the absence of EDTA (site-specific) which allows evaluation of the extracts’ ability to bind to Fe. It was observed that in general, all the extracts of B. monosperma displayed a protective effect depending on the concentration of the extracts. However, the effect was slightly more in the non-site-specific assay, which indicated the suppression of OH• radicals; this has been implicated as a highly damaging species in free radical pathology, capable of damaging almost every molecule found in living cells (Yasuda et al. Citation2000).
Figure 1. Scavenging of hydroxyl radicals by various extracts of B. monosperma in (a) site specific and (b) non-site specific deoxyribose degradation assay.
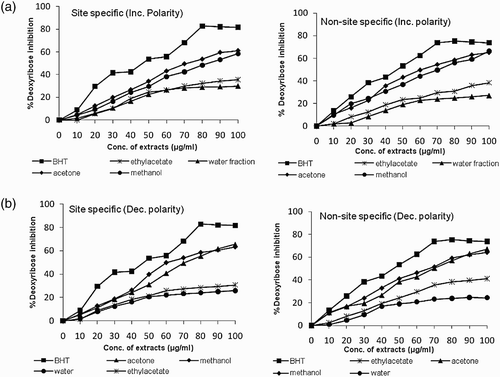
The ability of extracts to bind to Fe in site-specific assay varied from 29.9 to 61.1% and 25.8 to 65.5% in increasing and decreasing order of solvent polarities, respectively, at 100 μg ml−1 concentration, with acetone extracts being the most effective. Similarly the scavenging of OH• radicals by the extracts in non-site-specific assay varied from 27.1 to 66.5% and 24.4 to 67.1% in increasing and decreasing order of solvent polarities, respectively, at 100 μ g ml−1 concentration of acetone extracts. The IC50 values were found to be minimum (75.8 μg ml−1) in acetone extract and maximum (145.21 μg ml−1) in water fraction in increasing order of solvent polarities. In case of decreasing polarity extracts the IC50 value was minimum (71.04 μg ml−1) in the methanol extract whereas it was maximum (182.57 μg ml−1) in the water extract. The antioxidant potential of extracts displayed by deoxyribose degradation (site-specific and non-site-specific) assay was found to be highly correlated to their total phenolic content, showing a positive correlation in most of the extracts. The abilities of extracts in both the assays indicate their effectiveness as chelating agents, as well as their capacity to scavenge OH• radicals which are produced in solution from a Fe2+-EDTA chelate (Singh et al. Citation2007).
Fe2+ ion chelating activity
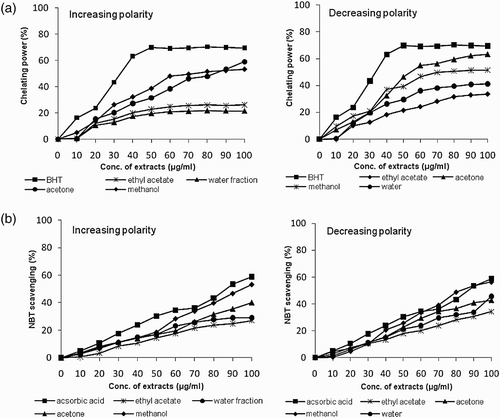
The results of chelating ability of various extracts of B. monosperma towards ferrous ion, the most effective pro-oxidant commonly found in food system (Yamaguchi et al. Citation1998) are presented in . The ferrous state of iron can stimulate lipid peroxidation by the Fenton reaction and is the most powerful pro-oxidant among various species of metal ions (Kuate et al. Citation2010). In this assay, per cent inhibition of formation of ferrous complex by extracts of B. monosperma was estimated. The results revealed that Fe2+-ion chelating activity of extracts in increasing order of solvent polarity was minimum (21.33%) in water fraction whereas maximum (58.87%) in acetone extract at 100 μg ml−1 concentration. Conversely, decreasing polarity extracts showed minimum inhibition (33.69%) in ethyl acetate fraction and maximum (63.44%) in acetone extract at 100 μ g ml−1 concentration. Further, it was observed that, in general, the Fe2+-ion chelating activity increased as the concentration of the extracts increased up to 60 μg ml−1, at which the effect levelled off even with a further increase in extract concentration (. Moreover, various potencies for ion-chelation activity with an IC50, ranging from 67.4 to 227.4 μg ml−1, were noted with various extracts depending on the procedure of extraction. In general, IC50 value was observed minimum (67.43 μg ml−1) in acetone extract of decreasing polarity. Furthermore, the antioxidant potential of acetone extracts evaluated by chelating power assay was also highly correlated (r 2=0.908 and r 2=0.954 for increasing and decreasing polarity extracts) to the total phenolic content.
NBT (superoxide scavenging) assay
Superoxide anion is a reduced form of molecular oxygen and is one of the most representative free radicals. In cellular oxidation reactions, superoxide radicals are normally formed first and their effects can be magnified because they lead to a cascade formation of other ROS and oxidizing agents in the cells (Liu and Ng Citation2000). O2• radicals can directly mediate protein damage and stimulate the formation of other entities involved in the damage of biomolecules such as lipids (Halliwell et al. Citation1995). In this study, the effect of B. monosperma extract to interact with O2• radicals was measured as a function of its inhibitory effect on the NBT reduction caused by these radicals. It is clear from the data that all extracts of B. monosperma react directly with O2• radicals in a dose dependent manner (10–100 μg ml−1) (. However, the methanol extracts showed maximum inhibition (53.06% and 56.35%) in increasing and decreasing order of solvent polarity, respectively, at 100 μg ml−1 extract concentration (. The ethyl acetate extracts had lowest scavenging activity. The IC50 value was determined as 88.5 μg ml−1 and 85.9 μg ml−1 in methanol extracts of increasing and decreasing polarity extracts, respectively, which was highly significant (p<0.05) compared to ethyl acetate extracts. The antioxidant potential of B. monosperma extracts evaluated by NBT assay when compared with total phenolic content was also found to be highly correlated showing a positive correlation in most of the extracts.
Electron paramagnetic resonance (EPR) studies
Keeping in view the potencies of extracts to scavenge free radicals as depicted by the above assays, EPR studies were carried out for acetone and methanol extracts of both increasing and decreasing solvent polarities. The characteristic experimental EPR spectrum of •DMPO-SO spin adduct recorded during the thermally initiated decomposition of K2S2O8 in DMSO at 333 K, along with its simulation (
mT,
mT,
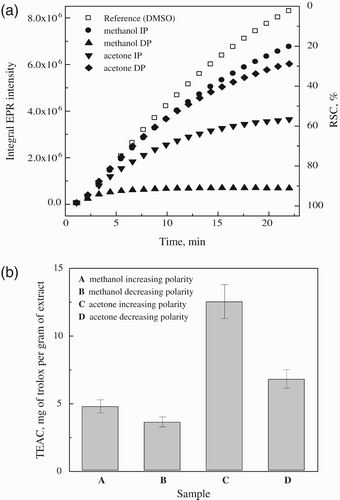
mT; g=2.0059) were studied. represents the original sets of 20 individual EPR spectra monitored in the presence of DMPO during heating of K2S2O8 at 333 K for the reference sample DMSO (200 μ l DMSO, 25 μl 0.2 M DMPO in DMSO, 25 μl 0.01 M K2S2O8 in DMSO) and for DMSO extracts of test samples (200 μl extract in DMSO, instead of DMSO in reference sample). It should be noted here that the decrease in the monitored EPR signal intensity of spin adducts is influenced by concentration of individual extracts (). shows a time dependence of EPR integral intensities (evaluated by double integration of sets of 20 individual EPR spectra for each measurement, e.g. ) representatively for the samples of B. monosperma extracts. EPR integral intensity after 22 minutes detected for the extracts was compared to that of the reference. The difference between these EPR intensities is proportional to the amount of radicals terminated by the scavengers present in the investigated extract sample (), and is called radical scavenging capacity (RSC, expressed in %). Finally, RSC values were recalculated to the TEAC values (molar amount of Trolox/1 g of dry extract inducing the identical changes in RSC) using calibration curve obtained under strictly identical conditions for Trolox solutions in K2S2O8/DMPO/DMSO systems (. The radical scavenging ability from EPR spin trapping assay of extracts ranged from 3.7 to 12.6 mg Trolox g−1 with highest RSC displayed by acetone extracts of increasing polarity (. In EPR experiments we generate the SO
with high redox potential, which are capable of reacting with a variety of other compounds present in the extracts (e.g. lignans, polysaccharides, anthocyanins, phytic acid, tannins and flavonoids) other than phenolics (Martínez-Tomé et al. Citation2004).
Figure 3. Time course of 20 individual EPR spectra obtained for samples of various extracts of B. monosperma dissolved in DMSO. All sets of 20 EPR spectra of DMPO adducts monitored during the thermal (333 K) decomposition of K2S2O8 in the presence of DMSO extracts were taken for 22 minutes under the same experimental conditions as for reference sample (DMSO, instead of DMSO bark extracts). Extracts concentrations: methanol I.P., acetone I.P. & D.P. (100 mg ml−1) and methanol D.P. (500 mg ml−1).
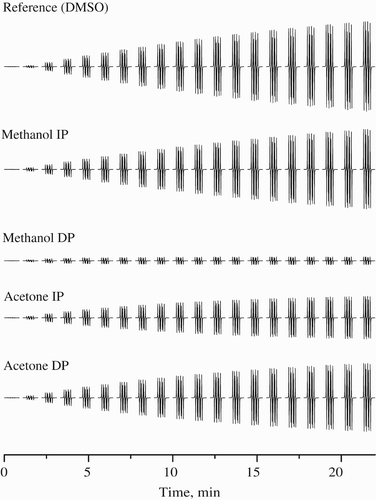
Figure 4. (a) Time course of EPR integral intensities of DMPO adducts recorded during the first 22 minutes of the thermal decomposition of K2S2O8 in the presence of DMSO extracts of B. monosperma bark and (b) radical scavenging capacities equated to actual dry weight of the extracts and expressed as TEAC g−1 of extracts.
Conclusions
In conclusion, the present investigation reported the antioxidant properties of bark of B. monosperma extracted according to increasing and decreasing solvent polarity. This study also reports the phenolic content and phytochemical profile of the various extracts. Significant variations were found in total phenolic content, antioxidant activity and release of phytochemicals depending on the solvent and method of extraction. Overall, acetone extracts of decreasing solvent polarity were found to be richer in antioxidant activity and phytochemicals. The amount of phenolic compounds detected was observed to be greater in acetone as compared to other solvents. Although other antioxidant compounds may be present in the extracts, radical scavenging activity investigated by various assays and the total phenolics content are highly correlated to each other. Further studies will be focused on cytotoxic effects, fractionation and purification of active components in acetone extracts of B. monosperma. This will most likely improve the antioxidant activity and other potential health benefits, promoting their use as natural antioxidant source.
Acknowledgements
The authors thank the Chairperson, Department of Biotechnology, Chaudhary Devi Lal University, Sirsa for providing necessary laboratory facilities. We also wish to thank Prof. Vlasta Brezova, Faculty of Chemical and Food Technology, Slovak University of Technology in Bratislava, Slovak Republic for her help in EPR studies.
References
- Aggarwal , A K , Tripathi , D M , Sahai , R , Gupta , N , Saxena , P P , Puri , A , Singh , M , Misra , R N , Dubey , C B and Saxena , K C . 1997 . Management of giardiasis by a herbal drug ‘Pippali Rasayana’: a clinical study . J Ethnopharmacol. , 56 : 233 – 236 .
- Amensour , A , Sendra , E , Abrini , J , Perez-Alvarez , J A and Fernandez-Lopez , J . 2010 . Antioxidant activity and total phenolic compounds of myrtle extracts . CyTA – J Food. , 8 : 95 – 101 .
- Aruoma , O I . 1999 . Free radicals, oxidative stress, and antioxidants in human health and disease . J Agric Food Chem. , 47 : 397 – 492 .
- Aruoma , O I , Grootveld , M and Halliwell , B . 1987 . The role of iron in ascorbate-dependent deoxyribose degradation . J Inorg Biochem. , 29 : 289 – 299 .
- Brand-Williams , W , Cuvelier , M E and Berset , C . 1995 . Use of a free radical method to evaluate antioxidant activity . Lebensmittel Wissenschaft und Technologie , 28 : 25 – 30 .
- Burli , A D and Khade , B A . 2007 . A comprehensive review on Butea monosperma (Lam.) Kuntze . Pharmacog Rev. , 1 : 333 – 337 .
- Choedon , T , Shukla , S K and Kumar , V . 2010 . Chemopreventive and anti-cancer properties of the aqueous extracts of flowers of Butea monosperma . J Ethnopharmacol. , 129 : 208 – 213 .
- Dinis , TC P , Madeira , VM C and Almeida , L M . 1994 . Action of phenolic derivatives (acetaminophen, salicylate, and 5-aminosalicylate) as inhibitors of membrane lipid peroxidation and as peroxyl radical scavengers . Arch Biochem Biophy. , 315 : 161 – 169 .
- Djeridane , A , Yousfi , M , Nadjemi , B , Boutassouna , D , Stocher , P and Vidal , N . 2006 . Antioxidant activity of some Algerian medicinal plants extracts containing phenolic compounds . Food Chem. , 97 : 654 – 660 .
- Hall , C A and Cuppett , S L . 1997 . “ Activities of natural antioxidants ” . In Antioxidant methodology in vivo and in vitro concepts , Edited by: Aruoma , O I and Cuppett , S L . 2 – 29 . Champaign , IL : AOCS Press .
- Halliwell , B , Aeschbach , R , Löliger , J and Aruoma , O I . 1995 . The characterization of antioxidants . Food Chem Toxicolo. , 33 : 601 – 617 .
- Halliwell , B and Gutteridge , J M . 1984 . Lipid peroxidation, oxygen radicals, cell damage, and antioxidant therapy . Lancet , 23 : 1396 – 1397 .
- Halliwell , B , Gutteridge , JM C and Aruoma , O I . 1987 . The deoxyribose method: a simple “test-tube” assay for determination of rate constants for reaction of hydroxyl groups . Ann Biochem. , 165 : 215 – 219 .
- Honda , K , Casadesus , G , Paterson , R B , Perry , G and Smith , M A . 2004 . Oxidative stress and redox iron in Alzheimer's disease . Ann New York Acad Sci. , 1012 : 179 – 182 .
- Jiménez , T J , Connell , S O , Lyons , H , Bradley , B and Hall , M . 2010 . Antioxidant, antimicrobial, and tyrosinase inhibition activities of acetone extract of Ascophyllum nodosum. . Chem Pap. , 64 : 434 – 442 .
- Kuate , D , Etoundi , BC O , Soukontoua , Y B , Ngondi , J L and Oben , J E . 2010 . Antioxidant characteristics of Dichrostachys glomerata spice extracts . CyTA – J Food. , 8 : 23 – 37 .
- Kujala , T S , Loponen , J M , Klika , K D and Pihlaja , K . 2000 . Phenolic and betacyanins in red beet root (Beta vulgaris) root: distribution and effects of cold storage on the content of total phenolics and three individual compounds . J Agric Food Chem. , 48 : 5338 – 5342 .
- Li , H , Hao , Z , Wang , X , Huang , L and Li , J . 2009 . Antioxidant activities of extracts and fractions from Lysimachia foenum-graecum Hance . Biores Technol. , 100 : 970 – 974 .
- Liu , F and Ng , T B . 2000 . Antioxidative and free radical scavenging activities of selected medicinal herbs . Life Sci. , 66 : 725 – 735 .
- Liu , F , Ooi , VE C and Chang , S T . 1997 . Free radical scavenging activities of mushroom polysaccharide extracts . Life Sci. , 60 : 763 – 771 .
- Liu , H , Qiu , N , Ding , H and Yao , R . 2008 . Polyphenol content and antioxidant capacity of 68 Chinese herbals suitable for medical or food uses . Food Res Int. , 41 : 363 – 370 .
- Martínez-Tomé , M , Murcia , M A , Frega , N , Ruggieri , S , Jiménez , A M , Roses , F and Parras , P . 2004 . Evaluation of antioxidant capacity of cereal brans . J Agric Food Chem. , 52 : 4690 – 4699 .
- Oyaizu , M . 1986 . Studies on product of browning reaction prepared from glucose amine . Jap J Nut. , 44 : 307 – 315 .
- Pandey , R and Mishra , A . 2010 . Antibacterial activities of crude extract of Aloe barbadensis to clinically isolated bacterial pathogens . App Biochem Biotechnol. , 160 : 1356 – 1361 .
- Rapta , P , Polovka , M , Zalibera , M , Breierova , E , Zitnanova , I , Marova , I and Certik , M . 2005 . Scavenging and antioxidant properties of compounds synthesized by cartenogenic yeasts stressed by heavy metals- EPR spin trapping study . Biophy Chem. , 116 : 1 – 9 .
- Scalbert , A and Williamson , G . 2085 . Dietary intake and bioavailability of polyphenols . J Nut. , 130 : 2073
- Sharma , N and Shukla , S . 2011 . Hepatoprotective potential of aqueous extract of Butea monosperma against CCl4 induced damage in rats . Exp Toxicol Pathol. , 63 : 671 – 676 .
- Singh , R , Singh , S , Kumar , S and Arora , S . 2007 . Free radical scavenging activity of acetone extract/fractions of Acacia auriculiformis . A. Cunn. Food Chem. , 103 : 1403 – 1410 .
- Somani , R , Kasture , S and Singhai , A K . 2006 . Antidiabetic potential of Butea monosperma in rats . Fititerpia. , 77 : 86 – 90 .
- Tawaha , K , Alali , F Q , Gharaibeh , M , Mohammad , M and El-Elimat , T . 2007 . Antioxidant activity and total phenolic content of selected Jordanian plant species . Food Chem. , 104 : 1372 – 1378 .
- Trease , G E and Evans , W C . 1996 . Pharmacognosy , 13 , 282 – 396 . London , , UK : Bailliere Tindall .
- Viuda-Martos , M , Ruiz-Navajas , Y , Fernandez-Lopez , J and Perez-Alvarez , J. A. 2008 . Antifungal activity of lemon (Citrus lemon L.), mandarin (Citrus reticulate L.), grape-fruit (Citrus paradisi L.) and orange (Citrus sinensis L.) essential oils . Food Cont. , 19 : 1130 – 1138 .
- World Health Organization [Internet]. 2008. Traditional medicine. Media centre; [cited 2009 Dec 8]. Available from: http://www.who.int/mediacentre/factsheet/fs/34/en/
- Yamaguchi , T , Takamura , H , Matoba , T and Terao , J . 1998 . HPLC method for evaluation of the free radical-scavenging activity of foods by using 1,1-dicrylhydrazyl . Biosci Biotechnol Biochem. , 62 : 1201 – 1204 .
- Yasuda , T , Inaba , A , Ohmori , M , Endo , T , Kubo , S and Ohsawa , K . 2000 . Urinary metabolites of gallic acid in rats and their radical scavenging effect on DPPH . J Nat Prod. , 63 : 1444 – 1446 .
- Yen , G C and Chen , H Y . 1995 . Antioxidant activity of various tea extracts in relation to their antimutagenicity . J Agric Food Chem. , 43 : 27 – 32 .
- Yen , G C and Duh , P D . 1993 . Antioxidant properties of methanolic extracts from peanut hulls . J Am Oil Chem Soc. , 70 : 383 – 386 .
- Zalibera , M , Rapta , P , Staško , A , Brindzová , L and Brezová , V . 2009 . Thermal generation of stable SO spin trap adducts with super-hyperfine structure in their EPR spectra: An alternative EPR spin trapping assay for radical scavenging capacity determination in dimethylsulphoxide . Free Rad Res. , 43 : 457 – 469 .