Abstract
Plant cell cultures provide an alternative means for producing secondary metabolites. The present study is an attempt to study the effect of some elicitors and precursor molecules on the production of L-Dopa (3, 4-dihydroxy-L-phenylalanine), which is a neurotransmitter precursor being used for symptomatic relief of Parkinson's disease. It is one of the most highly active allelochemicals present in Mucuna pruriens. Suspension cultures were established on Murashige and Skoog (MS) medium supplemented with 6-benzylaminopurine (BAP) and indole acetic acid (IAA). Elicitors such as methyl jasmonate, chitin, pectin, yeast extract and also a precursor L-tyrosine at different concentrations were supplemented to the suspension cultures at different concentrations, and their effect on L-Dopa production was studied. L-Dopa produced was quantified by high performance liquid chromatography (HPLC). Compared to the control set of suspension cultures a several-fold increase in L-Dopa concentration was observed in elicitors treated and precursor fed suspension cultures. Compared to elicitor treatments precursor feeding resulted in higher concentrations of L-Dopa production.
Introduction
The biochemical potential of plant cell cultures to produce specific secondary metabolites such as drugs, flavors, pigments, and agrochemicals is of considerable interest due to their uses in biotechnology (Chakraborty and Chattopadhyay Citation2008). In search for alternatives for the production of desirable medicinal compounds from plants, biotechnological approaches are found to have potential as a supplement to traditional agriculture in the industrial production of bioactive plant metabolites (Ramachandra Rao and Ravishankar Citation2002). Cell suspension culture systems could be used for large-scale culturing of plant cells from which secondary metabolites could be extracted. The advantage of this method is that it can ultimately provide a continuous, reliable source of natural products (Vanisree 2004).
Elicitation of in vitro cultures is a useful approach for enhancing and extending production of desirable products (Oksman-Caldentey and Inze Citation2004) within shorter production times (Discosmo and Misawa Citation1985). It has been proved to be an effective way to increase secondary metabolite production. Elicitors are signal molecules that activate the signal-transduction cascade and lead to the activation and expression of genes related to the biosynthesis of secondary metabolites (Zhao et al. Citation2005).
Exogenous supply of a biosynthetic precursor(s) to the culture medium may also increase the yield of the desired product. This approach is useful when the precursors are inexpensive. The concept is based on the idea that a compound which is an intermediate, either in or at the beginning of a secondary metabolite biosynthetic route, stands a good chance of increasing the yield of the final product. Attempts to induce or increase the production of plant secondary metabolites, by supplying precursor or intermediate compounds, have been effective in many cases (Moreno et al. Citation1993; Whitmer et al. Citation1998; Silvestrini et al. Citation2002).
Mucuna pruriens is a well-known medicinal plant, but the study of its pharmacological properties and the corresponding compounds still continues. The importance of M. pruriens as a medicinal plant is mainly due to the presence of L-Dopa (3, 4-dihydroxy-L-phenylalanine), a neurotransmitter precursor being used for symptomatic relief of Parkinson's disease. It is produced via the oxidation of tyrosine by the enzyme tyrosinase. Once formed L-Dopa can be converted into several neurologically important catecholamines such as the neurotransmitter dopamine and the important hormones adrenaline and noradrenaline. It is present in all parts of the plant, but in varying concentrations (Riley Citation1997).
Cell suspension cultures of M. pruriens were successfully established (Huizing et al. Citation1985; Wichers et al. Citation1985; Chattopadhyay et al. 1994) and the accumulation of L-Dopa in the cell cultures of Mucuna have been successfully investigated, in M. pruriens (Brain Citation1976; Wichers et al. Citation1993), M. hassjoo, M. pruriens, and M. deeringiana (Teramoto and Komamine Citation1988). Abiotic stresses like fluorescent light, nature of the nitrogen source, sucrose and phosphate in the culture medium have been shown to significantly influence the higher rate of L-Dopa production in suspension cultures (Wichers et al. Citation1985; Chattopadhyay et al. 1994). The effect of plant growth regulators in combination with salt stress was reported to influence the L-Dopa accumulation in callus cultures (Wichers et al. Citation1993). However, to date no attempts are reported in the literature on elicitor/precursor-enhanced production of L-Dopa in M. pruriens. Hence, the present study aims to evaluate the effect of elicitors and precursor not only on L-Dopa production, but also on cell growth and cell viability in cell cultures of M. pruriens.
Materials and methods
Callus induction and suspension cell cultures
The seeds of M. pruriens were procured from the University of Agriculture Sciences, Bangalore, surfaces were sterilized with 1% mercuric chloride for 5 min, followed by washing with sterile distilled water 5–6 times to remove traces of surface-sterilant. They were germinated on basal Murashige and Skoog (MS) medium (Murashige and Skoog Citation1962). The plant was identified by senior taxonomist Prof. S. B. Kamalakar, Department of Botany, Sahyadri Science College, Shivamogga. The plants grown were used as an explant source.
Callus was induced from in vitro leaf tissue explants cultured on MS medium supplemented with sucrose 3% (w/v), indole acetic acid (IAA) (11.41 μM) and 6-benzylaminopurine (BAP) (0.88 μM) (w/v) and solidified with 0.8% agar-agar; pH was adjusted to 5.8 prior to autoclaving at 121°C for 20 min. The culture tubes were incubated with a 16 h photoperiod provided by cool white fluorescent lamps (25 μmol m−2 s−1) at 25°C and sub-cultured every second week. Callus (2 g cell fresh weight (FW)/100 ml medium) was transferred into 20 ml MS liquid medium in Erlenmeyer flasks, supplemented with IAA (11.41 μM), BAP (0.88 μM) and 3% sucrose for proliferation. Suspension cultures were established by shaking on a rotary shaker (REMI, India) at 100 rpm at 22°C, under a 16 h photoperiod provided by cool white fluorescent lamps (25 μmol m−2 s−1).
Preparation and application of elicitors and precursor to cell cultures
Methyl jasmonate (MJ) (50, 100, 150, 200 and 250 μM), pectin and yeast extract (YE) (50, 100, 150, 200 and 250 mg l−1) were dissolved in sterile distilled water, chitin (50, 100, 150, 200 and 250 mg l−1) was solubilized in 1 or 2 drops of 50% H2SO4 and L-tyrosine (50, 100, 150, 200 and 250 mM) was prepared by dissolving initially with 1 or 2 drops of 1 N NaOH. Later both chitin and tyrosine solutions were diluted with sterile double distilled water to get the final concentrations, pH was adjusted to 5.8 and solution was filter-sterilized before use.
Measurement of M. pruriens cell growth
The cell growth was monitored at every third day (up to 15 days) by determining the fresh weight. Briefly the cells were harvested on every third day to collect cells by vacuum filtration, weighed for growth analysis and analyzed on the same day. The fresh weight of the cells was recorded with the help of a physical balance. Alternatively cell growth was measured by sedimented cell volume (SCV) measurement. Cell viability was determined by vital staining with methylene blue stain. All data were expressed as an average of three separate experiments.
Extraction and quantification of L-Dopa
Extraction of L-Dopa was carried out as described by Myhrman Citation(2002) with appropriate modifications as required. Approximately 1 g of cells were taken and crushed using pestle and mortar by adding 10 ml of distilled water. The extract was boiled for 10 min, cooled and centrifuged for about 10 min at 5000 rpm. The supernatant was collected, boiled and cooled, again centrifuged and the resulting supernatant was collected and used for the estimation.
L-Dopa content in the extract was estimated by high performance liquid chromatography (HPLC) analysis using a Waters model 2487 (Waters, USA), pump 1515 with 2487 dual absorbance UV detection and 2414 RI detectors, manual sampler injector using C-18 column and a guard pre-column was packed with material, as in the main column. Isocratic elution was carried out using water:methanol:phosphoric acid [975.5:19.5:1 (v/v)] as described by Siddhuraju and Becker Citation(2001). Separation was performed at room temperature (25°C) and after the injection of 20 μl, the column was operated with the flow rate of 1.2 ml−1. Absorbance was monitored at 282 nm and peak heights and areas were determined. Sample was eluted between the third and fourth minutes. The amount of L-Dopa present in the tissue extracts was calculated with the help of standard curve for L- Dopa (Sigma, St. Louis, MO., USA). Amount of L- Dopa present in tissue extract was expressed as mg g−1 tissue dry weight. All the samples were passed through a glass-filter (0.20 μm pore size, Millipore, USA) and analyzed by HPLC for quantitative determination of L-Dopa.
Statistical analysis
All experiments were conducted in replications. The data generated was subjected to statistical analysis using Microsoft Excel programme (MS Office, 2003) and represented as mean ± SE.
Results and discussion
Effect of elicitors and precursor on cell growth
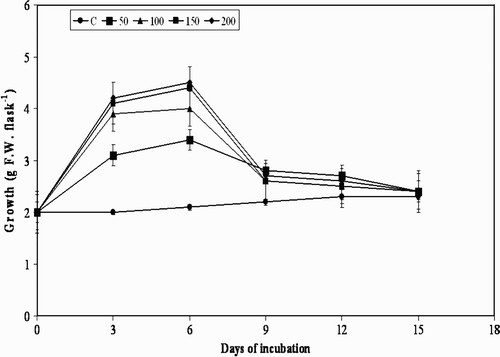
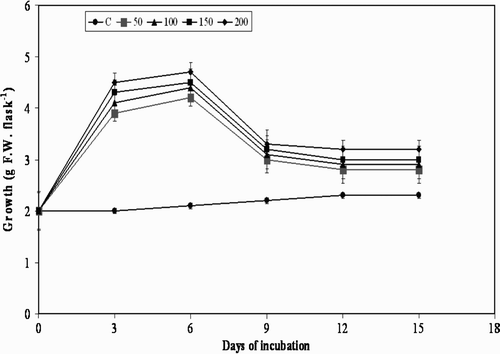
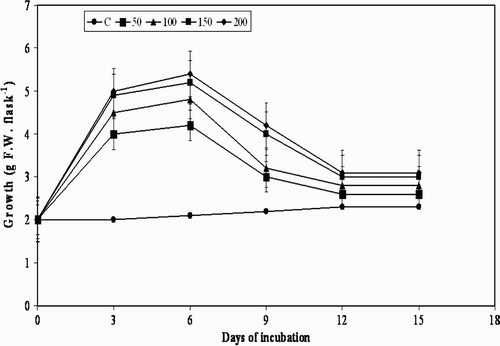
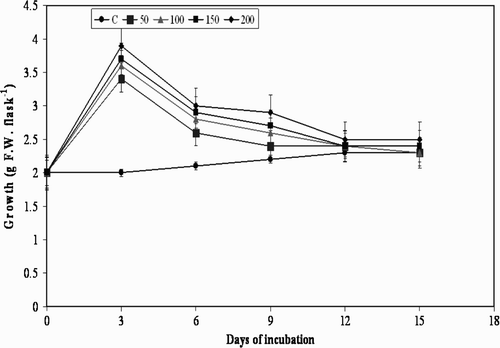
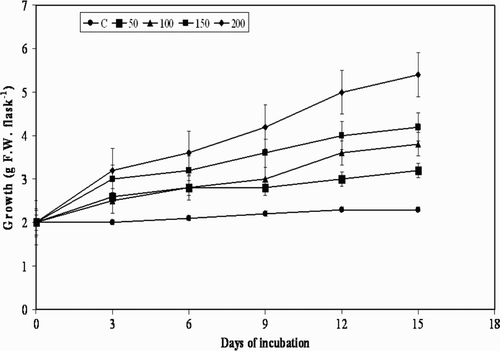
The treatment of plant cell cultures with MJ, pectin, and YE used in the present study is shown in . Although there was substantial increase in cell growth observed in the beginning i.e. from day 0 to day 3, and further to day 6, further elicitation to day 9 with all the elicitors decreased the cell growth, and resulted in moderate browning of the cells. A decrease in growth from day 9 was observed at all concentrations of the three elicitors, except for chitin, with which a similar effect was observed from day 6 (). Usually, when elicitors are added to in vitro cultures of plants they cease to grow temporarily or permanently, which may also lead to a defense response by switching away from primary metabolism (growth) to secondary metabolite production (Leon et al. Citation2001). The results of the present study are in agreement with the statement that there was cessation in the cell growth after day 9 to day 15. However, the results are not the same with the precursor L-tyrosine, with which there was gradual increase in the cell growth from day 3 to day 15 ().
Effect of elicitors and precursor on cell viability
The results of the present study show that long-term treatment with elicitors caused cell browning and had a significant effect on cell viability. From day 3 to day 6 for all the elicitors except for chitin (day 3) no significant variations in cell viability (80–90%) was observed; thereafter it was approximately 75%, which further progressively decreased by day 15. However, cell viability was affected less in YE-treated cell cultures than in MJ, chitin and pectin treatments, as determined by culture fresh weights. Decrease in L-Dopa accumulation in all the elicitor-treated cell cultures was noticed after day 9, except for chitin which showed similar results by day 6. Nevertheless, production of L-Dopa did not fall below the control levels determined in (2.36±0.12).
In contrast to the elicitors, in the cell cultures treated with precursor L-tyrosine there was increase in L-Dopa from day 3 to day 15 and no loss in cell growth and cell viability was observed. Similar observations were also reported by Whitmer et al. Citation(1998) and Baldi and Dixit Citation(2008).
Effect of elicitors on the accumulation L-Dopa
All the elicitors increased the accumulation of L-Dopa in the cell suspension cultures compared to the control group of cultures from day 3 to day 15. A several-fold increase in L-Dopa was obtained during day 3 to day 9 in MJ, YE and pectin elicited cultures; however, chitin showed similar results by day 6. Further, from day 9 to day 15, accumulation of L- Dopa was observed to decrease with all the four elicitors at all the concentrations studied ().
Table 1. Effect of elicitor methyl jasmonate on the production of L-Dopaα in suspension cultures of Mucuna pruriens L.
Table 2. Effect of elicitor chitin on the production of L-Dopaα in suspension cultures of Mucuna pruriens L.
Table 3. Effect of elicitor pectin on the production of L-Dopaα in suspension cultures of Mucuna pruriens L.
Table 4. Effect of elicitor yeast extract on the production of L-Dopaα in suspension cultures of Mucuna pruriens L.
A 19.46-fold ( mg g−1) increase in L-Dopa concentration was obtained in 200 μM MJ elicited cells on day 9 (). Chitin did not have much impact on the L-Dopa accumulation. Nevertheless, L-Dopa accumulation increased moderately (
mg g−1) at 200 mg l−1 with a 5.97-fold increase under the influence of this elicitor compared to the control set of cultures obtained on day 6 (). Pectin elicited the accumulation of L-Dopa (42.82±0.3 mg g−1) with an 18.14-fold increase on day 9 (). YE induced the highest concentration of L-Dopa (
mg g−1), with a 26.41-fold increase on day 9 at 200 mg l−1 ().
In the present study, of the four elicitors studied, YE proved to be more efficient than MJ, chitin and pectin in increasing the L-Dopa content. Pectin also significantly increased the L-Dopa production, but not as much as YE. Chitin and MJ also increased the L-Dopa compared to control set of cultures, but the extent of increase is comparatively lower than the other two elicitors, with chitin being the least efficient. Accumulation of various classes of secondary metabolite molecules in cell cultures of legumes by YE and MJ have been reported (Suzuki et al. Citation2002, Citation2005; Achnine et al. Citation2005; Broeckling et al. Citation2005; Farag et al. Citation2007; Naoumkina et al. Citation2007). Pectin has been reported to be an effective elicitor that has significantly increased menthol in Mentha piperita cell cultures (Chakraborty and Chattopadhyay Citation2008); similarly chitin has also been used as an elicitor in the study of its effect on Citrus aurantium (Gallao et al. Citation2007).
Although accumulation of L- Dopa was found to increase in the initial period of treatment with elicitors, long-term treatment (for 15 days in the present study) decreased the accumulation of L-Dopa and also resulted in loss of cell viability. This may be due to a cell dedifferentiation process that often leads to a lower productivity (Verpoorte et al. Citation2002). Similar results have also been reported by Rijhwani and Shanks (1988), Moreno et al. Citation(1993) and Negeral and Javelle Citation(1995).
Effect of precursor on the accumulation of L-Dopa
L-Dopa is produced via the oxidation of L-tyrosine by the copper-containing enzyme tyrosine hydroxylase in the presence of molecular O2 (Pattison et al. Citation2002). Hence, in the present study L-tyrosine was used as precursor to study its influence (supplementation in the medium) on L-Dopa production.
Accumulation of L-Dopa under the influence of precursor was rather different to that with the four elicitors used in the present study. L-tyrosine increased the L-Dopa production from day 3 to day 15 at all the concentrations tested (). The highest concentration of L-Dopa was obtained ( mg g−1) on day 15 at 200 mg l−1 with a 40.13-fold increase. Attempt to increase the production of desired molecules by supplementing precursors in the medium have been effectively studied in case of Solanum lyratum and Cistanche salsa (Lee et al. Citation2007; Liu et al. Citation2007). Production of anthocyanin and phenylethanoid glycosides was enhanced by feeding the precursor L-phenyl alanine and related precursors to cell cultures of strawberry and Cistanche deserticola respectively (Edahiro et al. Citation2005; Quyang et al. Citation2005).
Table 5. Effect of precurssor L-tyrosine on the production of L-Dopaα in suspension cultures of Mucuna pruriens L.
Plant tissue cultures have long been proposed as useful systems for the large-scale production of phytochemicals. The results of the present study indicate that treatments with elicitors for long periods caused browning of cell cultures and decreased cell growth, and had slight effect on cell viability and even on the production of L-Dopa. Of the four elicitors and precursor studied, tyrosine significantly increased the concentration of L-Dopa to a much greater extent than the elicitor molecules. Thus from the results of the present study, it could be concluded that precursor feeding is more advantageous than using elicitors and could be applied successfully to large-scale production of L-Dopa. As there are no reports available to date on the enhancement of L-Dopa by elicitors or precursor feeding in the M. pruriens, this study represents the first successful cell culture based approach for the production of L-Dopa.
Acknowledgements
We thank Dr Manjappa and Dr Suresh, Department of Chemistry, Bapuji Institute of Engineering and Technology, Davangere, for the help in HPLC analysis. We also thank the University of Agriculture Sciences, Bangalore.
References
- Achnine , L , Huhman , D V , Farag , M A , Sumner , L W , Blount , J W and Dixon , R A . 2005 . Genomics-based selection and functional characterization of triterpene glycol syltransferases from the model legume Medicago truncatula . Plant J. , 41 : 875 – 887 .
- Baldi , A and Dixit , V K . 2008 . Enhanced artemisinin production by cell cultures of Artemisia annua . Curr Trends Biotechnol Pharm. , 2 ( 2 ) : 341 – 348 .
- Brain , K R . 1976 . Accumulation of L-DOPA in cultures from Mucuna pruriens . Plant Sci Lett. , 7 : 157 – 161 .
- Broeckling , C D , Huhman , D V , Farag , M , Smith , J T , May , G D , Mendes , P , Dixon , R A and Sumner , L W . 2005 . Metabolic profiling of Medicago truncatula cell cultures reveals effects of biotic and abiotic elicitors on primary metabolism . J Exp Bot. , 56 : 323 – 336 .
- Chakraborty , A and Chattopadhyay , S . 2008 . Stimulation of menthol production in Mentha piperita . cell culture. In Vitro Cell Dev B. , 44 : 518 – 524 .
- Chattopadhyay , S , Datta , S K and Mahato , S B . 1995 . Production of L-Dopa from cell-suspension culture of Mucuna-pruriens-F-pruriens . Plant cell Plant Cell Rep. , 13 : 519 – 522 .
- Discosmo , F and Misawa , M . 1985 . Eliciting secondarymetabolism in plant cell cultures . Trends Biotechnol. , 3 : 318
- Edahiro , J I , Nakamura , M , Seki , M and Furusaki , S . 2005 . Enhanced accumulation of anthocyanin in cultured strawberry cells by repetitive feeding of L-phenylalanine into the medium . J Biosci Bioeng. , 99 ( 1 ) : 43 – 47 .
- Farag , M A , Huhman , D V , Dixon , R A and Sumner , L W . 2007 . Metabolomics reveals novel pathways, differential mechanistic and elicitorspecific responses in phenylpropanoid and isoflavonoid biosynthesis in Medicago truncatula . cell cultures. Plant Physiol. , 146 : 387 – 402 .
- Gallao , M I , Angelo , L , Cortelazzo , MP S and Fevereiro , E S . 2007 . Response to Chitin in suspension cultured citrus aurintum . cells. Brazilian. J Plant Physiol. , 19 ( 1 ) : 69 – 76 .
- Huizing , H J , Wijnsma , R , Batterman , S , Malingré , M and Wichers , H J . 1985 . Production of L-Dopa by cell suspension cultures of Mucuna pruriens I. Initiation and maintenance of cell suspension cultures of Mucuna pruriens and identification of L-Dopa . Plant Cell Tiss Org. , 4 : 61 – 73 .
- Lee , M H , Cheng , J J , Lin , C Y , Chen , Y J and Lu , M K . 2007 . Precursor feeding strategy for the production of solanine, solanidine and solasodine by a cell culture of Solanum lyratum . Process Biochem. , 42 ( 5 ) : 899 – 903 .
- Leon , J , Rojo , E and Sanchez-Serrano , J J . 2001 . Wound signaling in plants . J Exp Bot. , 52 : 1 – 9 .
- Liu , J Y , Guo , Z G and Zeng , Z L . 2007 . Improved accumulation of phenylethanoid glycosides by precursor feeding to suspension culture of Cistanche salsa . Biochem Eng J. , 33 ( 1 ) : 88 – 93 .
- Moreno , PR H , van der Heijden , R and Verpoorte , R . 1993 . Effect of terpenoid precursor feeding and elicitation on formation of indole alkaloids in cell suspension cultures of Catharanthus roseus . Plant Cell Rep. , 12 : 702 – 705 .
- Murashige , T and Skoog , F . 1962 . A revised medium for rapid growth and bioassays with tobacco tissue cultures . Physiol Plantarum , 15 : 473 – 497 .
- Myhrman , R . 2002 . “ Detection and removal of L-Dopa in the legume Mucuna ” . In Mucuna as a food and feed: Current uses and the way Forward Edited by: Flores , M , Eilittä , M , Myhrman , R , Carew , L and Carsky , R . 142 – 163 . Honduras Proceedings of International Workshop; Tegucigalpa, Honduras, CIDICCO.
- Naoumkina , M , Farag , M A , Sumner , L W , Tang , Y , Liu , C J and Dixon , R A . 2007 . Different mechanisms for phytoalexin induction by pathogen and wound signals in Medicago truncatula . 104 : 17909 – 17915 . Proc Natl Acad Sci USA.
- Negeral , J and Javelle , F . 1995 . Induction of phenyl propanoid and tyramine metabolism in pectinase or pronase elicited cell suspension culture of tobacco . Physiol Plantarum. , 95 : 569 – 574 .
- Oksman-Caldentey , K M and Inzé , D . 2004 . Plant cell factories in the post-genomic era: new ways to produce designer secondary metabolites . Trends Plant Sci. , 9 ( 433–440 )
- Pattison , D I , Dean , R T and Davis , M J . 2002 . Oxidation of DNA, proteins and lipids by DOPA, protein-bound DOPA, and related catechol (amine) s . Toxicology. , 177 : 23 – 37 .
- Quyang , J , Wang , X D , Zhao , B and Wang , Y C . 2005 . Enhanced production of phenylethanoid glycosides by precursor feeding to cell culture of Cistanche deserticola . Process Biochem. , 40 ( 11 ) : 3480 – 3484 .
- Ramachandra Rao , S and Ravishankar , G A . 2002 . Plant cell cultures: Chemical factories of secondary metabolites . Biotechnol Adv. , 20 : 101 – 153 .
- Rijhwani , S K and Shanks , J V . 1998 . Effect of elicitor dosage and exposure time on biosynthesis of indole alkaloids by Catharanthus roseus hairy root cultures . Biotechnol Progr. , 14 ( 3 ) : 442 – 449 .
- Riley , P A . 1997 . Melanin . Int J Biochem Cell Biol. , 29 : 1235 – 1239 .
- Siddhuraju , P and Becker , K . 2001 . Rapid reversed-phase high performance liquid chromatographic method for the quantification of L-Dopa (L-3,4-dihydroxyphenylalanine), non-methylated and methylated tetrahydroisoquinoline compounds form Mucuna beans . Food Chem. , 72 : 389 – 394 .
- Silvestrini , A , Pasqua , G , Botta , B , Monacelli , B , van der Heijden , R and Verpoorte , R . 2002 . Effect of alkaloid precursor feeding on Camptotheca acuminata cell line . Plant Physiol Biochem. , 40 : 749 – 753 .
- Suzuki , H , Achnine , L , Xu , R , Matsuda , S P and Dixon , R A . 2002 . A genomics approach to the early stages of triterpene saponin biosynthesis in Medicago truncatula . Plant J. , 32 : 1033 – 1048 .
- Suzuki , H , Reddy , M S , Naoumkina , M , Aziz , N , May , G D , Huhman , D V , Sumner , L W , Blount , J W , Mendes , P and Dixon , R A . 2005 . Methyl jasmonate and yeast elicitor induce differential genetic and metabolic re-programming in cell suspension cultures of the model legume Medicago truncatula . Planta. , 220 : 698 – 707 .
- Teramoto , S and Komamine , A . 1988 . “ Biotechnology in agriculture and forestry ” . In Bajaj YPS, editor. Medicinal and aromatic plants IV , 209 – 224 . Berlin : Heidelberg: Springer-Verlag .
- Verpoorte , R , Contin , A and Memelink , J . 2002 . Biotechnology for the production of plant secondary metabolites . Phytochem Rev. , 1 ( 1 ) : 13 – 25 .
- Whitmer , S , Canel , C , Hallard , D , Goncalves , C and Verpoorte , R . 1998 . Influence of precursor availability on alkaloid accumulation by transgenic cell lines of Catharanthus roseus . Plant Physiol. , 116 : 853 – 857 .
- Wichers , H J , Wijnsma , J F , Malingré , T H and Huizing , H J . 1985 . Production of L-Dopa by cell-suspension cultures of Mucuna. pruriens . Plant Cell Tiss Org. , 4 : 75 – 82 .
- Wichers , H J , Visser , J F , Huizing , H J and Pras , N . 1993 . Occurrence of L-DOPA and dopamine in plants and cell cultures of Mucuna pruriens and effects of 2, 4-D and NaCl on these compounds . Plant Cell Tiss Org. , 33 : 259 – 264 .
- Zhao , J , Davis , L C and Verpoorte , R . 2005 . Elicitor signal transduction leading to production of plant secondary metabolites . Biotechnol Adv. , 23 ( 4 ) : 283 – 333 .