Abstract
Curcumin possess anti-inflammatory activity. In this study, the binding of curcumin with PLA2 was studied using X-ray crystallography. Since the electron density found at the active site did not match with curcumin, 2-methoxycyclohexa-2-5-diene-1,4-dione (MCW) (the photodegraded product of curcumin) was fitted in the unexplained electron density. To understand the binding mode of actual curcumin, molecular docking study was carried out. The crystallographic and docked structures were superimposed with respect to the ligand position and it was found that curcumin was binding in the hydrophobic cavity of PLA2 with a binding energy of−16.81 Kcal/mol. The binding mode was of such a nature that it prevented the entry of the substrate to the hydrophobic active site. This study indicates that curcumin can act as an inhibitor of PLA2.
An animated Interactive 3D Complement (I3DC) is available in Proteopedia at http://proteopedia.org/w/Journal:FLS:1.
Introduction
Enzymes like cyclooxygenase (COX), lipoxygenase (LOX) and phospholipase A2 (PLA2) are involved in inflammatory disorders. Among these enzymes, PLA2 is important because it produces arachidonic acid, which is an important inflammatory mediator. PLA2 is a calcium-dependent enzyme with six or seven disulphide bridges. The catalytic mechanism of PLA2 has been proposed on the basis of its crystallographic and biochemical data (Dijkstra et al. Citation1983). It has been found that the catalytic mechanism of PLA2 requires millimolar amounts of Ca2+ as a catalytic cofactor (Verheij et al. Citation1980). Arachidonic acid initiates a number of physiologically important cellular processes, for example, inflammation and release of substances such as prostaglandins, thromboxanes, leukotrienes and platelet-activating factors (Anderson et al. Citation1997; Kudo and Murakami Citation2002). An imbalance in the level of arachidonic acid metabolism is linked to rheumatoid arthritis, neurological disorders, inflammation, asthma, psoriasis and tissue injury during myocardial ischemia (Julie et al. Citation1991; Pruzanski et al. Citation1993). Hence, the use of PLA2 inhibitors still remains as a potential approach to control inflammation.
Curcumin is a naturally occurring polyphenolic phytochemical isolated from the turmeric plant (Curcuma longa). The anti-inflammatory properties of curcumin have been explained by Chainani-Wu Citation(2003) and Menon and Sudheer Citation(2007). The binding of curcumin and its analogues at the active site of PLA2 has already been demonstrated by molecular modelling and docking studies (Nirmal et al. Citation2008; Dileep et al. Citation2011). Curcumin-induced inhibition of tumorigenesis in mice has been reported by Srimal and Dhawan Citation(1973) and Huang et al. Citation(1997). Curcumin has also been reported as an inhibitor of cyclooxygenase (COX) and lipoxygenase (LOX) (Chinthalapally Citation2007). Ewa et al. Citation(2000) in their study on lipoxygenase–curcumin co-crystals reported that curcumin is photosensitive and degrades when exposed to X-rays. In the present study, we have attempted to solve the crystal structure of PLA2 in complex with curcumin. However, only 2-methoxycyclohexa-2-5-diene-1,4-dione (MCW) was observed in the crystal structure, which is actually the photodegraded product of curcumin and is bound at the active site of PLA2. In our previous report (Dileep et al. Citation2011), we focused on comparing the binding energies of different curcumin analogues. In the present study, we have compared the binding mode of the docked curcumin structure with its photodegraded product.
Materials and methods
Crystallization
Porcine pancreatic PLA2 and curcumin were purchased from Sigma-Aldrich (Bengaluru, India). The enzyme was crystallized by hanging drop vapour diffusion method at a temperature of 294 K. The enzyme concentration was 60 mg/ml in 50 mM tris maleate buffer of pH 7.2 with 5 mM CaCl2 as additive. Drops of the enzyme mixed with equal volume of reservoir solution were equilibrated against the reservoir solution containing 15% of 2-methyl-2,4-pentanediol (MPD) in the same buffer. Crystals were formed in 2 weeks. These crystals were then carefully soaked in a 5 mg/ml solution of curcumin in MPD for 24 hours. The whole set-up was covered with thick aluminium foil to avoid exposure to light.
Data collection
X-ray diffraction data were collected at 100 K by using a MAR Research 345-mm imaging plate mounted on a Bruker AXS MICROSTAR rotating anode X-ray generator. The data were processed with iMosflm of CCP4 package (Leslie Citation2006). The data set obtained had 97.3% completeness at 2.50 Å of resolution. The space group was and the unit cell parameters were as follows: a=b=68.36 Å and c=70.12 Å. The overall data collection statistics are given in .
Table 1. Data collection and refinement statistics of PLA2–MCW complex crystal.
Structure solution and refinement
The crystal structure was solved by molecular replacement method by using MOLREP (Vagin and Teplyakov Citation2000), and the crystal structure of porcine pancreatic PLA2 at 2.6 Å (PDB ID: 1P2P) was used as the search model. This gave a distinct solution in rotation and translation search. A rigid body refinement gave an R factor of 0.437 for the data with a resolution range of 43.5–2.5 Å. Restraint refinement of atomic positions and refinement of isotropic temperature factors were also carried out. The R and free R ( reached 0.240 and 0.288, respectively. The model was fitted according to the electron density map by using COOT program (Emsley and Cowtan Citation2004). Water molecules and calcium ions were detected from the difference Fourier (
) maps and were included in the subsequent cycles of refinement. The R and R
free then dropped down to 0.203 and 0.254, respectively. At this stage, an unexplained electron density was observed at the active site of PLA2, but it did not match with curcumin (the expected ligand at the active site). It has already been reported that a photodegraded product of curcumin, that is 2-methoxycyclohexa-2-5-diene-1,4-dione (MCW), could be found at the active site when the crystal is exposed to X-rays (Ewa et al. Citation2000). Hence, MCW was fitted in the unexplained electron density. Further TLS & restraint refinement brought down the R and R
free to 0.180 and 0.229, respectively. Refinement statistics are shown in .
Molecular docking studies
Since the intact curcumin could not be detected at the active site of PLA2, an in silico molecular docking was carried out to understand the actual mode of binding. The final refined crystal structure was used as the target for docking studies. Water molecules in the crystal structure were removed, hydrogen atoms were added and energy was further minimised by applying CHARMm force field (Brooks et al. Citation1983). The dielectric constant was set as 78. A grid was set based on the crystallographic ligand. The following residues were set as flexible: Arg 6, Ile 9, Leu 19, Phe 22, Asn 23, Gly 30, His 48, Asp 49 and Tyr 69. The structure of curcumin was downloaded from the Pubchem database (http://pubchem.ncbi.nlm.nih.gov/). The docking of curcumin at the active site of PLA2 was carried out by using flexible docking module of Discovery Studio 2.5 (Accelrys Inc., San Diego, CA, USA) by setting default parameters. Finally, refinement was carried out by applying simulated annealing. The target temperature for heating and cooling were 700 K and 300 K, respectively. The best docked pose was selected based on the binding energy.
Results and discussion
The asymmetric unit of the crystal contains 1 molecule of PLA2, 1 molecule of MCW, 2 calcium ions and 32 water molecules. The final R and R free were 0.180 and 0.229, respectively. The root-mean-square deviations for bond lengths and angles were 0.020 Å and 1.90°, respectively. Ramachandran map showed that 91.8% residues were lying in the most favoured region. The average B value for all atoms is 23 Å2. The atomic coordinates have been deposited with Protein Data Bank (PDB ID: 3HSW).
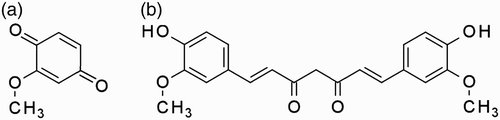
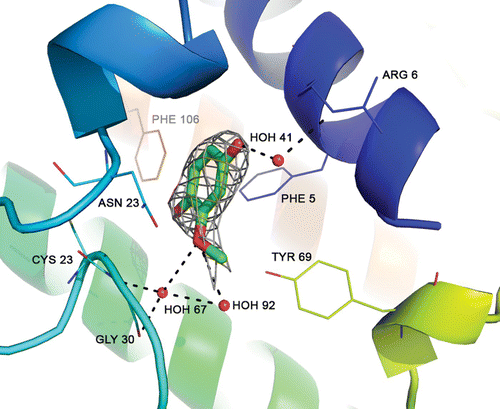
The degradation of curcumin to MCW as a result of exposure to X-rays has already been reported (Ewa et al. Citation2000). The chemical structures of curcumin and MCW are shown in . The electron density map of MCW bound at the active site of PLA2 is shown in . The MCW makes three water-mediated hydrogen bonds with enzyme residues of Arg 6, Cys 23 and Gly 30. It also makes 22 van der Waals’ contacts (≤4 Å) with surrounding residues of Arg 6, Ile 9, Pro 18, Leu 19, Phe 22, Tyr 28, Gly 30 and Tyr 69.
Figure 2. Electron density map of MCW bound at the active site of PLA2. Dashed lines indicate three water-mediated hydrogen bonds with residues of Arg 6, Cys 23 and Gly 30 of the enzyme.
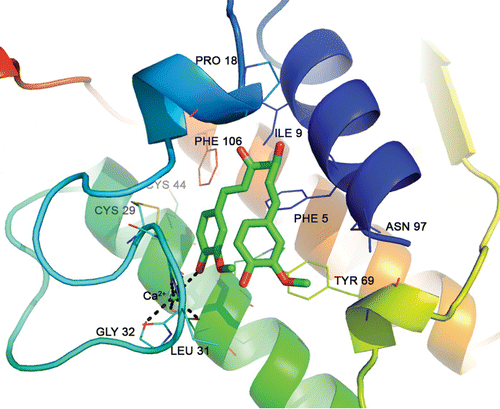
The probable binding mode of curcumin at the active site of the PLA2 was deduced by in silico docking studies and is shown in . In the docked structure, one-half portion of the curcumin molecule was located in the hydrophobic channel and the remaining half was protruded in the bulk solvent. This makes one coordinate bond with catalytic Ca2+ 72 van der Waals’ interactions (≤4 Å) with the surrounding enzyme atoms.
Figure 3. The best docked pose of curcumin at the active site of PLA2. Dashed lines indicate hydrogen bonds.
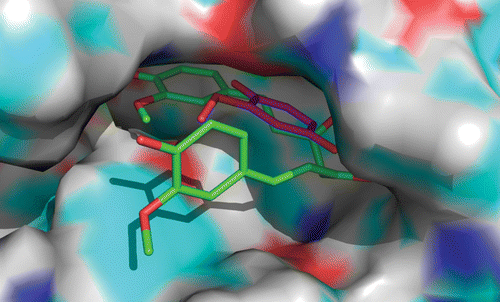
The relative position and orientation of curcumin and MCW are shown in . It was found that the aromatic ring of both curcumin and MCW are aligned in the same plane. Even though the aromatic ring of curcumin was found to pierce deeply into the active site cleft in comparison to that of MCW, the methoxy groups of both the ligands are oriented towards the catalytic calcium. The distance between methoxy groups was found to be 3.0 Å.
Conclusions
Residues such as His 48, Asp 99 and Ca2+ are involved in the catalytic mechanism of pancreatic sPLA2 (Berg et al. Citation2001). Any ligand that prevents the entry of the substrate to the active site can function as an inhibitor of PLA2. The binding of curcumin probably prevents the entry of the substrate into the active site and might be acting as an inhibitor of PLA2. Turmeric has a long history of use in the Ayurvedic medicines for the treatment of inflammatory disorders and has been reported to possess multifunctional bioactivity. This study gives a possible mechanism of anti-inflammatory activity of turmeric.
Acknowledgements
The authors gratefully acknowledge G.N. Ramachandran Protein Crystal Data Collection Facility, CAS in Crystallography & Biophysics, University of Madras, for the X-ray diffraction data collection and the Bioinformatics Infrastructure Facility (supported by DBT, Government of India) at the Department of Biotechnology and Microbiology, Kannur University, for the computational work. K.V. Dileep and P.K. Mandal thank ICMR and CSIR for their Senior Research Fellowships.
References
- Anderson , K M , Roshak , A , Winkler , J D , McCord , M and Marshall , L A . 1997 . Cytosolic 85-kDa phospholipase A2-mediated release of arachidonic acid is critical for proliferation of vascular smooth muscle cells . J Biol Chem. 272:30504–30511. ,
- Berg , O G , Gelb , M H , Tsai , M D and Jain , M K . 2001 . Interfacial enzymology: the secreted phospholipase A2-paradigm . Chem Rev. , 101 : 2613 – 2654 .
- Brooks , B R , Bruccoleri , R E , Olafson , B D , States , D J , Swaminathan , S and Karplus , M . 1983 . CHARMM: a program for macromolecular energy minimization and dynamics calculations . J Comput Chem. , 4 : 187 – 217 .
- Chainani-Wu , N . 2003 . Safety and anti-inflammatory activity of curcumin: a component of tumeric Curcuma longa . J Altern Complement Med. , 9 : 161 – 168 .
- Chinthalapally , V R . 2007 . Regulation of cox and lox by curcumin: the molecular targets and therapeutic uses of curcumin in health and disease . Adv Exp Med Biol. , 595 : 213 – 226 .
- Dileep , K V , Tintu , I and Sadasivan , C . 2011 . Molecular docking studies of curcumin analogs with phospholipase A2 . Interdiscip Sci. , 3 : 189 – 197 .
- Dijkstra , B W , Renetseder , R , Kalk , K H , Hol , W G and Drenth , J . 1983 . Structure of porcine pancreatic phospholipase A2 at 2.6 A resolution and comparison with bovine phospholipase A2 . J Mol Biol. , 168 : 163 – 179 .
- Emsley , P and Cowtan , K . 2004 . Coot: model-building tools for molecular graphics . Acta Crystallogr D. , 60 : 2126 – 2132 .
- Ewa , S J , McCabe , P N , Steven , H S and Jankun , J . 2000 . Curcumin inhibits lipoxygenase by binding to its central cavity: theoretical and X-ray evidence . Int J Mol Med. , 6 : 521 – 526 .
- Huang , M T , Newmark , H L and Frenkel , K . 1997 . Inhibitory effects of curcumin on tumorigenesis in mice . J Cell Biochem. , 27 : 26 – 34 .
- Julie , A G , Glenn , M S , Richard , B , Richard , L , Ken , Y H , Ivan , A R and Kieran , F S . 1991 . Circulating phospholipase A2 activity associated with sepsis and septic shock is indistinguishable from that associated with rheumatoid arthritis . Inflammation. , 15 : 355 – 367 .
- Kudo , I and Murakami , M . 2002 . Phospholipase A2 enzymes . Prostaglandins Other Lipid Mediat. , : 68 – 69:3–58 .
- Leslie , AG W . 2006 . The integration of macromolecular diffraction data . Acta Crystallogr D. , 62:48–57
- Menon , V and Sudheer , A R . 2007 . Antioxidant and anti-inflammatory properties of curcumin . Adv Exp Med Biol. 595:105–125. ,
- Nirmal , N , Om Prabha , G and Velmurugan , D . 2008 . Modeling studies on phospholipase A2-inhibitor complexes . Indian J Biochem Biophys. , 45 : 256 – 262 .
- Pruzanski , W , Vadas , P and Browning , J . 1993 . Secretory non-pancreatic group II phospholipase A2: role in physiologic and inflammatory processes . J Lipid Mediat. , 8 : 161 – 167 .
- Srimal , R C and Dhawan , B N . 1973 . Pharamacology of diferuloyl methane: a non steroidal anti-inflammatory drug . J Pharm Pharmacol. , 25:447
- Vagin , A and Teplyakov , A . 2000 . An approach to multi-copy search in molecular replacement . Acta Crystallogr D. , 56 : 1622 – 1624 .
- Verheij , H M , Volwerk , J J , Jansen , EHJ M , Puyk , W C , Dijkstra , B W , Drenth , J and de Haas , G H . 1980 . Methylation of histidine-48 in pancreatic phospholipase A2. Role of histidine and calcium ion in the catalytic mechanism . Biochem. , 19 : 43 – 50 .