Abstract
Plants and fungi are able to move to perform essential functions. When these functions rely on insects, e.g. the nutrition uptake of carnivorous plants such as Dionaea, Aldrovanda, and Utricularia or the cross-pollination mechanism of Stylidium, then these movements must be both very rapid (faster than the second time scale) and repetitive. This paper discusses the different strategies these plants have developed to achieve this goal, and is aimed at pointing out how intricate the involved biological, chemical and physical mechanisms are. Before presenting some recent advances in the modelling of these plants, the paper first describes the functioning of motor cells, which is based on the equilibration of osmotic pressures, as well as the electric signalling through the propagation of action potentials, by which the stimulation due to the insect is transmitted to these motor cells. The paper then discusses the mechanisms these plants use to perform movements well below the second time scale, which are essentially based on the physical phenomenon known as snap-buckling in material sciences. The Conclusions mention several points that are not well understood and would clearly deserve further attention.
Introduction
From the biological point of view, plant movements are usually classified according to four main characteristics: (1) their active (the plant physiologically drives its own movement) or passive (the surrounding imposes the movement to the plant) character, (2) their reversibility (the movement can be performed forth and back several times) or irreversibility (the movement can be performed only once in a given direction), (3) their nastic (the plant responds non-directionally to the stimulus) or tropic (the response of the plant depends on the position of the stimulus) character and (4) their characteristic times (from hours for the growth of shoots down to microseconds for the ejection of seeds and spores).
Not all of these characteristics are independent. In particular, there is a marked correlation between the time scale of the plant movement and its reversible or irreversible character. Indeed, fastest movements are usually obtained through explosive dehiscence, i.e. the spontaneous and very rapid opening at maturity of certain structures, such as fruits, anthers or sporangia. For example, the sandbox tree, Hura crepitans, can fling seeds as far as 100 m away with a discharge time of about 100 μs. These movements are exceedingly fast but take place only once (they are irreversible) because plant tissues are damaged during the process. Repetitive and reversible movements therefore necessarily rely on different mechanisms, which are order(s) of magnitude slower. For example, the centimetre-sized leaves and leaflets of Mimosa pudica can fold in about 1 s after reception of an external stimulus (e.g. touch, heat, light change, etc.) because of groups of antagonistic specialized cells that are located in ‘hinges’ called pulvini. Upon stimulation, the cells on one side of the pulvinus swell and those on the other side shrink, thereby actuating the movement (Tamiya et al. 1988).
Several functions, however, require movements that are both repetitive and faster than the second time scale. This is especially the case for those functions that rely on insects either as nutrition sources or as vectors to exchange pollen with other plants. For example, carnivorous plants that use active traps (as opposed to sticky ones) must obviously move faster than insects, in order to catch them, but each trap must generally also be able to trap several (many) insects (to extract enough resources from the preys so as to overcome the cost of building and maintaining the traps). This paper focuses precisely on these rapid and active reversible movements, which require (1) a mechanism of detection and transmission of a stimulus, (2) an internal active motor to change the equilibrium state of the plant, both for the rapid and the slow recovering state and (3) a mechanism to amplify the speed of the internal motion during the rapid phase. Discussion will focus on the examples of Dionaea (Droseraceae s.s.), Aldrovanda (Droseraceae s.s.), Utricularia (Lentibulariaceae s.s.) and Stylidium (Stylidiaceae s.s.), whose rapid, active and reversible movements have been the subject of detailed experimental studies. The paper emphasizes how intricate the involved biological, chemical and physical mechanisms are.
The remainder of this paper is organized as follows: the next section describes the fast movements of Dionaea, Aldrovanda, Utricularia and Stylidium. The ‘Active cells and the equilibration of osmotic pressures’ section describes the functioning of the active cells that enable these fast movements, which is based on the equilibration of osmotic pressures. Then the ‘Detection and electrical signalling’ section points out that these plants usually do not fire at random but rather when touched by an insect, which stimulation is transmitted to the motor cells by electrical signals. Next ‘Snap-buckling as a speed booster’ details the mechanisms these plants use to accelerate movements well below the second time scale, which are essentially based on the physical phenomenon known as snap-buckling in material sciences. Finally, ‘Conclusions’ mentions those points that to my knowledge are not (or less) understood and would clearly deserve further attention from the whole scientific community, including biologists, chemists and physicists.
Dionaea, Aldrovanda, Utricularia and Stylidium
Let us first discuss succinctly the four plants that are studied in this paper. Three of these, Dionaea muscipula, Aldrovanda vesiculosa and Utricularia, are carnivorous plants, which move fast in order to catch and trap insects. The fourth one, Stylidium, is only marginally carnivorous and its fast movement is actually a pollination mechanism.
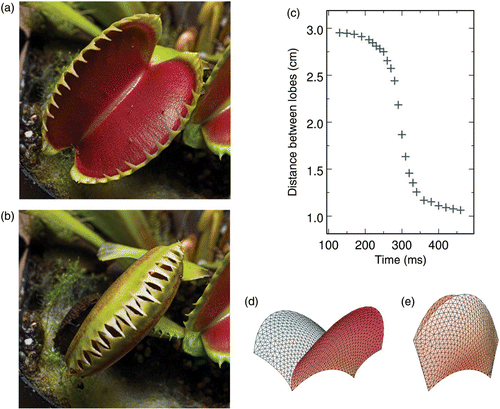
D. muscipula (Juniper et al. 1989), which was called as ‘one of the most wonderful plants in the world’ by Darwin (Darwin 1875), is certainly the best known one. It is a small perennial herb from North America, whose leaves are divided into a lower part for photosynthesis and an upper part for prey capture. The trap, i.e. the upper part of the leaf, has a size in the range of 2–6 cm and consists of a pair of trapezoidal lobes held together along a midrib ( and ). These lobes snap in about 100–700 ms when one of the trigger hairs located close to their centre is stimulated ().
Figure 1. (a, b) Photographs of the upper part of a Dionaea muscipula leaf in open and closed configurations, respectively (photographs © Dr Barry Rice, Sierra College, Rocklin, CA, USA). The trapezoidal lobes are approximately 4 cm wide. (c) Observed time evolution of the distance between the lobes after stimulation of the trigger hairs located close to their centre. (d, e) Models of the trap in open and closed configurations, respectively (adapted from Poppinga and Joyeux (2011)).
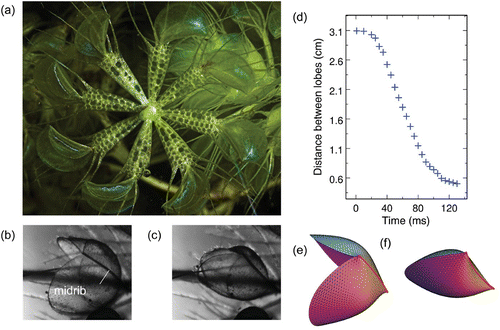
A. vesiculosa (Juniper et al. 1989) has repeatedly been described as ‘a miniature, aquatic Dionaea’ (Darwin 1875). Although both plants are indeed closely related from the evolutionary point of view (Cameron et al. 2002), this description is nevertheless misleading (discussed later in the paper). A. vesiculosa is a rootless, submerged aquatic herb with an almost worldwide distribution, which develops whorls of seven or eight leaves per node (). Each leaf consists of a pair of oval lobes that are 4–7 mm long ( and ); these lobes shut in about 100 ms upon excitation of one of the 20 sensitive hairs located on the inner surface of each lobe ().
Figure 2. (a) Photograph of a node of an Aldrovanda vesiculosa whorl with eight leaves (photograph © Dr Barry Rice, Sierra College, Rocklin, CA, USA). (b, c) Microphotographs of a trap in open and closed configurations, respectively. The oval lobes are approximately 5 mm long. (d) Observed time evolution of the distance between the lobes after excitation of one of the 20 sensitive hairs located on their inner surfaces. (e, f) Models of the trap in open and closed configurations, respectively. –2(f) are adapted from Poppinga and Joyeux (2011).
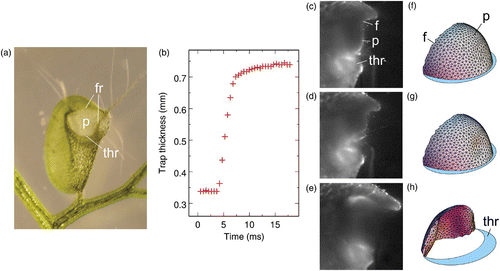
The Utricularia genus comprises more than 220 species with an almost worldwide distribution and exhibits different life forms, both terrestrial and aquatic. All species are completely rootless and all aquatic species feature submersed suction traps (Juniper et al. 1989). The lenticular Utricularia traps are millimetre-sized and have an opening, which is most of the time covered by a semi-circular curtain-like door (). When a small aquatic animal touches one of the trigger hairs attached close to the centre of the door, the door opens in a few milliseconds and water and the prey are engulfed inside the trap, and the door closes again (–3(e)).
Figure 3. (a) Photograph of a trap of Utricularia inflata in set configuration (adapted from Vincent et al. (2011)). The trap is approximately 3 mm wide. The concave curvature of the walls is due to the lower internal pressure in set configuration. (b) Observed time evolution of the trap thickness following manual stimulation of one of the trigger hairs. (c–e) High-speed photographs of the door opening of an Utricularia australis trap following manual stimulation of one of the trigger hairs. (d, e) Photographs recorded 7.6 and 8.6 ms after (c), respectively. The door is approximately 300 μm wide. (f–h) Models of the trap door in set condition, at the onset of buckling, and while swinging open, respectively. –3(h) are adapted from Joyeux et al. (2011). ’thr’, ’p’ and ’f’ denote the threshold, panel of the door and frame of the door, respectively.
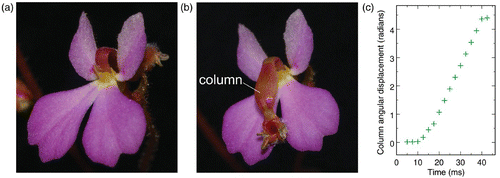
As all members of the small Stylidiaceae family, which is essentially confined to Australia, Stylidium (Darnowski 2002) is characterized by having the stamens fused to the style to form a single column. Following stimulation by a nectar-gathering insect, this column flips in 20–30 ms by 200–300° () from the anterior petal () to the posterior ones (). During this travel, the column exchanges pollen with the insect.
Figure 4. (a, b) Photographs of a Stylidium turbinatum flower, with the column in set configuration and just after firing, respectively (photographs © Holger Hennern, Botanischer Garten Wuppertal, Wuppertal, Germany). The flower is approximately 5 mm wide. (c) Observed time evolution of the angular displacement of the column after firing. Adapted from figure 8 of Findlay and Findlay (1975). The angle is 0° when the column rests against the labellum.
Active cells and the equilibration of osmotic pressures
Plants that are capable of rapid and reversible movements usually have active cells, whose functioning usually relies on the chemical concept of equilibration of osmotic pressures. Generally speaking, osmosis describes the movement of solvent molecules through a selectively permeable membrane into a region of higher solute concentration so as to equalize the solute concentration on the two sides. In plants, cell walls are usually permeable to water but not to ions such as K+ or Cl−. Active cells work by transporting such ions through their walls against a chemical gradient, which of course requires a continuous supply of metabolic energy. Water passively follows these ions to equilibrate the ion concentration. The role of active cells may, however, differ from one plant to another.
In Utricularia, active cells pump water out of the trap interior. This process, which lasts about 1 h, has two consequences. First, the hydrostatic pressure inside the trap drops below that outside the trap by about 10–20 kPa (Sydenham and Findlay 1973; Sasago and Sibaoka 1985a, 1985b). Second, the concave wall curvature due to the lower internal pressure results in elastic energy being stored in the wall (Joyeux et al. 2011; Vincent et al. 2011). When the door opens, the walls of the trap release the stored energy and relax to their equilibrium positions, thereby causing water and the prey to engulf in the trap. How these cells exactly work is still somewhat hypothetical, but it seems probable that certain glands secret salt (Sasago and Sibaoka 1985a) and that Cl− ions are actively transported through the walls of the trap thanks to a continuous supply of ATP (Sydenham and Findlay 1973). As already mentioned, water moves passively after chlorides, so that the volume of water inside the trap diminishes.
In Dionaea, Aldrovanda and Stylidium, bending movements are instead produced by changes in volume and length of certain motor cells relative to antagonistic motor cells or to neighbouring non-motor cells of fixed volume (Hill and Findlay 1981). In Aldrovanda and Stylidium, and maybe also in Dionaea, changes in volume and length are driven by the transport of K+ ions. Because water moves after these ions to keep the osmotic pressure constant, a net flux of K+ ions into a cell causes this cell to swell, whereas a net efflux conversely causes the cell to shrink. Motor tissues and transport of K+ ions have been described for all three plants, Dionaea (Iijima and Hagiwara 1987), Aldrovanda (Ashida 1934; Iijima and Sibaoka 1983), and Stylidium (Findlay and Findlay 1984). K+ transport across the cell membrane, and the associated variation of osmotic pressure, is an active, energy-consuming mechanism during the slow setting phases of these plants, i.e. during the time it takes for the lobes of Dionaea and Aldrovanda to (re)open and for the column of Stylidium to reset back to its original position (Findlay and Findlay 1981). In contrast, the fast snapping phase may (e.g. for Aldrovanda (Iijima and Sibaoka 1983)) or may not (e.g. for Stylidium (Findlay and Findlay 1984)) involve significant transport of ions through the membrane of motor cells. It is also probable that it does not require substantial supply of metabolic energy. Actually, for these three plants, just as for Utricularia, snapping rather appears as the mere release, upon stimulation, of the potential energy stored in the cellulose walls of the motor cells and the surrounding tissues during the slow, active setting phase and its conversion to kinetic energy for the moving part of the plant. How this conversion takes place and leads to such fast movements will be discussed next.
Detection and electrical signalling
In addition to active cells, plants capable of fast movements also have in common the fact that they do not fire at random, which would be highly inefficient, but instead, in most cases, when they are touched by an insect. Such stimulation must be transmitted to the motor cells, which are in charge of actuating the movement. The point is that chemical signalling by hormones, either by cytoplasmic streaming within cells or by diffusion between cells, would be much too slow for any practical purpose. Most of fast-moving plants therefore use electrical signalling.
The detection/signalization mechanism is rather elaborate in Dionaea and Aldrovanda. Indeed, for these two plants, perception is localized in sensitive hairs, which are located on the inner surfaces of the traps. There are three hairs per lobe for Dionaea and about 20 of them for Aldrovanda. At the base of each sensitive hair, a layer of 20–30 cells act as mechanoreceptors and produces a graded receptor potential upon stimulation (Benolken and Jacobson 1970). If this receptor potential becomes larger than a certain threshold, it gives rise to an action potential that, once evoked, spreads without attenuation from cell to cell throughout the entire trap (Iijima and Sibaoka 1982; Hodick and Sievers 1988). Such electric signals transmit information over ‘long’ distances at speeds up to 25 cm s−1, i.e. much more rapidly than chemical signals. It has furthermore been suggested that the influx of Ca2+ ions associated with membrane excitation by an action potential might activate the membrane ATP-ase, which is responsible for K+ transport and therefore motor cells activation (Iijima and Sibaoka 1985; Sibaoka 1991).
The case of Utricularia is less clear. There are several trigger hairs attached close to the centre of the door, and the stimulation of which provokes the opening of the door and the firing of the trap. What is unclear, however, is whether these hairs act as mechanical levers, which deform the thin door, or as mechanoreceptors similar to those of Dionaea and Aldrovanda traps, which trigger a receptor potential and eventually an action potential when bent (Juniper et al. 1989).
At last, it appears that, as in Mimosa, no differentiated receptor tissue is found in Stylidium. In most species, the firing of the column is simply initiated by the rubbing and sliding of the proboscis of the insect against the posterior side of the column while it reaches for nectar at the base of the corolla tube (Findlay and Findlay 1975). It is tempting to hypothesize that, as in Mimosa, an action potential is nevertheless evoked by this stimulation and propagates in the column.
Snap-buckling as a speed booster
After having established that fast-moving plants do have active cells and are able to detect and eventually propagate stimulation signals, let us consider the mechanisms they have developed to move faster than allowed by the simple swelling/shrinking mechanism of antagonistic motor cells. In most, but not all (see later), cases, this mechanism relies on snap-buckling. Generally speaking, buckling is characterized by the very sudden failure of an object submitted to a stress smaller than the ultimate stress that it is capable of withstanding. Snap-buckling is observed, e.g. for an elastic column with fixed ends forming a shallow arch and submitted to a perpendicular load. The column deforms little up to a critical load, while it rapidly snaps to an inverted curvature above this threshold. The cap of a sliced tennis ball provides yet another example of snap-buckling, which is rather close to the one displayed by the carnivorous plants discussed in this paper. When held by the circular edge and submitted to thumb pressure, the initially convex (dome-shaped) cap deforms little up to a critical pressure, while it flips rapidly to the concave (bowl-shaped) state above this threshold.
At this point, we shall consider that plant tissues are made of a homogeneous, thin, solid and elastic material. Let us recall briefly that such a material is characterized by its Young's modulus E (also known as the elastic modulus or modulus of elasticity), which is a measure of its stiffness, and is defined as the ratio of the uniaxial stress over the uniaxial strain in the range of strain where the linear Hooke's law holds. According to the point of view of cellular materials (Gibson and Ashby 1999; Gibson et al. 2010), the Young's modulus of the tissue scales as the bulk modulus of the cells it is made of, i.e. as the parameter that characterizes how changes in cell volume are related to turgor pressure variations. Both ϵ and E have typical values in the range of 1–50 MPa (Steudle et al. 1977; Cosgrove 1988). The elastic energy of a homogeneous thin solid shell with Young's modulus E can be decomposed into a strain contribution, which arises from in-plane deformations and is proportional to Eh, and a curvature contribution, which arises from out-of-plane deformations and is proportional to Eh
3, where h is the thickness of the shell (Niordson 1985). Plant tissues are also characterized by a poroelastic time
, which denotes the time it takes for water to cross a length ℓ of the porous tissue diffusely, and may be interpreted as the fastest possible water-driven movement at the tissue level (Skotheim and Mahadevan 2005). Because for plant tissues the typical values of the Darcy permeability k and water viscosity η are of the order of 10−20–10−19 m2 and 10−3 Pa s, respectively, this indicates that plant movements faster than 1 s imply water displacements ℓ shorter than about 50 μm.
Aldrovanda’s mechanism is certainly the simplest one among the four plants that are discussed in this paper. (Ashida 1934) suggested that motor tissues of this plant are located on both sides of the midrib and parallel to it. An action potential triggers a rapid migration of K+ ions (and therefore water) from the interior of these cells to outside water (Iijima and Sibaoka 1983). The active motor cells consequently lose their turgor, which allows the other cell layers to bend the lobes. Note that water initially enclosed in the motor cells does not have to travel a long way: it indeed only has to cross the membrane of motor cells for the movement to take place. Conversely, the uptake of K+ ions and water by motor cells during the resetting phase forces the lobes to open against the opposite bending force exerted by the remainder of the tissue, a slow process that requires energy supply (Iijima and Sibaoka 1983).
We recently confirmed this scenario by modelling Aldrovanda’s trap as a thin solid shell with a leaf-like geometry described by a triangulated surface (Poppinga and Joyeux 2011). The midrib that separates the lobes is bent inward in the closed configuration ( and ). Starting from the initial value, the curvature of the midrib was regularly decreased (this is a simple and convenient way to account for the action of the motor cells located on both sides of the midrib) and the minimum energy configuration was sought for each different curvature. It was found that the two lobes open rather quickly with decreasing midrib curvature ( and ) and that the potential energy is a smooth function thereof. Closure of the lobes consequently follows essentially the same pathway in the reverse direction. The model therefore strongly suggests that snapping in Aldrovanda is not based on buckling and that Aldrovanda should indeed be classed in the set of swelling and shrinking dominated plants (Skotheim and Mahadevan 2005), although active motor cells all work together instead of being divided into two antagonistic groups, as in Mimosa pulvini. The model moreover points out that snapping of the lobes of Aldrovanda’s traps is greatly accelerated by a unique feature, namely the kinematic amplification of the bending deformation of the midrib. It indeed appears that the geometry of the leaves is optimized in order that a minute displacement of the midrib is sufficient to trigger a large opening or closing of the lobes.
Although other mechanisms have been proposed (e.g. Williams and Bennet 1982), it is probable that the traps of Dionaea snap according to the mechanism already proposed by Darwin (Darwin 1875). Darwin indeed established that the upper leaves of Dionaea include two distinct cell layers – a point confirmed by several authors (Mozingo et al. 1970; Fagerberg and Allain 1991) – and that snapping of the traps is due to the differential behaviour of these two layers, which causes the leaf curvature to transform from convex to concave ( and ). More specifically, Darwin proposed that, upon stimulation, the motor cells at the inner surface of the lobes release a certain amount of water and shrink, while the motor cells at the outer surface take up this water and swell (Darwin 1875; Brown 1916). More recent experiments suggest instead that the inner epidermis of the Dionaea trap lobes does not shrink and that the expansion of the outer surface is in part irreversible (Hodick and Sievers 1989; Forterre et al. 2005), but this actually results in a comparable change in curvature. The point, anyway, is that upper Dionaea leaves are about 400 μm thick. It is therefore probable that the swelling/shrinking mechanism is in itself not sufficient to explain the closure of the traps in about half a second, while irreversible growth is even much slower. On the basis of experimental observations, as well as the predictions of a homogeneous paraboloid model with quadratic energy, it was recently suggested that closure times shorter than the poroelastic time may actually be allowed by an actively controlled snap-buckling instability (Forterre et al. 2005). This suggestion has, however, been questioned (Markin et al. 2008; Volkov et al. 2008).
To solve this question, we also modelled the trap of Dionaea as a thin solid shell with a leaf-like geometry described by a triangulated surface (Poppinga and Joyeux 2011). The fact that each lobe contains two different cell layers mechanically connected to each other was modelled by building two virtual meshes separated from the original one by h/2, on both sides of the normal to the surface at the original vertices. Moreover, the effect of turgor pressure variations proposed by Darwin was introduced in the form of a strain parameter s, which states that the equilibrium dimensions of the outer virtual mesh are multiplied by a factor √ 1−s/2, and those of the inner one by a factor √ 1+s/2, compared to the reference configuration (note that we could equivalently have used a factor √ 1−s for the outer layer and a factor 1 for the inner layer to account for the alternative scenario proposed by Forterre et al. (2005)). In this model, elastic strain and curvature energies are consequently coupled, because for negative s, the shell tends to bend outwards in order to increase the surface of the outer layer and eventually decrease that of the inner layer (and conversely for positive s). The open configuration, which is reached during the slow setting phase, is convex ( and ). Stimulation of a trigger hair leads to a decrease of s (because of a water flow from the inner to the outer motor cell layers, according to Darwin's questioned proposition). The lobes consequently tend to bend outwards, but their convex geometry opposes this trend and thus an increasing amount of strain energy is stored in the leaf. This first step is not very fast (takes several hundreds of milliseconds). However, at some given value of s, the buckling threshold is reached and the convex geometry becomes unstable. The leaves buckle and release most of the strain energy they had stored by changing their curvature from convex to concave (). Although it accounts for the largest part of the displacement of the leaves, this step is much faster than the first one (around 10 ms, ) because it is driven essentially by snap-buckling. A third step, driven as the first one by an active diminution of s, is necessary to finish closing the door (). Curves showing the time evolution of the curvature of the lobes obtained with this model are in fair agreement with experimental ones (Poppinga and Joyeux 2011). The elastic model therefore confirms that the buckling instability indeed plays a major role in the fast snapping of Dionaea’s traps.
Suction traps, which are found exclusively in Utricularia, certainly belong to the most complex leaf structures described till now. As already mentioned, the thin walls of the bladders become concave and elastic energy is stored in the shell during the slow setting phase, where active cells pump water from the inside to the outside of the utricule (). As the door opens following stimulation of the trigger hairs, the sudden release of this energy enables water to be sucked inside the trap. The entire mechanism therefore rests on the ability of the door to open very rapidly upon stimulation. Keeping with the woodwork terminology used in our previous study (Joyeux et al. 2011), the door consists of three essential parts, namely the frame, the threshold and the moving panel ( and ). The sides of the panel are continuously attached to the frame, while its bottom rests freely on the threshold in set conditions. The threshold and the frame form a massive thickening, which prevents any distortion of the shape of the opening during deflation and resists crumpling in set conditions. Moreover, the walls of the trap are thinner on the line where they connect to the frame and the threshold so that they anyway transmit little stress to the door during the deflation phase (Juniper et al. 1989). The door can therefore be considered as an isolated system with fixed boundaries, which is submitted only to pressure and internal elastic forces. High-speed videos show that opening of the door is exceedingly fast. For example, –3(e) indicate that it takes less than 1 ms for the panel of an Utricularia australis trap to switch from the closed to the open configuration (Joyeux et al. 2011; Vincent et al., 2011) – and yet the traps of U. australis certainly do not belong to the fastest ones of the Utricularia species (see also Singh et al. (2011)). Closer examination of suggests that the swinging of the panel is preceded by localized curvature inversion and that the door therefore acts as a flexible valve that buckles under the effect of pressure forces rather than as a panel articulated on hinges.
In order to confirm this hypothesis, we modelled the panel of the door as a thin solid shell having the shape of a quarter of an ellipsoid. One of the limiting ellipses represents the frame and is kept fixed in the simulations. The other limiting ellipse represents the free edge of the panel. At equilibrium, this edge rests on a planar surface, which represents the threshold (). The pressure inside the door was decreased slowly with respect to the pressure outside. For a Young's modulus E close to 3 MPa, we observed that the door deforms very little up to a pressure difference of about 16 kPa, in agreement with experimental results (Sydenham and Findlay 1973; Sasago and Sibaoka 1985a, 1985b), while for larger pressure differences, the panel buckles, slides on the threshold and finally swings wide open (Joyeux et al. 2011; Singh et al. 2011). shows the first indentation that appears in the panel, while shows the panel opening wide. The model therefore strongly supports the hypothesis that the door acts as a flexible valve rather than a door articulated on hinges. In real Utricularia traps, the pressure difference remains almost constant (just below the critical value) once the trap is set and the mechanism is usually fired by a small animal touching the trigger hairs. As already mentioned, the question of whether the onset of buckling is purely mechanical (the hairs deform slightly the panel tissue) or is driven by an action potential and the subsequent softening of the panel tissue is still debated. It is also interesting to note that the door deforms very little up to the critical pressure, while the trap walls deform continuously and become concave when the pressure inside the trap is decreased. This marked difference is due to the respective geometries of the body and the door. At equilibrium, the door is indeed convex everywhere and behaves much like a sphere, which sustains pressure without deforming much up to the critical pressure where it buckles. In contrast, the walls of the trap display regions with negative curvature, which act as seeds from which deflation propagates continuously when water is pumped outside the trap.
Finally, it should be mentioned that the fast movement of the column of Stylidium () is still not completely understood. It has been shown that there are considerable difficulties in accounting for the 20–30 ms swinging of the column on the basis of the sole variation of osmotic pressure in particular cells of the motor tissues (Hill and Findlay 1981). This indicates that snap-buckling is certainly involved, as for Dionaea and Utricularia. It has, however, also been shown that the transport of K+ ions in motor tissues is quite intricate (Findlay and Findlay 1984). Any realistic mechanical model of this plant should be able to cope with these results. We are currently working on this question.
Conclusions
This paper focused on fast and repetitive plant movements, from the stimulation by an insect or a small aquatic animal to the movement actuation by active cells and its eventual acceleration by snap-buckling. Although a few questions could recently be dealt with thanks to the collaboration between biologists and physicists, many of them remain unsolved. Just to quote a few open questions, related to this paper, the mechanism for generating the receptor potential has, e.g. not been established. Similarly, the origin of the ‘memory’ of Dionaea, which allows it to move only when the first stimulation is followed by a second one less than 20 s later, is not clear. More generally, the transport of K+ ions through the cell membrane is poorly understood, as well as its activation by an electric potential. It should also be stressed that, although it is very fast, the closing dynamics of all the plants discussed in this article is still much slower than the predictions of purely inertial thin shell models (Joyeux et al. 2011; Poppinga and Joyeux 2011). This indicates that the closure movement is strongly damped by passive flows, which are induced inside the porous tissue by the bending motion and create viscous resistance (Forterre et al. 2005). Hydraulic movements at the cellular level, in connection with the material properties of cell walls, is certainly a field that would deserve more attention (Dumais and Forterre 2012).
A recent biological review on carnivorous plants (Krol et al. 2012) also concluded with a similar list of questions under scrutiny, including negative excitability–photosynthesis coupling, enzyme secretion, nutrient absorption, food web relationship and phylogenetic and intergeneric relationships. I am therefore convinced that a comprehensive understanding of these fascinating osmotic machines will require combined efforts from all fields of sciences, biology, chemistry and physics.
References
- Ashida , J . 1934 . Studies on the leaf movement of Aldrovanda vesiculosa L. I. Process and mechanism of the movement . Mem Coll Sci Univ Kyoto Ser. B , 9 : 141 – 244 .
- Benolken , R M and Jacobson , S L . 1970 . Response properties of a sensory hair excised from Venus’ fly-trap . J Gen Physiol. , 56 : 64 – 82 .
- Brown , W H . 1916 . The mechanism of movement and the duration of the effect of stimulation in the leaves of Dionaea . Am J Bot. , 3 : 68 – 90 .
- Cameron , K M , Wurdack , K J and Jobson , R W . 2002 . Molecular evidence for the common origin of snap-traps among carnivorous plants . Am J Bot. , 89 : 1503 – 1509 .
- Cosgrove , D J . 1988 . “ In defense of the cell volumetric elastic modulus ” . In Plant Cell Environ Vol. 10 , 67 – 69 .
- Darnowski , D W . 2002 . “ Triggerplants ” . Dural , , Australia : Rosenberg Publishing .
- Darwin , C . 1875 . “ Insectivorous plants ” . London : John Murray .
- Dumais , J and Forterre , Y . 2012 . “ ‘Vegetable dynamicks’: the role of water in plant movements ” . In Annu Rev Fluid Mech Vol. 44 , 453 – 478 .
- Fagerberg , W R and Allain , D . 1991 . A quantitative study of tissue dynamics during closure in the traps of Venus's flytrap . Dionaea muscipula Ellis. Am J Bot. , 78 : 647 – 657 .
- Findlay , G P and Findlay , N . 1975 . Anatomy and movement of the column in Stylidium . Aust J Plant Physiol. , 2 : 597 – 621 .
- Findlay , G P and Findlay , N . 1981 . Respiration-dependent movements of the column of . Stylidium graminifolium. Aust J Plant Physiol. , 8 : 45 – 56 .
- Findlay , N and Findlay , G P . 1984 . Movement of potassium ions in the motor tissue of Stylidum . Aust J Plant Physiol. , 11 : 451 – 457 .
- Forterre , Y , Skotheim , J M , Dumais , J and Mahadevan , L . 2005 . How the Venus flytrap snaps . Nature. , 433 : 421 – 425 .
- Gibson , L J and Ashbyn , M F . 1999 . “ Cellular solids: structure and properties ” . Cambridge : Cambridge University Press .
- Gibson , L J , Ashbyn , M F and Harley , B A . 2010 . “ Cellular materials in nature and medicine ” . Cambridge : Cambridge University Press .
- Hill , B S and Findlay , G P . 1981 . The power of movement in plants: the role of osmotic machines . Q Rev Biophy. , 14 : 173 – 222 .
- Hodick , D and Sievers , A. 1988 . The action potential of Dionaea muscipula Ellis . Planta. , 174 : 8 – 18 .
- Hodick , D and Sievers , A . 1989 . On the mechanism of trap closure of Venus flytrap (Dionaea muscipula Ellis) . Planta. , 179 : 32 – 42 .
- Iijima , T and Hagiwara , S . 1987 . Voltage-dependent K channels in protoplasts of trap-lobe cells of . Dionaea muscipula.J Membr Biol. , 100 : 73 – 81 .
- Iijima , T and Sibaoka , T . 1982 . Propagation of action potentials over the trap-lobes of Aldrovanda vesiculosa . Plant Cell Physiol. , : 679 – 688 .
- Iijima , T and Sibaoka , T . 1983 . Movements of K+ during shutting and opening of the trap-lobes in Aldrovanda vesiculosa . Plant Cell Physiol. , 24 : 51 – 60 .
- Iijima , T and Sibaoka , T . 1985 . Membrane potentials in excitable cells of Aldrovanda vesiculosa trap-lobes . Plant Cell Physiol. , 26 : 1 – 13 .
- Joyeux , M , Vincent , O and Marmottant , P . 2011 . Mechanical model of the ultrafast underwater trap of Utricularia . Phys Rev E. , 83 : 021911
- Juniper , B E , Robins , R J and Joel , D M . 1989 . “ The carnivorous plants ” . London : Academic Press .
- Krol , E , Plachno , B J , Adamec , L , Stolarz , M , Dziubinska , H and Trebacz , K . 2012 . Quite a few reasons for calling carnivores ‘the most wonderful plants in the world’ . Ann Bot. , 109 : 47 – 64 .
- Markin , V S , Volkov , A G and Jovanov , E . 2008 . Active movements in plants. Plant Signal Behav . 3 : 778 – 783 .
- Mozingo , H N , Klein , P , Zeevi , Y and Lewis , E R . 1970 . Venus's flytrap observations by scanning electron microscopy . Am J Bot. , 57 : 593 – 598 .
- Niordson , F I . 1985 . “ Shell theory. New York ” . North-Holland
- Poppinga , S and Joyeux , M . 2011 . Different mechanics of snap-trapping in the two closely related carnivorous plants Dionaea muscipula and Aldrovanda vesiculosa . Phys Rev E. , 84 : 041928
- Sasago , A and Sibaoka , T . 1985a . Water extrusion in the trap bladders of Utricularia vulgaris. I. A possible pathway of water across bladder wall . Bot Mag Tokyo. , 98 : 55 – 66 .
- Sasago , A and Sibaoka , T . 1985b . “ Water extrusion in the trap bladders of Utricularia vulgaris. II. A possible mechanism of water outflow ” . In Bot Mag Tokyo , Vol. 98 , 113–124 .
- Sibaoka , T . 1991 . “ Rapid plant movements triggered by action potentials ” . In Bot Mag Tokyo , Vol. 104 , 73–95 .
- Singh , A K , Prabhakar , S and Sane , S P . 2011 . The biomechanics of fast prey capture in aquatic bladderworts . Biol Lett. , 7 : 547 – 550 .
- Steudle , E , Zimmermann , U and Lüttge , U . 1977 . Effect of turgor pressure and cell size on the wall elasticity of plant cells . Plant Physiol. , 59 : 285 – 289 .
- Skotheim , J M and Mahadevan , L . 2005 . Physical limits and design principles for plant and fungal movements . Science. , 308 : 1308 – 1310 .
- Sydenham , P H and Findlay , G P . 1973 . “ The rapid movement of the bladder of Utricularia spp ” . In Aust J Biol Sci , Vol. 26 , 1115– 1126 .
- Tamiya , T , Miyazaki , T , Ishikawa , H , Iriguchi , N , Maki , T , Matsumoto , J J and Tsuchiya , T . 1988 . Movement of water in conjunction with plant movement visualized by NMR imaging . J Biochem. , 104 : 5 – 8 .
- Vincent , O , Weisskopf , C , Poppinga , S , Masselter , T , Speck , T , Joyeux , M , Quilliet , C and Marmottant , P . 2011 . Ultra-fast underwater suction traps . Proc R Soc B Biol Sci. , 278 : 2909 – 2914 .
- Volkov , A G , Adesina , T , Markin , V S and Jovanov , E . 2008 . Kinetics and mechanism of Dionaea muscipula trap closing . Plant Physiol. , 146 : 694 – 702 .
- Williams , S E and Bennet , A B . 1982 . Leaf closure in the Venus flytrap: an acid growth response . Science. , 218 : 1120 – 1122 .