Abstract
In order to contribute to the knowledge of plants from Galician (northwest of the Iberian Peninsula), in the present study, 35 Galician endemic and subendemic plants were examined for their antioxidant activity, total phenolic composition, UV absorption, and skin care properties. The radical scavenging capacity, ferric-reducing antioxidant power, protection against oxidation of the β-carotene/linoleic acid emulsion, antiradical protection of irradiated human skin fibroblast were used to evaluate the antioxidant activity. In several cases, the results of this study strongly indicate in vitro antioxidant activity, which may be due to the presence of a high total phenolic content. Antiradical protection of irradiated human skin fibroblast, stimulation of human skin fibroblast proliferation, and significant absorption of UV radiation were also observed. In order to identify active principles, some extracts were submitted to fractionation and genistein was isolated from Pterospartum tridentatum subsp. tridentatum. The results suggest that some Galician plants extracts have promising skin care properties and could be used as natural antioxidants in cosmetics, pharmaceutical, and food materials.
Introduction
Oxidative stress results from the imbalance between the production of free radicals and the antioxidative defense system in favor of free radicals. Because of the high occurrence of potential biological targets for oxidative damage, skin is very susceptible to such reactions and the functions of fibroblasts as well as the physical and chemical nature of the supporting macromolecules is affected (Kohen Citation1999). Photooxidative damage by UV-induced reactive oxygen species (ROS) has been implied in several pathological states including photoaging and photocarcinogenesis of the skin (Scharffetter-Kochanek et al. Citation1997). A decrease in the ROS load due to the effect of efficient protective agents, such as phenolic compounds, may represent a promising strategy to prevent, or at least minimize, ROS-induced cutaneous pathological states (Ropke et al. Citation2005). The antioxidant activity of phenolic compounds is determined by their molecular structure and the ability to delocalize unpaired electrons, which are used to stabilize the phenoxyl radicals formed after reaction with ROS (Fernández-Panchón et al. Citation2008). Another antioxidant mechanism of phenolic compounds involves their ability to chelate transition metal ions and to alter peroxidation kinetics by modifying lipid packing order and decreasing membrane fluidity (Blokhina et al. Citation2003).
There is currently great worldwide interest in finding new and safe antioxidants from natural sources to prevent or minimize oxidative damage to living cells. On the other hand, there is also interest in new natural products capable of reversing the histological changes induced by UV irradiation and of recovering the dermal structure by increasing fibroblast activity and collagen deposition, e.g. retinoids, resveratrol (Rona et al. Citation2004), aloe vera extracts (Aburjai & Natsheh Citation2003), and Carica papaya seed extracts (Nayak et al. Citation2012).
Mediterranean herbs and aromatic plants, e.g. spike lavender (Lavandula latifolia), common melilot (Melilotus officinalis), fennel (Foeniculum vulgare), milfoil (Achillea millefolium), tarragon (Artemisia dracunculus), and Lavandin cv. Super (Lavandula latifolia × Lavandula angustifolia), have been studied for their radical scavenging and antioxidant activity (Parejo et al. Citation2002). The antioxidant capacity of another species traditionally used in Spain by the rural population has also been studied (López et al. Citation2008).
The aim of this study was to carry out a phytochemical investigation and determine the total phenolic content, UV absorption, and antiradical and antioxidant activity of 35 Galician plants, which are northwest of the Iberian Peninsula endemic or subendemic plants. Because the distribution of phenolic compounds in plants at the tissue, cellular, and subcellular levels is not uniform (Maisuthisakul et al. Citation2008), in several cases, different parts of the plant were studied.
In addition, the human skin fibroblast (HSF) stimulation activity of some Galician plants was also studied.
Materials and methods
Plant material
Armeria pubigera, Linaria polygalifolia, subsp. polygalifolia, Iberis procumbens subsp. microcarpa, and Silene littorea subsp. littorea were harvested fresh from A Lanzada (O Grove, A Coruña, Spain, February 2008). Adenocarpus lainzii and Ulex gallii subsp. breoganii were harvested fresh from O Castrove (Poio, Pontevedra, Spain, February 2008). Pterospartum tridentatum subsp. tridentatum, Sagina merinoi, Saxifraga spathularis, Thymus caespititius, Carex durieui, Carex asturica, Cirsium filipendulum, Phalacrocarpum oppositifolium subsp. oppositifolium (June 2008), and Thymelaea broteriana (March 2009) were harvested fresh from O Candan (Forcarei, Pontevedra, Spain). Armeria merinoi, Santolina melidensis, Xolantha globulariifolia, Alyssum serpyllifolium subsp. lusitanicum, and Centaurea janeri subsp. gallaecica were harvested fresh from Melide (A Coruña, Spain, March 2009). Linaria triornithophora was harvested fresh from Vilasobroso (Pontevedra, Spain, May 2009). Centaurea ultreiae, Thymelaea coridifolia subsp. coridifolia, Erica erigena, and Euphorbia flavicoma subsp. occidentalis were harvested fresh from Monte Castelo (Coristanco, A Coruña, Spain, February 2010). Centaurea borjae, Rumex scutatus subsp. gallaecicus, Leucanthemum pluriflorum, Erica mackaiana, and Angelica pachycarpa were harvested fresh from Punta Candieira (Cedeira, A Coruña, Spain, July 2010). Koeleria maritima and Linaria polygalifolia subsp. aguillonensis were harvested fresh from Cedeira (A Coruña, Spain, July 2010). Echium rosulatum subsp. rosulatum was harvested fresh from Ponteareas (Pontevedra, Spain, May 2011). Armeria transmontana, and Echinospartum ibericum were harvested fresh from Viana do Bolo (Ourense, Spain, June 2011). Samples of the herbarium specimens are deposited in the Lourizán Herbarium (LOU, Centro de Investigación Forestal de Lourizán, Xunta de Galicia, Pontevedra, Spain).
Chemicals
The following chemicals were used for the experimental study: (+/−)-1,3-butanediol, gallic acid, 2,2-diphenyl-1-picrylhydrazy (DPPH) radical, butylated hydroxytoluene (BHT), butylated hydroxyanisole (BHA), 2,2′-azino-bis 3-ethylbenzothiazoline-6-sulfonic acid (ABTS), 6-hydroxy-2,5,7,8-tetramethylchroman-2-carboxylic acid (Trolox), Tween 40, linoleic acid, l-ascorbic acid, phosphate buffer (pH 6.6), α-tocopherol, 2,4,6-tris(2-pyridyl)-s-triazine (TPZ), Trypan blue solution (0.4%), M4655 medium, amino acid solution (M7145), l-glutamine (20 mM, G7513), fetal calf serum (FCS, F7524), penicillin, and streptomycin (P0781); from Sigma Aldrich Chemical Co.; Folin–Ciocalteu's phenol reagent and β-carotene from Fluka; and potassium hexacyanoferrate solution from Riedel de Haën.
Solvent extraction
The plants were frozen using liquid N2 and lyophilized. Twenty-five grams of dried and finely powdered parts of the plants was extracted with 750 mL of ethanol–water (1:1) solution by heating under reflux for 3 h. The extracts were filtered and concentrated by vacuum drying and lyophilized. This extract was the dried extract. One gram of this crude extract was redissolved in 100 mL of 1,3-butanediol–water (1:1) and the materials were extracted by maceration for 7 days. The extracts were filtered to obtain hydroglycolic extracts suitable for cosmetic formulations.
Analytical methods
Determination of total phenolic content
The amount of total phenolic content (TPC) of the plant extracts was determined according to the Folin–Ciocalteu method (Singleton & Rossi Citation1965) and expressed as gram of gallic acid equivalents (GAE) per gram of dry weight of extract. The reaction mixture was composed of 0.5mL of plant extracts (0.250 mg/mL), 3.75 mL of distilled water, 0.25 mL of the Folin–Ciocalteu's phenol reagent, and 0.5 mL of 10% sodium carbonate. The mixture was kept in the dark for 1 h and its absorbance measured at 765 nm in an Agilent 8453E UV–visible spectrophotometer (Agilent Technologies, USA). The percentage of TPC for each plant extract was calculated from calibration curve of gallic acid.
α,α-diphenyl-β-picrylhydrazyl radical scavenging assay
The antioxidant activity of plant extracts was measured in terms of hydrogen donation or free radical scavenging ability by using the α,α-diphenyl-β-picrylhydrazyl (DPPH) stable radical (von Gadow et al. Citation1997). Two milliliters of a 6×10−5 M methanolic solution of DPPH radical was added to 50 μL of a methanolic solution of the antioxidant, and the decrease in the absorbance at 515 nm after 16 min was recorded in an Agilent 8453E UV–visible spectrophotometer. Inhibition percentages (IP) of the DPPH radical for each plant extract were determined according to the following equation:
Ferric-reducing antioxidant power assay
The ability of plant extracts to reduce Fe (III)/tripyridyltriazine complex was assessed by ferric-reducing antioxidant power (FRAP) assay (Benzie & Strain Citation1996). The reactive solution was prepared with 25 mL of 300 mmol/L acetate buffer, 2.5 mL of a 10 mmol/L TPTZ solution in 40 mmol/L HCl and 20 mmol/L FeCl3·6H2O in distilled water. The reactive solution was always prepared fresh and was used as a blank. A 100 μL aliquot plant extract was mixed with 3 mL of FRAP reagent. Deionized water was used as control. The absorbance reading at 593 nm was taken after standing for 6 min. Aqueous solutions of ascorbic acid (vitamin C) of 0.1–1 mM were used for calibration and the reductive activities of the plant extracts have been expressed as ascorbic acid equivalents (AAE) in millimoles of ascorbic acid per gram of dry weight of extract.
Trolox equivalent antioxidant capacity assay
Determination of antioxidant activity with the Trolox equivalent antioxidant capacity (TEAC) assay is based on the reaction between ABTS [2,2′-azinobis(3-ethylbenzothiazoline-6-sulfonate)] and potassium persulfate giving blue/green ABTS radical (ABTS+) (Re et al. Citation1999). ABTS was dissolved in water to a concentration of 7 mM and reacted with 2.45 mM potassium persulfate (final concentration) at a molar ratio of 2:1 to form the ABTS+ radical, and left in dark at room temperature for 12–16 h. The ABTS+ solution was then diluted with phosphate buffer saline (PBS) (pH 7.4) to an absorbance of 0.70 at 734 nm and equilibrated at 30°C. After adding 1.0 mL of diluted ABTS+ solution to 10 μL of plant extracts and Trolox in ethanol, the absorbance reading was taken 1min after initial mixing and up to 10 min. Appropriate solvent blanks were run in each assay. The results have been expressed as gram of Trolox per gram of dry weight of the extract and as gram of Trolox per gram of GAE.
β-carotene bleaching assay
The antioxidant activity of plant extracts and BHA was determined according to a β-carotene bleaching method (Miller Citation1971). The antioxidant activity is measured as the ability of a compound to minimize the coupled oxidation of linoleic acid and β-carotene in an emulsified aqueous system, which loses its orange color when reacting with radicals. An emulsion was prepared as follows: 1 mL β-carotene solution (200 μg/mL chloroform) was added to a volumetric flask, together with 20 mg of linolenic acid and 200 mg of Tween 40. Chloroform was evaporated under vacuum and 50 mL of distilled and oxygenated water was added to the flask under vigorous stirring. Next, 5 mL aliquots of the emulsion was transferred into test tubes containing 0.2 mL of plant extracts, and the test tubes and the control tubes were stoppered and placed in a water bath at 50°C. Absorbance readings at 470 nm were taken at regular intervals until complete decoloration. BHT was used as a reference synthetic antioxidant. The antioxidant activity coefficient (AAC) was calculated according to the following equation:
Reducing power assay
The ability of plant extracts to reduce Fe (III) to Fe (II) was assessed by the method of Oyaizu (Oyaizu Citation1986). This method is relevant due to the role of this cation as an initiator of oxidation processes in organisms. One milliliter of the plant extracts and ascorbic acid dissolved in H2O was mixed with 2.5 mL of 0.2 M phosphate buffer (pH 6.6) and 2.5 mL of 1% (w/v) potassium hexacyanoferrate solution. After 20 min of incubation at 50°C, 2.5 mL of 10% (w/v) trichloroacetic acid (TCA) was added and the mixture was centrifuged for 10 min. Finally, 2.5 mL of the upper layer was mixed with 2.5 mL of water and 0.5 mL of 0.1% (w/v) ferric chloride and the absorbance was recorded at 700 nm. Reductive activities of the plant extracts have been expressed as AAE in millimoles of ascorbic acid per gram of GAE.
Trypan blue exclusion assay
Stimulation of HSF proliferation was measured by trypan blue exclusion assay (Paduch et al. Citation2007). For tryptan blue exclusion test, cells were seeded into wells of sterile 24-well plate in 1mL of culture medium (3×104 cells/mL) with a M4655 medium supplemented with 1% amino acid solution, 1% l-glutamine, 2.5% FCS (v/v) and 1% antibiotics (penicillin and streptomycin). After 24 h of incubation at 37°C in humidified atmosphere with 5% CO2, the culture was changed for 1 mL of culture medium containing 2.5% of FCS and plant extract. As negative control for cells viability, microtiter wells contained only HSF cells suspended in 1 mL of culture medium supplemented with 2.5% FCS. As positive control for cells viability, microtiter wells contained only HSF cells suspended in 1 mL of culture medium supplemented with 10% FCS. All HSF cells were cultured at 37°C for 24, 48, 72, and 168 h. After detaching cells from the tissue culture flask by trypsinization, a cell suspension was mixed with trypan blue solution. Trypan blue is a stain that enters only across the membranes of nonviable/dead cells. Dye-excluding (viable) cells were counted in a hemocytometer and the percentage of viable cells was determined according to the following equation:
Antiradical activity in irradiated HSF assay
Protective effects of antioxidants against damage skin fibroblast can be measured by in vitro assays using UV radiation (Noel-Hudson et al. Citation1990; Emonet-Piccardi et al. Citation1998). HSF alone (control) and HSF containing different concentrations of plant extract (extract) were irradiated with 5–40 J/cm2 (from a solar simulador Suntest CPS+, Atlas Material Testing Technology, USA). α-Tocopherol (vitamin E) was used as positive control. The percentage of antiradical activity of extracts was calculated, measuring optical density (OD) at 550 nm after addition of DMSO, by the following equation:
Ultraviolet light absorption activity
The ultraviolet (UV-A = 400–325 nm, UV-B = 315–280 nm, and UV-C = 280–100 nm) light absorption activity of water extracts (100 ppm) was measured using a Hewlett Packard 8452A spectrophotometer.
1H/13C NMR and EI-MS spectroscopic analysis
In order to identify active principles, the dried extract of Pterospartum tridentatum subsp. tridentatum leaves was submitted to fractionation. Isolated compounds were identified by 1H/13C NMR spectroscopy and chemical ionization mass spectrometry (CIMS). NMR spectra were acquired with TMS as internal standard at 500.14 MHz (1H) and 125.77 MHz (13C) using a Bruker AMX500 500MHz spectrometer and at 250.13 MHz (1H) and 62.90 MHz (13C) using a Bruker AMX500 250 MHz spectrometer. The mass spectra of compounds were obtained on a HP5988A electrospray triple quadrupole mass spectrometer.
A portion (1 g) of dried extract of Pterospartum tridentatum subsp. tridentatum leaves (Pt1) was fractionated by column chromatography on Sephadex LH-20 (2×25 cm), MeOH, 4 mL/min; tr: 120–140 mL; detection of the eluates by TLC (SiO2, CH2Cl2/MeOH (95:5), Hannesian stain), R f : 0.40. This portion of Pterospartum tridentatum subsp. tridentatum leaves (Pt2) (0.625 g) was fractionated by column chromatography on silica gel (40–63 μm, 3×25 cm), (2.5×11 cm), CH2Cl2/MeOH (95:5)→CH2Cl2/MeOH (8:2), 4 mL/min; tr: 60–80 mL; detection of the eluates by TLC (SiO2, CH2Cl2/MeOH (95:5), Hannesian stain), R f : 0.40. The other portion of Pterospartum tridentatum subsp. tridentatum leaves (Pt3) (5 mg) was fractionated by preparative chromatography (PLC) on silica gel (60 F254, 1 mm), CH2Cl2/MeOH (95:5) → CH2Cl2/MeOH (90:10); R f : 0.40. Genistein (4′,5,7-trihydroxyisoflavone) (1) (1 mg, 0.1%, w/w, on dry plant) was obtained. The spectral data of genistein isolated from Pterospartum tridentatum subsp. tridentatum leaves was in agreement with the values in the literature (Mabry et al. Citation1970; Breitmaier & Voelter Citation1990).
Genistein (4′,5,7-trihydroxyisoflavone) (1)
1H NMR (MeOD, 250.13 MHz, δ ppm): 8.02 (1H, s, =CH–OR), 7.34 (2H, d, J=8.7 Hz, 2×Ar), 6.82 (2H, d, J=8.7 Hz, 2×Ar), 6.31 (1H, d, J=2.3 Hz, Ar), 6.20 (1H, d, J=2.3 Hz, Ar). | |||||
13C NMR (MeOD, 62.90 MHz, δ ppm): 182.2 (C˭O), 166.2 (C‒OH), 163.8 (C‒OH), 159.7 (C‒O), 158.8 (C–OH), 154.8 (CH–O), 131.4 (2×CH), 124.7 (C), 123.3 (C), 116.3 (2×CH), 106,2 (C), 100.2 (CH), 94.9 (CH). IQ/MS m/e: 271 [(M+1)+, 100 . | |||||
Results and discussion
In this study, 35 endemic and subendemic plants from Galician (northwest of the Iberian Peninsula) have been studied. The geographical distribution of these plants is given in (Castroviejo Citation1986–2011).
Table 1. Geographical distribution of Galician plants studied in this work.
The extraction yield on dry weight of extracts, the TPC, the DPPH radical scavenging activity, and the reductive Fe (III)/tripyridyltriazine complex activity (FRAP assay) of 43 hydroglycolic extracts studied are given in . When the IP of the DPPH radical of the extracts at 500 ppm were higher than 30%, EC50 were calculated ().
Table 2. Extraction yield, total phenolic content, DPPH radical scavenging, and FRAP activity.a
The plants analyzed showed important differences in their phenolic content, ranging between 0.516 for Xolantha globulariifolia to almost zero gram of GAE per gram of dry extract for Sagina merinoi or Angelica pachycarpa. They also differed in the antioxidant activity parameters analyzed. The radical scavenging capacity varied between very high values around 90% inhibition of DPPH radical at 500 ppm for Xolantha globulariifolia and Armeria pubigera and very low levels close to zero for Alyssum serpyllifolium subsp. lusitanicum, Linaria triornithophora, and Angelica pachycarpa. The FRAP of the extracts was comparable to that obtained for chestnut (Vázquez et al. Citation2012). FRAP ranged from values higher than 2.000 for Xolantha globulariifolia and Erica erigena to 0.103 mmol AAE per gram of dry extract for Linaria polygalifolia subsp. polygalifolia.
A significant linear correlation was found between TPC and antioxidant capacity by the DPPH radical and FRAP method, with coefficient values r=0.709 and r=0.685, respectively. This result indicates that phenolic components contribute to the antioxidant capacity of extracts but are not solely responsible. Other substances in plants, including protein and carbohydrate, also contribute to the antioxidant capacity (Amarowicz & Shahidi Citation1997; Wang et al. Citation2012). There was also a significant correlation observed between DPPH and FRAP data (r=0.828). Nevertheless, in some cases good FRAP values were obtained even for low DPPH values, e.g. for Pterospartum tridentatum subsp. tridentatum or Armeria transmontana, and, on the opposite, a very good DPPH value (86%) corresponded with a medium FRAP (0.927) value in the case of Armeria pubigera. The mode of action of antioxidants is complex and may be dependent upon a wide range of variables (Craft et al. Citation2012).
Xolantha globulariifolia, Euphorbia flavicoma subsp. occidentalis, Armeria pubigera, Erica erigena, Thymelaea broteriana, Erica mackaiana, Saxifraga spathularis, Thymelaea coridifolia subsp. coridifolia, Rumex scutatus subsp. gallaecicus, Carex asturica, Centaurea ultreiae, Thymus caespititius, Centaurea borjae, Cirsium filipendulum, Pterospartum tridentatum subsp. tridentatum, Armeria transmontana, and Phalacrocarpum oppositifolium subsp. oppositifolium hydroglycolic extracts showed at 500 ppm a radical scavenging capacity higher than 30% and EC50 comparable to those of standard synthetic antioxidants, such as BHA and BHT, which are widely used in food, cosmetic, and pharmaceutical industries, natural antioxidants (Moure et al. Citation2001; Rubilar et al. Citation2007), and medicinal and aromatic plant extracts (Miliauskas et al. Citation2004).
On the basis of all these results, we selected six plant extracts for additional studies as very promising ones considering simultaneously the results obtained for the three parameters analyzed: TPC, DPPH, and FRAC assays.
TEAC value equivalent to Trolox, the antioxidant potency according to a β-carotene bleaching assay, and the reductive Fe(III) to Fe(II) activity of Xolantha globulariifolia, Armeria pubigera, Thymelaea broteriana, Pterospartum tridentatum subsp. tridentatum, Saxifraga spathularis, and Erica erigena hydroglycolic extracts are given in .
Table 3. TEAC value equivalent to Trolox, antioxidant potency according to a β-carotene bleaching assay, and reductive Fe(III) to Fe(II) activity.a
Xolantha globulariifolia, Armeria pubigera, Thymelaea broteriana extracts were found to have TEAC values higher than that of Rosmarinus officinalis extract (Celiktas et al. Citation2007), whereas Pterospartum tridentatum subsp. tridentatum, Saxifraga spathularis, and Erica erigena extracts were found to have TEAC values similar to that of this natural antioxidant extract. Moreover, Xolantha globulariifolia, Armeria pubigera, Thymelaea broteriana, Pterospartum tridentatum subsp. tridentatum, Saxifraga spathularis, and Erica erigena hydroglycolic extracts were found to have higher antioxidant potency according to a β-carotene bleaching assay than that of the synthetic antioxidant BHT.
Furthermore, the results of reducing power assay show similar reductive activity of Xolantha globulariifolia, Armeria pubigera, Thymelaea broteriana, Pterospartum tridentatum subsp. tridentatum, Saxifraga spathularis, and Erica erigena hydroglycolic extracts than another antioxidant as Origanum syriacum, O. minutiflorum, O. onites, and Majorana hortensis extracts (Dorman et al. Citation2004).
Armeria pubigera, Saxifraga spathularis, and Erica erigena hydroglycolic extracts () were found to stimulate HSF proliferation and their activity values were comparable than other natural fibroblast stimulators (Nualkaew et al. Citation2012). Saxifraga spathularis hydroglycolic extract was also able to protect irradiated HSF. The results are given in . Antiradical activities of this extract at 15.63–31.25 μ g/mL concentrations were a little smaller than vitamin E activities at the same concentration.
Table 4. Stimulation of HSF proliferation measured by Trypan blue exclusion assay.
Table 5. Antiradical activity of Saxifraga spathularis aqueous-glycolic extract against damage in HSF.a
On the other hand, the ultraviolet–visible absorption spectrum of all plant extracts was also measured. The absorbance bands in the ultraviolet regions (UV–A = 400–315 nm, UV–B = 315–280 nm) are given in (10 ppm in water). The accumulation of UV-absorbing compounds as phenolics is a mechanism of adaptation to and protection from the damage by solar radiation developed by photoautotrophic organisms (Solovchenko & Merzlyak Citation2008).
Table 6. UV absorption bands.a
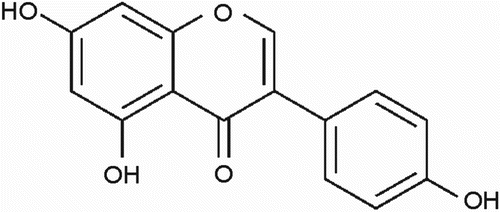
Finally, as a result of phytochemical investigation of Pterospartum tridentatum subsp. tridentatum leaf extract, the isoflavone genistein (4′,5,7-trihydroxyisoflavone) (1) was isolated. The structure of compound 1 is shown in . Genistein is a phytoestrogen with a wide variety of pharmacological effects in animal cells, including tyrosine kinase inhibition, antioxidant activity, chemoprevention of breast and prostate cancers, and cardiovascular disease (Dixon & Ferreira Citation2002).
Conclusions
The present study contributes to the knowledge of endemic and subendemic plants from Galician. Some extracts of these plants were found to have a high phenolic content, radical scavenging capacity, ultraviolet radiation absorption capacity, antiradical protection, and stimulation on HSF.
Therefore, natural antioxidant extracts of Xolantha globulariifolia, Euphorbia flavicoma subsp. occidentalis, Armeria pubigera, Erica erigena, Thymelaea broteriana, Erica mackaiana, Saxifraga spathularis, Thymelaea coridifolia subsp. coridifolia, Rumex scutatus subsp. gallaecicus, Carex asturica, Centaurea ultreiae, Thymus caespititius, Centaurea borjae, Cirsium filipendulum, Pterospartum tridentatum subsp. tridentatum, Armeria transmontana, and Phalacrocarpum oppositifolium subsp. oppositifolium could have promising skin care properties, could protect the human body from free radicals, as well as could retard lipid oxidative rancidity in cosmetics, pharmaceutical, and food materials.
Acknowledgements
The authors would like to acknowledge financial support from CarOi'Line Cosmética and from Xunta de Galicia through INCITE Research Projects 07MRU009E and 09MRU001E. Galician plants were harvested with authorization from Xunta de Galicia (Servicio de Conservación da Biodiversidade, Dirección Xeral de Conservación da Natureza). The authors also thank Herminia Domínguez, Enma Conde, and Andrés Moure (Department of Chemical Engineering, University of Vigo, Spain) for TEAC, β -carotene, and reducing power measurements. 1H/13C NMR and CI-MS spectroscopic analysis were performed at Servizo de Espectrometría de Masas and Servizo de Resonancia Magnética at RIAIDT, University of Santiago de Compostela, Spain.
References
- Aburjai , T and Natsheh , F M . 2003 . Plants used in cosmetics . Phytother Res. , 17 : 987 – 1000 . (doi:10.1002/ptr.1363)
- Amarowicz , R and Shahidi , F . 1997 . Antioxidant activity of peptide fractions of capelin protein hydrolysates . Food Chem. , 58 : 355 – 359 . (doi:10.1016/S0308-8146(96)00229-4)
- Benzie , IF F and Strain , J J . 1996 . The reducing ability of plasma as a measure of ‘antioxidant’ power – the FRAP assay . Anal Biochem. , 239 : 70 – 76 . (doi:10.1006/abio.1996.0292)
- Blokhina , O , Virolainen , E and Fagerstedt , K V . 2003 . Antioxidant, oxidative damage and oxygen deprivation stress: a review . Ann Bot. , 91 : 179 – 194 . (doi:10.1093/aob/mcf118)
- Breitmaier , E and Voelter , W . 1990 . Carbon-13 NMR spectroscopy , New York (USA) : VCH Publishers .
- Castroviejo S, editor. 1986–2011. Flora ibérica. Plantas vasculares de la Península Ibérica e Islas Baleares, Consejo Superior de Investigaciones Científicas. Madrid (Spain): Real Jardín Botánico.
- Celiktas , O Y , Bedir , E and Sukan , F V . 2007 . In vitro antioxidant activities of Rosmarinus officinalis extracts treated with supercritical carbon dioxide . Food Chem. , 101 : 1457 – 1464 . (doi:10.1016/j.foodchem.2006.03.055)
- Craft , B D , Kerrihard , A L , Amarowicz , R and Pegg , R B . 2012 . Phenol-based antioxidants and the in vitro methods used for their assessment . Compr Rev Food Sci F. , 11 : 148 – 173 . (doi:10.1111/j.1541-4337.2011.00173.x)
- Dixon , R A and Ferreira , D . 2002 . Genistein . Phytochem. , 60 : 205 – 211 . (doi:10.1016/S0031-9422(02)00116-4)
- Dorman , H JD , Bachmayer , O , Kosar , M and Hiltunen , R . 2004 . Antioxidant properties of aqueous extracts from selected Lamiaceae species grown in Turkey . J Agric Food Chem. , 52 : 762 – 770 . (doi:10.1021/jf034908v)
- Emonet-Piccardi , N , Richard , M J , Ravanat , J L , Signorini , N , Cadet , J and Béani , J C . 1998 . Protective effects of antioxidants against UVA-induced DNA damage in human skin fibroblasts in culture . Free Radical Res. , 29 : 307 – 313 . (doi:10.1080/10715769800300341)
- Fernández-Panchón , M S , Villano , D , Troncoso , A M and García-Parrilla , M C . 2008 . Antioxidant activity of phenolic compounds: from in vitro to in vivo evidence . Crit Rev Food Sci Nutr. , 48 : 649 – 671 . (doi:10.1080/10408390701761845)
- von Gadow , A , Joubert , E and Hansmann , C F . 1997 . Comparison of the antioxidant activity of rooibos tea (Aspalathus linearis) with green, oolong and black tea . Food Chem. , 60 : 73 – 77 . (doi:10.1016/S0308-8146(96)00312-3)
- Kohen , R . 1999 . Skin antioxidants: their role in aging and in oxidative stress – new approaches for their evaluation . Biomed Pharmacother. , 53/4 : 181 – 192 . (doi:10.1016/S0753-3322(99)80087-0)
- López , V , Akerreta , S , Casanova , E , García-Mina , J M , Cavero , R Y and Calvo , M I . 2008 . Screening of Spanish medicinal plants for antioxidant and antifungal activities . Pharm Biol. , 46 : 602 – 609 . (doi:10.1080/13880200802179634)
- Mabry , T J , Markham , K R and Thomas , M B . 1970 . The systematic identification of flavonoids , 3 – 15 . Berlin (Germany) : Springer-Verlag .
- Maisuthisakul , P , Pasuk , S and Ritthiruangdej , P . 2008 . Relationship between antioxidant properties and chemical composition of some Thai plants . J Food Compos Anal. , 21 : 229 – 240 . (doi:10.1016/j.jfca.2007.11.005)
- Miliauskas , G , Venskutonis , P R and van Beek , T A . 2004 . Screening of radical scavenging activity of some medicinal and aromatic plant extracts . Food Chem. , 85 : 231 – 237 . (doi:10.1016/j.foodchem.2003.05.007)
- Miller , H E . 1971 . A simplified method for the evaluation of antioxidants . J Am Oil Chem Soc. , 48 : 91 (doi:10.1007/BF02635693)
- Moure , A , Cruz , J M , Franco , D , Domínguez , J M , Sineiro , J , Domínguez , H , Núñez , M J and Parajo , J C . 2001 . Natural antioxidants from residual sources . Food Chem. , 72 : 145 – 171 . (doi:10.1016/S0308-8146(00)00223-5)
- Nayak , B S , Ramdeen , R , Adogwa , A , Ramsubhag , A and Marshall , J R . 2012 . Wound-healing potential of etanol extract of Carica papaya (Caricaceae) seeds . Int Wound J. , 9 : 650 – 655 . (doi:10.1111/j.1742-481X.2011.00933.x)
- Noel-Hudson , M S , Cornelis , M , Lindenbaum , A and Wepierre , J . 1990 . Screening test for free radical scavengers on cutaneous cultured cells . Int J Cosmet Sci. , 12 : 105 – 114 . (doi:10.1111/j.1467-2494.1990.tb00525.x)
- Nualkaew , N , Wang , R and Hensel , A . 2012 . Stimulation of cellular proliferation of human fibroblasts keratinocytes cell by Elipta prostata and Litsea glutinosa extracts . Planta Med. , 11 : 78 – PI166 .
- Oyaizu , M . 1986 . Studies on product of browning reaction prepared from glucosamine . Jpn J Nutr. , 44 : 307 – 315 . (doi:10.5264/eiyogakuzashi.44.307)
- Paduch , R , Wójciak-Kosior , M and Matysik , G . 2007 . Investigation of biological activity of Lamii albi flos extracts . J Ethnopharmacol. , 110 : 69 – 75 . (doi:10.1016/j.jep.2006.09.004)
- Parejo , I , Viladomat , F , Bastida , J , Rosas-Romero , A , Flerlage , N , Burillo , J and Codina , C . 2002 . Comparison between the radical scavenging activity and antioxidant activity of six distilled and nondistilled Mediterranean herbs and aromatic plants . J Agric Food Chem. , 50 : 6882 – 6890 . (doi:10.1021/jf020540a)
- Re , R , Pellegrini , N , Proteggente , A , Pannala , A , Yang , M and Rice-Evans , C . 1999 . Antioxidant activity applying an improved ABTS radical cation decolorization assay . Free Radical Biol Med. , 26 : 1231 – 1237 . (doi:10.1016/S0891-5849(98)00315-3)
- Rona , C , Vailati , F and Berardesca , E . 2004 . The cosmetic treatment of wrinkles . J Cosmet Dermatol. , 3 : 26 – 34 . (doi:10.1111/j.1473-2130.2004.00054.x)
- Ropke , C D , Sawada , T CH , da Silva , V V , Michalany , N S and de Moraes Barros , S B . 2005 . Photoprotective effect of Pothomorphe umbellata root extract against ultraviolet radiation induced chronic skin damage in the hairless mouse . Clin Exp Dermatol. , 30 : 272 – 276 . (doi:10.1111/j.1365-2230.2005.01749.x)
- Rubilar , M , Pinelo , M , Shene , C , Sineiro , J and Nuñez , M J . 2007 . Separation and HPLC-MS identification of phenolic antioxidants from agricultural residues: almond hulls and grape pomace . J Agric Food Chem. , 55 : 10101 – 10109 . (doi:10.1021/jf0721996)
- Scharffetter-Kochanek , K , Wlaschek , M , Brenneisen , P , Schauen , M , Blaudschun , R and Wenk , J . 1997 . UV-induced reactive oxygen species in photocarcinogenesis and photoaging . Biol Chem. , 378 : 1247 – 1257 .
- Singleton , V L and Rossi , J A . 1965 . Colorimetry of total phenolics with phosphomolybdic-phosphotungstic acid reagents . Am J Enol Vitic. , 16 : 144 – 158 .
- Solovchenko , A E and Merzlyak , M N . 2008 . Screening of visible and UV radiation as a photoprotective mechanism in plants . Russ J Plant Phys. , 55 : 719 – 731 . (doi:10.1134/S1021443708060010)
- Vázquez , G , Fernández-Agulló , A , Gómez-Castro , C , Freire , M S , Antorrena , G and González-Álvarez , J . 2012 . Response surface optimization of antioxidants extraction from chestnut (Castanea sativa) bur . Ind Crops Prod. , 35 : 126 – 134 . (doi:10.1016/j.indcrop.2011.06.022)
- Wang , R , Chen , P , Jia , F , Tang , J , Ma , F and Xu , B . 2012 . Characterization and antioxidant activities of polysaccharides from Panax japonicus C.A . Meyer. Carbohyd Polym. , 88 : 1402 – 1406 . (doi:10.1016/j.carbpol.2012.02.026)