Abstract
The antimicrobial effects of silver nanocomposite particles (Ag-ZnO NC) on microorganisms, the antimicrobial mechanism and applications in medical devices are not yet clear. Stable Ag-ZnO NC were prepared and their morphological sizes and shapes were characterized by scanning electron microscopy. The effect of Ag-ZnO NC was tested on Bacillus thuringiensis, Escherichia coli and Pseudomonas aeruginosa in antibacterial tests including growth kinetics, antimicrobial susceptibility (disc diffusion) and minimal inhibitory concentration (MIC). Different concentrations of nanocomposites (i.e. 10, 20, 50,100, and 200 μg) showed concentration-dependant efficacy on all three tested microorganisms. E. coli was fairly sensitive in 200 μg of NC, forming a ∼15 mm inhibition zone; followed by B. thuringiensis, having ∼9 mm of inhibition zone, while P. aeruginosa, a pathogenic bacterium, showed negligible inhibition zone with Ag-ZnO NC. Growth of E. coli under Ag-ZnO NC treatment was significantly delayed with an extended lag phase of 2 hrs and 30 mins. Scanning electron microscopy confirmed the bacteriostatic effect of Ag-ZnO NC, which was manifested in cell division arrest with significant cell elongations compared to the control. The free radical generation effect of Ag-ZnO NC was tested against all these organisms. The results suggest that Ag-ZnO NC can be used effectively against microbial growth. This may be of use in diverse medical devices for antimicrobial control and can be a proper substitute for antibiotics in curing human health.
Introduction
The increasing resistance of microbial organisms to multiple antibiotics, and continuing emphasis on healthcare costs, has led researchers to develop new antimicrobial substances that are effective against drug resistance. This has led to the use of Ag-based or Zn-based antiseptics that may have broad-spectrum activity with minimal microbial resistance (CitationJones et al. 2004). The antibacterial effects of Ag salts have been noticed since antiquity and Ag is currently used to control bacterial growth in a variety of applications, including dental work, catheters, and burn wounds (CitationSilver & Phung 1996; CitationCrabtree et al. 2003; CitationCatauro et al. 2004). Reducing the particle size of materials is an efficient and reliable tool for improving their biocompatibility. Nanotechnology helps in overcoming the limitations of size and can globally change the view regarding science (CitationMirkin & Taton 2000). The efficiency of nanomaterials can be optimized for application in fields such as bioscience and medicine. Infectious diseases remain one of the greatest challenges to global health as the causative strains of bacteria are fast developing resistivity to current antibiotics (CitationKyriacou et al. 2004) and are now ineffectively managed with current medications. Research by pharmaceutical companies for new antibacterial agents is in progress worldwide. Nanoscale materials have emerged as novel antimicrobial agents owing to their effectiveness in small doses, minimal toxicity and lack of side effects (CitationLara et al. 2010, Citation2010a). Ag ions and Ag-based compounds are highly toxic to microorganisms, showing strong biocidal effects on as many as 12 species of bacteria including Escherichia coli (CitationZhao & Stevens 1998). They also have an exceptionally broad spectrum of bacteriocidal activity (CitationRatte 1999; CitationSilver 2003; CitationSilver et al. 2006; CitationLuoma & Rainbow 2008). Silver nanoparticles, containing about 10,000–15,000 silver atoms (CitationOberdörster et al. 2005; CitationWarheit et al. 2007), are a promising alternative to silver salts and bulk metal because salts may possess quick and uncontrolled silver release while the bulk metal is very slow and inefficient in releasing silver (CitationVertelov et al. 2008). Recently it was shown that both ions and nanoparticles are sources of the toxicity of nanosilver (CitationVertelov et al. 2008). Recent studies have demonstrated that antimicrobial formulations comprising nanoparticles dispersed onto certain nanoscale metal oxides are effective bactericidal materials (CitationGade et al. 2008). Like silver, Zn metal oxides such as ZnO have received increasing attention as antibacterial materials in recent years, because of their stability under harsh processing conditions; for several decades they have been used in medicine as mild topical astringents, and antibacterial agents against eczema, slight excoriations, in wounds and for hemorrhoids (CitationSweetman 2005). An improved antimicrobial activity of silver nanoparticles in combination with Zn was reported by CitationKawashita et al. (2000), and CitationPak and Jang (2003). It was inferred that silver coating the surface of Zn nanoparticles has the ability to include the electrons produced through photocatalytic reactions of Zn nanoparticles; this increases electron isolation, forming gaps in the cell membrane so there is an increase of antimicrobial activity (CitationJafari et al. 2011). However, mechanism of action of the silver and zinc nanoparticles is not yet fully established (CitationJayesh et al. 2007; CitationReddy et al. 2007).
E. coli, the most extensively studied organism, is an opportunistic pathogen causing gastroenteritis, urinary tract infections and neonatal meningitis. Pseudomonas aeruginosa, a multiresistant Gram-negative, rod shaped bacterium is emerging as an opportunistic pathogen in humans. Various factors such as resistance to multiple antibiotics, production of extracellular toxins, enzymes, exopolysaccharides and biofilm formation, etc., are involved in the establishment of the infection. In this study, an attempt has been made to study scanning electron microscopic (SEM) properties of silver and zinc oxide formulated nanocomposite (silver-zinc-nano), and its anti-microbial properties including growth kinetics, antimicrobial susceptibility (disc diffusion) and minimal inhibitory concentration (MIC) on the clinically important bacteria Bacillus thuringiensis, Escherichia coli and Pseudomonas aeruginosa. Moreover, the possibility role of free-radical ions generated around Ag-Zn nanocomposite in microbial inhibition was measured.
Materials and methods
Preparation of Ag-ZnO nanocomposite
Microwave assisted reactions in ethylene glycol medium were performed to form the product. In a typical reaction, the desired amount of zinc acetate and thiourea (as per oxidizer to fuel ratio) were dissolved in minimum amount of distilled water and mixed with a calculated amount (2 to 3 drops) of ethylene glycol. The reaction was exposed to microwave irradiation followed by reflux condensation to form the product. After centrifugation the sample was washed with distilled water and alcohol in order to remove any organic impurity and finally the sample was dried in an oven at for 6 h. For silver-modified ZnO, 1 mol % of silver nitrate solution was sonicated with the above prepared ZnO sample followed by addition of NaBH4 solution. Finally, the Ag-capped ZnO nanocrystals were separated by centrifugation.
SEM characterization of silver-nano composites (Ag-ZnO NC)
The silver nanocomposite, i.e. silver zinc oxide nanocomposite (Ag-ZnO NC) was well dispersed in MilliQ water in a sonicator at 20 kHz for 20 min and centrifuged at 10,000 rpm for 20 min, followed by subsequent dehydration in 50%, 70%, 90% and 100% ethanol, intermittently followed by freeze drying. Samples were coated with a thin layer of gold, and the images were taken using a JEOL, JSM-5600LV SEM (Tokyo, Japan) with an acceleration voltage of 15 kV. Photographs were taken at different magnifications and used for morphological characterization of nanoparticles.
Microorganisms and their maintenance
The bacterial strains, B. thuringiensis, E. coli (MTCC-9537) and P. aeruginosa (MTCC 2453) were obtained from IMTECH, Chandigarh, India, and cultured on sterile nutrient agar (NA) plates (Hi-Media, Mumbai, India). Broth cultures (18–24 h) of the three types of microbial strain showing the OD of 0.129–0.134 at 625 nm wavelength (i.e. equivalent to 0.5 McFarland solution) were used for testing antimicrobial activity.
Preparation of Ag-ZnO NC working solution and growth kinetics study
To examine the effect of silver nanoparticles on the growth of bacteria and its bactericidal activity, the Gram-negative bacteria E. coli and P. aeruginosa, and the Gram-positive bacterium B. thuringiensis were tested. In each case, around 106–107 cells were inoculated in 100 ml NB and incubated for 2 h at with continuous agitation at 150 rpm. Following this, 100 μg of the nanocomposite dose was charged in one set each of microbial culture while the other set was kept without the NC, as a negative control. Spectrophotometric absorbance was recorded at 625 nm for both the bacterial concentrations for 6 h at 1 h intervals. The OD values were converted into concentration of bacterial cells (CFU per milliliter) following the procedure of CitationSondi and Salopek-Sondi (2004). Susceptibility of the test organisms was tested in increasing doses of the silver nanocomposites.
Testing antimicrobial activity by disc diffusion assay
A solid assay was performed on NA plates for which fresh overnight cultures were prepared as a working bacterial suspension in NB (concentration of 105 or 107 CFU ml−1 of each organism). Lawn cultures were prepared from NB suspensions on freshly prepared NB plates and incubated for 2 h at (E. coli and P. aeruginosa) and at
for B. thuringiensis. Filter paper disks (6 mm) were impregnated with different concentrations of the silver NC and were placed on each plate along with a standard of ofloxacin (equivalent to each dose of silver NC) and incubated at their respective temperatures for 24 h. Inhibition zone diameter values were determined by E-testing and comparative analysis of the inhibition zones were made with control. All the readings were taken in triplicate and the experiments were done in triplicate.
Determination of MIC
The MIC value is considered as the lowest concentration of the sample of nanocomposite which inhibits the growth of a microbe. The MIC of the Ag-ZnO NC was determined by the microbroth dilution method according to the British Society for Antimicrobial Chemotherapy (BSAC) guidelines (CitationAndrews 2001). Fresh NB cultures maintained at an optical density of 0.1 at 600 nm (OD of 0.1 corresponds to a concentration of 108 CFU ml−1) was used for the MIC test. Each 2 ml of broth culture was supplemented with 10, 20, 50, 100 or of silver NC solution. Culture broths used without silver NC served as control. All treatments and controls were incubated in their respective temperatures for 24 h. The experiments were carried out in triplicate. Evaluation of the MIC was carried out by the spread plate technique to detect the microbial colony growth at different concentrations of silver nanoparticles and a number of colonies were converted to the concentration of bacterial cells (CFU ml−1).
SEM studies on bacterial morphology
Treated ( Ag-ZnO NC) and untreated samples of E. coli were centrifuged at 10,000 rpm for 20 min and serially dehydrated with 50%, 70%, 90% and 100% of ethanol and lyophilized. The lyophilized samples were coated with a thin layer of gold, and the images were taken using a JEOL, JSM-5600LV SEM with an acceleration voltage of 15 kV. Photographs were taken at different magnifications and used for morphological characterization of nanoparticles.
Statistical analysis
Each experiment was carried out in triplicate and the results were represented as the mean of the triplicates. Standard error (SE) of mean values were calculated for the data.
Results
Characterization of Ag-ZnO NC through SEM
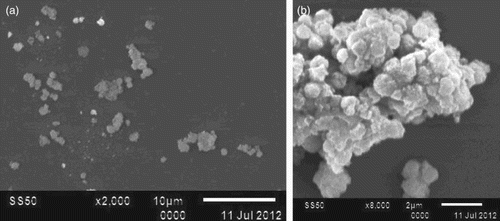
The nanocomposite aqueous suspension showed globular or spherical shaped particles, in a dispersed and an agglomerated condition. Approximately 0.5 μm of silver NC were observed and that confirmed the particles to be the nanoparticles ((a, b)).
Disc diffusion assay
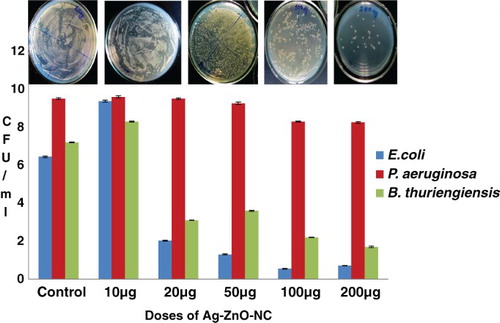
It was found that E. coli was significantly affected at 20 μ g of the ZnO NC concentration and it was found significantly higher in 100 μg of the nanoparticle with maximum anti-microbial inhibition (21 mm). P. aeruginosa did not show any sensitivity at this concentration and successfully persisted until the higher concentration, i.e. 200 μ g, which showed a little inhibition (6 mm). Comparative data with ofloxacin as a standard antibiotic showed higher zone of inhibition (17.0 mm) as compared to 200 μg of antibiotic (, (a–d)). B. thuringiensis showed higher inhibition at 200 μg (9 mm), which explains the non-specific mode of action of silver nanoparticles irrespective of pathogenicity of the tested microbes. A correlation was noticed when ofloxacin was used as a standard. The liquid assay at the same dosage level also revealed a dose of 100 μg of AgZnO NC to be the MIC for E. coli; it produced the minimum number of bacterial colonies (0.5 CFU ml−1) and displayed an inhibition of 91.4% (). However, the organism showed little difference in its zone of inhibition in 50–200 μg of AgZnO NC. It was followed by B. thuringiensis with 76% as the reduction rate of the organism with its minimum inhibition at 200 μg.
Figure 2. Comparative analysis of the antimicrobial/killing effect of Ag-ZnO NC on E. coli and B. thuriengensis showing zone of inhibition. 2a-c - Zone of inhibition on E. coli. 2d - Zone of inhibition on B. thuriengiensis.
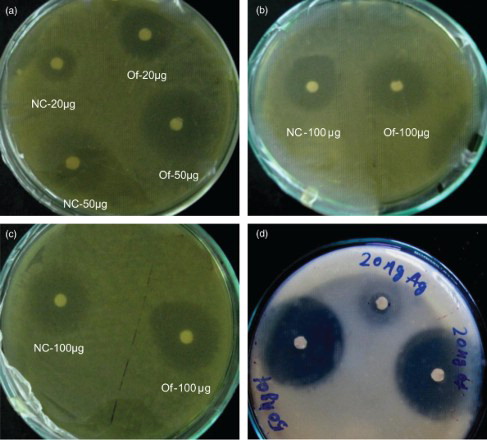
Table 1. Effect of Ag-ZnO NC on different test organisms.
P. aeruginosa showed a negligible reduction in its population due to anti-microbial action with only 13% of the bacterial inhibition at a 200 μg Ag ZnO NC. The spread count plates of the corresponding nano-exposed (increasing gradient) culture stocks showed a prominent decrease in the number of colonies of E. coli, the lowest of which, CFU at a dose of 100 μ g, was in line with the highest inhibition zone value of 21 mm at 100 μg. The bacterial population was markedly reduced at the corresponding dose of 100 μg by approximately 91%, followed by B. thuringiensis by 76% (
CFU) and then P. aeruginosa with only a 13% reduction, with a population of
CFU.
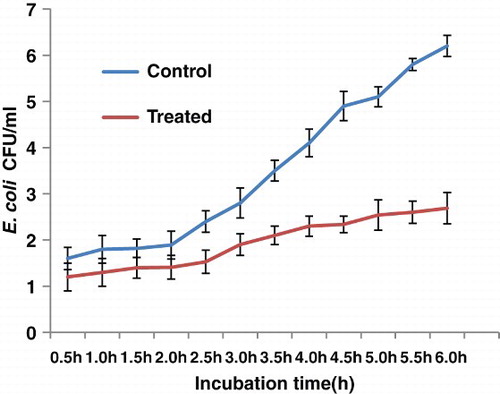
Monitoring the bacterial growth kinetics of E. coli at its MIC dose, 100 μ g in 100 ml of NB medium, clearly showed that unlike the control culture, where the lag phase was sustained for 2 h, the nanoparticles caused a delay in their growth with a prolonged lag phase until 4.5 h, extending for the next 2.5 h. Hence, the exponential phase was greatly reduced to almost negligible. The physiological effect studied at the optimum dosage of 100 μg, supplemented with 107 CFU ml−1 of E. coli and the B. thuringiensis, revealed a prominent delay in the initial growth phase, termed the ‘lag phase’, that extended for more two hours as shown clearly on the growth curve. B. thuringiensis also showed a delayed lag phase but it was shorter than that in E. coli ()
Figure 3. Graphical representation of MIC assay in terms of CFU ml-1 of E. coli in different doses of Ag-ZnO-NC.
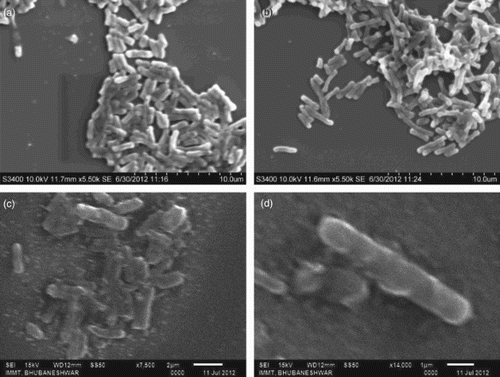
SEM photographs of E. coli with the control and treatment conditions exposed at 100 μg clearly revealed changes in cellular dimension ((a–d)). The length of the normal cell, i.e. 10 μ m, was elongated to 12–15 μm in treated cells; this was inferred to be due to arrest of the cell division, thereby inhibiting the cell growth. Hence, it can be suggested that Ag-ZnO NC is most effective on E. coli, least effective on P. aeruginosa, and B. thuringiensis showed a moderate sensitivity, which might be due to the Gram-positive character of that microorganism.
Discussion
Studies on metallic particles of nanodimension less than 100 nm has been of immense application in various fields including medicine, dentistry, food industry, etc., with the most highlighted activity in sweeping off microorganisms. The objective of this study was to monitor the anti-microbial action on B. thuringiensis, E. coli and P. aeruginosa. B. thuriengensis was selected to investigate the non-specific mode of killing action, to reveal any undesirable action on harmless microbes. SEM images showing the agglomerated globular particles of nanodimension of less than 5 nm confirmed the nanoscale composite.
In order to demonstrate bacterial elimination, the anti-microbial assay was studied at the surface as well as in the liquid. For this, five doses of Ag-ZnO nanocomposites were considered –10, 20, 50, 100 and 200 μg – with an inoculum count of 107 CFU/ml. The response of E. coli in the disk diffusion test showed that almost all doses were very effective, with little difference in anti-microbial inhibition. The highest inhibition zone value was 21 mm for 100 μ g. Noticeable inhibition began to be seen from the dosage level of 20 μg, with a range of values lying between 18 and 21 mm, which shows the sensitivity of the organism towards the nanocomposite. In contrast, P. aeruginosa showed a negligible response towards any of the test dosages up to 200 μg, where a faint zone of inhibition was observed. Further, inhibitory activity of Ag-ZnO NC was similar to ofloxacin, which was taken as a reference. The latter showed a higher magnitude due to the synergistic action of two different antibiotics. Evidently, the extent of the killing effect of Ag-ZnO NC may be on a par with ofloxacin with an increasing concentration of nanosilver. Moreover, during the course of the experiment, a very important observation was noted: the anti-microbial action of the nanoparticles was greatly enhanced when they were charged just after the incubation of the test organism for two hours at . This might be due to the nano action amidst the process of active cell division during the logarithmic phase. Moreover, a similar study performed by CitationMorones et al. (2005) on the interaction of E. coli on different concentrations of Ag-ZnO NC at its mid-log phase found the bacteria to show a good sensitivity at lower concentration (CitationKim et al. 2007).
Although growth on agar plates is a more easy means of observing the antimicrobial properties of silver nanoparticles, the liquid-growth experiments generated more definite results. Interestingly, the results showed a similar trend as that found in the plate study. The broth experiments carried out in 100 ml of NB medium were supplemented with the nanoparticles. Although increasing concentrations of nanoparticles progressively inhibited the growth of the organism, the prominent reduction was seen in the case of E. coli with the reduction percentage of , at 100 μg of the Ag-ZnO NC as compared to 200 μ g of Ag-ZnO NC. It was followed by B. thuringiensis and P. aeruginosa with 76% and 13% reduction respectively, both at the highest concentration of AgNP, i.e. 200 μ g. Hence, as expected from earlier research carried out on these organisms, E. coli was found to be significantly sensitive to the Ag-ZnO composites, while P. aeruginosa responded at 200 μ g with an insignificant anti-microbial effect of only 13%. In contrast, a lower MIC in P. aeruginosa was observed with combined nanoparticles of Ag-ZnO, with
showing the highest inhibitory effect. The probable reason for the reduced bacterial elimination might be due to the nanosilver concentration and also largely due to the resistivity of P. aeruginosa. From growth kinetics study of the studied organisms E. coli and B. thuringiensis was selected for growth curve study. The organisms were conditioned with 100 μ g of nanoagent; following 2 h of incubation, the growth pattern was spectrophotometrically recorded at the optimum wavelength of 600 nm, with a time interval of 1 h. By comparing the kinetics with the control condition, growth in the nano-exposed condition was found to be delayed in the lag phase for the next two hours, although the observation was only half completed until 6 h owing to technical difficulties. This could be attributable to greater stability of the nanoparticles used in this study. In contrast, B. thuringiensis, a Gram-positive bacteria, showed a shorter lag phase than E. coli as revealed in the growth curve studies. In fact, a shorter lag phase was observed in the growth of Gram-positive bacteria treated with nanoparticles, which contrasted with the Gram-negative strains (CitationShrivastava et al. 2007). Suitable captures using SEM were obtained to visualize the morphological status of the nano-exposed E. coli at the optimum dose of 100 μg as well as in the control conditions. A considerable difference in the cellular dimension of the treatment and the normal condition was recorded in Ag-ZnO NC treated cell as compared to the untreated cells with a normal cellular length of 5 μm; the treated cells were clearly found elongated as compared to the cells in the control condition. This might be due to bacteriostatic effect of the Ag-ZnO NC that might be act upon the inhibition of peptidoglycan synthesis during cell division. The hypothesis is in support of the above statement that the bacterial cells had actively responded to the nanocomposites, when the latter were charged in to the cell culture at their logarithmic phase. Hence, the silver-ZnO NC have acted upon the bacterial cell wall of E. coli, inhibiting its synthesis. According to CitationLara et al. (2010a), bactericidal action based on the inhibition of cell wall synthesis could be another possible mechanism. This was further supported by the hypothesis that the bacteria following nano treatments become non-culturable due to inhibition of further cell division. Although the anti-microbial principles and mechanisms showed by nanosilver are diverse, they are not yet clear. The main action of the particle might be the nanoparticle–bacterial interaction owing to the cell surface negative charge that leads to easy internalization of nanoparticles, into the bacterial walls. The penetration of Ag-ZnO NC into the bacterial cells become easier in the growing stage. These might inhibit cell wall synthesis, thereby arrest the cell division and subsequently inhibit its growth, so that they remains in dividing stage. Although it is assumed that Ag-ZnO NC are involved in some sort of binding mechanism on the cell wall, the mechanism of the interaction between Ag-ZnO NC and components of the outer membrane is still unclear. Some studies showed that silver nanoparticles attack Gram-negative bacteria by anchoring and penetrating the cell wall, and as a consequence, the leading structural change in the cell membrane takes place and cell permeability increases. Hence, uncontrolled transport through cytoplasmic membrane followed by cell death results (CitationSondi & Salopek-Sondi 2004). However, we did not find any bacterial cells showing evidence of cell lysis or any sort of cellular leakages in their surroundings, due to cellular death. Hence, the image data indicates the silver-ZnO nanocomposites are strongly bacteriostatic rather than being bacteriocidal at the dosage of 100 μg. In addition, a dose of 200μg is also cell-inhibiting for the other two organisms, P. aeruginosa and B. thuringiensis. Interestingly, B. thuringiensis also showed a noticeable inhibition response to Ag ZnO NC as compared to its control condition, effective with 20–200 μg of the Ag ZnO NC.
Hence, whatever the anti-microbial action, the dispersed nano silver in ZnONP (Zinc oxide nano particle) is unsafe for use in agriculture as it is able to non-specifically act on microbes like B. thuringiensis. Our data are in agreement with studies carried out by CitationKim et al. (2007) on the Gram-negative bacteria E. coli and Gram-positive Staphylococcus aureus. These authors reported the highest antibacterial activity against E. coli, mostly due to the difference between the cell wall of Gram-negative and Gram-positive microorganisms (CitationKim et al. 2007). The structural difference lies in the organization of peptidoglycan, which is the key component of membrane structure. Gram-negative bacteria exhibit a thin layer of peptidoglycan (about 2–3 nm) between the cytoplasmic membrane and the outer cell wall. The outer membrane of E. coli cells is predominantly constructed from tightly packed lipopolysaccharide (LPS) molecules, which are composed of covalently linked lipids and polysaccharides, and lack strength and rigidity, providing an effective permeability barrier (CitationLiu et al. 2009). The overall negative charge on the bacterial cell surface at biological pH values is negative because of excess number of carboxylic groups, which upon dissociation makes the cell surface negative. It is therefore agreed that the opposite charges of bacteria and nanoparticles make them bind effectively due to electrostatic forces. Because Gram-negative bacteria have a relative abundance of negative charges, the growth of Gram-negative bacteria is more profoundly affected by the silver nanoparticles than Gram-positive organisms. On the other hand, Gram-positives have a thick peptidoglycan layer (30 nm) and they lack the outer membrane (CitationFeng et al. 2000). Peptidoglycan, consisting of linear polysaccharide chains cross-linked by short peptides, forms a three-dimensional rigid structure (CitationBaron 1996) that imparts rigidity; extended cross-linking not only endows the cell walls with fewer anchoring sites for the silver nanoparticles but also makes them difficult to penetrate.
Pseudomonas, was more resistant than E. coli that might be due to certain differences in the cell wall composition. Further, CitationLiu et al. (2009) indicated that ZnONP may distort and damage bacterial cell membranes, resulting in a leakage of intracellular contents and eventually the death of bacterial cells; the inhibitory effects against E. coli O157:H7 increase as the concentration of ZnONP is increased.
We conclude that Ag-ZnO nanocomposite was most effective against E. coli as well as B. thuringiensis, although non-specific in action. Pseudomonas aeruginosa was resistant even at the highest dosage considered, i.e. 200 μg. Further, the anti-microbial agent proved to be bacteriostatic in action; it was confirmed by SEM reports that it acts on cells by arresting their division. Ag-ZnO NC could be superior to third generation commercial antibiotics, as it is very powerful even at the lowest concentration, while NPs, even used alone, could be as efficient as ofloxacin if the nanocomposite is made more effective in its size, shape and stability.
In conclusion, the Ag-ZnO nanocomposite showed great promise as antimicrobial agents. There may be applications of Ag nanoparticles in various fields such as medical devices and antimicrobial systems against E. coli as well as B. thuringiensis, although they are non-specific in action. Pseudomonas aeruginosa was resistant even at the highest dosage considered, i.e. . The lower efficacy of the Ag nanoparticles against P. aeruginosa may derive from the difference in membrane structure. To confirm this hypothesis, further comparative study between various Gram-negative and Gram-positive bacterial species is needed. The peptidoglycan layer is a specific membrane feature of bacterial species and not mammalian cells. Therefore, if the antibacterial effect of Ag nanoparticles is associated with the peptidoglycan layer, it will be easier and more specific to use Ag nanoparticles as an antibacterial agent.
Acknowledgements
We acknowledge help with the SEM study from Prof. M. Panda, Central Instrumentation Laboratory, OUAT, Bhubaneswar and Director, IMMT, Bhubanewar.
References
- Andrews JM. 2001. Determination of minimum inhibitory concentrations. J Antimicrob Chemoth. 48:5–16. doi: 10.1093/jac/48.suppl_1.5
- Baron S. 1996. Medical microbiology. 4th ed. Galveston: University of Texas Medical Branch, Galveston, Texas.
- Catauro M, Raucci MG, De Gaetano F, Marotta A. 2004. Antibacterial and bioactive silver-containing Na2O-CaO-2SiO2 glass prepared by sol-gel method. J Mater Sci Mater Med. 15(7):831–837. doi: 10.1023/B:JMSM.0000032825.51052.00
- Crabtree JH, Burchette RJ, Siddiqi R, Huen IT, Handott LL, Fishman A. 2003. The efficacy OF silver-ion implanted catheters in reducing peritoneal dialysis-related infections. Perit Dial Int. 23(4):368–374.
- Feng QL, Wu J, Chen GQ, Cui FZ, Kim TM, Kim JO. 2000. A mechanistic study of the antibacterial effect of silver ions on Escherichia coli and Staphylococcus aureus. J Biomed Mater Res. 52:662–668. doi: 10.1002/1097-4636(20001215)52:4<662::AID-JBM10>3.0.CO;2-3
- Gade AK, Bonde P, Ingle AP, Marcato PD, Duran N, Rai MK. 2008. Exploitation of Aspergillus niger for synthesis of silver nanoparticles. J Biobased Mater Bioenergy. 2:243–247. doi: 10.1166/jbmb.2008.401
- Jafari IA, Ghane M, Arastoo S. 2011. Synergistic antibacterial effects of nano zinc oxide combined with silver nanocrystales. African J Microbiology Research. 5(30):5465–5473.
- Jayesh P, Chatterjee AK, Duttagupta SP, Mukherji S. 2007. Strain specificity in antimicrobial activity of silver and copper nanoparticles. Nanomedicine. 3:95–101. doi: 10.1016/j.nano.2006.12.001
- Jones SA, Bowler PG, Walker M, Parsons D. 2004. Controlling wound bioburden with a novel silver-containing Hydrofiber® dressing. Wound Repair Regen. 12(3): 288–294. doi: 10.1111/j.1067-1927.2004.012304.x
- Kawashita M, Tsuneyama S, Miyaji F. 2000. Antibacterial silver containing silica glass prepared by sol-gel method. Biomaterials. 21:393–398. doi: 10.1016/S0142-9612(99)00201-X
- Kim JS, Kuk E, Yu KN, Kim JH, Park SJ, Lee HJ, Kim SH, Park YK, Park YH, Hwang CY, Kim YK, Lee YS, Jeong DH, Cho MH. 2007. Antimicrobial effects of silver nanoparticles. Nanomedicine. 3:95–101. doi: 10.1016/j.nano.2006.12.001
- Kyriacou SV, Brownlow WJ, Xu XHN. 2004. Using nanoparticle optics assay for direct observation of the function of antimicrobial agents in single live bacterial cells. Biochemistry. 43:140–147. doi: 10.1021/bi0351110
- Lara HH, Ayala-Nuñez NV, Ixtepan-Turrent L, Rodriguez-Padilla C. 2010. Bactericidal effect of silver nanoparticles against multidrug-resistant bacteria. World J Microbiology and Biotechnology. 26:615–621. doi: 10.1007/s11274-009-0211-3
- Lara HH, Ixtepan-Turrent L, Garza-Trevino EN, Rodriguez-Padilla C. 2010a. PVP coated silver nanoparticles block the transmission of cell-free and cell associated HIV-1 in human cervical culture. J Nanobiotechnology. 8(15):1–11. doi: 10.1186/1477-3155-8-1
- Liu Y, He L, Mustapha A, Li H, Hu ZQ, Lin M. 2009. Antibacterial activities of zinc oxide nanoparticles against Escherichia coli O157:H7. J Appl Microbiol. 107:1193–1201. doi: 10.1111/j.1365-2672.2009.04303.x
- Luoma SN, Rainbow PS. 2008. Metal contamination in aquatic environments: science and lateral management. Cambridge: Cambridge University Press.
- Mirkin CA, Taton TA. 2000. Semiconductors meet biology. Nature. 405:626–627. doi: 10.1038/35015190
- Morones JR, Elechiguerra JL, Camacho A, Holt K, Kouri JB, Ramírez JT. 2005. The bactericidal effect of silver nanoparticles. Nanotechnology. 16:2346–2353. doi: 10.1088/0957-4484/16/10/059
- Oberdörster G, Oberdörster E, Oberdörster J. 2005. Nanotoxicology: an emerging discipline evolving from studies of ultrafine particles. Environ Health Perspect. 113: 823–839. doi: 10.1289/ehp.7339
- Pak S, Jang Y. 2003. Preparation and characterization of activated carbon fibers supported with silver metal for antibacterial behavior. J Colloid Interf Sci. 261:238–243. doi: 10.1016/S0021-9797(03)00083-3
- Ratte HT. 1999. Bioaccumulation and toxicity of silver compounds: a review. Environ Toxicol Chem. 18:89–108. doi: 10.1002/etc.5620180112
- Reddy KM, Feris K, Bell J, Wingett DG, Hanley C, Punnoose A. 2007. Selective toxicity of zinc oxide nanoparticles to prokaryotic and eukaryotic systems. Appl Phys Lett. 90:2139021–2139023.
- Silver S. 2003. Bacterial silver resistance: molecular biology and uses and misuses of silver compounds. FEMS Microbiol Rev. 27:341–353. doi: 10.1016/S0168-6445(03)00047-0
- Silver S, Phung LT. 1996. Bacterial heavy metal resistance: new surprises. Annu Rev Microbiol. 50:753–789. doi: 10.1146/annurev.micro.50.1.753
- Shrivastava S, Bera T, Roy A, Singh G, Ramachandrarao P, Dash D. 2007. Characterization of enhanced antibacterial effects of novel silver nanoparticles. J Nanotechnol. 18: 225103–12. doi: 10.1088/0957-4484/18/22/225103
- Silver S, Phung LT, Silver G. 2006. Silver as biocides in burn and wound dressings and bacterial resistance to silver compounds. J Ind Microbiol Biotechnol. 33:627–634. doi: 10.1007/s10295-006-0139-7
- Sondi I, Salopek-Sondi B. 2004. Silver nanoparticles as antimicrobial agent: a case study on E. coli as a model for Gram-negative bacteria. J Colloid Interface Sci. 275:177–182. doi: 10.1016/j.jcis.2004.02.012
- Sweetman SC. 2005. Martindale, the complete drug reference. 34th ed. p. 1163. London, UK: Pharmaceutical Press.
- Vertelov GK, Krutyakov YA, Efremenkova OV, Olenin AY, Lisichkin GV. 2008. A versatile synthesis of highly bactericidal Myramistin® stabilized silver nanoparticles. Nanotechnology. 19:355–707. doi: 10.1088/0957-4484/19/35/355707
- Warheit DB, Borm PJ, Hennes C, Lademann J. 2007. Testing strategies to establish the safety of nanomaterials: conclusions of an ECETOC workshop. Inhalat Toxicol. 19: 631–643. doi: 10.1080/08958370701353080
- Zhao G, Stevens SEJr. 1998. Multiple parameters for the comprehensive evaluation of the susceptibility of Escherichia coli to the silver ion. Biometals. 11:27–32. doi: 10.1023/A:1009253223055