Abstract
The present study was undertaken to explore the comparative phytochemical analysis and immunomodulating activities of various successively extracted leaf extracts of Stevia rebaudiana (Bertoni) in albino rats. Phytochemical screening conducted on various organic leaf extracts of S. rebaudiana revealed the presence of several biologically active molecules that include sterols, saponins, alkaloids, glycosides, phenols, tannins, flavonoids and resins. The immunomodulatory activities were determined by neutrophil adhesion test, haemagglutination antibody (HA) titre delayed type hypersensitivity (DTH) response and phagocytic activity. The response produced by oral administration of ethanolic and aqueous leaf extracts showed a significant increase in neutrophil adhesion and DTH response. The augmentation of humoral immune response to sheep red blood cells by ethanolic and aqueous extracts at 300–500 mg kg–1 was higher than when an extract dose of 200 mg kg–1 was used, as shown by a dose-related increase in antibody titres in experimental rats. This study demonstrates potent immunomodulatory activities and therapeutic potential of phytochemical compounds extracted from S. rebaudiana leaf to combat against immunological disorders.
Introduction
Medicinal plants are a very rich source of bioactive substances which are claimed to improve immunity (Gaur et al. Citation2009). Ayurveda, the Indian traditional medicine system, emphasizes the promotion of health by strengthening host defence systems against various diseases (Gaur et al. Citation2009). Diagnosis and drug development in Ayurveda are based on Tridosha theories, which include Vayu, Pitta and Kapha (Sastri Citation1996). The principle of balance and counter balance is fundamental to these theories. Ayurveda (ayu = life, veda = knowledge) extensively uses plant-derived compound formulations for the treatment of various ailments after a careful study into the type of disease. Plant extracts are complex mixtures of compounds and no single compound can provide the desired activity (Jagetia et al. Citation2004).
The immune system is involved in the aetiology as well as in the pathophysiologic mechanism of many diseases. Modulation of the immune response to alleviate diseases, has been of interest for many years and the concept of ‘Rasayana’ and ‘Ayurveda’ are based on related principles (Sharma et al. Citation1983). The immune system uses various endogenous molecules to control invaders. It is important that these molecules can distinguish ‘self’ from ‘non-self’ and mount defence reactions in a very selective and specific manner (Steven et al. Citation2002). Natural adjuvants, synthetic agents and antibody reagents are used as immunosuppressive and immunostimulative agents. However, there are major limitations to the use of these agents, such as increased risk of infection and generalized effects throughout the immune system (Tripathi et al. Citation2012). Synthetic cyclophosphamide has been extensively studied as an immunosuppressant (Shand & Howard Citation197Citation9). However, the major undesirable drawback of this drug is myelosuppression. Immunostimulation and immunosuppression both need to be handled to regulate immune functions normally. Therefore, some stimulatory or suppressive agents have been shown to possess the ability to normalize or modulate pathophysiological processes, and are hence called immunomodulatory agents (Wagner Citation1983). It has been claimed that a number of Indian medicinal plants and ‘Rasayana’ possess immunomodulatory activities; these are believed to promote health, immunity and longevity (Patwardhan et al. Citation1990; Diasio and LoBuglio Citation1996).
Stevia rebaudiana Bertoni is a herbaceous perennial plant of the Asteraceae family. It is native of Paraguay, where it grows wild in sandy soil. Stevia leaf extracts are used in Japan, Korea and certain countries of South America to sweeten soft drinks, soju, soy sauce, yogurt and other foods, whereas in the USA it is used as a dietary supplement. Recently, cultivation of S. rebaudiana has begun in India and Bangladesh (Hossain et al. Citation2010). The leaf extracts of S. rebaudiana have been used traditionally in the treatment of diabetes (Shivanna et al. Citation2013). The main sweet component in the leaves of S. rebaudiana is stevioside (Geuns Citation2000). It has been suggested that Stevia sweetener extracts have beneficial effects on human health, including antihypertensive, antihyperglycaemic, noncariogenic and anti-human rota virus activities (Chan et al. Citation2000; Lee et al. Citation2001). The present study reports comparative screening of immunomodulatory activities of successive S. rebaudiana leaf extracts in order to confirm the active compounds responsible for their possible immunotherapeutic potential.
Materials and methods
Plant material
The leaves of Stevia rebaudiana were collected in March 2006 from Jeevan Agro farms, Sagar, MP, India. A voucher specimen no. Bot/H/3352 was submitted at the herbarium of Department of Botany, Dr. H. S. Gour University, Sagar, MP, India.
Preparation of the extracts
The air-dried leaves of S. rebaudiana (100 g) were extracted with 500 ml of various solvents (petroleum ether, benzene, acetone, chloroform, ethanol and water) successively, on the basis of their polarity for about 40–50 complete cycles using a Soxhlet apparatus. The crude extracts were filtered and evaporated under reduced pressure to give a viscous dark mass. Each extract was preserved in a separate vial and kept at 4°C before use. The yields of all the extracts were related to the initial dry plant material ().
Table 1. Phytochemical screening of various organic solvent extracts obtained from S. rebaudiana leaves.
Drugs
Accurately weighed quantities of various organic extracts were suspended in 1% sodium carboxy methylcellulose (SCMC) to prepare suitable dosages. Cyclophosphamide was used as a standard immunosuppressant drug.
Experimental animals
Animal use protocol was approved by Dr. Hari Singh Gour University, Sagar, MP, India (Animal Eths Comm/IE/98/Reg No 379/01/ab/CPCSEA) and was in accordance with international standard on the care and use of experimental animals (CCAC Citation1993). Swiss albino rats of either sex weighing between 100–125 g were used for the study. Animals were housed under standard conditions of temperature (25°C), 12 h/12 h light/dark cycles and fed with standard pellet diet and tap water.
Antigen
Fresh blood was collected from sheep sacrificed in the local slaughter house. Sheep red blood cells (SRBCs) were washed three times in large volumes of pyrogen free 0.9% normal saline and adjusted to a concentration of 0.5 × 109 cells ml–1 for immunization and challenge.
Preliminary phytochemical screening
To identify the phytochemical constituents present in organic extracts of S. rebaudiana leaves, preliminary screening was performed using the Draggendorff's and Mayer's test, Liebermann-Burchard test, foam formation test, lead acetate test, Molisch's and Felhing's test and ferric chloride test, to determine alkaloids, terpenes, steroids, saponins, flavonoids, polysaccharides and tannins, respectively (Harborne Citation1973; Trease and Evans Citation1983).
Toxicity study
To assess the toxicity, various leaf extracts of S. rebaudiana were dissolved in water or suspended in 1% sodium carboxy methylcellulose and administered orally to different groups of rats in doses of 100–1000 mg kg–1 for the LD50 study using the modified method (Ghosh Citation1971). There was no lethality in any of the groups after seven days of treatment.
Neutrophil adhesion test
Wilkinson's (Citation1978) method was employed for the neutrophil adhesion test. Rats of group I were used as control and received 10 ml kg–1 normal saline, whereas groups II, III, IV and V were pretreated with different concentrations of organic leaf extracts of S. rebaudiana (200–500 mg kg–1, orally). On the 14th day of drug treatment, blood samples were collected by puncturing retro-orbital plexus into heparinized vials and analysed for total leukocyte cell (TLC) and differential leukocyte cell (DLC) counts. After initial counts, blood samples were incubated with nylon fibres for 15 min at 37°C. The incubated blood samples were again analysed for TLC and DLC respectively to give the neutrophil index of blood samples. The percentage neutrophil adhesion was calculated by the following formula:
Haemagglutination antibody (HA) titre
Puri et al. (Citation1993) described the method for haemagglutination antibody titre. The animals were immunized by injecting 0.1 ml of SRBC suspension containing 0.5 × 109 cells intra-peritoneally on day 0. Blood samples were collected in micro centrifuge tubes from individual animal by retro-orbital puncture on day 7. The blood samples were centrifuged and serum was obtained. Antibody levels were determined by the haemagglutination technique. Equal volumes of individual serum samples of each group were pooled. Two-fold serial dilutions of pooled serum samples made in 25 µl volume of normal saline in micro-titration plates were added to 25 µl of 1% suspension of SRBCs in saline. After mixing, the plates were incubated at 37°C for 1 h and examined for haemagglutination under a microscope. The reciprocal of the highest dilution of the test serum agglutination was taken as the antibody titre.
Delayed-type hypersensitivity (DTH) response
In this assay, the rats were challenged by injection of 0.5 × 109 cells SRBCs in the right hind foot pad. Foot thickness was measured after 24 and 48 h of this challenge. The differences obtained for pre- and post-challenge foot thickness were taken for the measurement of DTH and were expressed in mm. The extracts were administered orally on day 0 and continued until day 7 of challenge (Shivaprasad et al. Citation2006).
Phagocytic response
A method described by Cheng et al. (Citation2005) was adopted for phagocytic response assay. The animals were treated from day 0 to day 7 with different concentrations (200–500 mg kg–1, orally) of the various organic leaf extracts. On day 7, all the animals of the entire group received the treatment of an intravenous injection of 0.3 ml per 30 g Indian ink dispersion (pre-warmed at 37°C). A 50 µl sample of blood was collected from each animal by retro orbital bleeding at intervals of 2 and 10 min after the injection of ink dispersion. Blood samples were added to 4 ml of 0.1% sodium carbonate solution to lyse the erythrocytes. Absorbance of these samples was measured at 675 nm using a spectrophotometer. After 10 min of blood collection, animals were sacrificed and their livers and spleens were collected and weighed. Rate of carbon clearance (K) and phagocytic index (α) was calculated by using the following formula and compared with the control animals:
Statistical analysis
Data were expressed as the mean ± standard deviation (SD) of the means and statistical analysis was carried out employing one-way analysis of variance (ANOVA). Differences between the data were considered significant at p < 0.05.
Results
Phytochemical screening
The five extracts of Stevia rebaudiana leaves, i.e. petroleum ether extract (PTSR), chloroform extract (CLSR), acetone extract (ACSR), ethanolic extract (ALSR) and aqueous extract (AQSR), were analysed for their phytochemical profile and immunomodulatory potential. S. rebaudiana leaf extracts showed the presence of different phytochemicals depending on the solubility of the compounds and the solvent used. The petroleum ether leaf extract mainly showed the presence of steroids. In addition, steroids, cardiac glycosides and tannins were found to be present in the chloroform extract. Acetone and ethanolic extracts contained alkaloids, saponins, cardiac glycosides, tannins and sugars, whereas the aqueous extract showed the presence of alkaloids, saponins, cardiac glycosides, anthraquinone glycosides, tannins and sugars ().
Neutrophil adhesion test
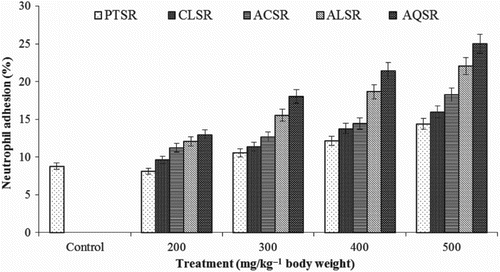
Percentage neutrophil adhesion was performed by the adhesion of neutrophils to nylon fibres and the results were compared with that of the control group. Ethanolic and aqueous extracts (at doses of 200–500 mg kg–1 orally) significantly (p < 0.05) enhanced the adhesion of neutrophils to nylon fibres, which correlates to the process of margination of cells in blood vessels. The neutrophil adhesion was found to be higher with ethanolic and aqueous extracts (), and was also related to the increase in the percentage of neutrophils, indicating possible immunostimulant effect. Percentage neutrophil adhesion for ethanolic extracts of S. rebaudiana leaves was higher (12.10 ± 0.95, 15.55 ± 1.26, 18.69 ± 0.67 and 22.08 ± 0.98%) at different doses (200, 300, 400 and 500 mg kg–1 body wt, respectively), when compared to the control group (8.82 ± 1.71). Aqueous extracts of S. rebaudiana leaves at various doses of 200, 300, 400 and 500 mg kg–1 body wt showed 12.98 ± 1.29, 18.03 ± 0.97, 21.45 ± 1.38 and 25.02 ± 1.20% neutrophil adhesion, respectively (). No significant increase in neutrophil adhesion was observed at a dose of 200 mg kg–1. However, extract dose concentrations ranging from 300–500 mg kg–1 revealed significant increase in neutrophil adhesion, compared to the control ().
DTH response
The cell-mediated immune response of various organic extracts of S. rebaudiana leaves was assessed by DTH reaction, i.e. foot pad reaction. DTH response to SRBC was calculated as a measure of paw oedema thickness (mm) for 200, 300 and 400 mg kg–1 body weight of each animal after treatment with different extracts, and in comparison with the control. After 24 h, animals treated with ethanol extract doses (200, 300, 400 and 500 mg kg–1 body wt) showed DTH values of 0.33 ± 0.09, 0.41 ± 0.01, 0.50 ± 0.01 and 0.58 ± 0.01 mm, respectively, while, in control group it was 0.18 ± 0.01 mm (). However, paw oedema thickness values after 72 h were 0.27 ± 0.01, 0.29 ± 0.09, 0.34 ± 0.01 and 0.37 ± 0.01 mm, while in control it was 0.15 ± 0.01 mm (). In aqueous extract, after 24 h, the DTH response was 0.42 ± 0.01, 0.46 ± 0.01, 0.56 ± 0.01 and 0.63 ± 0.01 mm, while in the control group it was 0.18 ± 0.01 mm (). Finally, after 72 h, the values were found to be reduced significantly (0.30 ± 0.01, 0.36 ± 0.01, 0.38 ± 0.01 and 0.43 ± 0.02 mm), compared to the control group (0.15 ± 0.01 mm) (). All the extracts of S. rebaudiana showed an increase in DTH in all the treated groups when compared to the vehicle control group. Animals treated with aqueous and ethanol extracts of S. rebaudiana leaves showed more potent results with significant difference to the control group. The mean paw oedema values due to the DTH reaction are shown in Tables and . Increases in the DTH reaction in rats in response to cell-dependent antigens revealed the stimulatory effect of ethanolic and aqueous leaf extracts of S. rebaudiana on T cells.
Table 2. Effect of S. rebaudiana leaf extracts on delayed type hypersensitivity (DTH) response after 24 h.
Table 3. Effect of S. rebaudiana leaf extracts on delayed type hypersensitivity (DTH) response after 72 h.
HA titre
The HA titre was used to assess humoral immune response. Humoral antibody response to SRBC challenge was found to be significantly improved by all the extracts of S. rebaudiana, when analysed by measuring agglutination titre against SRBC antigens at various serum dilutions. The mean value of HA titre of all the extracts was compared to vehicle control. The effect was found to be dose dependent. Ethanolic and aqueous extracts showed superior activity compared to the others. The humoral antibody titre for ethanolic and aqueous extracts of S. rebaudiana for the highest dose value (500 mg kg–1) was found to be 69.64 ± 7.09 and 94.83 ± 2.72, respectively (). The augmentation of humoral response to SRBCs by ethanolic and aqueous leaf extracts is evidenced by an increase in the antibody titres in the blood of rats. Administration of higher doses, i.e. 300, 400 and 500 mg kg–1, produced significant increases in HA titre as evident from haemagglutination after incubation of serum with SRBCs.
Table 4. Effect of S. rebaudiana leaf extracts on haemagglutination antibody (HA) titre.
Phagocytic response
The carbon clearance assay suggested that the administration of plant extracts for seven days significantly increased the phagocytic index, compared to vehicle control animals. Rate of carbon clearance is a measurement of the competency of the reticulo-endothelial system (RES) and its granulopoetic activity. Faster removal of carbon particles has been correlated with enhanced phagocytic activity. Petroleum ether, chloroform, acetone, ethanol and aqueous extracts of S. rebaudiana leaves at different doses (200, 300, 400 and 500 mg kg–1 body wt) were tested in four different groups (III, IV, V and VI) of animals, 10 min prior to carbon injection. Different levels of elevation in the phagocytic index were observed in S. rebaudiana after seven days of oral administration (), and there were dose-related increases in the clearance rate of carbon by the cells of the RES. All extracts of S. rebaudiana showed dose-dependent increases in the phagocytic index. Ethanolic and aqueous extracts of the S. rebaudiana showed better phagocytic activity in comparison to other extracts (petroleum ether, chloroform and acetone extracts).
Table 5. Effect of S. rebaudiana leaf extracts on phagocytic index.
The ethanolic leaf extract at the doses of 200, 300, 400 and 500 mg kg–1 body weight showed phagocytic index as 3.89 ± 0.24, 4.72 ± 0.22, 6.41 ± 0.11 and 8.00 ± 0.43, respectively. In addition aqueous extract showed highest phagocytic index as 4.58 ± 0.47, 5.00 ± 0.34, 6.95 ± 0.27, 7.98 ± 0.48 with respective doses of 200, 300, 400 and 500 mg kg–1 body weight/oral dose for 7 days. The phagocytic index of control group was 3.12 ± 0.49 ().
Discussion
The results obtained in this study support the claims that Stevia rebaudiana, an important plant in Indian indigenous medicine, has medicinal use. The immunomodulatory activities of various organic leaf extracts of S. rebaudiana were explored. Shivanna et al. (Citation2013) reported the levels of proteins, sugar, phenols and flavonoids in S. rebaudiana leaves to be 16 g/100 g, 46.1 g/100 g, 91 mg g–1 and 23 mg g–1, respectively. A high level of total phenols is a positive indication for the antioxidant and antidiabetic properties of S. rebaudiana (Shivanna et al. Citation2013). Previous studies have also noted the importance of hypoglycaemic components of S. rebaudiana due to the presence of rebaudioside A and stevioside that are concentrated in leaves (Wheeler et al. Citation2008).
Modulation of the immune response through stimulation or suppression may help in maintaining homeostasis of the body. Agents that activate host defence mechanisms in the presence of an impaired immune response can provide supportive therapy to conventional chemotherapy (Wagner Citation1984). In this study, adherence to nylon fibres increased in the ethanolic and aqueous extracts of S. rebaudiana immunized group. This may be due to the upregulation of β2 integrins and decreased corticosterone levels (Benschop et al. Citation1994). Increased glucocorticoids level may also lead to this decrease in the neutrophil adherence (Benschop et al. Citation1994). Phagocytosis by neutrophils is an essential part of host defence against foreign antigens. Ethanol and aqueous extracts of S. rebaudiana showed significant amount of percentage neutrophil adhesion effect, which differed in each extract. This may be due to the concentration of active phytochemicals present in the plant extracts. The neutrophil, unable to divide and with limited capacity for protein synthesis is, nevertheless, capable of a wide range of responses, in particular chemotaxis, phagocytosis, exocytosis and both intracellular and extracellular killing (Dale and Foreman Citation1984). In the present study, ethanolic and aqueous extracts (300–500 mg kg–1, orally) of C. bonducella and S. rebaudiana evoked significant results in percentage neutrophil adherence. Similar findings were also noticed by Benacerraf (Citation1978). In addition, Ghule et al. (Citation2006) also reported that Capparis zeylanica showed dose-dependent immunostimulatory activity in both alcohol and aqueous extracts. Thakur et al. (Citation2006) observed that sapogenin of ethanolic extract of Chlorophytum borivillanum induced stimulation of immune response in treated animals. In the present investigation, ethanolic and aqueous extracts of S. rebaudiana showed potent results, proving their immunostimulatory activities and showing that they could be effective candidates for the prevention of autoimmune diseases.
Cell-mediated immunity is a part of the processes of graft rejection, tumour immunity and immunity to many intracellular infections or to microorganisms, which may cause chronic diseases (Sahu et al. Citation2013). Ethanolic and aqueous extracts of S. rebaudiana exhibited potent increase in paw oedema in response to SRBC. The initial response in the first hour resulted in the infiltration of CD4 lines of T lymphocytes, diapedesis of mononuclear macrophages and liberation of oedema producing substances, i.e. serotonin, prostaglandilin E and cytokines, etc. The infiltration of lymphocytes may be due to the compounds distorting the endothelium and enabling different types of lymphocytes to accumulate. This process indicats the cell-mediated immune response (Sahu et al. Citation2013). Extracts of plants possess some compounds that may support the cell-mediated immune response. This indicates that ethanolic and aqueous extracts of S. rebaudiana containing saponins and tannins may activate the T-cells leading to release of vasoactive amines and multiple hormone substances such as lymphokines. These substances then probably function as mediators of the ensuing hypersensitivity response particularly by attracting and activating macrophages (Roitt Citation1998; Ray et al. Citation1996). S. rebudiana dervied extracts are considered rich sources of saponin like phytoconstituents which have shown potent immunostimulating effects. (Shibata Citation1977; Liu et al. Citation1995; Verotta et al. Citation2001). Reduction in paw oedema may be due to the action of various enzymes and hormones that increase phagocytosis, which activate macrophages on the invader. This may be due to saponins and similar compounds increasing the metabolic activity of the neighbouring cells to release serine proteases and immunohormones, including cytokines (Shukla et al. Citation2011). These metabolites and activated macrophages eliminate the causative agents, hence the oedema gradually reduces (Shukla et al. Citation2011).
Antibodies, product of B lymphocytes and plasma cells, are central to the humoral immune responses (Miller Citation1991). IgG and IgM are the major immunoglobulins which are involved in complement activation, opsonization, neutralization of toxins, etc. (Miller Citation1991). The augmentation of the humoral immune response to SRBCs by plant extracts, shown by an increase in the antibody titre in rats, indicated the enhanced responsiveness of T and B lymphocyte subsets (Benacerraf Citation1978). The high values of haemagglutination antibody titre obtained with ethanolic and aqueous leaf extracts of Stevia rebaudiana indicated that immunostimulation was achieved through humoral immunity. Bafna and Mishra (Citation2006) reported that animals treated with different doses of methanol extract of Curculigo orchioides showed an increase in the haemagglutination antibody titre in a dose-dependent manner; such results reveal the significance of the present study. Similar findings with alcoholic extract of Isatis cappadocica (Rezaeipoor et al. Citation2000) and aqueous and alcoholic extracts of Echinacea purpurea (Freier et al. Citation2003) have been reported, with increased titres of IgM. Aqueous extract of Clausena excavate has also been found to show similar results in case of DTH response and HA titre (Manosroi et al. Citation2005).
Phagocytic activity is a parameter that can be used to measure the competency of the RES and its granulopoetic activity (Mishra Citation2005). Phagocytosis and killing of invading microorganisms by macrophages constitute the body's primary line of defence against infection (Van Furt Citation1982). An increase in phagocytic index, i.e. clearance of carbon particles from the blood, suggests that there is activation of white blood cells (WBC) (Rastogi et al. Citation2008). Stimulation of activated macrophages secretes a number of cytokines, which in turn stimulate other immune cells (Nose et al. Citation1998). In the present study, S. rebaudiana aqueous extract was found to be more potent than ethanolic extract which showed significant immunomodulatory activities. In the present study, petroleum ether, chloroform and acetone extracts did not produce any significant increase or decrease in the phagocytic activity, which suggests that the active substances that stimulate the immune system are either absent or present in low concentrations. Ethanolic and aqueous extracts of plant significantly influence and activate macrophages, confirming that both ethanolic and aqueous leaf extracts of S. rebaudiana may have phytochemicals (alkaloids, saponins, terpenoids, etc.) which exert such activities. Thakur et al. (Citation2006) observed similar findings with the root extract of Chlorophytum borivilianum. The oral administration of ethanolic and water extracts of Capparis zeylanica at doses of 150–300 mg kg–1 body wt showed a similar dose-related increase in the clearance rate of carbon by the cells of the RES (Ghule et al. Citation2006). Sehar et al. (Citation2008) also tested stevioside isolated from S. rebaudiana leaves for its immunomodulatory activity on different parameters of the immune system in mice at three different doses (6.25, 12.5 and 25 mg kg–1), and it was found that it acted as a promising immune drug by stimulating both humoral as well as cellular immunity and phagocytic function.
The present observations appear to give some support to the use of Stevia rebaudiana in traditional medicine for the treatment of autoimmune diseases. The extracts not only produced a non-specific immune response, but also improved humoral as well as cell-mediated immunity effectively. Thus, from the results obtained, it can be concluded that S. rebaudiana has therapeutic potential and could be served as a safe and effective immunomodulatory agent in the treatment of immunological disorders. Further studies on the mechanism of action of S. rebaudiana derivatives and its purified molecules, in order to establish its therapeutic potential for the prevention of autoimmune diseases, are being planned. This study validates the traditional use of S. rebaudiana as a ‘Rasayana’ in Ayurvedic system of medicine.
Acknowledgements
The authors are grateful to Department of Botany and Pharmaceutical Sciences, Dr. H. S. Gour University, Sagar, MP, India for providing laboratory facilities and Madhya Pradesh Science and Technology, Bhopal, MP, India for providing financial assistance.
References
- Bafna AR, Mishra SH. 2006. Immunostimulatory effect of methanol extract of Curculigo orchioides on immunosuppressed mice. J Ethnopharmacol. 104:1–4. doi: 10.1016/j.jep.2005.06.048
- Benacerraf B. 1978. A hypothesis to relate the specificity of T lymphocytes and the activity of I region specific Ir genes in macrophages and borrower lymphocytes. J Immunol. 120:1809–1832.
- Benschop RJ, Oostveen FG, Heijnen CJ, Ballieux RE. 1994. The effects of beta-adrenoceptor stimulation on adhesion of human natural killer cells to cultured endothelium. Br J Pharmacol. 113:1311–1316. doi: 10.1111/j.1476-5381.1994.tb17141.x
- CCAC. 1993. Guide to the care and used of experimental animals, vol. 1. The Canadian Council on Animal care, http://www.ccac.ca/
- Chan P, Tom Linson B, Chen Y-J, Liu J-C, Hsieh M-H, Cheng J-T. 2000. A double-blind placebo-controlled study of the effectiveness and tolerability of oral stevioside in human hypertension. Br J Clin Pharmacol. 50:215–220. doi: 10.1046/j.1365-2125.2000.00260.x
- Cheng W, Li J, You T, Hu C. 2005. Anti-inflammatory and immunomodulatory activites of the extracts from the inflorescence of Chrysanthemum indicum Linn. J Ethnopharmacol. 101:334–337. doi: 10.1016/j.jep.2005.04.035
- Dale MM, Foreman JC. 1984. Text book of immunopharmacology. London, UK: Blackwell, p. 5254.
- Diasio RB, LoBuglio AF. 1996. Immunomodulator: immunosuppressive agents and I immunostimulants. In: Goodman and Gilman's, editor. The pharmacological basis of therapeutics. 9th ed. The New York: McGraw-Hill, p. 1291–1307.
- Freier DO, Wright K, Klein K, Voll D, Dabiri K, Cosulich GR. 2003. Enhancement of the humoral immune response by Echinacea purpurea in female swiss mice. Immunopharmacol Immunotoxicol. 25:551–560. doi: 10.1081/IPH-120026440
- Gaur K, Kori ML, Nema RK. 2009. Comparative screening of immunomodulatory activity of hydro-alcoholic extract of Hibiscus rosasinensis Linn. and ethanolic extract of Cleome gynandra Linn. Glob J Pharmacol. 3:85–89.
- Geuns JMC. 2000. Safety of stevia and stevioside. Recent Res Dev Phytochem. 4:75–88.
- Ghosh MN. 1971. Fundamentals of experimental pharmacology. Calcutta, India: Scientific Book Agencies, p. 84–88.
- Ghule BV, Murugananthan G, Nakhat PD, Yeole PG. 2006. Immunostimulant effects of Capparis zeylanica Linn. leaves. J Ethnopharmacol. 108:311–315. doi: 10.1016/j.jep.2006.03.041
- Harborne JB. 1973. Pytochemical methods. London, UK: Chapman and Hall, Ltd., p. 49–188.
- Hardman JG, Limbird LE, Molinoff PB, Ruddon RW, Gilman AG. 2006. Alkylating agents. In: New York Academy of Sciences, editor. Goodman and Gilman's the pharmacological basis of therapeutics. New York: McGraw-Hill, p. 1216–1218.
- Hossain MA, Siddique AB, Rahman SMM, Hossain MA. 2010. Chemical composition of the essential oils of Stevia rebaudiana Bertoni leaves. Asian J Trad Med. 5:56–61.
- Jagetia GC, Malagi KJ, Baliga MS, Venkatesh P, Veruva RR. 2004. Triphala, an ayurvedic rasayana drug, protects mice against radiation-induced lethality by free-radical scavenging. J Alt Comp Med. 10:971–978. doi: 10.1089/acm.2004.10.971
- Lee C-N, Wong K-L, Lin J-C, Chen Y-J, Chen J-T, Chan P. 2001. Inhibitory effect of stevioside on calcium influx to produce antihypertension. Plant Med. 67:796–799. doi: 10.1055/s-2001-18841
- Liu J, Wang S, Lui H, Yang L, Nan G. 1995. Stimulatory effect of saponin from Panax ginseng on immune function of lymphocyte in the elderly. Mech Ageing Dev. 83:43–53. doi: 10.1016/0047-6374(95)01618-A
- Majumder UK, Gupta M, Mukhopadhyay DK. 1997. Effect of mycotoxins isolated from Penicillium nigricans on glucose-6-phasphate dehydrogenase. Indian J Exp Biol. 35:1233–1236.
- Manosroi A, Saraphanchotiwitthaya A, Manosroi J. 2005. In vivo immunomodulating activity of wood extracts from Clausena excavate Burm. J Ethnopharmacol. 102:5–9. doi: 10.1016/j.jep.2005.04.033
- Miller LE. 1991. Manual of laboratory immunology. London, UK: Lea and Febiger, p. 1–18.
- Mishra SN. 2005. Bhaisajya Ratnavali. 1st ed. Varansi, India: Chaukambha Surbharti Prakashan. p. 1008–1333.
- Nose M, Terawaki K, Oguri K, Ogihara Y, Yoshimatsu K, Shimomura K. 1998. Activation of macrophages by crude polysaccharide fractions obtained from shoots of Glycyrrhiza glabra and hairy roots of Glycyrrhiza uralensis in vitro. Biol Pharm Bull. 21:1110–1112. doi: 10.1248/bpb.21.1110
- Patwardhan B, Kalbag D, Patki PS, Nagsampagi BA. 1990. Search of immunomodulatory agents: a review. Indian Drugs. 28:348–358.
- Puri A, Saxena R, Saxena RP and Saxena KC. 1993. Immunomodulant agents from Andrographis paniculata. J Nat Prod. 56:995–999. doi: 10.1021/np50097a002
- Rastogi B, Tiwari U, Dubey A, Bawara B, Chauhan NS, Saraf DK. 2008. Immunostimulatory activity of Cocculus Hirsutus in immunosuppressed rat. Pharmacologyonline. 3:38–57.
- Ray A, Banerjee BD, Sen P. 1996. Modulation of humoral and cell mediated immune response by Azadirachta indica in mice. Indian J Exp Biol. 34:698–701.
- Rezaeipoor R, Sanei Ziaei L, Kamalinejad M. 2000. Effect of Isatis cappadocica on humoral immune responses. Fitoterapia. 71:14–18. doi: 10.1016/S0367-326X(99)00092-1
- Roitt I. 1998. EssentiaL immunology. 5th ed. Oxford: Blackwell Scientific Publication, London.
- Sahu MS, Sahu RA, Verma A. 2013. Immunomodulatory activity of alcoholic extract of Habenaria intermedia in mice. Int J Pharm Sci. 5:406–409.
- Sastri VS. 1996. Tridosha theory. India: Arya Vaidya Sala Publication Kottakkal, India, p. 3–6.
- Sehar I, Kaul A, Bani S, Pal HC, Saxena AK. 2008. Immune up regulatory response of a non-caloric natural sweetener, stevioside. Chemico-Biol Interac. 173:115–121. doi: 10.1016/j.cbi.2008.01.008
- Shand FL, Howard JG. 1979. Induction in-vitro of reversible immunosuppression and inhibition of B cell receptor regeneration by defined metabolites of cyclophosphamide. Eur J Immunol. 9:17–21. doi: 10.1002/eji.1830090105
- Sharma P, Charaka S, Asthana C. 1983. Chaukhamba Orientalia Publication. Varanasi, India, p. 54.
- Shibata S. 1977. Saponins with biological and pharmacological activity in: new natural products and plant drugs with pharmacological, biological or therapeutical activity. Berlin: Springer, p. 177–196.
- Shivanna N, Naika M, Khanum F, Kaul VK. 2013. Antioxidant, anti-diabetic and renal protective properties of Stevia rebaudiana. J Diabetes Comp. 27:103–113. doi: 10.1016/j.jdiacomp.2012.10.001
- Shivaprasad HN, Kharya MD, Rana AC, Mohan S. 2006. Preliminary immunomodulatory activities of aqueous extract of Terminalia chebula. Pharm Biol. 44:32–34. doi: 10.1080/13880200500530542
- Shukla S, Mehta A, Kim M, Ayyadurai N. 2011. In vivo immunomodulatory activities of ethanolic leaf extract of Stevia rebaudiana in albino rats. Res J Biotechnol. 6:27–31.
- Steven MH, Vizi E. 2002. Immunomodulation. Curr Opin Pharmacol. 2:425–427. doi: 10.1016/S1471-4892(02)00188-1
- Thakur M, Bhargava S, Dixit VK. 2006. Immunomodulatory activity of Chlorophytum borivilium Sant. Comp Alt Med. 13:1–5.
- Trease GE, Evans MC. 1983. Textbook of pharmacognosy, 12th ed. London, UK: Balliere, Tindall, p. 343–383.
- Tripathi S, Maurya AK, Kahrana M, Kaul A, Sahu RK. 2012. Immunomodulatory property of ethanolic extract of Trigonella Foenum-Graeceum leaves on mice. Der Pharm Lett. 4:708–713.
- Van Furt R. 1982. Current view on the mononuclear phagocyte system. Immunobiol. 161:178–185. doi: 10.1016/S0171-2985(82)80072-7
- Verotta L, Guerrini M, El-Sebakhy Asaad AM, Toaima SM, Abou-Sheer ME, Luo YD, Pezzuto JM. 2001. Cycloartane saponins from Astragalus peregrinus as modulators of lymphocyte proliferation. Fitoterapia. 72:894–905. doi: 10.1016/S0367-326X(01)00339-2
- Wagner H. 1983. Immunomodulatory agents. Proc Alfred Benzon Symp. 20:0–559.
- Wagner H. 1984. Economic and medicinal plant research, Vol. I. London, UK: Academic Press, p. 113–153.
- Wheeler A, Boileau AC, Winkler PC, Compton JC, Prakash I, Jiang X. 2008. Pharmacokinetics of rebaudioside A and stevioside after single oral doses in healthy men. Food Chem Toxicol. 46:S54–S60. doi: 10.1016/j.fct.2008.04.041
- Wilkinson PC. 1978. Neutrophil adhesion test. In: Vane JK, Ferreria SH editors. Handbook of experimental pharmacology, I, 1st ed. Berlin: Springer Verlag, p. 109.