Abstract
Methanol and 70% acetone extracts from Veronica jacquinii, Veronica urticifolia and Veronica teucrium were evaluated regarding their neuroprotective/antioxidant effects on the human neuroblastoma SH-SY5Y cell line. All extracts exhibited modest protective activity by increasing cell survival in cells stressed with sodium nitroprusside (9–12%) and hydrogen peroxide (16–21%) compared to non-treated cells. These activities were accompanied by reductions in the amount of superoxide radicals and index of lipid peroxidation. Extracts were further analysed for the content of total phenolics, phenylpropanoid glycosides and iridoids, and the major compounds acteoside and aucubin. The highest amount of total phenolics was observed in V. jacquinii, while V. teucrium was richest in total iridoids, acteoside and aucubin. Angiogenic properties on EA.hy926 human endothelial cells were also examined. At the highest non-toxic concentration (25 µg/ml), the tested extracts inhibited spontaneous tube formation of endothelial cells on the extracellular matrix, implying their possible antiangiogenic activity. The most potent inhibitory effects were shown by methanol extract of V. jacquinii and aqueous acetone extract of V. teucrium. The extracts did not significantly change the adhesive or migratory capacity of EA.hy926 cells. Considering the traditional use of Veronica species, these results suggest a need for further assessment of their supposed wound-healing properties.
Introduction
The genus Veronica (Plantaginaceae) is represented by 450 species found in temperate regions of both hemispheres. In Asia and Europe, Veronica species have been used in folk medicine for the treatment of certain respiratory diseases, wound healing and problems of the nervous system, and as a diuretic (Küpeli et al. Citation2005). Despite their widespread traditional use, there is a scarcity of physiological evidence to support any claim of therapeutic value for Veronica species. A few studies have confirmed that certain Veronica species have considerable bioactivity, such as antibacterial (Abu Ziada et al. Citation2008; Gusev et al. Citation2012; Stojković et al. Citation2013), antioxidant (Živković et al. Citation2012), anti-inflammatory (Küpeli et al. Citation2005) and cytotoxic effects (Saracoglu et al. Citation2011). Wound healing is a complex process that involves a series of biochemical and cellular reactions, which include an inflammatory response to injury, cell proliferation, formation of fibrin, production of collagen, angiogenesis and re-epithelialization (Martin Citation1996). Angiogenesis is the formation of new blood vessels from the pre-existing vascular network, and stimulation of angiogenesis may improve wound healing by providing nutrients to support the active cells, promoting granulation of tissue formation and facilitating the clearance of debris (Kleinman & Malinda Citation2000). In contrast, pathological angiogenesis occurs in many diseases such as rheumatoid arthritis, diabetic retinopathy and cancer (Fotsis et al. Citation1993). The formation of new blood vessels is critical for the growth of tumours, and therefore the therapeutic inhibition of angiogenesis could serve as a novel means to treat cancer. A wide range of plants contains active compounds with angiogenesis-modulating properties (Fan et al. Citation2006).
Oxidative stress is involved in neurodegenerative diseases such as Parkinson's disease, Alzheimer's disease and amyotrophic lateral sclerosis (Andersen Citation2004). Neurons are very susceptible to oxidative stress owing to their high consumption of oxygen and high content of unsaturated fatty acids (Wang & Michaelis Citation2010). The human body keeps the levels of reactive oxygen species (ROS) under control through the endogenous antioxidant system (enzymes and non-enzyme antioxidants) and exogenous antioxidants ingested in the diet. The mammalian brain contains glutathione and the enzymes glutathione peroxidase, glutathione reductase and superoxide dismutase to defend itself against oxidative stress, but their levels in the brain are low compared with their content in other organs. Good tolerability and low risk for developing side-effects have led to the emerging interest in herbal antioxidants as a potentially useful tool in reducing oxidative stress-induced cellular injury.
In this study, the neuroprotective and wound healing/angiogenic potentials of selected Veronica species were investigated. Methanol and 70% acetone extracts from three Veronica species (Veronica jacquinii Baumg., Veronica urticifolia Jacq. and Veronica teucrium L.) were prepared and evaluated regarding their neuroprotective/antioxidant effects on the human neuroblastoma SH-SY5Y cell line. Furthermore, their influence on angiogenic properties was estimated on EA.hy926 human endothelial cells by cell adhesion, scratch and three-dimensional tube formation assays. Extracts were also analysed for their content of total phenolics, phenylpropanoid glycosides and iridoids, as well as the major compounds acteoside and aucubin.
Materials and methods
Plant material and chemicals
Aerial flowering parts of V. teucrium, V. urticifolia and V. jacquinii were collected during the flowering period in 2010 at three mountains in Serbia: Stol (1000 m), Goč (1100 m) and Vršačke planine (600 m). Extracts were prepared by maceration with methanol, 70% aqueous acetone or water (solid:solvent ratio 1:20) at room temperature for 48 h, followed by vacuum evaporation. All extracts had low solubility in dimethyl sulfoxide (DMSO), and the highest concentration of extract that could be applied within the DMSO concentration range without significant influence on the cell viability was 0.5% (v/v). This concentration was thus used in the experiments. Media and supplements cell lines were mostly obtained from PAA (Pasching, Austria), except for Dulbecco's modified Eagle's medium (DMEM; Gibco [New York, USA]). Matrigel was a product of BD Biosciences (San Jose, USA). All other chemicals were from Sigma Chemical Co. (St Louis, MO, USA).
Determination of total phenolics
The concentration of total phenolic compounds in the extracts was determined spectrophotometrically using the Folin–Ciocalteu method with slight modifications (Waterman & Mole Citation1994). Two hundred microlitres of each extract (5 mg/ml) was added to 1 ml of 1:10 diluted Folin–Ciocalteu reagent. After 4 min, 800 ml of sodium carbonate (75 g/l) was added. After 2 h of incubation at room temperature, the absorbance at 765 nm was measured. Gallic acid (0–100 mg/l) was used for calibration of a standard curve. The results were expressed as milligrams of gallic acid equivalents per gram of dry weight of plant material (mg GAE/g DW). Triplicate measurements were taken and mean values were calculated.
Determination of total iridoids
The content of iridoids was determined according to the colorimetric method based on a Trim–Hill reaction (Gálvez et al. Citation2005). Each extract (100 µl) was mixed with 1 ml of Trim–Hill reagent (acetic acid–0.2% CuSO4–conc. HCl, 10:1:0.5). Samples were heated at 100°C for 5 min, then absorbance was measured at 609 nm; a blue colour indicated the presence of iridoids. The amount of iridoids was calculated using the calibration curve of aucubin (0.1–1 mg/l). Results were expressed as the mean value of three replicates.
Determination of total phenylpropanoid glycosides
Determination of phenylpropanoids was performed according to the colorimetric method based on estimation of o-dihydroxycinnamic derivatives (European Pharmacopoeia Citation6.Citation0 Citation2008). One millilitre of each sample was added to 2 ml 0.5 M HCl, 2 ml of 10% (w/v) aqueous solution of sodium nitrite and 10% (w/v) aqueous solution of sodium molybdate, and 2 ml of aqueous 2 M NaOH. The solution was adjusted to 10 ml with water. The compensation solution consists of 1 ml of each sample, 2 ml 0.5 M HCl and 2 ml of aqueous 2 M NaOH. The solution was diluted to 10 ml with water. Absorbance at 525 nm was measured, with a purple colour indicating the presence of phenylpropanoids. The concentration was calculated using the equation: [A × 1000]/185 × m, where A is absorbance of the sample and m is the mass of the dry plant material. The results were expressed as acteoside equivalents. All test analyses were run in triplicate. Data are presented as the mean ± standard deviation (SD).
High-performance thin-layer chromatography analysis
High-performance thin-layer chromatography (HPTLC) analysis was performed according to the method described previously, with slight modification (Biringanine et al. Citation2006). A Camag HPTLC system with a Linomat 5 sample applicator was used for the analysis. Sample solutions (2 µl) and 0.3, 0.6, 1, 1.5, 2 and 3 µl standard solution of acteoside (500 µg/ml) were applied to precoated 20 × 10 cm silica gel 60 HPTLC plates (0.25 mm layer thickness; Merck [Darmstadt, Germany]) as 7 mm bands. The plate was developed with ethyl acetate–water–formic acid–acetic acid (100:27:11:11, v/v) in saturated chambers (20 min with filter paper) over a distance of 7 cm. After development the plate was dried at 120°C for 10 min. For post-chromatographic derivatization, the plate was dipped in anisaldehyde–sulfuric acid reagent for a few seconds and then heated at 105°C for 4 min. Bands were scanned at 520 nm, and evaluation was via peak areas with polynomial regression. As acteoside is present in Veronica extracts at different concentration levels, the application of a polynomial calibration function allows measurements over a wide detection range. Results were expressed in milligrams per extract.
Cell cultures and treatments
The human neuroblastoma cell line SH-SY5Y was obtained from the American Type Culture Collection, and EA.hy926 cells were a kind gift from Dr Cora Jean Edgell (University of North Carolina, Chapel Hill, NC, USA). The cultures were maintained in a humidified atmosphere containing 5% CO2 at 37°C and prepared for the experiments using the conventional trypsinization procedure with trypsin/ethylenediaminetetraacetic acid (EDTA). The SH-SY5Y cell line was grown in modified Eagle's medium (MEM) and F12 cell culture medium (1:1) supplemented with 10% foetal bovine serum (FBS), 2 mM L-glutamine, non-essential amino acids and antibiotic/antimycotic solution. For the measurement of acid phosphatase activity (cell viability) or the amount of superoxide ion, SH-SY5Y cells were incubated in 96-well flat-bottom plates (20 × 103 cells per well). The cells grown in 25 ml flasks were used for the malondialdehyde assay. Undifferentiated cells were rested for 24 h, and treated with sodium nitroprusside (SNP), hydrogen peroxide (H2O2) and/or different extracts. Cell viability was determined after 24 h of treatment. To determine the effects of the extracts on cells exposed to oxidative stress, extracts were added as pretreatment 30 min before 1 mM SNP/100 µM H2O2.
EA.hy926 cells were cultured in DMEM supplemented with 10% FBS, 2 mM L-glutamine, 100 µg/ml streptomycin, 100 U/ml penicillin and HAT media supplement (1 mM hypoxathine, 4 µM aminopterin, 0.16 mM thymidine). The cells were incubated in 96-well flat-bottom plates for the cell adhesion assessment (2.5 × 104 cells/well) and tube formation on Matrigel (5 × 104 cells/well) or 24-well plates (2 × 105 cells/well) for the scratch assay. For the cell adhesion assessment, EA.hy926 cells were incubated with the highest non-toxic concentrations of tested extracts (25 µg/ml) 24 h before the experiment. For the tube formation and wound healing assays, the cells were treated immediately after plating on Matrigel or following the formation of scratch, respectively.
Assessment of cell viability
Cell viability was determined by measuring the activity of acid phosphatase (Connolly et al. Citation1986). At the end of incubation, the cultivating medium was removed and 10 mM p–nitrophenyl phosphate was added as a substrate for acid phosphatase. The reaction was stopped after 1 h of incubation at 37°C by adding 0.1 M NaOH. The colour development, which correlates with the number of viable cells, was monitored by an automated microplate reader at 405 nm (Multiscan spectrum, Thermo Electron Corporation [Madison, USA]). After subtracting the background value (NaOH alone), the results were presented as a percentage of the viability of untreated cells, which represented 100% viable.
Index of lipid peroxidation
The index of lipid peroxidation was measured spectrophotometrically as the amount of thiobarbituric acid reactive species (TBARS). SH-SY5Y cells were treated with SNP/H2O2 and/or different extracts and were collected using the trypsinization procedure with trypsin/EDTA. After centrifugation, supernatants were discarded and cells were lysed by freezing and thawing twice in deionized water. The content of TBARS formed spontaneously was measured upon treating the samples with cold thiobarbituric acid–reagent (10% trichloroacetic acid, 0.6% thiobarbituric acid) and subsequent heating at 100°C (Rehncrona et al. Citation1980). The absorbance was measured at 520 nm.
Superoxide production and measurement
The amount of superoxide ion () was measured by the reduction of nitroblue tetrazolium (NBT), as previously described (Song et al. Citation2011). A coloured formazan product formed from NBT was quantified spectrophotometrically. Reduction of NBT was measured at 560 nm.
Cell adhesion assay
The influence of Veronica extracts on the adhesive capacity of endothelial cells was assessed by the cell adhesion assay. The wells of 96-well plates were covered with 50 µl of Matrigel (20 µg/ml) and maintained at 4°C overnight. After 24 h, the plates were carefully washed three times with phosphate-buffered saline (PBS). Pretreated endothelial cells were seeded on Matrigel-coated plates at the indicated density and incubated for 15 min at 37°C. To remove unbound cells, the wells were gently washed three times with PBS following incubation. The cells that remained attached were fixed with paraformaldehyde solution (4%) and stained using crystal violet dye. In brief, cells were incubated with crystal violet solution (0.5%) for 30 min, excessive dye was removed by intensive washing with water and bound colour was dissolved using acetic acid (33%). The optical density was measured at 570 nm using a microplate reader (Multiskan spectrum, ThermoScientific, Madison, USA).
Wound healing assay/scratch assay
The migratory ability of endothelial cells was evaluated using a wound healing migration assay. The EA.hy926 cells were plated 24 h before the experiment and confluent monolayers were wounded with 1000 µl pipette tips on the day of analysis. After removing any cellular debris by washing with PBS, the cells were supplied with the complete medium in the absence (controls) or the presence of plant extracts (25 µg/ml). The wounds were observed by light microscopy using a computer-based Carl Zeiss Axiovision microscope, and images were taken at 0, 6, 12 and 24 h after creation of the scratches. The width of the wound was analysed at each time interval by digitally drawing lines (using CorelDraw) between the migrating cells at the wound edges. The data were expressed as a percentage of scratch width covered by proliferating and/or migrating cells, where the healing capacity of the untreated control cells after 24 h was set at 100%.
Tube formation assay
A tube formation assay on Matrigel was used to estimate the influence of Veronica plant extracts on the spontaneous rearrangement of endothelial cells into capillary-like structures. The bottom of a 96-well plate was overlaid with 50 µl of Matrigel (20 mg/ml, thawed on ice overnight) and incubated for 1 h at 37°C to allow polymerization. After 1 h the wells were carefully washed with PBS and endothelial cells were seeded at the indicated density in the absence (control) or presence of tested extracts (25 µg/ml). Tube formation was examined and photographed using a camera-supplied light microscope (Carl Zeiss, Germany) after 12 h incubation. The number of formed loops was counted visually in each well. Each coherent and closed network was counted as a loop.
Statistical analysis
All experiments were conducted with between three and five repetitions. Each assay was performed in triplicate under identical conditions. Data were expressed as means ± SEM. The statistical analyses were performed by GraphPad Prism software (v.4.0) and the significance of differences between groups was assessed by one-way analysis of variance (ANOVA). Results were considered statistically significant at p < 0.05. When appropriate, subsequent statistical comparisons were performed by the Bonferroni test.
Results
Determination of phenolics, iridoids and phenylpropanoid glycosides
The contents of total phenolics, iridoids and phenylpropanoid glycosides in different extracts of Veronica species are shown in Table . The highest amount of total phenolics was observed in V. jacquinii extracts (195–201 mg GAE/g), while V. teucrium and V. urticifolia extracts contained similar levels of these compounds. The highest total phenylpropanoid content was determined in V. teucrium extracts (26–38 mg acteoside/g). The extracts most abundant in iridoids were from V. urticifolia (270–359 mg aucubin/g), followed by V. teucrium (231–322 mg aucubin/g) and V. jacquinii (167–249 mg aucubin/g). The results indicate that the contents of total phenolics, iridoids and phenylpropanoids are affected by the solvents used for extraction. In all tested species aqueous acetone solvent was more effective than methanol in extracting these classes of compounds. The high extraction efficiency of aqueous solvent could be primarily due to the water-soluble nature of plant phenolics and glycosides enhanced by the presence of an organic solvent, which facilitates solubilization through penetration in plant tissues (Moure et al. Citation2001).
Table 1. Total phenolics, iridoids and phenylpropanoid glycosides in Veronica species.
Acteoside and aucubin contents
The HPTLC method, as a rapid and reliable technique, was performed for the quantification of acteoside and aucubin in selected Veronica extracts, and the results are summarized in Table . It can be observed that both extracts of V. urticifolia contained a significantly higher amount of acteoside (88–108 mg/g) than did V. teucrium and V. jacquinii extracts, where it ranged from 29 to 38 mg/g. Aucubin was detected only in V. urticifolia extracts. With regard to the solvent efficiency, methanol was found to be a more effective solvent than aqueous acetone for the extraction of aucubin and acteoside from Veronica species.
Table 2. Acteoside and aucubin contents in Veronica species.
Protection of SH-SY5Y cells against sodium nitroprusside-induced neurotoxicity
Six Veronica extracts were initially evaluated for cytotoxicity by acid phosphatase assays performed on the SH-SY5Y cell line. The extracts were evaluated for their neuroprotective activity at the optimal concentrations of SNP and H2O2, causing 60–70% of cell viability compared with the non-treated cells. The influence of ligands on the survival of stressed cells was evaluated by the combined treatment with 1 mM SNP/100 µM H2O2 and the highest non-toxic concentration of extracts. All extracts exhibited modest protective activity by increasing cell survival in cells stressed with SNP (9–12%) and H2O2 (16–21%) in comparison to the non-treated cells. Since the lower concentrations of the extracts did not show neuroprotective effects and the higher concentrations could not be applied because of the interfering influence of the DMSO concentrations, all assays were performed using a single effective concentration of the extracts. The increase in cell viability is presented as the percentage of the basal level of viability (100%) observed in non-treated cells (Table ).
Table 3. Influence of Veronica extracts on the viability of SH-SY5Y cells stressed with sodium nitroprusside (SNP) and hydrogen peroxide (H2O2).
Antioxidative effects of Veronica extracts on SH-SY5Y cells treated with sodium nitroprusside
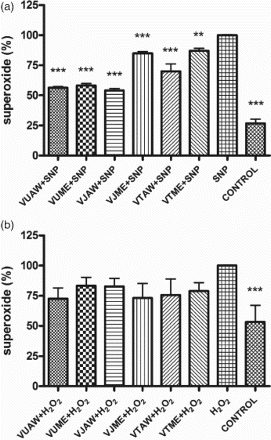
The index of lipid peroxidation was measured spectrophotometrically as the amount of TBARS, to evaluate the level of oxidative stress in SH-SY5Y cells induced by SNP/H2O2 treatment. The amount of TBARS in the cells pretreated with extracts was considerably lower in the cells stressed with SNP (22–34%, p < 0.0001), while in the cells treated with H2O2 these changes were not statistically significant (Figure ). The level of malondialdehyde accumulation ranged between 333 and 353 nmol/mg and between 55 and 110 nmol/mg in the cells stressed by 1 mM SNP and 100 µM H2O2, respectively. These results prompted the authors to analyse the amount of to check whether the examined extracts had direct interaction with this deleterious radical, released during SNP/H2O2 decomposition. The pretreatment with extracts lowered the amount of released
radicals in the SNP-treated cells (13–46%, p < 0.0001), whereas in the H2O2-treated cells extracts it did not cause significant changes in
levels (Figure ).
Figure 1. Influence of Veronica extracts on the amount of thiobarbituric acid reactive species (TBARS) in the cells stressed with sodium nitroprusside (SNP)/hydrogen peroxide (H2O2). Cells were pretreated with 50 µg/ml of extracts 30 min before addition of the stressor: (a) 1 mM SNP or (b) 100 µM H2O2. The index of lipid peroxidation (ILP) was measured 24 h after the treatment. The results are presented as a percentage of the amount of TBARS produced in cells treated only with stressors, which represented 100%. Presented values are mean ± SEM obtained from at least three experiments. VU = Veronica urticifolia; VJ = Veronica jacquinii; VT = Veronica teucrium; AW = acetone–water; ME = methanol.
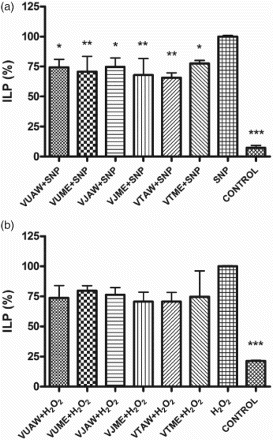
Figure 2. Influence of Veronica extracts on the amount of released superoxide () radicals in the cells stressed with sodium nitroprusside (SNP)/hydrogen peroxide (H2O2). Cells were pretreated with 50 µg/ml of extracts 30 min before addition of the stressor: (a) 1 mM SNP (A) or (b) 100 µM H2O2. The nitroblue tetrazolium (NBT) test was performed 24 h after the treatment. The results are presented as a percentage of the amount of
radicals released by cells treated only with stressors, which represented 100%. Presented values are mean ± SEM obtained from at least three experiments. VU = Veronica urticifolia; VJ = Veronica jacquinii; VT = Veronica teucrium; AW = acetone–water; ME = methanol.
Influence of Veronica plant extracts on endothelial cell adhesion and migratory capacity of EA.hy926 cells
The initial stages of angiogenesis are accompanied by changes in the adhesive properties of the endothelial cells, which allow them to interact with remodelled extracellular matrix (ECM). To establish the possible influence of the investigated Veronica plant extracts on the adhesive ability of human endothelial cells, a cell adhesion assay was performed. The results clearly show that pretreatment with methanol and aqueous acetone extracts of Veronica species did not change the ability of endothelial cells to adhere (or attach) to components of the ECM (Table ).
Table 4. Influence of Veronica extracts on endothelial cell adhesion.
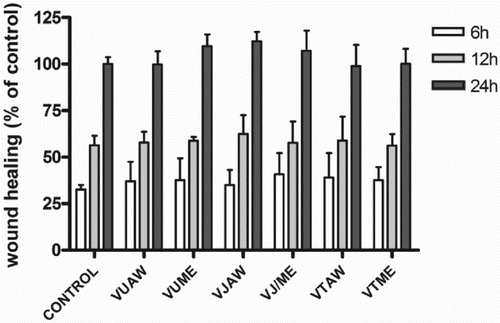
A wound healing assay, which evaluated the next step in the angiogenic process, demonstrated that the extracts had no significant effects on the ability of EA.hy926 endothelial cells to migrate into a denuded area at any monitored interval (Figure ).
Figure 3. Influence of Veronica extracts on the migratory capacity of EA.hy926 cells. Confluent monolayers of EA.hy926 cells were wounded and immediately after formation of scratches treated with investigated extracts (25 µg/ml). The healing process was monitored at indicated time-points and data are expressed as a percentage of scratch width covered by proliferating and/or migrating cells, where the healing capacity of the untreated control cells after 24 h was set at 100%. VU = Veronica urticifolia; VJ = Veronica jacquinii; VT = Veronica teucrium; AW = acetone–water; ME = methanol.
Antiangiogenic properties of Veronica extracts
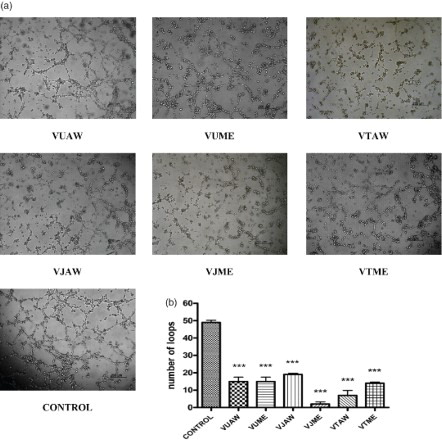
The capacity of Veronica plant extracts to affect the spontaneous differentiation of EA.hy926 cells into tube structures on Matrigel was estimated. This assay is a powerful tool for the screening of potential antiangiogenic or proangiogenic agents (Staton et al. Citation2009). All extracts at their highest non-toxic concentration inhibited spontaneous tube formation on Matrigel (Figure a). The highest inhibitory effect was exhibited by the methanol extract of V. jacquinii and aqueous acetone extract of V. teucrium, both of which almost completely prevented the formation of tubular structures. The other Veronica extracts decreased the number of formed loops by 60–70% compared to the control (Figure b).
Figure 4. Influence of Veronica extracts on tube formation. The cells were seeded on Matrigel and treated with the investigated extracts (25 µg/ml). After 12 h of incubation, (a) photographs were taken, and (b) the number of formed loops was counted and compared. VU = Veronica urticifolia; VJ = Veronica jacquinii; VT = Veronica teucrium; AW = acetone–water; ME = methanol.
Discussion
Phytochemical investigation of the genus Veronica revealed the presence of iridoid glucosides, mainly aucubin, catalpol and 6-O-catalpol esters, and phenylpropanoid and flavonoid glycosides (Harput et al. Citation2011). Although many studies have reported the detection and identification of the chemical constituents of Veronica species, data concerning the contents of these constituents are scarce. Total iridoid and phenylpropanoid amounts have not been studied previously; only total phenolic content has been reported. Nikolova (Citation2011) estimated that the total phenolic content in V. teucrium, V. jacquinii and V. urticifolia varied between 32 and 37 mg GAE/g, which is lower than the values obtained in this study. These quantitative differences could be the consequence of different solvents being used for extraction.
Aucubin is one of the major iridoid glucosides in the families Scrophulariaceae, Plantaginaceae and Rubiaceae (Marin Citation2003). There are only a few reports regarding aucubin content in Veronica species. In V. spicata and V. chamaedrys relative amounts of aucubin have been determined by micellar electrokinetic capillary chromatography–mass spectrometry (Suomi et al. Citation2001), whereas Crişan et al. (Citation2010) used liquid {chromatography}/ mass spectrometry for the quantification of aucubin in several Veronica species. Compared to the present results, they reported a much lower concentration of aucubin in V. urticifolia.
Although acteoside is a characteristic phenylpropanoid glucoside for Veronica species (Johansen et al. Citation2007), to the authors’ knowledge, this is the first report on the acteoside quantification in these species. In the literature data on neuroprotective activity of phenylpropanoids, acteoside and its aglycones, caffeic acid and 3′,4′-dihydroxylphenylethanol, significantly protected primary cultures of rat cortical cells from the toxicity induced by glutamate. They also restored the level of glutathione and the activities of antioxidative enzymes reduced by glutamate (Koo et al. Citation2006). In another study, acteoside inhibited neuronal death induced by 1-methyl-4-phenylpyridinium ions (Li et al. Citation2008). Wang et al. (Citation2009) demonstrated that acteoside significantly and dose-dependently prevented SH-SY5Y cells from Aβ25–35-induced neuronal cell injury, and this effect might be explained by the antioxidant and antiapoptotic mechanisms. Acteoside has been previously characterized as an effective scavenger of biologically active free radicals and an inhibitor of lipid peroxidation (Lee et al. Citation2004). Numerous studies have also found iridoids to exhibit neuroprotective effects. Xue et al. (Citation2008) observed that aucubin prevents the death of hippocampal CA1 region neurons by changing the antioxidative ability and expression of Bcl-2 and Bax in rats with diabetic encephalopathy. Aucubin also showed a significant protective effect against H2O2-induced damage in PC12 cells.
During the progression of neurodegenerative diseases there is a significant increase in the production of ROS in the brain, especially the non-radical H2O2. This compound can easily enter the cells owing to its high membrane permeability, and it is indicated as a potential source of hydroxyl radicals, some of the most unstable and damaging radicals involved in tissue injury (Custódio et al. Citation2013). In this work, the neuroprotective effect of the investigated extracts was evaluated against H2O2-induced cytotoxicity on SH-SY5Y human neuroblastoma cells. All extracts showed higher activity in oxidative stress-induced neurodegeneration than in nitrosative stress-induced damage of neuronal cells, without statistically significant differences in the effects of the investigated extracts. According to these results, direct free radical scavenging activity was responsible for the neuroprotective activity against nitrosative stress, while this was not the case for the activity shown for the damage induced by oxidative stress. No significant correlation was found between acteoside and aucubin content and the neuroprotective activity shown by the investigated extracts. Also, no specific relationship existed between total phenolics, total phenylpropanoids or total iridoids and this activity. These results suggest that these compounds can act synergistically to produce various effects.
There are also reports on the wound healing activity of iridoids and phenylpropanoids. Shim et al. (Citation2007) showed that aucubin may be useful for oral wound healing and can be applied as a topical agent to oral wounds. In addition, it was previously found that extracts that are rich in acteoside significantly accelerate the wound healing process. In the same investigation these extracts possessed remarkable anti-inflammatory action in the excision wound model (Korkina et al. Citation2007).
Some Veronica plants are used as wound healing remedies in traditional Turkish medicine (Harput et al. Citation2011). The healing potential of phytomedicines is often associated with angiogenesis, which is a critical step in wound healing. The influence of the tested extracts on epithelial cell adhesion, cell migration and cell differentiation was evaluated by three in vitro models: the cell adhesion assay, scratch assay and three-dimensional tube formation assay, respectively. According to the results obtained in this study, Veronica extracts did not have an influence on the early phases of angiogenesis, such as the adhesion and migration of endothelial cells, but they influenced a later phase of angiogenesis, differentiation, which was observed as the inhibition of the formation of capillary-like tubes on the ECM. All tested extracts inhibited spontaneous tube formation on Matrigel in the highest non-toxic concentration, and these results suggest that the investigated Veronica extracts showed antiangiogenic potential.
Despite their traditional use in wound healing, the therapeutic value of Veronica species has not been scientifically tested and the mechanisms are not known. According to the results obtained in this study, the tested Veronica extracts may modulate wound healing not through neovascularization but via indirect effects such as antimicrobial, astringent and anti-inflammatory activity. In relation to this assumption, it has been indicated that Veronica plant extracts are responsible for relieving symptoms in skin complaints such as ulcers, eczema and skin burns (PDR for Herbal Medicines Citation2000). Different reports indicate that several Veronica species have antibacterial activity against Staphylococcus aureus and Pseudomonas species, which are commonly found in contaminated cutaneous wounds (Abu Zaida et al. Citation2008; Gusev et al. Citation2012). Furthermore, it has been shown that extracts of various Veronica species exhibit anti-inflammatory activity owing to the presence of iridoid glucosides (Küpeli et al. Citation2005). A high content of phenolic compounds such as flavonoids and phenylpropanoids, which are responsible for radical scavenging activity, may contribute to the prevention of the oxidative degradation of endothelial cells induced by ROS. Therefore, the possible antimicrobial and anti-inflammatory activity of Veronica extracts may have a role in the wound healing activity of these species. However, the exact mechanism underlying the therapeutic effects of these species should be elucidated in future investigations.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Funding
This research was funded by Ministry of Education, Science and Technological Development of the Republic of Serbia [projects no. III41030 and 31089].
References
- Abu Ziada ME, Mashaly IA, Abd El-Monem M, Torky M. 2008. Economic potentialities of some aquatic plants growing in north east Nile delta, Egypt. J Appl Sci. 8:1395–1405. doi: 10.3923/jas.2008.1395.1405
- Andersen J. 2004. Oxidative stress in neurodegeneration: cause or consequence? Nat Rev Neurosci. 10:S18–S25. doi: 10.1038/nrn1434
- Biringanine G, Chiarelli MT, Faes M, Duez P. 2006. A validation protocol for the HPTLC standardization of herbal products: application to the determination of acteoside in leaves of Plantago palmata Hook. f.s. Talanta. 69:418–424. doi: 10.1016/j.talanta.2005.10.007
- Connolly DT, Knight MB, Harakas NK, Wittwer AJ, Feder J. 1986. Determination of the number of endothelial cells in culture using an acid phosphatise assay. Anal Biochem. 152:136–140. doi: 10.1016/0003-2697(86)90131-4
- Crişan G, Vlase L, Balica G, Muntean D, Ştefănescu C, Păltinean R, Tămaş M, Leucuţa S. 2010. LC/MC analysis of aucubin and catalpol of some Veronica species. Farmacia. 58:237–242.
- Custódio L, Patarra J, Alberício F, Neng NR, Nogueira JM, Romano A. 2013. Extracts from Quercus sp. acorns exhibit in vitro neuroprotective features through inhibition of cholinesterase and protection of the human dopaminergic cell line SH-SY5Y from hydrogen peroxide-induced cytotoxicity. Ind Crop Prod. 45:114–120. doi: 10.1016/j.indcrop.2012.12.011
- European Pharmacopoeia 6.0 (Ed.). 2008. Council of Europe. Strasbourg-Cedex, France.
- Fan T-P, Yeh J-C, Leung KW, Yue P, Wong R. 2006. Angiogenesis: from plants to blood vessels. Trends Pharmacol Sci. 27:297–309. doi: 10.1016/j.tips.2006.04.006
- Fotsis T, Pepper M, Adlercreutz H, Fleischmann G, Hase T, Montesano R, Schweigerer L. 1993. Genistein, a dietary-derived inhibitor of in vitro angiogenesis. Proc Natl Acad Sci USA. 90:2690–2694. doi: 10.1073/pnas.90.7.2690
- Gálvez M, Martín-Cordero C, Houghton PJ, Ayuso MJ. 2005. Antioxidant activity of methanol extracts obtained from Plantago species. J Agric Food Chem. 53:1927–1933. doi: 10.1021/jf048076s
- Gusev NF, Nemereshina ON, Petrova GV, Sychev MV. 2012. Evaluation of biologically active substances and antibacterial activity of herbal drugs from Veronica L. Russ J Biopharm. 4:17–22.
- Harput US, Genc Y, Khan N, Saracoglu I. 2011. Radical scavenging effects of different Veronica species. Rec Nat Prod. 5:100–107.
- Johansen M, Larsen TS, Mattebjerg MA, Gotfredsen CH, Jensen SR. 2007. Chemical markers in Veronica sect. Hebe. Biochem Syst Ecol. 35:614–620. doi: 10.1016/j.bse.2007.04.010
- Kleinman HK, Malinda KM. 2000. Role of angiogenesis in wound healing. In: Mousa SA, editor. Angiogenesis inhibitors and stimulators: potential therapeutic implications. Georgetown (TX): Landes Biosciences. p. 102–110.
- Koo KA, Kim SH, Oh TH, Kim YC. 2006. Acteoside and its aglycones protect primary cultures of rat cortical cells from glutamate-induced excitotoxicity. Life Sci. 79:709–716. doi: 10.1016/j.lfs.2006.02.019
- Korkina LG, Mikhal'chik EV, Suprun MV, Pastore S, Dal Toso R. 2007. Molecular mechanisms underlying wound healing and anti-inflammatory properties of naturally occurring biotechnologically produced phenylpropanoid glycosides. Cell Mol Biol. 53:84–91.
- Küpeli E, Harput S, Varel M, Yesilada E, Saracoglu I. 2005. Bioassay-guided isolation of iridoid glucosides with antinociceptive and anti-inflammatory activities from Veronica anagallis-aquatica L. J Ethnopharmacol. 102:170–176. doi: 10.1016/j.jep.2005.05.042
- Lee KJ, Woo ER, Choi CY, Shin DW, Lee DG, You HJ, Jeong HG. 2004. Protective effect of acteoside on carbon-tetrachloride-induced hepatotoxicity. Life Sci. 74:1051–1064. doi: 10.1016/j.lfs.2003.07.020
- Li Y, Lu J, Li Q, Zhao Y, Pu X. 2008. Pedicularoside a from Buddleia lindleyana inhibits cell death induced by 1-methyl-4-phenylpyridinium ions (MPP+) in primary cultures of rat mesencephalic neurons. Eur J Pharmacol. 579:134–140. doi: 10.1016/j.ejphar.2007.10.052
- Marin P. 2003. Biochemical and molecular systematics of plants [Biohemijska i molekularna sistematika biljaka]. Beograd: NNK Internacional.
- Martin A. 1996. The use of antioxidants in healing. Dermatol Surg. 22:156–160.
- Moure A, Cruz JM, Franco D, Domnguez JM, Sineiro J, Domnguez H, Jose Nunez M, Parajo JC. 2001. Natural antioxidants from residual sources. Food Chem. 72:145–171. doi: 10.1016/S0308-8146(00)00223-5
- Nikolova M. 2011. Screening of radical scavenging activity and polyphenol content of Bulgarian plant species. Pharmacognosy Res. 3:256–259. doi: 10.4103/0974-8490.89746
- PDR (Physicans Desk References) for Herbal Medicines. 2000. 2nd ed. Monvale (NJ): Medicinal Economics Company.
- Rehncrona S, Smith DS, Akesson B, Westerberg E, Siesjo BK. 1980. Peroxidative changes in brain cortical fatty acids and phospholipids, as characterized during Fe2+ and ascorbic acid-stimulated lipid peroxidation in vitro. J Neurochem. 34:1630–1638. doi: 10.1111/j.1471-4159.1980.tb11254.x
- Saracoglu I, Oztunca FH, Nagatsu A, Harput US. 2011. Iridoid content and biological activities of Veronica cuneifolia subsp. cuneifolia and V. cymbalaria. Pharm Biol. 49:1150–1157. doi: 10.3109/13880209.2011.575790
- Shim KM, Choi SH, Jeong MJ, Kang SS. 2007. Effects of aucubin on the healing of oral wounds. In Vivo. 21:1037–1042.
- Song JX, Lin X, Wong RNS, Sze SCW, Tong Y, Shaw PC, Zhang YB. 2011. Protective effects of dibenzocyclooctadiene lignans from Schisandra chinensis against beta-amyloid and homocysteine neurotoxicity in PC12 cells. Phytother Res. 25:435–443.
- Staton CA, Reed MWR, Brown NJ. 2009. A critical analysis of current in vitro and in vivo angiogenesis assays. Int J Exp Pathol. 90:195–221. doi: 10.1111/j.1365-2613.2008.00633.x
- Stojković DS, Živković J, Soković M, Glamočlija J, Ferreira ICFR, Janković T, Maksimović Z. 2013. Antibacterial activity of Veronica montana L. extract and of protocatechuic acid incorporated in a food system. Food Chem Toxicol. 55:209–213. doi: 10.1016/j.fct.2013.01.005
- Suomi J, Wiedmer S, Jussila M, Reikkola M-L. 2001. Determination of iridoid glycosides by micellar electrokinetic capillary chromatography–mass spectrometry with use of the partial filling technique. Electrophoresis. 22:2580–2587. doi: 10.1002/1522-2683(200107)22:12<2580::AID-ELPS2580>3.0.CO;2-7
- Wang X, Michaelis E. 2010. Selective neuronal vulnerability to oxidative stress in the brain. Front Aging Neurosci. 2:1–13.
- Wang H, Xu Y, Yan J, Zhao X, Sun X, Zhang Y, Guo J, Zhu C. 2009. Acteoside protects human neuroblastoma SH-SY5Y cells against β-amyloid-induced cell injury. Brain Res. 1283:139–147. doi: 10.1016/j.brainres.2009.05.101
- Waterman P, Mole S. 1994. Analysis of phenolic plant metabolites. Oxford: Blackwell. p. 16.
- Xue H, Jin L, Jin L, Zhang P, Li D, Xia Y, Lu Y, Xu Y. 2008. Neuroprotection of aucubin in primary diabetic encephalopathy. Sci China Life Sci 51:495–502. doi: 10.1007/s11427-008-0069-x
- Živković J, Ćebović T, Maksimović Z. 2012. In vivo and in vitro antioxidant effects of three Veronica species. Cent Eur J Biol. 7:559–568. doi: 10.2478/s11535-012-0041-4
