Abstract
Two edible seaweeds, Sargassum polycystum and Sargassum wightii, were investigated for their antidiabetic potential using in vitro enzyme inhibitory assays. Among the various extracts, petroleum ether and ethyl acetate extracts of S. wightii showed significant inhibitory effects against α-amylase (IC50 378.3 µg/ml) and α-glucosidase (IC50 314.8 µg/ml). Methanol extract of S. wightii showed the highest inhibition against dipeptidyl peptidase-IV (DPP-IV) (IC50 38.27 µg/ml) and moderate antioxidant activity was observed in acetone extract of S. wightii (44%). Similarly, ethyl acetate extract of S. polycystum showed the highest inhibition against α-amylase (IC50 438.5 µg/ml) and methanol extract of S. polycystum showed maximum inhibition against α-glucosidase (IC50 289.7 µg/ml) and DPP-IV (36.94 µg/ml). The antioxidant activity was poor (22%). The extracts were investigated for in vitro cytotoxicity, DNA fragmentation in macrophages and haemolytic activity against erythrocytes, but no notable toxicity was observed with any of the tested extracts. Gas chromatography–mass spectrometry revealed the presence of the antidiabetic compound fucosterol and other major bioactive compounds, giving an insight into the antidiabetic and antioxidant properties of these algae. This study reveals the possible mechanisms of antidiabetic action in vitro, and these two seaweeds may also have an antidiabetic action in vivo.
Introduction
Diabetes mellitus is a complex disease characterized by chronic hyperglycaemia due to deficiency in insulin secretion or resistance (Nickavar & Yousefian Citation2011). The currently available medication acts through the stimulation and enhancement of endogenous insulin secretion, and by inhibiting common dietary enzymes such as α-amylase and α-glucosidase (Rang et al. Citation2003). Enzymes such as α-amylase and α-glucosidase act synergistically to digest starch in the human body through the breakdown of starch by pancreatic α-amylase and the absorption of glucose by intestinal α-glucosidase (Kwon et al. Citation2007). Pancreatic α-amylase is a key enzyme that determines the rate of starch digestion by the hydrolysis of inner α-1,4-glucosidic linkages, and produces linear and branched malto-oligosaccharides (Hizukuri et al. Citation1996). These are then acted on by α-glucosidases, which play a role in the conversion of carbohydrates into glucose, and this may lead to postprandial hyperglycaemia (Sudha et al. Citation2011). An effective strategy for reducing postprandial hyperglycaemia is by inhibition of pancreatic α-amylase and α-glucosidase, which delays the carbohydrate digestion and glucose absorption significantly (Tarling et al. Citation2008). Antioxidant compounds also play a major role in the control of oxidative stress-related diseases including diabetes, cancer and cardiovascular diseases (Wu & Hansen Citation2008).
Dipeptidyl peptidase-IV (DPP-IV) is a new class of oral medication that works by inhibiting the rapid degradation of incretin hormones, which prevents postprandial hyperglycaemia (Creutzfeldt Citation2005). Incretins are a group of gastrointestinal hormones secreted by the gut in response to food intake. The two hormones largely accountable for the effects of incretins are glucagon-like peptide (GLP-1) and glucose-dependent insulinotropic polypeptide (GIP). These, in turn, have a direct effect on pancreatic cells by the secretion of insulin from the β-cells and the suppression of glucagon release from the α-cells. GLP-1 can also act at peripheral tissues to improve insulin utilization and GIP is also involved in glucose metabolism via insulin secretion (Al-Masri et al. Citation2009). Both of these incretin hormones have very short half-lives because of their rapid degradation by DPP-IV. Therefore, inhibiting DPP-IV can prevent the rapid degradation of incretin hormones which, in turn, increases the levels of biologically active incretins (GLP-1 and GIP), which reduce glucose production by inhibiting glucagon from the α-cells of the pancreas and increasing insulin production (Havale & Pal Citation2009). Commercially available antidiabetic drugs such as sitagliptin and saxagliptin are used for DPP-IV inhibition (Bharti et al. Citation2012). Although powerful synthetic α-amylase, α-glucosidase and DPP-IV inhibitors including acarbose, voglibose, sitagliptin and saxagliptin are available, they are known to cause hepatic disorders and other gastrointestinal symptoms (Murai et al. Citation2002).
The edible marine macroalgae Sargassum polycystum and Sargassum wightii have been used as food and as folk medicine in eastern as well as western countries (Chan et al. Citation2006). Natural marine products contain a wide range of bioactive compounds with various bioactivities including antidiabetic and antioxidant properties. Seaweeds are classified based on their pigmentation into brown (phaeophyta), red (rhodophyta) and green (chlorophyta), and contain many bioactive components which have potential health benefits (Yuan et al. Citation2005; MacArtain et al. Citation2007). Marine algae have gained importance because they synthesize a wide variety of secondary metabolites, and compounds from these could play a crucial role in the management of diabetes (Newman et al. Citation2003). Bioactive compounds from edible seaweeds have significant roles in the modulation of glucose-induced oxidative stress and inhibition of starch digestive enzymes (Lee et al. Citation2008).
The aim of this study was to determine the antidiabetic, antioxidant and toxicological effects of two edible seaweeds, S. polycystum and S. wightii, collected from the southern coast of India, using in vitro assays.
Materials and methods
Collection of seaweeds
Fresh brown seaweeds, S. wightii and S. polycystum (Phaeophyceae), were collected from the Mandapam coastal region (latitude 9°17′N, longitude 79°11′E), in the Gulf of Mannar, Tamilnadu, southern India, during October 2012. The collected specimens were identified and authenticated by Dr P. Kaladharan, Principal Scientist and Scientist in Charge, Calicut Regional Centre of Central Marine Fisheries Research Institute. The fresh brown seaweeds were cleaned with water to remove debris, and epiphytes and holdfasts were removed. Before extraction the seaweeds were shade-dried, powdered and stored in air-tight containers at −20°C.
Preparation of seaweed extracts
Powdered samples (25 g) were extracted using 250 ml of various solvents such as petroleum ether, benzene, ethyl acetate, acetone and methanol for 24 h using Soxhlet apparatus. Each filtrate was evaporated to dryness under reduced pressure using a rotary evaporator (Super Fit Rotavap, model PBU-6, India). Finally, the samples were lyophilized using a freeze-dryer (Lark, Penguin Classic Plus, Mumbai, India) and stored at −20°C for use in subsequent experiments.
Preliminary phytochemical screening
Preliminary phytochemical screening was carried out following the standard protocols of Harborne (Citation1998).
DPPH free radical scavenging activity
The free radical scavenging activity of seaweed extracts was measured according to the method of Mensor et al. (Citation2001). In brief, 500 µl of a 0.3 mM methanolic solution of 1,1-diphenyl-2-picrylhydrazyl (DPPH; HiMedia, Mumbai, India) was added to test samples of various concentrations (250–1000 µg/ml). Samples were incubated in the dark for 30 min and reduction of DPPH concentration was measured at 518 nm in an ultraviolet (UV)–visible spectrophotometer (Systronics AU-2700, Ahmedabad, India) using methanol as a blank and the synthetic antioxidant butylated hydroxytoluene (BHT) as a positive control. The experiments were performed in triplicate and the percentage of reduction was calculated as follows:
In vitro α-amylase inhibition study
The α-amylase inhibitory activity of seaweed extracts was determined according to the modified method described by Jayasri et al. (Citation2009). In brief, 250 µl of algal extracts at varying concentrations (250–1000 µg/ml) and 250 µl of 0.5 mg/ml α-amylase (porcine pancreatic α-amylase; Sigma, St Louis, MO, USA) dissolved in 0.02 M sodium phosphate buffer (pH 6.9 with 0.006 M sodium chloride) were incubated for 10 min at 25°C. After preincubation, 250 µl of 1% starch solution in 0.02 M sodium phosphate buffer (pH 6.9 with 0.006 M sodium chloride) was added to each tube and samples were incubated at 25°C for a further 10 mins. The reaction was stopped with 500 µl dinitrosalicylic acid (Sigma, St Louis, MO, USA) colour reagent and tubes were incubated in a boiling water bath for 5 min. Once samples had cooled to room temperature, the reaction mixture was diluted by adding 5 ml of distilled water and absorbance was measured at 540 nm in the UV–visible spectrophotometer (Systronics AU-2700, Ahmedabad, India). Acarbose was used as a positive control and all experiments were performed in triplicate.
The α-amylase inhibitory activity was calculated as follows:
In vitro α-glucosidase inhibition study
The α-glucosidase inhibitory activity of seaweed extracts was determined according to the modified method described by Jayasri et al. (Citation2009). A volume of 50 µl of algal extracts at varying concentrations (250–1000 µg/ml) and 100 µl of α-glucosidase (SRL, Mumbai, India) solution (1.0 U/ml) in 0.1 M phosphate buffer (pH 6.9) was mixed in 96-well microplates and incubated at 25°C for 10 min. After preincubation, 50 µl of 5 mM p-nitrophenyl α-D-glucopyranoside (Sigma, St Louis, MO, USA) in 0.1 M phosphate buffer (pH 6.9) was added to each well and incubated at 25°C for 5 min. The absorbance was measured at 405 nm using a plate reader (BioTek, Mumbai, India) and results were compared to the control. Acarbose was used as a positive control and all experiments were performed in triplicate.
The α-glucosidase inhibitory activity was calculated as follows:
In vitro dipeptidyl peptidase-IV inhibition study
The DPP-IV inhibitory activity of seaweed extract was determined according to the modified method of Al-Masri et al. (Citation2009). Standard diprotin A (Sigma, St Louis, MO, USA) was diluted to various concentrations (0.2, 0.4, 0.8, 1.6, 3.2 and 6.4 µg/ml) using Tris–HCl buffer (50 mM, pH 7.5), the final volume was made up to 35 µl, and 15 µl of DPP-IV enzyme (Sigma, St Louis, MO, USA) was added to the mixture. One unit of enzyme activity was defined as the amount of enzyme that catalysed the release of 1 µmol pNA from the substrate per minute under assay conditions.
After addition of the enzyme, the mixture was preincubated for 10 min at 37°C to enhance the binding capacity of the inhibitor. This was followed by the addition of 50 µl of Gly-pro-p-nitroanilide (GPPN; Sigma, St Louis, MO, USA) 0.2 mM in Tris–HCl as a substrate. Final incubation was carried out at 37°C for 30 min and the reaction was terminated by the addition of 25 µl of 25% glacial acetic acid. The absorbance was measured at 405 nm using a microtitre plate reader (BioTek, Mumbai, India). Experiments were performed in triplicate and the results were compared with a negative control.
Dipeptidyl peptidase-IV inhibition assay of seaweed extract
Seaweed extract was dissolved in dimethyl sulphoxide (DMSO) to make a stock concentration of 1000 µg/ml. From the stock, the following concentrations were prepared: 2.5, 10, 40 and 80 µg/ml in Tris–HCl buffer (50 mM, pH 7.5) in a total volume of 100 µl/well. The assay was performed in triplicate according to the standardized procedure of diprotin A. The percentage of DPP-IV inhibition was calculated as follows:
Assessment of cell viability
J774 (mouse macrophage) cell lines obtained from NCCS (Pune, India) were grown in Dulbecco's modified Eagle's medium (DMEM) containing 10% foetal bovine serum, 2 mM L-glutamine and 100 U/ml of penicillin/streptomycin with 5% CO2 at 37°C in a humidified incubator. The cells were seeded at 5×105 cells/ml into 96-well plates. After 24 h, the cells were exposed to various concentrations of extracts (1000–250 µg/ml) and incubated for 24 h at 37°C/5% CO2. At the end of incubation, cytotoxicity was determined by the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay. In brief, 20 µl of MTT (5 mg/ml) reagent was added per well and incubated for 4 h at 37°C/5% CO2. The formazan crystals formed were solubilized in DMSO after aspirating the medium. Absorbance was measured at 630 nm with a microplate reader (BioTek, Mumbai, India). Cell viability was expressed against the time period with various concentrations of the compound.
DNA fragmentation assay
To investigate the ability of the extracts to induce characteristic nuclear changes and apoptotic body formation, the cells were assessed using fluorescent microscopy by the acridine orange and ethidium bromide staining method. The cells, seeded in six-well plates along with cover slips at a seeding density of 5×105 cells/well, were treated with the test extracts (1000 µg/ml) for 24 h. Cells grown on cover slips were loaded with acridine orange–ethidium bromide (4 µg/ml) solutions for 15 min at 37°C in the dark. Cover slips were inverted on to slides and viewed under a Weswox LED fluorescent microscope (FM-3000) with attached camera, and photographs were taken at 40× and 100 × magnification under fluorescent conditions.
In vitro haemolytic activity
The haemolytic activity of seaweed extracts was determined according to the method described by Malagoli (Citation2007). Human red blood cells (RBCs) were obtained from the peripheral blood (A positive) of a healthy donor, using ethylene diaminetetraacetic acid (EDTA) as an anticoagulant. The collected blood was washed with sterile NaCl solution, the cells were pelleted by centrifugation (150× g for 5 min) and the supernatant was discarded. This procedure was repeated three times, until the supernatant turned colourless. The pellet obtained was diluted 1/9 (v/v) in sterile 0.9% NaCl saline solution then 1/24 (v/v) in sterile Dulbecco's phosphate-buffered saline (D-PBS) pH 7.0, containing 0.5 mM boric acid and 1 mM calcium chloride. The crude extracts were assayed for their haemolytic activity under in vitro conditions in 96-well plates. In brief, each well received 100 µl of 0.85% NaCl solution containing 10 mM CaCl2. The negative control was 100 µl of normal saline, 0.1% Triton X-100 served as a positive control, and test extracts of various concentrations (250–1000 µg/ml) were added to each well. Then, each well received 100 µl of human erythrocytes diluted in D-PBS. After 30 min of incubation at room temperature, the samples were centrifuged and the supernatants obtained were used to measure the absorbance of liberated haemoglobin at 630 nm. The morphology of the erythrocytes was recorded by smearing the extracts treated with RBCs, and the morphological changes were recorded under the microscope (Weswox LED fluorescent microscope, FM-3000).
Gas chromatography–mass spectrometry analysis
Gas chromatography–mass spectrometry (GC/MS) (Perkin Elmer, [Chennai, India] Clarus 680 GC coupled to a Clarus 600 MS) analysis was performed for the detection of major compounds present in various extracts of S. wightii and S. polycystum. The column used in GC/MS was an Elite-5MS (30.0 m, 0.25 mm ID, 250 µm df). The carrier gas used was helium, at a constant flow rate of 1 ml/min. Electron impact ionization (EI) mode was used, with electron energy set at 70 eV. After 1 µl of extract diluted with methanol was injected into the GC/MS, the compounds were identified based on the molecular structure, molecular mass and calculated fragment ratio of resolved spectra compared with those of mass spectra available from the library. Spectral data were interpreted using the database of the National Institute Standard and Technology (NIST).
Statistical analysis
All data are expressed as mean ± SEM. Statistical analysis was performed using two-way analysis of variance (ANOVA). Values were considered to be significantly different when the p value was < 0.0001 compared to the baseline values.
Results
Phytochemical analysis
Phytochemical analysis of seaweed extracts () showed the presence of alkaloids, phenols, flavanoids, proteins, lipids, carbohydrates, glycosides and tannins.
Table 1. Phytochemical screening of various extracts of Sargassum polycystum (SP) and Sargassum wightii (SW).
DPPH free radical scavenging assay
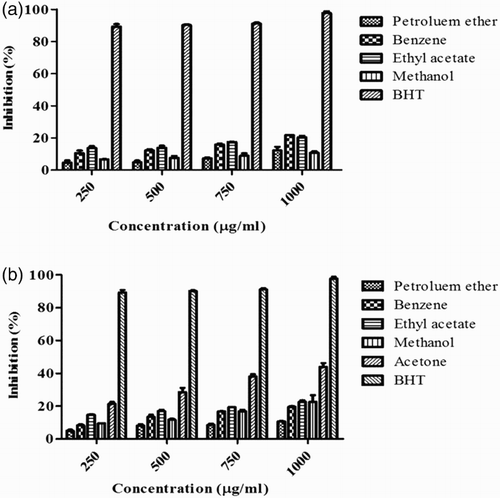
The results of the DPPH free radical scavenging activity assay showed that there was a reduction in the concentration of DPPH due to the scavenging ability of the algal extracts. At a concentration of 1000 µg/ml, the acetone extract of S. wightii showed a satisfactory scavenging effect (43%) and the benzene extract of S. polycystum exhibited an effect of 22% in DPPH compared with that of the standard BHT (97%). The results obtained are shown in .
Figure 1. 1,1-Diphenyl-2-picrylhydrazyl (DPPH) radical scavenging activity of Sargassum polycystum and Sargassum wightii. (A) DPPH radical scavenging activity of petroleum ether, benzene, ethyl acetate and methanol extracts of S. polycystum. (B) DPPH radical scavenging activity of petroleum ether, benzene, ethyl acetate, methanol and acetone extracts of S. wightii. Butylated hydroxytoluene (BHT) was used as a positive control and absorbance was measured at 517 nm. Values are means ± SD (n = 3); ***p < 0.0001 is considered significant.
In vitro α-amylase inhibition study
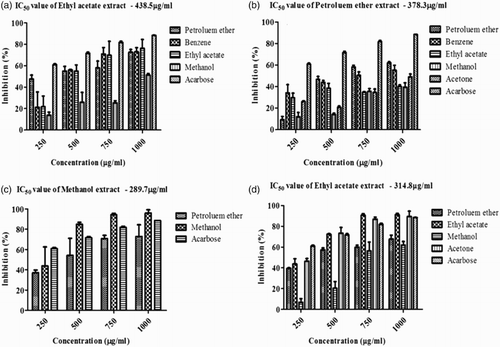
The inhibitory effects of S. polycystum and S. wightii on α-amylase are shown in (A) and (B). Among the five extracts studied (petroleum ether, benzene, ethyl acetate, methanol and acetone), all the tested concentrations (250, 500, 750 and 1000 µg/ml) showed significant α-amylase inhibition compared with that of acarbose. Ethyl acetate extract of S. polycystum showed maximum percentage inhibition of 77%, with an IC50 of 438.5 µg/ml, and petroleum ether extract of S. wightii showed maximum inhibition of 62%, with an IC50 of 378.3 µg/ml. All the results were compared with acarbose (88%) at a maximum concentration of 1000 µg/ml.
Figure 2. α-Amylase and α-glucosidase inhibitory activity of Sargassum polycystum and Sargassum wightii. (a) In vitro α-amylase inhibitory activity of petroleum ether, benzene, ethyl acetate and methanol extracts of S. polycystum. (b) In vitro α-amylase inhibitory activity of petroleum ether, benzene, ethyl acetate, methanol and acetone extracts of S. wightii. Acarbose was used as a positive control and absorbance was measured at 540 nm. Values are means ± SD (n = 3); ***p < 0.0001 is considered significant. (c) In vitro α-glucosidase inhibitory activity of petroleum ether and methanol extracts of S. polycystum. (d) In vitro α-glucosidase inhibitory activity of petroleum ether, ethyl acetate, methanol and acetone extracts of S. wightii. Acarbose was used as a positive control and absorbance was measured at 405 nm. Values are means ± SD (n = 3); ***p < 0.0001 is considered significant.
In vitro α-glucosidase inhibition study
The in vitro α-glucosidase inhibition study revealed that S. polycystum and S. wightii showed significant α-glucosidase inhibition at all the tested concentrations (250, 500, 750 and 1000 µg/ml) for the different solvent extracts (petroleum ether, benzene, ethyl acetate, methanol and acetone). Methanol extract of S. polycystum showed maximum inhibition of 96%, with an IC50 of 289.7 µg/ml, and ethyl acetate extract of S. wightii showed maximum inhibition of 91%, with an IC50 of 314.8 µg/ml. Notably, methanol extract of S. polycystum and ethyl acetate extract of S. wightii had higher inhibitory activity than acarbose (88%) at a concentration of 1000 µg/ml. The results obtained are shown in (C) and (D).
In vitro dipeptidyl peptidase-IV inhibition study
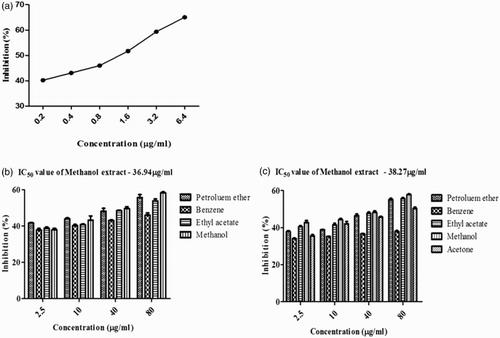
The effectiveness of various extracts of S. polycystum and S. wightii on DPP-IV inhibition was compared with diprotin A (positive control). Five different solvent extracts of S. polycystum and S. wightii showed significant DPP-IV inhibition at all the tested concentrations. Methanol extracts of both S. polycystum and S. wightii showed maximum percentage inhibition of 58% at a concentration of 80 µg/ml, with IC50 values of 36.94 and 38.27 µg/ml, respectively. The results obtained are shown in .
Figure 3. Dipeptidyl peptidase-IV (DPP-IV) inhibitory activity of Sargassum polycystum and Sargassum wightii. (a) DPP-IV inhibitory activity of diprotin A as positive control. (b) DPP-IV inhibitory activity of petroleum ether, benzene, ethyl acetate and methanol extracts of S. polycystum. (c) DPP-IV inhibitory activity of petroleum ether, benzene, ethyl acetate, methanol and acetone extracts of S. wightii. Absorbance was measured at 405 nm. Values are means ± SD (n = 3); ***p < 0.0001 is considered significant.
Assessment of cell viability
The effects of various algal extracts on macrophage (J774) viability were determined by the MTT assay. Following 24 h exposure, extracts of both S. polycystum and S. wightii showed poor to moderate cytotoxicity (A). At a lower concentration of 250 µg/ml, maximum cells remained viable for all the extracts. The cytotoxicity was found to be 3.2% and 6.48% when cells were treated with 250 µg/ml of S. polycystum and S. wightii methanol extract, respectively. It was observed that 39.36% cell death was seen after 24 h when treated with 1000 µg/ml of S. polycystum methanol extract. Similarly, 46.96% cell death was seen after 24 h when treated with 1000 µg/ml of S. wightii methanol extract (F, G).
Figure 4. Measurement of cell viability and haemolytic activity of Sargassum polycystum and Sargassum wightii. (a) Cell viability determined by MTT assay. J774 cells were treated with 1000, 750, 500 and 250 µg/ml of ethyl acetate and methanol extracts of S. polycystum (SP), or methanol and petroleum ether extracts of S. wightii (SW). Absorbance was measured at 630 nm. (b) Haemolytic activity of ethyl acetate and methanol extracts of S. polycystum (SP) and methanol and petroleum ether extracts of S. wightii (SW). Absorbance was measured at 630 nm. Values are means ± SD (n = 3); ***p < 0.0001 is considered significant.
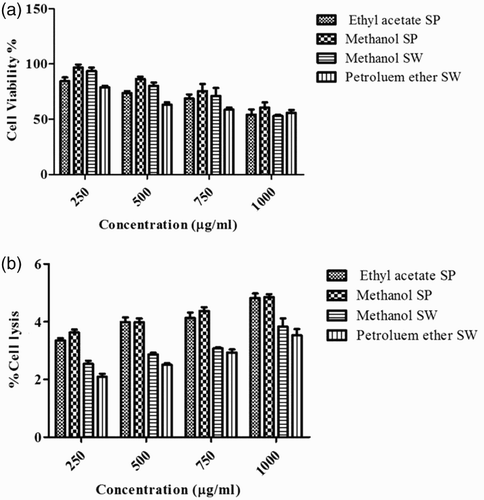
Figure 5. In vitro haemolytic activity, cytotoxicity and DNA fragmentation studies of Sargassum polycystum and Sargassum wightii. (A–E) Microscopic images (40× magnification) of in vitro haemolytic activity of various extracts of S. polycystum and S. wightii: (A) control, erythrocytes treated with normal saline, (B) erythrocytes treated with test extract, S. polycystum ethyl acetate extract (SP EA), (C) S. polycystum methanol extract (SP M), (D) S. wightii methanol extract (SW M), (E) S. wightii petroleum ether extract (SW PE). (F, G) Phase-contrast microscopic images of J774 cells treated with media alone: (F) control and (G) cells treated with test extracts. (H–L) Fluorescent photomicrographs (100× and 40× magnification) of J774 cells treated without seaweed extracts: (H) control, (I) J774 cells treated with S. polycystum ethyl acetate extract (SP EA), (J) J774 cells treated with S. polycystum methanol extract (SP M), (K) J774 cells treated with S. wightii methanol extract (SW M), (L) J774 cells treated with S. wightii petroleum ether extract (SW PE). Each experiment was performed in triplicate (n = 3) and generated similar morphological features.
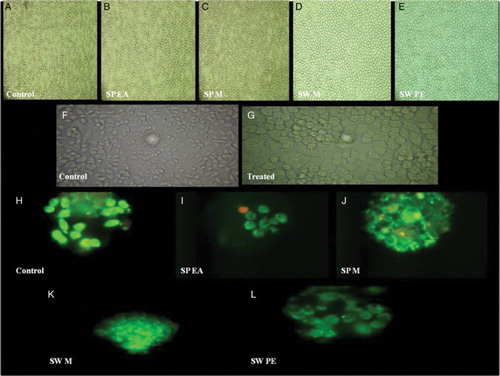
DNA fragmentation study
The proportions of viable, apoptotic and necrotic J774 cells were evaluated after 24 h treatment with various seaweed extracts. Cells treated with a maximum concentration of 1000 µg/ml of seaweed extracts were found to be viable, and no DNA fragmentation or nuclear changes were observed (H–L).
In vitro haemolytic activity
The results of haemolytic activity of S. polycystum and S. wightii extracts are shown in (B). The crude extracts of S. polycystum and S. wightii did not cause any lysis of human erythrocytes. Ethyl acetate (4.82%) and methanol (4.85%) extracts of S. polycystum, and methanol (3.83%) and petroleum ether (3.53%) extracts of S. wightii caused less than 5% lysis at 1000 µg/ml. The extracts did not result in any erythrocyte membrane damage at any of the tested concentrations (A–E).
Gas chromatography–mass spectrometry analysis
The methanol extract of S. polycystum and ethyl acetate extract of S. wightii were analysed using GC/MS to identify the major compounds (a, b). As shown in , GC/MS analysis identified 18 major compounds with relevant biological activity. The major bioactive compounds identified in the methanol extract of S. polycystum were hexanoic acid trimethylsilyl ester, heptanoic acid 6-oxo-trimethylsilyl ester, (.±.)-3-hydroxybutyric acid trimethylsilyl ether trimethylsilyl est, 3,3,6,6-tetramethyl-1,2,3,4,5,6,7,8-octahydro-1,8-acridinedione and 9,10-anthracenedione 1,3,8-trihydroxy-6-methyl. Similarly, the ethyl acetate extract of S. wightii confirmed the presence of Z,Z-6,28-heptatriactontadien-2-one, 3,7,11,15-tetramethyl-2-hexadecen-1-ol, fumaric acid dodecyl tetradec-3-enyl ester, n-hexadecanoic acid, l-(+)-ascorbic acid 2,6-dihexadecanoate, octadecanoic acid (stearic acid), tetradecanoic acid (myristic acid), dodecanoic acid (lauric acid), undecanoic acid, eicosanoic acid (arachidic acid), oleic acid, hentriacontane and fucosterol.
Figure 6. (a) Gas chromatogram of the methanol extract of Sargassum polycystum: A= 3,3,6,6-tetramethyl-1,2,3,4,5,6,7,8-octahydro-1,8-acridinedione; B= 9,10-anthracenedione, 1,3,8-trihydroxy-6-methyl. (b) Gas chromatogram of the ethyl acetate extract of Sargassum wightii: A= Z,Z-6,28 heptatriactontadien-2-one; B= n-hexadecanoic acid; C= hentriacontane; D= fucosterol.
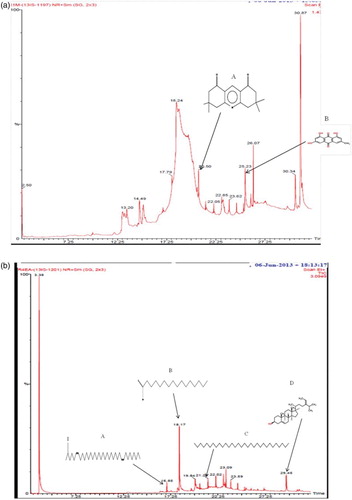
Table 2. Chemical composition of the methanol and ethyl acetate extracts from Sargassum polycystum and Sargassum wightii.
Discussion
Natural compounds that can reduce postprandial hyperglycaemia by inhibiting certain enzymes, such as α-amylase, α-glucosidase and DPP-IV, have been found to be useful in controlling diabetes (Layer et al. Citation1986). Marine algae have been used as a source for many pharmacological studies, based on traditional knowledge. However, such traditional knowledge has not gained much importance in drug discovery because of a lack of systematic studies and scientific evidence. Recently, several studies have been carried out on Sargassum species owing to their diverse pharmacological properties and structurally novel bioactive compounds (Liu et al. Citation2012). Although various phytochemical and pharmacological studies have been conducted on Sargassum species, to the authors’ knowledge the antidiabetic activity of S. polycystum and S. wightii has not been reported previously.
In this study, the in vitro antidiabetic and antioxidant potential of two edible seaweeds, S. polycystum and S. wightii, collected from Mandapam coastal region in the Gulf of Mannar, Tamilnadu, on the south-east coast of India, was investigated. More than one-third of the seaweed resources of the Indian Ocean are found on the coast of Tamilnadu, especially in the Gulf of Mannar (Muthukrishnan et al. Citation2013). The protein, lipids, minerals, vitamins and various other phytocompounds present in the seaweed vary with species, geographical location, season, temperature and stress tolerance (Norziah & Ching Citation2000; Wong & Cheung Citation2000).
Sargassum polycystum and S. wightii were serially extracted and screened for the presence of both non-polar and polar compounds. The preliminary phytochemical screening revealed the presence of major bioactive compounds such as alkaloids, phenols, flavanoids, glycosides, lipids, proteins and carbohydrates. These bioactive compounds have been shown to possess a wide range of pharmacological properties, including antidiabetic and antioxidant activity (Liu et al. Citation2012). Therefore, the seaweed extracts were further analysed for antidiabetic and antioxidant activity. The advantage of using DPPH is that the reducing free radical can be directly measured using a spectrophotometric assay and it gives reliable information regarding the antioxidant ability of the tested compounds. The acetone extract of S. wightii showed maximum inhibition of DPPH (43%) and the benzene extract of S. polycystum showed maximum inhibition of 22% at a concentration of 1000 µg/ml. There was also a reduction in the concentration of DPPH owing to the free radical scavenging ability of acetone extract of S. wightii and benzene extract of S. polycystum. The natural defence system of the body can be overwhelmed by reactive oxygen species, which cause oxidative damage to various cellular and extracellular macromolecules, leading to tissue injury (Halliwell & Auroma Citation1991). The results indicate that the compounds present in S. wightii and S. polycystum play a significant role in scavenging free radicals and thus preventing oxidative damage. As previously reported, S. polycystum alcoholic extract contains various phytochemicals such as flavanoids, terpenoids and phenolic compounds that possess excellent free radical scavenging ability (Matanjun et al. Citation2008). Moreover, Sargassum species contain sulphated polysaccharides such as alginates, fucoidan and laminaran, which have antioxidant potential that can prevent the destruction of pancreatic β-cells that leads to diabetes (Rioux et al. Citation2010).
The present study provides the first report on the antidiabetic properties of various extracts of S. polycystum and S. wightii using in vitro assays. The seaweed extracts are known to be effective inhibitors of α-amylase, α-glucosidase and DPP-IV owing to the presence of various phytochemicals which bind to the active site and alter the catalytic activity of the enzymes. In this study, various extracts of S. polycystum and S. wightii showed a concentration-dependent α-amylase, α-glucosidase and DPP-IV inhibitory activity. The tested ethyl acetate extract of S. polycystum (77%) and petroleum ether extract of S. wightii (62%) showed significant α-amylase inhibition. The IC50 values for S. polycystum and S. wightii were 438.5 µg/ml and 378.3 µg/ml, respectively, for α-amylase inhibition. Similarly, the methanol extract of S. polycystum (96%) and ethyl acetate extract of S. wightii (91%) showed more effective inhibition of α-glucosidase than the standard drug, acarbose. The IC50 values of S. polycystum and S. wightii were 289.7 µg/ml and 314.8 µg/ml, respectively. The percentage inhibition and IC50 values show that the ethyl acetate and methanol extracts of S. polycystum and the petroleum ether and ethyl acetate extracts of S. wightii are potent inhibitors of both α-amylase and α-glucosidase, which synergistically play a major role in the control of postprandial hyperglycaemia. DPP-IV comprises a new class of drug that inhibits the rapid degradation of incretin hormones (GLP-1 and GIP), which prevents postprandial hyperglycaemia. All of the solvent extracts of both species showed significant DPP-IV inhibition. Methanol extracts of S. polycystum and S. wightii (58%) showed equal preference for DPP-IV inhibition. The IC50 values obtained for S. polycystum and S. wightii were 36.94 µg/ml and 38.27 µg/ml, respectively.
The above results supplement previous work on S. polycystum, which was reported to reduce blood glucose and plasma insulin levels in diabetic/obese mice (Maeda et al. Citation2007). The phytochemicals and soluble fibres present in the extracts of S. polycystum have cholesterol-lowering effects and affect intestinal glucose absorption (Taskinen Citation2002; Vaugelade et al. Citation2000). The aqueous extract of S. polycystum contains mostly sulphated polysaccharides, which can reduce hyperglycaemia, dyslipidaemia and oxidative stress by increasing insulin sensitivity in rat models of type 2 diabetes (Motshakeri et al. Citation2013). Along with these reported studies, the present investigation adds strong supporting evidence that members of the species Sargassum (S. polycystum and S. wightii) are an excellent source of α-amylase, α-glucosidase and DPP-IV inhibitors. Consumption of these extracts could tentatively reduce the activity of intestinal enzymes (α-amylase and α-glucosidase) and inhibit DPP-IV enzyme; this could delay the degradation of incretin hormones (GLP-1 and GIP), which could stimulate insulin and reduce glucagon release, and stimulate the regeneration and differentiation of β-cells, which could improve glucose homeostasis and prevent hyperglycaemia.
This study was extended to investigate the various in vitro toxicological parameters which could lead to the validation of these extracts as a functional food ingredient. The J774 cells treated with various concentrations (250–1000 µg/ml) of seaweed extracts were found to be viable at the lower concentration of 250 µg/ml. No considerable morphological changes occurred at this concentration. Acridine orange–ethidium bromide staining revealed that no cell death, nuclear changes or DNA fragmentation occurred due to the treatment of extracts, even at higher concentration (1000 µg/ml). In vitro haemolytic assays showed lysis of less than 5% against RBCs at a higher concentration of 1000 µg/ml and did not show any erythrocyte membrane damage at any of the tested concentrations. Assessment of cell membrane stability is a vital process during the screening process for new drugs, and RBCs represent a good model for the study of membrane stability since their lysis releases haemoglobin, which can be easily detected using spectrophotometry. The mechanical stability of the erythrocytic membrane is a good indicator of the effect of various compounds and the present results show that the tested extracts do not induce haemolysis at even high concentrations. These results are similar to those reported by Lordan et al. (Citation2013), who found that seaweeds (A. nodosum, F. serratus, F. vesiculosus and P. canaliculata) in the class Phaeophyceae show potent α-amylase and α-glucosidase inhibition but are non-toxic under in vitro conditions. The overall findings of the current study suggest that extracts for α-amylase, α-glucosidase and DPP-IV inhibition are far below cytotoxic levels and do not contain any toxic substances that could induce membrane instability.
Since the methanol and ethyl acetate extracts of S. polycystum and S. wightii exhibited the highest antidiabetic activity, they were analysed by GC/MS to identify the major compounds present, using retention time and mass spectra available in the data library of the NIST. Ethyl acetate extract of S. wightii showed the presence of fucosterol, which has already been reported to have antidiabetic properties (Lee et al. Citation2004). Fucosterol is a natural compound, mainly present in marine algae, which is used in the treatment of diabetes by inhibiting glycogen breakdown in the liver. This may also significantly delay carbohydrate digestion and glucose absorption. The presence of fucosterol, and its antidiabetic, hepatoprotective and antioxidant activity, has been reported in the marine algae Pelvetia (Lee et al. Citation2004). The identified compounds in the extracts of both algal species showed the presence of major saturated and unsaturated fatty acids. Previous reports state that most of the identified compounds in the extracts exhibit various biological activities, including antioxidant, anti-inflammatory, antibacterial, antifungal and larvicidal activity (Agoramoorthy et al. Citation2007; Aparna et al. Citation2012; Huang & Wang Citation2004; Rahuman et al. Citation2000). Ascorbic acid, esters of fumaric acid and oleic acid are present in ethyl acetate extract of S. wightii. These compounds are well documented for various treatments such as wound healing, in the treatment of Huntington's disease and neurodegenerative diseases, and for the prevention of atherosclerosis (Ellrichmann et al. Citation2011; Okwu & Ighodaro Citation2010; Parthasarathy et al. Citation1990). The GC/MS results indicate that fucosterol and various other bioactive compounds present in the algal extracts are responsible for the potential antidiabetic and antioxidant activity.
In summary, it can be concluded that the extracts of S. polycystum and S. wightii have significant effects in inhibiting major carbohydrate-hydrolysing enzymes such as α-amylase and α-glucosidase, and inhibiting incretin-degrading enzymes such as DPP-IV, which can delay carbohydrate digestion and glucose absorption and prevent postprandial hyperglycaemia. Owing to their strong inhibitory properties, these seaweed extracts have potential for use in functional food applications to lower hyperglycaemia. The in vitro toxicological studies indicate that none of the extracts is toxic. GC/MS confirms the presence of major antidiabetic and antioxidant compounds including fucosterol. Further research on the in vivo antidiabetic properties of these extracts and the identification of active compounds is in progress. These studies should reveal the possible therapeutic uses of these algal species.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Agoramoorthy G, Chandrasekaran M, Venkatesalu V, Hsu MJ. 2007. Antibacterial and antifungal activities of fatty acid methyl esters of the blind-your-eye mangrove from India. Braz J Microbiol. 38:739–742. doi: 10.1590/S1517-83822007000400028
- Al-Masri IM, Mohammad MK, Tahaa MO. 2009. Inhibition of dipeptidyl peptidase IV (DPP IV) is one of the mechanisms explaining the hypoglycemic effect of berberine. J Enzyme Inhib Med Chem. 24:1061–1066. doi: 10.1080/14756360802610761
- Aparna V, Dileep KV, Mandal PK, Karthe P, Sadasivan C, Haridas M. 2012. Anti-inflammatory property of n-hexadecanoic acid: Structural evidence and kinetic assessment. Chem Biol Drug Des. 80:434–439. doi: 10.1111/j.1747-0285.2012.01418.x
- Bharti SK, Krishnan S, Kumar A, Rajak KK, Murari K. 2012. Antihyperglycemic activity with DPP-IV inhibition of alkaloids from seed extract of Castanospermum australe, Investigation by experimental validation and molecular docking. Phytomedicine. 20:24–31. doi: 10.1016/j.phymed.2012.09.009
- Chan CX, Ho CL, Phang SM. 2006. Trends in seaweed research. Trends Plant Sci. 11:165–166. doi: 10.1016/j.tplants.2006.02.003
- Creutzfeldt W. 2005. The (pre) history of the incretin concept. Regul Pept. 128:87–91. doi: 10.1016/j.regpep.2004.08.004
- Ellrichmann G, Petrasch-Parwez E, Lee DH, Reick C, Arning L, Saft C, Gold R, Linker RA. 2011. Efficacy of fumaric acid esters in the R6/2 and YAC128 models of Huntington's disease. PLoS One. 6(1):e16172. doi: 10.1371/journal.pone.0016172
- Halliwell B, Auroma OI. 1991. DNA damage by oxygen-derived species its mechanism and measurements in mammalian systems. FEBS Lett. 281:9–19. doi: 10.1016/0014-5793(91)80347-6
- Harborne JB. 1998. Methods of extraction and isolation. In: Phytochemical Methods (3 ed.). Chapman and Hall, London; p. 60–66.
- Havale SH, Pal M. 2009. Medicinal chemistry approaches to the inhibition of dipeptidyl peptidase-4 for the treatment of type 2 diabetes. Bioorg Med Chem. 17:1783–1802. doi: 10.1016/j.bmc.2009.01.061
- Hizukuri S, Abe JI, Hanashiro I. 1996. Starch analytical aspects. In Eliasson AC (ed) Carbohydrates in food. Marcel Dekker, Inc., New York; p. 347–429.
- Huang HL, Wang BG. 2004. Antioxidant capacity and lipophilic content of seaweeds collected from the Qingdao coastline. J Agric Food Chem. 52:4993–4997. doi: 10.1021/jf049575w
- Jayasri MA, Radha A, Mathew TL. 2009. α-amylase and α-glucosidase inhibitory activity of Costus pictus D. Don in the management of diabetes. J. Herb. Med. Toxicol. 3:91–94.
- Kwon YI, Apostolidis E, Kim YC, Shetty K. 2007. Health benefits of traditional corn beans and pumpkin In vitro studies for hyperglycemia and hypertension management. J Med Food. 10:266–275. doi: 10.1089/jmf.2006.234
- Layer P, Rizza RA, Zinsmeister AR, Carlson GL, Dimagno EP. 1986. Effect of a purified amylase inhibitor on carbohydrate tolerance in normal subjects and patients with diabetes mellitus. Mayo Clin Proc. 61:442–447. doi: 10.1016/S0025-6196(12)61978-8
- Lee SH, Li Y, Karadeniz F, Kim MM, Kim SK. 2008. α-Glucosidase and α-amylase inhibitory activities of phloroglucinol derivatives from edible marine brown alga, Ecklonia cava. J. Sci. Food Agric. 89:1552–1558. doi: 10.1002/jsfa.3623
- Lee YS, Shin KH, Kim BK, Lee S. 2004. Anti-diabetic activities of fucosterol from Pelvetia siliquosa. Arch Pharm Res. 27:1120–1122. doi: 10.1007/BF02975115
- Liu L, Heinrich M, Myers S, Dworjanyn SA. 2012. Towards a better understanding of medicinal uses of the brown seaweed sargassum in traditional Chinese medicine. A phytochemical and pharmacological review. J Ethnopharmacol. 142:591–619. doi: 10.1016/j.jep.2012.05.046
- Lordan S, Smyth TJ, Soler-Vila A, Stanton C, Ross RP. 2013. The alpha-amylase and alpha-glucosidase inhibitory effects of Irish seaweed extracts. Food Chem. 141:2170–2176. doi: 10.1016/j.foodchem.2013.04.123
- MacArtain P, Gill CI, Brooks M, Campbell R, Rowland IR. 2007. Nutritional value of edible seaweeds. Nutr Rev. 65:535–543. doi: 10.1111/j.1753-4887.2007.tb00278.x
- Maeda H, Hosokawa M, Sashima T, Miyashita K. 2007. Dietary combination of fucoxanthin and fish oil attenuates the weight gain of white adipose tissues and decreases blood glucose in obese diabetic KK-Amice. J Agric Food Chem. 55:7701–7706. doi: 10.1021/jf071569n
- Malagoli D. 2007. A full-length protocol to test hemolytic activity of palytoxin on human erythrocytes. Inv Surv J. 4:92–4.
- Matanjun BP, Mohamed S, Mustapha NM, Muhammad K, Ming CH. 2008. Antioxidant activities and phenolics content of eight species of seaweeds from north Borneo. J Appl Phycol. 20:367–373. doi: 10.1007/s10811-007-9264-6
- Mensor LL, Menezes FS, Leitao GG, Reis AS, Santos TC, Coube CS, Leitao SG. 2001. Screening of Brazilian plant extracts for antioxidant activity by the use of DPPH free radical smethod. Phytother Res. 15:127–130. doi: 10.1002/ptr.687
- Motshakeri M, Ebrahimi M, Goh YM, Matanjun P, Mohamed S. 2013. Sargassum polycystum reduces hyperglycaemia, dyslipidaemia and oxidative stress via increasing insulin sensitivity in a rat model of type 2 diabetes. J Sci Food Agric. 93:1772–1778. doi: 10.1002/jsfa.5971
- Murai A, Iwamura K, Takada M, Ogawa K, Usui T, Okumura J. 2002. Control of postprandial hyperglycaemia by galactosyl maltobionolactone and its novel anti-amylase effect in mice. Life Sci. 71:1405–1415. doi: 10.1016/S0024-3205(02)01844-1
- Muthukrishnan A, Aruchamy S, Banukumar K, Alaguraja P. 2013. Climatic balance on coastal ecosystems in Gulf of Mannar, geoclimatic techniques. International Journal of Geomatics and Geosciences. 3:668–691.
- Newman DJ, Cargg GM, Snader KM. 2003. Natural products as source of new drugs over the period 1981–2002. J Nat Prod. 66:1022–1037. doi: 10.1021/np030096l
- Nickavar B, Yousefian N. 2011. Evaluation of α-amylase inhibitory activities of selected antidiabetic medicinal plants. J. Verbr. Lebensm. 6:191–195. doi: 10.1007/s00003-010-0627-6
- Norziah MH, Ching CY. 2000. Nutritional composition of edible seaweeds Gracilaria changgi. Food Chem. 68:69–76. doi: 10.1016/S0308-8146(99)00161-2
- Okwu ED, Ighodaro UB. 2010. GC-MS evaluation of bioactive compounds and antibacterial activity of the oil fraction from the leaves of Alstonia boonei De Wild. Der Pharma Chemica. 2(1):261–262.
- Parthasarathy SJ, Khoo C, Miller E, Barnett J, Witztum JL, Steinberg D. 1990. Low density lipoprotein enriched in oleic acid is protected against oxidative modification: implications for dietary prevention of atherosclerosis. Proc Natl Acad Sci USA. 87:3894–3898. doi: 10.1073/pnas.87.10.3894
- Rahuman AA, Gopalakrishnan G, Ghouse BS, Arumugam S, Himalayan B. 2000. Effect of Feronia limonia on mosquito larvae. Fitoterapia. 71:553–555. doi: 10.1016/S0367-326X(00)00164-7
- Rang HP, Dale MM, Ritter JM, Moore PK. 2003. Pharmacology (5th ed). Churchill Livingstone, London. Ch: 382.
- Rioux LE, Turgeon SL, Beaulieu M. 2010. Structural characterization of laminaran and galactofucan extracted from the brown seaweed Saccharina longicruris. Phytochemistry. 71:1586–1595. doi: 10.1016/j.phytochem.2010.05.021
- Sudha P, Smita ZS, Shobha BY, Ameeta KR. 2011. Potent α-amylase inhibitory activity of Indian Ayurvedic medicinal plants. BMC Complement Altern Med. 11:5. doi: 10.1186/1472-6882-11-5
- Tarling CA, Woods K, Zhang R, Brastianos HC, Brayer GD, Andersen RJ, Withers SG. 2008. The search for novel human pancreatic α-amylase inhibitors: high-throughput screening of terrestrial and marine natural product extracts. ChemBioChem. 9:433–438. doi: 10.1002/cbic.200700470
- Taskinen MR. 2002. Diabetic dyslipidemia. Atheroscler Suppl. 3:47–51. doi: 10.1016/S1567-5688(01)00006-X
- Vaugelade P, Hoebler C, Bernard F, Guillon F, Lahaye M, Duee PH. 2000. Non-starch polysaccharides extracted from seaweed can modulate intestinal absorption of glucose and insulin response in the pig. Reprod Nutr Dev. 40:33–47. doi: 10.1051/rnd:2000118
- Wong KH, Cheung PCK. 2000. Nutritional evaluation of some subtropical red and green seaweeds: Part I – proximate composition, amino acid profiles and some physico-chemical properties. Food Chem. 71:475–482. doi: 10.1016/S0308-8146(00)00175-8
- Wu XJ, Hansen C. 2008. Antioxidant capacity, phenolic content, polysaccharide content of Lentinus edodes grown in whey permeate based submerged culture. J Food Sci. 73:434–438. doi: 10.1111/j.1750-3841.2008.00675.x
- Yuan YV, Carrington MF, Walsh NA. 2005. Extracts from dulse (Palmaria palmata) are effective antioxidants and inhibitors of cell proliferation in vitro. Food Chem Toxicol. 43:1073–1081. doi: 10.1016/j.fct.2005.02.012
