Abstract
This study investigated the process of preparing fermented medicines as prescribed in Ayurveda, the traditional Indian system of medicine. Berberine, an alkaloid, was used as a model compound. Berberine is the active constituent of Berberis aristata and is alleged to have anti-inflammatory effect. Biotransformation of berberine was studied by the phospholipase A2 assay in fermented products prepared in traditional and commercially available brewer's yeast-induced environments. Sugar and alcohol levels were estimated to indicate the culmination of fermentation. It was confirmed that traditional fermentation biotransforms berberine to a greater extent than commercially available brewer's yeast-induced fermentation. Therefore, fermentation induced by commercially available yeast is no substitute for the ethnopharmacological and traditional fermentation prescribed in the traditional Indian system of medicine.
Introduction
Fermentation is prescribed as a method for the preparation of medicines in Ayurveda, the traditional Indian system of medicine (Chaudary et al. Citation2011). No other system of medicine uses fermentative technology as extensively as Ayurveda. However, no specific theoretical basis is provided in any of the Ayurvedic classics for adopting fermentative technology, explaining where and when it is to be applied (Mishra Citation2008). Neither it is explained explicitly why it is adopted, notwithstanding the rigorous tradition. The texts only provide recipes to make the medicines (Jadava Citation2002; Sastry Citation2002). In addition, seldom does the recipe call for the use of a starter culture for fermentation (Arkaprakasam Citation1995), except for the use of Woodfordia fruticosa L. Kurz. flowers to enhance the process (Chaudhary et al. Citation2011). This must be understood against the background of the documented practice of fermentation and distillation of preparations, for medicinal and other uses, with different forms of starter culture, as described elsewhere (Arkaprakasam Citation1995). The only instruction to use a starter culture is given in the recipe for the preparation of purgatives (Valiathan Citation2007). This may be the only context where the use of a starter culture in alcoholic preparations for oral medicine is advised. Therefore, such a preparation may not be intended for medical material that is to be metabolized, but only for use as a purgative.
It was inferred from a previous study that the fermentative process adopted for Ayurvedic medicine preparations, such as ‘asava’ and ‘arishta’, may transform various compounds present in the materials used in the preparation of polyherbal fermented drugs (Naveen Chandra et al. Citation2012). Such biotransformations may increase the desired properties of such polyherbal preparations and the specific activity of certain compounds present in these preparations (Naveen Chandra et al. Citation2012). The fermentative step may mimic the processes of ‘digestive transformation and metabolic transformation’, as described in classical texts of the Science of Ayurveda (Valiathan Citation2007). This may supply more bioactive, medically operational compounds to the body more rapidly, reducing the time taken for metabolic transformation. Hence, the body may be provided with more active medicines for a longer period than with non-fermented medicines.
However, the use or non-use of starter cultures and the longer period of fermentation for the fermentative production of Ayurvedic drugs are not explained in any of the texts. To understand the prescription of no specific starter cultures for preparing fermented Ayurvedic drugs and the longer period of fermentation, the model described in the previous study was employed (Naveen Chandra et al. Citation2012).
Berberine, an isoquinoline alkaloid, is found in various medicinal plants in varying amounts. This alkaloid has a wide range of pharmacological activities (Birdsall and Kelly Citation1997). Berberine is an inhibitor of the proinflammatory enzyme phospholipase A2 (PLA2) (Naveen Chandra et al. Citation2011). It is converted mainly to dihydroxyberberine by microbial transformation, as found in Fourier transform infrared spectroscopy, proton nuclear magnetic resonance and electrospray ionization–mass spectrometry studies (Naveen Chandra et al. Citation2012). This compound displays an enhanced level of PLA2 inhibition as a result of its inverted binding with the enzyme enabling the formation of more hydrogen bonds (Naveen Chandra et al. Citation2012).
The change in the inhibition of PLA2 by the combined effect of berberine and biotransformed berberine generated by alcoholic fermentation was assessed in the present study. The process was designed to simulate the traditional Ayurvedic system of fermented medicinal preparations, with or without the yeast inoculum (wine-model or traditional fermentation respectively).
Materials and methods
All reagents were obtained from E. Merck India (Mumbai, India). Berberine chloride was from Fluka Chemicals (USA). The optical density for biochemical estimations was recorded on a Hitachi double-beam spectrophotometer. Brewer's yeast and W. fruticosa L. Kurz. flowers were purchased locally from a traditional Indian medicine market. A voucher specimen was deposited in the herbarium of the Department of Biotechnology and Microbiology, Kannur University. PLA2 was purified from commercial porcine pancreatin obtained from Loba Chemie (Mumbai, India), using a previously described method (Tsao et al. Citation1973).
Fermentation of berberine
Berberine was selected as the model compound for this study. A 0.1% w/v solution of berberine chloride in water was prepared and nutrients for fermentation, such as sodium chloride (0.1%), ammonium sulphate (0.1%), potassium dihydrogen phosphate (0.1%) and sucrose (10%), were added. The medium was poured into six conical flasks (400 ml each) and autoclaved. Three of them were inoculated with brewer's yeast cultured in nutrient broth. The yeast starter culture was standardized to have identical growth phases in both the traditional and the yeast-mediated fermentation systems. The other three flasks were inoculated with 1 g W. fruticosa L. Kurz. flowers per flask. The flasks were kept in a platform shaker and shaken periodically. Samples of 25 ml were withdrawn aseptically every alternate day up to 14 days. A final sample was taken on day 28. Samples for the preparation and estimation of berberine/biotransformed berberine, sugar and alcohol were taken from different flasks by ensuring that sufficient amounts of the samples were collected.
Preparation of samples
Purification of berberine and its derivatives for enzyme kinetic studies
Berberine and its biotransformed derivatives were purified by ion-exchange chromatography using CM-Sephadex. The collected sample was passed through CM-Sephadex swollen in water in a chromatography column. The column was washed with distilled water. Berberine and its derivatives were eluted out with 2 N HCl and evaporated to dryness over a water bath to yield crystals.
Preparation of samples for sugar and alcohol estimation
Samples were filtered through a Whatman no. 1 filter paper and then used for the estimation of sugar. For the estimation of alcohol, samples were distilled to dryness in a small distillation flask. The distillate, which was devoid of sugar, was used for the estimation of alcohol.
Estimation of alcohol
The potassium dichromate–sulphuric acid method was used to estimate alcohol. In an acidic environment, alcohol is oxidized by dichromate to acetic acid, dichromate being reduced to green chromic sulphate and spectrophotometrically measured at 600 nm (Fletcher and Studus Citation2003). The experiment was repeated three times, and the mean and standard deviation were calculated using Microsoft Excel software.
Estimation of sugar
The dinitrosalicylic acid method was used for the estimation of sugar. The samples were mixed with an equal volume of 2 N HCl and kept on a water bath at 60°C for 1 h, to hydrolyse sucrose to reducing sugars. Dinitrosalicylic acid is reduced to red aminonitrosalicylic acid by reducing sugars. The red colour produced was spectrophotometrically measured at 510 nm (Marsden et al. Citation1982). The amount of sugar was calculated by comparison with a standard. The experiment was repeated three times, and the mean and standard deviation were calculated using Microsoft Excel.
Assay of phospholipase A2 inhibition
PLA2 from porcine pancreas was used to estimate the inhibitory effect of the model compound berberine and its biotransformed derivatives. PLA2 from porcine pancreas is structurally similar to human PLA2, which is involved in inflammatory responses (Berg et al. Citation1995). A spectrophotometric method was used to assess PLA2 activity. PLA2 produces organic acids from the substrate used in this assay, soya lecithin. This was reacted with hydroxylamine to produce a hydroxamic acid derivative, which produced a pink complex with ferric chloride in acidic medium. This was measured spectrophotometrically at 570 nm and its intensity was proportional to the activity of PLA2 in the solution (Bhat and Gowda Citation1989). The assay was carried out in 500 mM Tris.HCl buffer (pH 7.2) containing 10 mM calcium chloride. The substrate was a 2% aqueous suspension of soya lecithin emulsified with 1.5% bile salts in water.
Then, 700 µl of the above buffer was dispensed into test-tubes, and equal volumes of soya lecithin suspension and bile salt solution were added. Next, 100 µl of the enzyme solution with a concentration of 1 mg/ml was added to this mixture and the time noted on a stopwatch. The reaction was allowed to proceed for exactly 3 min. At the end of this period, 100 µl of the reaction mixture was pipetted into a test-tube containing 1.5 ml of 25% ether-alcohol, 200 µl of 2 N aqueous hydroxylamine HCl and 200 µl of 14% sodium hydroxide solution to arrest the reaction and to convert the fatty acid produced to hydroxamic acid derivative. This mixture was treated with 600 µl of 3 N HCl and 300 µl of 10% ferric chloride for the development of the coloured complex. A blank was carried out by following the same procedure as above, but substituting the enzyme solution with distilled water. Optical density was subsequently measured at 570 nm, using the blank for correction. The value gave the measure of activity of the enzyme.
The experiment was repeated with PLA2 incubated with various samples of berberine, purified from equal volumes of samples fermented traditionally and with yeast, keeping the enzyme to inhibitor ratio constant at 1:10. The difference in the optical density value was noted in each case. This corresponded to the degree of inhibition of the enzyme by the inhibitor, berberine, and its derivatives formed by fermentation. The experiment was repeated three times, and the mean and standard deviation of the values were calculated using Microsoft Excel.
Results
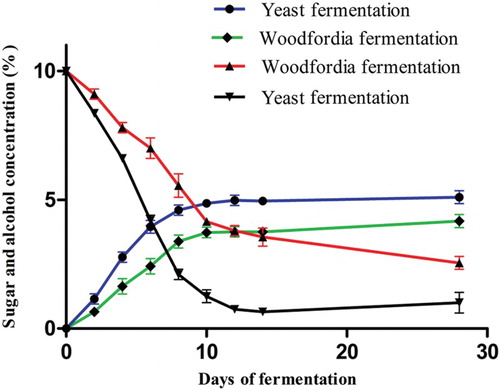
In the case of traditional fermentation, 3.53% alcohol was produced within 14 days (). After this, it was fairly constant, rising only to 3.92% on day 28 of fermentation. Depletion of sugar was gradual at first, from 10% to 8.96% on day 2 (). It was reduced to 3.20% on day 14. On day 28 there was nearly 2.3% sugar unutilized in the medium.
Figure 1. Sugar utilization and alcohol production in traditional and brewer's yeast fermentation. The descending curves show depletion of sugar and ascending curves show production of alcohol in the medium. The values are the average of three concurrent sets of experiments.
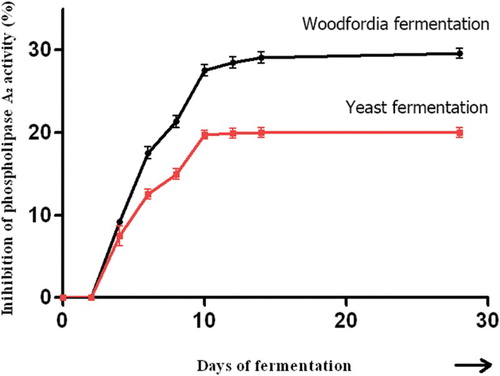
No rise in PLA2 inhibitory activity was discerned in the first 2 days, which implied that berberine was not converted at all by the organisms at higher sugar levels. Thereafter, a 9.20% rise in activity was seen, which rose steadily to 28.40% at the end of day 14, showing the rapid conversion of berberine at intermediate sugar levels. On day 28, activity rose marginally to 29% compared to 28.40% on day 14, which proved that biotransformation was not taking place at lower levels of sugar in the fermentation medium ().
Figure 2. Enhancement of phospholipase A2 inhibition due to berberine/biotransformed berberine by traditional and brewer's yeast fermentation. The curves denote the average of three concurrent sets of experiments.
With regard to brewer's yeast fermentation, alcohol generation was rapid, being 0.95% on day 2 (). It rose rapidly during days 4 and 6, to 2.57% and 3.70%, respectively. At the end of day 14 it was 4.80%. Alcohol rose only marginally to 4.85% until day 28 of fermentation. Depletion of sugar also took place at a higher rate in yeast fermentation. Sugar utilization was from 10% to 8.21% on day 2 and the sugar content was rapidly reduced to 1.98% on day 8. After that, the sugar utilization was reduced, as in the stationary phase ().
No significant difference in PLA2 inhibition was found during the first couple of days. It may be inferred that the biotransformation, which produced enhanced PLA2 inhibition, did not occur during the above period. The biotransformation of berberine occurred only from day 4 onwards, showing an enhancement in the inhibition of PLA2 of approximately 7.50% (). Thereafter, the increase in PLA2 inhibition was in the log phase, ending on day 10, with PLA2 inhibition at 19.2% and 26.9% in yeast-fermented and traditionally fermented systems, respectively. Further biotransformation with yeast fermentation occurred only at a negligible rate, 19.30% and 19.40% on days 12 and 14, respectively. The log phase of the berberine biotransformation continued for a couple more days in the traditionally fermented system, showing 28.40% of PLA2 inhibition on day 14. On day 28 of fermentation, the inhibition of PLA2 was 19.40% in the yeast-fermented system and 29.00% in the traditionally fermented system ().
Discussion
The sugar utilization, alcohol production and increase in PLA2 inhibition caused by berberine and biotransformed berberine showed almost identical lag, log and stationary phases of the microbial growth pattern. However, the traditional fermentation had a longer log phase than the brewer's yeast fermentation. This difference was continued into the stationary phase to produce more of the PLA2 inhibitory activity in the traditional fermentation. During the initial lag phase, up to 2 days, no increase in PLA2 inhibitory activity was observed in either case. On day 4, traditionally fermented berberine showed 9.20% enhancement while the yeast-fermented system showed only 7.50%, the difference being 1.70%. This difference became more pronounced as fermentation proceeded further. The difference was 9.00% on day 14 and 9.60% on day 28 (). Traditional fermentation with W. fruticosa L. Kurz. flowers produced PLA2 inhibitory activity enhanced by approximately 10% more than brewer's yeast fermentation (). It was obvious from the results that brewer's yeast utilized sugar at a higher rate and produced more alcohol; nearly 1% more than produced by the natural microflora of W. fruticosa L. Kurz. flowers. It may be a result of the rapid depletion of sugar in the medium and generation of alcohol that the brewer's yeast fermentation stopped early, leaving less possibility for the biotransformation of berberine. Traditional fermentation, in contrast, generated less alcohol and was significantly slower, taking more than 14 days to complete alcohol production. It may also be inferred that the continued storage of the brewer's yeast-fermented system may not elicit more biotransformed compounds, to reach the level of traditionally fermented products. This shows that active, biotransformed constituents in the fermented Ayurvedic medicinal preparations may be present in higher amounts if the traditional prescriptions are followed. Further investigations with the microbial consortium present in the W. fruticosa L. Kurz. flowers and brewer's yeast are necessary to show how the traditional system provides more effective fermentation to transform the compounds present in the fermented Ayurvedic medicinal preparations. This observation is in agreement with the traditional prescription to use only W. fruticosa L. Kurz. flowers to ferment Ayurvedic medicines and to use the sediment of the previous fermentation (enriched yeast) only for fast fermentation of alcoholic beverages (Arkaprakasam Citation1995).
The microbial consortium of W. fruticosa L. Kurz. flowers may be responsible for the more pronounced biotransformation of berberine, the model compound selected for this study. Traditionally fermented Ayurvedic products with W. fruticosa L. Kurz. flowers may also have the benefits of being rich in probiotics (Kumar et al. Citation2011). The role of these flowers in the production of fermented Ayurvedic medicines and their associated microflora was studied earlier (Atal et al. Citation1982). Therefore, it may be suggested that brewer's yeast-induced fermentation, as employed by many manufacturers of Ayurvedic medicines to reduce production costs, is no substitute for the traditional fermentation described in the ancient Ayurvedic literature. Such practices may not produce authentic fermented Ayurvedic medicines.
Acknowledgements
Discussions with Professor M.S. Valiathan, former president of the Indian National Science Academy, and Dr C. Ramankutty, Publications Department, Arya Vaidya Sala Kottakkal, Kerala, are gratefully acknowledged.
Disclosure statement
No potential conflict of interest was reported by the author(s).
References
- Arkaprakasam. 1995. Krishnadas academy. Varanasi, India: Oriental Publishers & Distributors.
- Atal C, Bhatia A, Singh R. 1982. Role of Woodfordia fruticosa flowers in the preparation of Asavas and Aristas. J Res Ayur Sidd. 3:193–199.
- Berg BV, Tessari M, de Haas GH, Verhiji HM, Boelens R, Kaptein R. 1995. Solution structure of porcine pancreatic phospholipase A2. EMBO J. 14:4123–4131.
- Bhat MK, Gowda TV. 1989. Purification and characterization of a mycotoxic phospholipase A2 from Indian cobra venom. Toxicon. 27:861–873. doi: 10.1016/0041-0101(89)90098-6
- Birdsall TC, Kelly GS. 1997. Berberine: therapeutic potential of an alkaloid found in several medicinal plants. Alt Med Rev. 2:94–103.
- Chaudhary A, Singh N, Dalvi M, Wele A. 2011. A progressive review of sandhana kalpana (biomedical fermentation): an advanced innovative dosage form of Ayurveda. Ayu. Journal. 32:408–416. doi: 10.4103/0974-8520.93925
- Fletcher PJ, Stadus JFV. 2003. Determination of ethanol in distilled liquors using sequential injection analysis with spectrophotometric detection. Analyt Chim Acta. 499:123–128. doi: 10.1016/j.aca.2003.07.005
- Jadava V. 2002. Susrutha samhitha with commentary of Dalhana. Varanasi, India: Chakhambha Orientalia.
- Kumar H, Rangrez AY, Dayananda KM, Atre AN, Patole AS, Shouche YS. 2011. Lactobacillus plantarum (VR1) isolated from an Ayurvedic medicine (Kutajarista) ameliorates in vitro cellular damage caused by Aeromonas veronii. BMC Microbiol. 11:152–156. doi:10.1186/1471-2180-11-152
- Marsden WL, Gray PP, Grej JN, Quinlan MR. 1982. Evaluation of the DNS method for analysing lignocellulosic hydrolysates. J Chem Technol Biotechnol. 32:1016–1022. doi: 10.1002/jctb.5030320744
- Mishra SK. 2008. Bhashjya Kalpana Vigyanam. Varanasi, India: Chakhambha Orientalia.
- Naveen Chandra D, Prasanth GK, Singh Nagendra, Kumar Sanjit, Jithesh O, Sadasivan C, Sujatha Sharma, Singh TP, Haridas M. 2011. Identification of a novel and potent inhibitor of phospholipase A2 in a medicinal plant: crystal structure at 1.93 Å and surface plasmon resonance analysis of phospholipase A2 complexed with berberine. Biochim Biophys Acta. 1814:657–663. doi: 10.1016/j.bbapap.2011.03.002
- Naveen Chandra D, Abhilash Joseph, Prasanth GK, Sabu A, Sadasivan C, Haridas M. 2012. Inverted binding due to a minor structural change in berberine enhances its phospholipase A2 inhibitory effect. Int J Biol Macromol. 50:578–585. doi: 10.1016/j.ijbiomac.2012.01.029
- Sastry PP. 2002. Sharangadhar samhitha with commentary of adhmallas dipika and Kashirams gudartha dipika. Varanasi, India: Chakhambha Orientalia.
- Tsao FHC, Cohen Hershel, Synder WR, Kezdy FJ, Law JH. 1973. Multiple forms of porcine pancreatic phospholipase A2: isolation and specificity. J Supramol Struct. 98:490–497. doi: 10.1002/jss.400010605
- Valiathan MS. 2007. The legacy of Susrutha. Hyderabad, India: Orient Longman.
