Abstract
The main aim of this study was to identify lipase-producing bacteria from bovine milk samples. The strain possessing the highest lipase activity was identified by physiological and molecular characterization. 16S rRNA phylogenetic analysis showed highest similarity with Pseudomonas sp. and the strain was named Pseudomonas sp. VITSDVM1. The lipase productivity was improved by varying the utilization of substrates, i.e. banana and potato peel extracts, in the production medium. The results of optimization studies showed that lipase activity was maximum with groundnut oil, compared with the other carbon sources used, and with peptone as a nitrogen source. The optimum pH and temperature were 8 and 40°C, respectively. The optimum substrate concentration was 20% for the incubation period of 72 h with a maximum activity of 4.66 U mL−1. The purified lipase enzyme analyzed by high-performance liquid chromatography revealed a retention time of 3.6 min. Hence, lipase production using agricultural waste such as banana and potato peels could be used in industrial applications.
Introduction
Lipases are water-soluble extracellular enzymes that catalyze the hydrolysis of triacylglycerols to liberate free fatty acids and glycerol. Lipases are an important group of biotechnologically relevant enzymes, and properties such as their ability to perform specific biotransformations including esterification and transesterification and their stability have made them increasingly popular in the food, pharmaceutical, detergent, cosmetic and oleochemical industries (Park et al. Citation2005; Grbavcic et al. Citation2007; Gupta et al. Citation2007). For many industrially important biotransformations, biotechnological interest in these enzymes has increased owing to these distinctive features (Chowdary et al. Citation2001; Hamsaveni et al. Citation2001; Krishna et al. Citation2001; Rao and Divakar Citation2001; Zhang et al. Citation2001; Castro et al. Citation2004; Carvalho et al. Citation2005; Fucinos et al. Citation2005).
The most suitable sources of lipases are bacteria, fungi and yeast. Because of their large potential in industrial applications such as in cosmetics and pharmaceuticals, the production of microbial lipases has increased in the past few decades (Elibol and Ozer Citation2000; Kamini et al. Citation2000; Fucinos et al. Citation2005). Microbial lipases have good substrate specificity, stability and selectivity, owing to which they have gained special attention for industry (Dutra et al. Citation2008; Griebeler et al. Citation2011). Bacterial lipases are more economical to produce and more stable than those derived from fungi and yeast. The production of microbial lipases is greatly influenced by the composition of the medium, as well as by physicochemical factors such as pH and temperature. It has been reported that the carbon source is the major factor in the expression of lipase activity. Expensive carbon substrates such as dextrin, fructose, glucose, lactose, maltose and starch can be replaced in the medium with cheaper agricultural by-products (Ghosh and Chandra Citation1984) or industrial amylaceous substances. Characterization of a novel Pseudomonas sp. has been reported, with potential applications using lipases (Lehmann et al. Citation2014). Agro-industrial residues are generally considered to be the best substrate for enzyme production (Singh and Kumar Citation2009). Banana peels and potato peels are cheap agricultural wastes. Bananas are the largest fruit crop produced in India, and this country contributes to 27% of worldwide banana production Bananas are cultivated in 130 countries, mainly in the tropical and subtropical regions of the southern hemisphere. Potatoes are also produced and used extensively all over the world. Therefore, large amounts of peels from both crops are produced. Asia is the largest producer of bananas and potatoes. At present, India is the biggest potato producer, with an output of over 20 million tonnes (Andreas et al. Citation2009). Banana and potato peels are organic wastes that are rich in carbohydrates and other basic nutrients that support microbial growth (Andreas et al. Citation2009). In Asia, about 20,000 tonnes of banana peels are discarded annually from processing plants, posing serious environmental and health problems. It has been estimated that banana peels contain 14.6% glucose and 56% sucrose.
The main objective of the present study was to utilize banana and potato peels as substrates for lipase production by a novel Pseudomonas sp. and thus enable a substantial reduction in environmental agro-industrial pollution.
Materials and methods
Isolation and screening
Ten cow's milk samples were collected from Vellore district of Tamil Nadu, India, and serially diluted up to 10−7. Diluted samples were plated on tributyrin agar and incubated at 37°C for 24 h. Lipolytic colonies were selected and screened by qualitative plate assay. Isolates were grown on tributyrin agar plates and incubated at 37°C for 2 days. Zones of clearance were observed. A clear zone indicates the production of lipase. Potent isolates showing the maximum zones of clearance were selected and maintained on nutrient agar at 4°C.
Strain selection and identification
The potent bacterial strain VITSDVM1 isolated from cow's milk was characterized based on its cultural, morphological and biochemical properties according to Bergey's manual of determinative bacteriology (Holt et al. Citation1994). Pure cultures of the identified strain was maintained on nutrient agar plates and stored at 4°C.
Molecular characterization
The most significant step in the analysis of phylogenetic relationships between species is DNA sequence comparison (Thabit et al. Citation2013). The individual colonies of the potent isolate were picked up with a sterilized toothpick, suspended in 0.5 µL of sterile saline in a 1.5 mL centrifuge tube and centrifuged at 10,000 rpm for 10 min. The pellet was suspended in 0.5 µL of InstaGene Matrix (Bio-Rad, USA) and incubated at 56°C for 30 min, then heated at 100°C for 10 min. After heating, the supernatant was used for polymerase chain reaction (PCR). DNA amplification was carried out using universal forward and reverse primers, 27F (5′-AGAGTTTGATCMTGGCTCAG-3′) and 1492R (5′-TACGGYTACCTTGTTACGACTT-3′), respectively. The conditions for amplification were 35 cycles at 94°C for 45 s, 55°C for 60 s and 72°C for 60 s (Gutell et al. Citation1990). The PCR products were sequenced using forward and reverse primers, 518F (5′-CCAGCAGCCGCGGTAATACG-3′) and 800R (5′-TACCAGGGTATCTAATCC-3′), respectively. Sequencing was performed using the BigDye Terminator cycle sequencing kit (Applied Biosystems, USA). Sequencing products were resolved on an Applied Biosystems model 3730XL automated DNA sequencing system (Applied Biosystems, California, USA). The 16S rRNA sequences were integrated into the database with the automatic alignment tool. A phylogenetic tree was generated by distance matrix analysis using the neighbor-joining method (Saitou and Nei Citation1987). A database search and comparisons were carried out with the BLAST search using the National Center for Biotechnology Information (NCBI) database (Altschul et al. Citation1990).
Substrate preparation
Fresh banana peels and potato peels were used as substrates in this study. Peels were washed and chopped into small pieces and dried in hot air oven at 100°C for 24 h. After drying, peels were ground and 25% peel extract was prepared. It was slightly boiled and then filtered to obtain a pure 25% peel extract.
Inoculum preparation
The culture was inoculated into nutrient broth and incubated for 24 h. The optical density of the suspension was standardized to 0.5 (2.1×106 cfu/mL) at 530 nm. It was used as the seed inoculum for enzyme production.
Lipase production
For the submerged fermentation the culture was grown in 500 mL Erlenmeyer flasks containing 120 mL of production medium (glucose 1.0%, peptone 2.0%, yeast extract 0.5%, olive oil 1.0%, NaNO3 0.1%, KH2PO4 0.1%, MgSO4.7H2O 0.05% in phosphate buffer 0.1 M at pH 6.5). The medium was supplemented with potato and banana substrate 10% (p/v) in separate flasks, inoculated with 24-h-old culture, and incubated at 30°C and 150 rpm for 5 days, to evaluate the influence on lipase activity.
Enzyme extraction
After fermentation, the culture broth was filtered and centrifuged at 8000 rpm for 10 min at room temperature. The clear supernatant obtained was taken to be crude enzyme.
Lipase assay
Substrate emulsion was prepared, with a composition of (g/100 mL) 70 mL of emulsification reagent (NaCl 1.79, KH2PO4 0.04, glycerol 54 mL and gum arabic 1.0) mixed with 30 mL olive oil. A 0.2 M potassium phosphate buffer (pH 6.0) was prepared by mixing 6.96 g of K2HPO4 in 200 mL of distilled water and 5.44 g of KH2PO4 in 200 mL of distilled water. Then, 1 mL of substrate emulsion, 0.8 mL of 0.2 M potassium phosphate buffer and 200 µL of sample were placed in a 250 mL conical flask. The flask was placed on a shaker at 120 rpm for 15 min. This reaction was terminated by adding 2 mL of acetone–ethanol mixture (1:1, v/v) and 4 drops of phenolphthalein blue indicator. A solution of 0.05 N NaOH was prepared by mixing 20 g of NaOH in 1000 mL of distilled water (Mustranta Citation1992). The amount of fatty acid liberated was determined by titration with 0.05 N NaOH and the lipase activity was calculated. One unit (U) of lipase activity is defined as the amount of enzyme required to liberate one micromole equivalent fatty acid min−1 mL−1 under the above assay conditions.
Total protein estimation
The total extracellular protein obtained from the culture filtrate was partially purified by ammonium sulfate precipitation and estimated by Lowry's method (Lowry et al. Citation1951).
Optimization of media parameters
The production of lipase depends on several process parameters. These parameters vary according to the microbial strain used for the production, and therefore conditions such as incubation time, pH, temperature, carbon, nitrogen and substrate concentration were optimized to ensure maximum enzyme production. The experiments were carried out systematically in such a way that the parameter optimized in one experiment was maintained at its optimum level in the subsequent experiments.
Incubation time
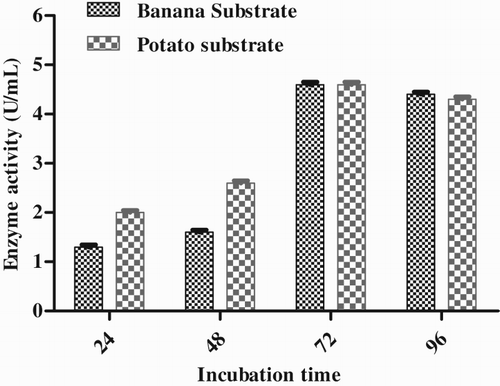
The Pseudomonas sp. was cultured in the fermentation growth medium with both banana and potato substrate. It was kept at 37°C for 3–5 days. Enzyme activity was determined every 24 h.
pH
The optimum pH for lipase enzyme production was selected by optimizing the growth media over a range of pH (4.0–8.0) while keeping other parameters unaltered. The buffer used in the media was different for different pH. Buffers used were sodium acetate buffer (pH 4.0 and 5.0), sodium phosphate buffer (pH 6.0 and 7.0) and Tris–HCl (pH 8.0). The medium was kept at 37°C for 72 h with constant agitation at 150 rpm.
Temperature
The optimum temperature for lipase enzyme production was selected by varying the temperature of the growth medium (4°C, 30°C, 40°C, 50°C, 60°C and 70°C) at pH 7.0 while other parameters were unaltered. The medium was kept at 37°C for 72 h with constant agitation at 150 rpm.
Carbon sources
Several carbon sources (coconut oil, sunflower oil, groundnut oil, ghee and tributyrin) were added as substitutes for olive oil in growth media at a final concentration of 1% (w/v), keeping all other parameters the same. Olive oil was used as a control with the same parameters. Enzyme activity was checked after 72 h.
Nitrogen sources
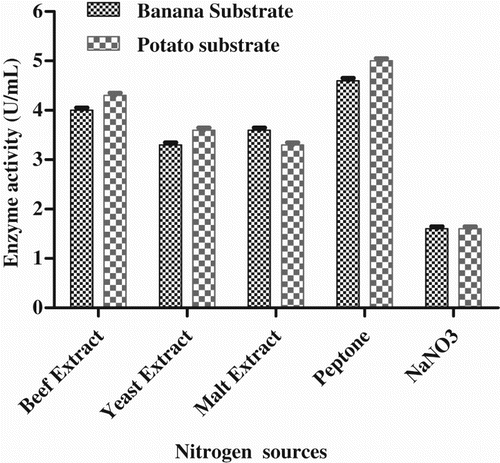
Different nitrogen sources (beef extract, yeast extract, malt extract, peptone and NaNO3) were added to the growth medium at a final concentration of 1% (w/v), keeping all other parameters the same. Enzyme activity was checked after 72 h.
Substrate concentration
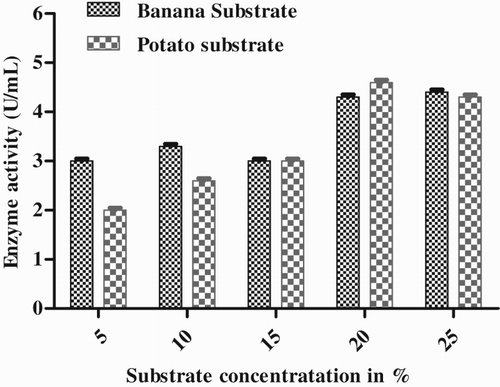
The growth medium was optimized by varying the concentrations of substrate (5–25%). Media with different concentrations of each substrate, such as potato and banana peels, were inoculated separately with Pseudomonas sp. and incubated for 72 h at 37°C and pH 7.0.
Purification of enzyme
Solid ammonium sulfate was added to the crude enzyme extract at 4°C and the mixture was subjected to centrifugation. The precipitate was dissolved in Tris–Cl. The solution was dialyzed against Tris–Cl buffer (pH 7.0) overnight using a dialysis bag. The dialyzed solution was concentrated in a vacuum and applied to a column of Sephadex-100, which was pre-equilibrated with citrate buffer and eluted with the same buffer. The flow rate was adjusted to 1 mL/3 min and 30 fractions were collected and analyzed.
High-performance liquid chromatographic analysis
The high-performance liquid chromatographic (HPLC) separation of the partially purified lipase enzyme was carried out on an LC-10 AT VP model HPLC using a 250×4.60 mm Rheodyne column (C18). The proteins were eluted with 70% (v/v) acetonitrile as the mobile phase at a flow rate of 1.0 mL/h at 280 nm with a C18 column (3.0×300 mm).
Results and discussion
Isolation and screening
Ten different cow's milk samples were screened in this study. Five of the 32 isolates showed a zone of hydrolysis. These five isolates were further screened for lipase production. Only one isolate (VITSDVM1) showed a clear zone of clearance with the efficient hydrolysis of tributyrin ().
Morphological and biochemical characterization
The isolate VITSDVM1 was found to be motile, Gram-negative rods. The cultural characteristics were observed as small, round, mucoid, creamy-white, fast growing colonies, and the biochemical features were catalase (+), oxidase (+), citrate (+), methyl red (–), Voges-Proskauer (–), nitrate (+) and indole (+), and the ability to assimilate glucose and lactose. Based on the morphological and biochemical characterization, strain VITSDVM1 was identified as Pseudomonas sp.
Molecular characterization
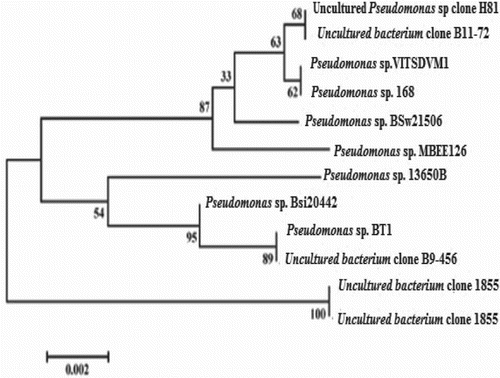
The 16S rRNA sequencing was performed by exporting to the database and checking for the homologous alignment. Based on the alignment results, strain VITSDVM1 showed 98% similarity with other Pseudomonas spp. The partial 16S rRNA sequences were deposited in GenBank under accession number KJ775757 and the phylogenetic tree was constructed ().
Effect of temperature
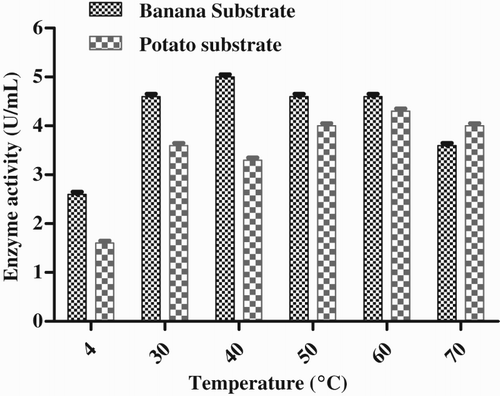
The optimum temperature for the lipase activity was determined using different temperatures for the fermentation process, of 4°C, 30°C, 40°C, 50°C, 60°C and 70°C. The maximum stability of the enzyme was observed in the temperature range 30–60°C. The enzyme activity was found to be good in the range 30–60°C and maximum at 40°C, i.e. 5.0 U mL−1 with banana substrate and 3.3 U mL−1 with potato substrate (). The enzyme was found to have a protein content of 10.6 mg mL−1. The enzyme activity decreased after an increase in temperature to above 40°C, 50°C and 60°C. Similar results were reported for Pseudomonas xinjiangensis, for which the maximum lipase production was at 37°C (Khemika et al. Citation2012). It was also reported that the growth and lipase enzyme production of Pseudomonas fluorescens were maximum at 36°C (Kulkarni and Gadre Citation2002). The minimum enzyme activity was found at 4°C, i.e. 2.6 U mL−1 with banana substrate and 1.6 U mL−1 with potato substrate. Lipase from Pseudomonas aeruginosa EF2 was found to work optimally at 50°C (Gilbert et al. Citation1991).
Effect of pH
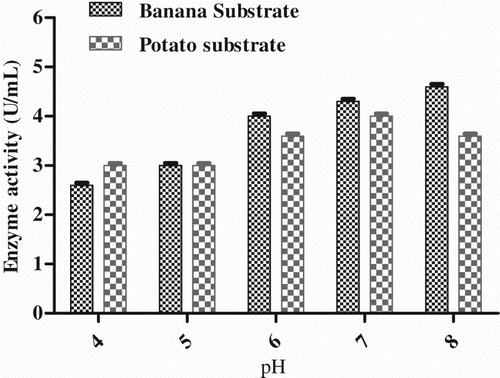
The effect of pH on the lipase enzyme activity was studied at various pH values ranging from pH 4 to 8. The maximum enzyme activity was found in slightly alkaline conditions, at pH 8 with banana substrate (4.6 U mL−1) and pH 7 with potato substrate (4.0 U mL−1) (). The lipase of P. aeruginosa EF2 worked optimally at pH 8.5 (Gilbert Citation1993). The protein content in the enzyme was found to be 11.7 mg mL−1.
Effect of carbon source
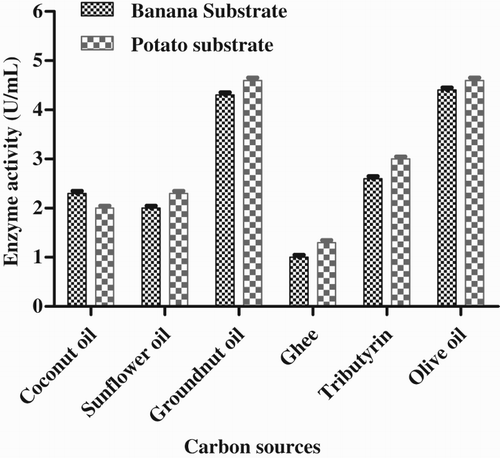
Groundnut oil was found to be the best of the six carbon sources used in this study, producing the maximum enzyme activity, i.e. 4.66 U mL−1 with potato substrate and 4.33 U mL−1 with banana substrate. The lipase enzyme activity was the same in olive oil as in groundnut oil. The protein content in the enzyme produced with groundnut oil was 11.3 mg mL−1. The minimum enzyme activity was found in ghee, i.e. 1.33 U mL−1 with potato substrate and 1.0 U mL−1 with banana substrate (). It was previously reported that lipase production was higher in vegetable oils, olive oil and sunflower oil (Gilbert et al. Citation1991).
Effect of nitrogen source
Among the different nitrogen sources, the maximum enzyme activity was found in peptone, i.e. 5.0 U mL−1 with potato substrate and 4.6 U mL−1 with banana substrate (). Similar results have been reported for organic nitrogen sources for P. fluorescens NS2 W (Khemika et al. Citation2012).
Effect of substrate concentration
The optimum substrate concentration for the media was selected by making different concentrations of both substrates (5%, 10%, 15%, 20% and 25%). The lipase activity of Pseudomonas sp. was found to be maximum at the 20% substrate concentration after 72 h, i.e. 4.6 U mL−1 with potato substrate and 4.33 U mL−1 with banana substrate ().
Effect of incubation time
Enzyme activity and incubation time have a significant correlation and enzyme synthesis is related to cell growth. The isolate was inoculated into the medium and checked every 24 h. The maximum lipase activity was observed after 72 h during the exponential growth phase, i.e. 4.6 U mL−1 in both substrates (). The enzyme was found to have a protein content of 12.2 mg mL−1. Further incubation led to a decrease in activity. The activity of the enzyme was slightly decreased after 96 h.
Purification and high-performance liquid chromatographic analysis
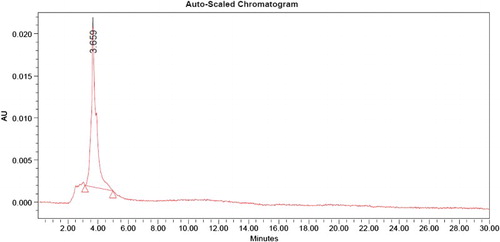
The maximum lipase activity was 4.6 U mL−1 after 72 h. Once the maximum lipase activity had been reached, the enzyme was salted out using ammonium sulfate at 60% saturation. Precipitates were found to have lipase activity of 7.66 U mL−1 and protein content of 6.02 mg mL−1. The precipitate was dissolved in Tris–HCl buffer, dialyzed against the same buffer and loaded on to the Sephadex-100 column, and the enzyme was further purified by gel filtration chromatography. Fraction numbers 13, 14, 15 and 16 were found to have maximum lipase activity. These fractions were pooled and the purified enzyme was found to have lipase activity of 9.33 U mL−1 and protein content of 1.07 mg mL−1. The HPLC chromatogram for purified enzyme produced from Pseudomonas sp. VITSDVM1 indicates the presence of lipase enzyme with a retention time of 3.659 min ().
Conclusion
In this study, lipase activity was found to be higher when using potato peel than when using banana peel as a substrate. Hence, potato peel waste could be used as a cheap substrate for the large-scale production of lipase from Pseudomonas sp. Furthermore, the strain could be exploited on an industrial scale as a potential enzyme producer, which could be successfully applied in the detergent, dairy, chemical and cosmetics industries.
Acknowledgement
We are greatly indebted to Vellore Institute of Technology for the constant encouragement, help and support in extending necessary facilities.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Altschul SF, Gish W, Miller M, Myers EW, Lipman DJ. 1990. Basic local alignment search tool. J Mol Biol. 215(3):403–410. doi: 10.1016/S0022-2836(05)80360-2
- Andreas S, Marleny DA, Saldana A. 2009. Potato peels: a source of nutritionally and pharmacologically interesting compounds – a review. Global Science Books.
- Carvalho PO, Calafatti SA, Marassi M, Silva DNJ, Contesini FJ, Bizaco R, Macedo GA. 2005. Potencial de biocatáliseenantiosseletiva de lipases microbianas. Química Nova. 28(4):614–621. doi: 10.1590/S0100-40422005000400012
- Castro HF, Mendes AA, dos Santos JC. 2004. Modificação de óleos e gordurasporbiotransformação. Química Nova. 27(1):146–156. doi: 10.1590/S0100-40422004000100025
- Chowdary GV, Ramesh MN, Prapulla JC. 2001. Enzymic synthesis of isoamylisovalerate using immobilized lipase from Rhizomucormiehei: a multivariate analysis. Process Biochem. 36:331–339. doi: 10.1016/S0032-9592(00)00218-1
- Dutra JCV, Terzi SC, Bevilaqua JV, Damaso MCT, Couri S, Langone MAP. 2008. Lipase production in solid state fermentation monitoring biomass growth of Aspergillus niger using digital image processing. Appl Biochem Biotechnol. 147:63–75. doi: 10.1007/s12010-007-8068-0
- Elibol M, Ozer D. 2000. Influence of oxygen transfer on lipase production by Rhizopusarrhizus. Process Biochem. 36:325–329. doi: 10.1016/S0032-9592(00)00226-0
- Fucinos P, Abadín CM, Sanromán A, Longo MA, Pastrana L, Rua ML. 2005. Identification of extracellular lipases/esterases produced by Thermusthermophilus HB27: partial purification and preliminary biochemical characterization. J Biotechnol. 117:233–241. doi: 10.1016/j.jbiotec.2005.01.019
- Ghosh SB, Chandra AK. 1984. Zbl. Mikrobiol. 139:293–304.
- Gilbert EJ. 1993. Pseudomonas lipase biochemical properties and molecular cloning. Enzyme Microb Technol. 15:634–645. doi: 10.1016/0141-0229(93)90062-7
- Gilbert EJ, Cornish A, Jones CW. 1991. Purification and properties of extracellular lipase from Pseudomonas aeruginosa EF2. J. Gen. Microbiol. 137:2223–2229. doi: 10.1099/00221287-137-9-2223
- Grbavcic SZ, Dimitrijevic-Brankovic SI, Bezbradica DI, Siler-Marinkovic SS, Knezevic ZD. 2007. Effect of fermentation conditions on lipase production by Candida utilis. J Serb Cheml Soc. 72(8–9):757–65. doi: 10.2298/JSC0709757G
- Griebeler N, Polloni AE, Remonatto D, Arbter F, Vardanega R, Cechet JL, Di Luccio M, de Oliveira D, Treichel H, Cansian RL, Rigo E, Ninow JL. 2011. Isolation and screening of lipase producing fungi with hydrolytic activity. Food and Bioprocess Technology. 4:578–586. doi: 10.1007/s11947-008-0176-5
- Gupta N, Shai V, Gupta R. 2007. Alkaline lipase from a novel strain Burkholderia multivorans: statistical medium optimization and production in a bioreactor. Process Biochem. 42(2):518–526. doi: 10.1016/j.procbio.2006.10.006
- Gutell RR, Schnare MN, Gray MW. 1990. A compilation of large (23S-like) ribosomal RNA sequences presented in a secondary structure format. Nucleic Acids Res. 18:2319–2330. doi: 10.1093/nar/18.suppl.2319
- Hamsaveni DR, Prapulla SG, Divakar S. 2001. Response surface methodological approach for the synthesis of isobutyl isobutyrate. Process Biochem. 36:1103–1109. doi: 10.1016/S0032-9592(01)00142-X
- Holt JG, Krieg NR, Sneath PHA, Staley JT, Williams ST. 1994. Group 17. Gram-positive coccij. Bergey's manual of determinative bacteriology. Baltimore, MD: Williams & Wilkins, 9th ed. p. 527–533.
- Kamini NR, Fuji T, Kurosu T, Iefuji H. 2000. Production, purification and characterization of an extracellular lipase from the yeast, Cryptococcus spS2. Process Biochem. 36:317–324. doi: 10.1016/S0032-9592(00)00228-4
- Khemika L, Angkhameen B, Hataichanoke N. 2012. Investigation of isolated lipase producing Bacteria from oil-contaminated soil with proteomic analysis of its proteins responsive to lipase inducer. J Biol Sci. 12:161–167. doi: 10.3923/jbs.2012.161.167
- Krishna SH, Sattur AP, Karanth NG. 2001. Lipase-catalyzed synthesis of isoamylisobutyrate – optimization using central composite rotatable design. Proc Biochem. 37:9–16. doi: 10.1016/S0032-9592(01)00161-3
- Kulkarni N, Gadre RV. 2002. Production and properties of an alkaline, thermophilic lipase from Pseudomonas fluorescens NS2 W. J Ind Microbiol Biotechnol. 28:344–348. doi: 10.1038/sj.jim.7000254
- Lehmann S, Maraite A, Steinhagen M, Ansorge-Schumacher M. 2014. Characterization of a novel Pseudomonas stutzeri lipase/esterase with potential application in the production of chiral secondary alcohols. Adv Biosci Biotechnol. 5:1009–1017. doi: 10.4236/abb.2014.513115
- Lowry OH, Rosebrough NJ, Farr NJ, Randall RJ. 1951. Protein measurement with folin phenol reagent. J. Biol Chem. 193:265–275.
- Mustranta A. 1992. Use of lipase in the resolution of raceme ibuprofen. Appl Microbiol Biotechnol. 38:61–66. doi: 10.1007/BF00169420
- Park H, Lee K, Chi Y, Jeong S. 2005. Effects of methanol on the catalytic properties of porcine pancreatic lipase. J Microbiol Biotechnol. 15(2):296–301.
- Rao P, Divakar S. 2001. Lipase catalyzed esterification of a-terpineol with various organic acids: application of the Plackett–Burman design. Process Biochem. 36:1125–1128. doi: 10.1016/S0032-9592(01)00154-6
- Saitou N, Nei M. 1987. The neighbor-joining method: a new method for reconstructing phylogenetic trees. Mol Biol Evol. 4:406–425.
- Singh RK, Kumar S. 2009. Production of α amylase from agricultural byproducts by Humicolalangginosa in solid state fermentation. Curr Trends Biotechnol Pharm. 3(2):172–180. 20.
- Thabit K, Al-Ghuribi SM, Al-Aswadi FN. 2013. DNA sequence comparisons using codons. Arab J Sci Eng. doi:10.1007/s13369-013-0760-5
- Zhang LQ, Zhang YL, Xu L, Li XL, Yang XC, Xu GL, Wu XX, Gao HY, Du WB, Zhang XT, Zhang XZ. 2001. Lipase catalyzed synthesis of RGD diamide in aqueous water-miscible organic solvents. Enzyme Microb Technol. 29:129–135. doi: 10.1016/S0141-0229(01)00345-3