Abstract
E-selectin-mediated leukocyte adhesion on endothelial cells is a critical event in the inflammatory response. Site density determination of E-selectin on the substrate surface is the primary step in understanding the mechanism of E-selectin-induced cell adhesion in a parallel plate flow chamber. So far, the fluorescent method cannot measure low molecular density surfaces because of its weak sensitivity, and the traditional 125I radioiodination method can only detect the radioactivity of a solution in a tube. In this study, to measure low site density surfaces, a new method was constructed by combining 125I radioiodination with a GE Infinia Hawkeye 4 ECT. A saturation curve was obtained for the relationship between site densities and incubated concentrations of E-selectin, and a linear correlation was found within the range of low site density. Site densities were 9 and 140 sites/µm2 when polystyrene surfaces were incubated with E-selectin concentrations of 10 and 40 ng/ml, respectively. Under these densities, HL-60 cells could tether and roll on the substrates of the flow chamber. The radioiodination method developed in this study is an ideal detection method for even low-density surfaces because of its high sensitivity, which will provide better understanding of cell adhesion using a flow chamber.
Introduction
In the process of inflammation and blood coagulation, circulating leukocytes will first tether to and roll on vascular surfaces, then migrate through the damaged tissue, and finally kill pathogens and repair the damage (Davis et al. Citation2003). Adhesion is a complex, multistep cascade that requires interactions with the respective ligands of selectins, which mediate tethering and rolling adhesion, and integrins, which mediate firm adhesion and migration (Chen et al. Citation1997). As one important member of the selectin family, E-selectin is highly expressed on endothelial cells when it is activated by cytokines or chemokines. The interaction of E-selectin with its ligand P-selectin glycoprotein ligand-1 (PSGL-1) is involved in the tethering and rolling of leukocytes (Finger et al. Citation1996; Hidalgo et al. Citation2007). The speed and strength of this interaction can usually be quantified by two-dimensional reaction kinetics parameters.
Of the numerous methods for measuring two-dimensional reaction kinetics parameters, the parallel plate flow chamber (PPFC) is an ideal technique owing to its advantage of simulating the real physiological environment. During a PPFC experiment, the cells are driven by fluid flow through a matrix covered by E-selectins. The adhesive behavior of a flowing cell, which has the opportunity to interact, will be determined by the E-selectin site density on the substrate surface. In a low site density situation, cells tether on the substrate for the rapid formation and breakage of single E-selectin–ligand bonds. In contrast to tethering, cells continuously roll in high site density conditions, since the formation of new bonds at the leading edge can balance the dissociation of existing bonds at the rear edge of the contact area (Li et al. Citation2012). As the site density plays a decisive role in E-selectin-mediated cell adhesion processes, site density determination of E-selectin on the substrate is the primary event in understanding the mechanism of selectin-induced cell adhesion in flow chamber experiments (Kayani et al. Citation2014).
The molecular site density of the suspension cell surface can be measured using fluorescence or radioiodination methods. For example, the site densities of PSGL-1 on the surface of neutrophils and HL-60 cells are 36 and 49 sites/µm2, respectively. Similarly, these two methods can be used to measure the molecular site density of the substrate surface. In association with a laser confocal microscope or fluorescence microplate reader (Tecan Sunrise), the fluorescence method can be used to quickly and safely measure high-density protein on the surface. However, the fluorescent method cannot handle low-density situations, such as 10 sites/µm2, for a tethering event (Li et al. Citation2012). The high sensitivity of the 125I radioiodination method, combined with a γ-counter, may provide a solution. A traditional γ-counter can be used to detect the amount of solution radioactivity in the tube, but not the radioactivity on the surface. To solve the problem of surface density measurement, this research uses multifunctional nuclide imaging equipment (Infinia Hawkeye 4 ECT) to carry out single-photon emission computed tomography (SPECT), which can test surface radioactivity. In addition, this technique can analyze multiple samples simultaneously, which will greatly improve the detection efficiency (Surhio et al. Citation2014).
In this study, the E-selectin site density on the substrate surface was measured by monoclonal antibody (mAb) labeling with 125I radioiodination, detection with SPECT equipment and verification with PPFC experiment. The results showed that the relationship between the site density and the incubating concentration is a saturation curve, and a linear correlation was observed under the condition of low site density. The site densities of polystyrene surfaces were 9 and 140 sites/µm2, incubated with 10 and 40 ng/ml E-selectin suspensions, respectively. Accordingly, HL-60 cells could tether and roll on the substrate of the PPFC (Khaskheli et al. Citation2015).
Materials and methods
125I Radioiodination and purification of monoclonal antibody
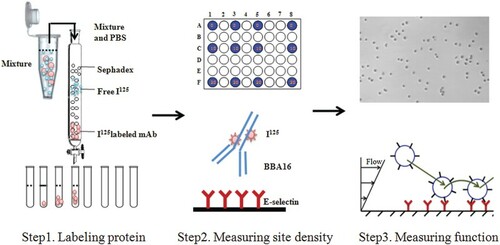
The extracellular domain of human E-selectin was fused with the 6-histidine-tagged Fc region of human immunoglobulin G1 via a polypeptide linker. E-selectin and its mAb BBA16 were purchased from R&D Systems (Minneapolis, MN, USA). For radioiodination labeling, first, 100 µl of 0.1 M phosphate buffer (pH 7) was placed in a clear plastic tube, and 10 µl (1.0 µCi) of Na125I was added using a microsyringe, followed by incubation for 5 min at room temperature. Then, 50 µg of mouse anti-human E-selectin mAb BBA16 (dissolved in 100 µl phosphate buffer) was added, followed by incubation for 6–9 min at room temperature, and 50 µl of scavenging buffer (10 mg tyrosine/ml in Tris iodination buffer) was added and mixed, then incubated for 5 min to terminate the reaction. Then, the reaction mixture was applied on a chromatographic column filled with 10 ml Sephadex G-25 (, step 1), Tris buffer solution was dropped into the chromatography column, and a 0.5–0.8 ml fraction was collected in each tube. Finally, the amount of radioactivity was measured using an Infinia Hawkeye 4 ECT (Davis et al. Citation2003).
Density determination of E-selectin using SPECT immunoradiometric assay
Polystyrene 48-well plates (Corning, NY, USA) were coated with different concentrations of E-selectin solution (0, 0.04, 0.08, 0.16, 0.32, 0.64 0.75, 1, 2 and 4 µg/ml) to form different density surfaces of E-selectin (, step 2). Each concentration of E-selectin was diluted in 4 × 130 µl solution, directly adsorbed to four interval wells (0.8 cm2) and incubated at 4°C in a refrigerator overnight, for 16 h. Then, each well was incubated with Hank's balanced salt solution (HBSS) containing 1% bovine serum albumin (BSA) at 4°C for 1 h to prevent non-specific adhesion. The adsorbent solution was sucked out and labeling 125I E-selectin mAb BBA16 was added and incubated for 30 min at 4°C. The mAb solution was extracted and washed three times with HBSS containing 1% BSA. Next, samples were placed in a GE Infinia Hawkeye 4 (GE Healthcare [Guangzhou, China]) and the 125I radiation intensity was measured by SPECT. After detection, the radiation quantity was calculated using the CardiIQFusion software (Tabassum et al. Citation2014).
E-selectin-mediated HL-60 cell adhesion by the parallel plate flow chamber assay
A coating region (5 mm × 5 mm) was outlined in the center of a 35 mm polystyrene dish (Corning, NY, USA), and E-selectin was diluted in 10 and 40 ng/ml solution and directly adsorbed to the dish, and incubated at 4°C in a refrigerator for 12 h. After washing three times with HBSS and blocking with HBSS containing 2% BSA at 4°C for 2 h, the dishes were ready for the flow chamber assay. HL-60 cells were cultured in RPMI 1640 medium supplemented with 100 units/ml penicillin, 10 mg/ml streptomycin and 10% fetal bovine serum, and the cell suspension solution (0.5 or 1 × 106 cells/ml in HBSS containing 1% BSA) was perfused over substrates coated with 10 and 40 ng/ml E-selectin in the flow chamber at a flow shear stress of 0.4 dyn/cm2 (, step 3). The flow shear stress was controlled with a syringe pump. Cellular behavior was magnified with an inverted microscope and captured with a high-speed CMOS acquisition system at a specific speed of 1280 pixels × 1024 pixels × 100 frames/s. At low densities of selectins incubated at 10 ng/ml, transient HL-60 cells tether but do not roll or skip at this wall shear stress (Li et al. Citation2012). Under the appropriate densities of selectins, such as 40 ng/ml, continuous rolling of HL-60 cells could be observed. The images of tethering and rolling HL-60 cells were captured and individual cells were tracked frame by frame with Image-Pro Plus software (version 6.0). The instantaneous velocity was estimated by the distance of the HL-60 cell center in two continuous frames in the flow direction. The accumulative distance can be calculated on the basis of the appointed start and stop times (Qureshi et al. Citation2015).
Results and discussion
125I Radioiodination and purification of E-selectin monoclonal antibody
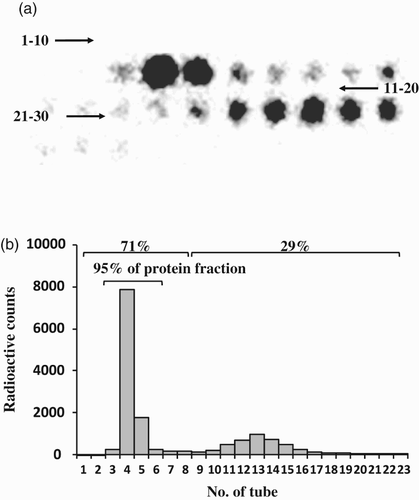
Mouse anti-human E-selectin mAb BBA16 was labeled with 125I and purified using a chromatographic column filled with Sephadex G-25. The radiation intensities of the collected fractions are shown in . The 23 eluted fractions from the liquid chromatography were placed in order on the detecting platform. A radioactive intensity image is shown in (A). The strength of the radioactivity is indicated by the gray level, with deeper gray representing stronger radioactivity. To quantify the strength of radioactivity, radioactive counts of each fraction in the image were calculated and made into a histogram (B). The tubes are numbered in order of the sequence of elution liquid running out from the chromatography. Two remarkable peaks were found. The first one was the 125I-labeled mAb protein peak. The radiation amount accounted for 71%, and it was mainly included in tubes no. 4 and 5. The second one was the free 125I peak, which was wide and flat. The radiation amount of this peak accounted for 29%, and it was mostly included in tubes 10–16. The obvious boundary between the two peaks indicated the successful separation of 125I-labeled mAb and free 125I. The labeling efficiency of the mAb protein was high enough (incorporation was 95%) to collect appropriate fractions (in this case, tubes 4 and 5), which would be used to bind E-selectin and detect its density.
Site density determination of E-selectin on a polystyrene surface
In this study, E-selectins were coupled on the polystyrene surface directly with random molecular orientation. In fact, interaction of E-selectin with its ligand PSGL-1 only occurred on the lectin domain of the N-terminal, and only the exposed active head could interact with the ligand to mediate cell adhesion. To obtain the effective site density of E-selectin in the right orientation, blocking mAb BBA16 was labeled with 125I and then the 125I-labeled BBA16 was used to bind E-selectin. Here, 5 ml 125I-labeled E-selectin was collected, and the total radioactive count was 31.79 ps. According to the total protein, 50 µg, and the molecular weight of 106 kDa for the E-selectin/Fc chimera, the radioactive count for one 125I-labeled E-selectin mAb is estimated to be 1.58 × 10−13 per second.
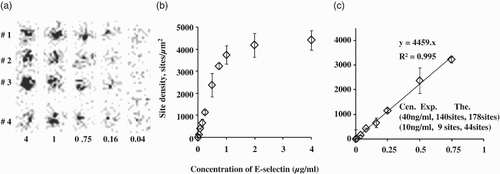
Polystyrene 48-well plates were coated with different concentrations of E-selectin solution overnight, for at least 16 h. After washing three times with 1% BSA in HBSS, the 48-well plates were placed on a GE Infinia Hawkeye 4 to detect radioactivity by SPECT. The radiation images indicated that the adsorption quantity of E-selectin was increased with increasing concentration (0.08, 0.16, 0.75, 1, 4 µg/ml) (A). Site densities were calculated from radioactive counts per well divided by single 125I-labeled E-selectin mAb, supposing that the ratio of E-selectin mAb to E-selectin was 1:1. The relationship between site density and concentration of E-selectins was a saturation curve (B). A linear correlation was found in the range of low site densities (C). The amount of absorbed E-selectins on the surface reached a maximum when gradient diluted E-selectin concentrations exceeded 1 µg/ml. In the flow chamber experiment, HL-60 cells could adhere to E-selectin substrates incubated with 10 ng/ml and 40 ng/ml solutions. Accordingly, site densities were 9 sites/µm2 and 140 sites/µm2 by real experimental measurement. However, the theoretical values were higher, at 44 sites/µm2 and 178 sites/µm2 by linear fitting in (C). Therefore, the observed data were smaller than the theoretical calculation. The reason for this may be the low detection sensitivity for E-selectin surface.
Figure 3. Site density measurement of E-selectin coated on a polystyrene surface. (a) Single-photon emission computed tomography (SPECT) radiation images of 125I-labeled monoclonal antibody (mAb) binding with adsorbed E-selectin at various concentrations (0.08, 0.16, 0.75, 1, 4 µg/ml), with four repeats for each concentration; (b) saturation curve of E-selectin site densities with different incubated concentrations; (c) linear fitting curve of E-selectin site densities under the low range of incubated concentrations; mean and standard deviation calculated from four repeats.
Site density determination verified by the parallel plate flow chamber assay
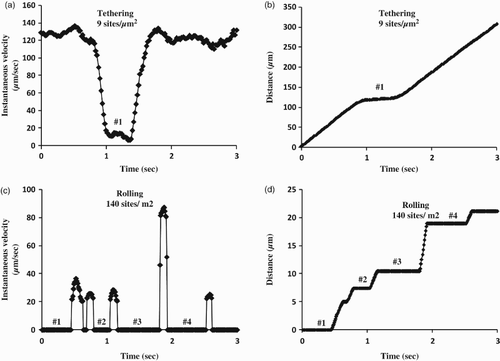
To investigate E-selectin-mediated tethering and rolling adhesion, this study examined the continuous tethering and rolling of HL-60 cells, recorded unceasingly at a high temporal resolution of 100 fps. The representative instantaneous velocity profiles (A, C) and accumulative distances (B, D) illustrated HL-60 cells tethering and rolling on E-selectin-coated substrates for 3 s at wall shear stresses of 0.4 dyn/cm2. At 9 sites/µm2, flowing HL-60 cells transiently adhered on the E-selectin substrate and then quickly detached. The instantaneous velocity remained high, apart from one brief low speed close to zero (Sarangapani et al. Citation2004). Correspondingly, there was a continuous increase in accumulative distances, interrupted by a small plateau in (B). At 140 sites/µm2, the instantaneous velocity profile exhibited a go–stop step cycle of cell rolling, from which a minimal model was derived under the assumption that rolling occurs by alternating two tether bonds, one at the front and the other at the rear of the cell. As soon as the rear bond dissociates, the cell enters a ‘go' phase by pivoting about the front bond until that becomes the rear bond. The cell then enters a ‘stop' phase during which a new front bond forms, until the rear bond breaks to start a new cycle (Li et al. Citation2012). The circulation of acceleration and deceleration in the instantaneous velocity profile (C) was consistently matched by a circulation pattern of increasing plateaux in the accumulative distances profile (D). To sum up, tethering and rolling behaviors of HL-60 cells on 9 and 140 sites/µm2 E-selectin prove the accuracy and reliability of the radioiodination method to measure surface site densities.
Figure 4. Instantaneous velocity and accumulative distance of HL-60 cell tether and roll on 9 or 140 sites/µm2 E-selectin on a polystyrene surface. (a, b) Instantaneous velocity and accumulative distance of one representative HL-60 cell tethered on 9 sites/µm2 E-selectin coated with 10 ng/ml solution at 0.3 dyn/cm2; (c, d) instantaneous velocity and accumulative distance of one representative HL-60 cell rolled on 140 sites/µm2 E-selectin coated with 40 ng/ml solution at 0.3 dyn/cm2. Data were recorded at 100 fps. The instantaneous velocities were set as zero when their values were below 20 µm/s. The numbers show the corresponding stops and plateaux in these two kinds of figure.
Conclusions
The above results illustrate that SPECT can accurately and efficiently detect radioactivity on the surface. The site density of E-selectin showed a linear correlation with the adsorbing concentration under the low-density condition. The 125I radioiodination method, combined with a γ-counter or SPECT equipment, is the ideal solution for low molecular density measurement because of its high sensitivity.
Fluorescent methods, combined with a laser confocal microscope or fluorescence microplate reader, can quickly and safely measure high site density protein on the surface, but cannot handle low-density conditions.
The application of the PPFC experiment proved the accuracy and reliability of radioiodination methods because HL-60 cells were able to roll on the substrate (40 ng/ml) at this density (100–250 sites/µm2).
In this study, a new method was designed by linking 125I radioiodination with SPECT equipment, which could quickly and easily observe and confirm the surface density. The application of this method to flow chamber essays could improve the understanding of cell adhesion mechanisms in inflammatory responses, thrombus diseases and tumor metastasis.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Chen S, Alon R, Fuhlbrigge RC, Springer TA. 1997. Rolling and transient tethering of leukocytes on antibodies reveal specializations of selectins. Proc Natl Acad Sci U S A. 94(7):3172–3177. doi:https://doi.org/10.1073/pnas.94.7.3172
- Davis C, Fischer J, Ley K, Sarembock IJ. 2003. The role of inflammation in vascular injury and repair. J Thromb Haemost. 1(8):1699–1709. doi: https://doi.org/10.1046/j.1538-7836.2003.00292.x
- Finger EB, Puri KD, Alon R, Lawrence MB, von Andrian UH, Springer TA. 1996. Adhesion through L-selectin requires a threshold hydrodynamic shear. Nature. 379(6562):266–269. doi:https://doi.org/10.1038/379266a0
- Hidalgo A, Peired AJ, Wild MK, Vestweber D, Frenette PS. 2007. Complete identification of E-selectin ligands on neutrophils reveals distinct functions of PSGL-1, ESL-1, and CD44. Immunity. 26(4):477–489. doi: https://doi.org/10.1016/j.immuni.2007.03.011
- Kayani S, Ahmad M, Zafar M, Sultana S, Khan MPZ, Ashraf MA, Hussain J, Yaseen G. 2014. Ethnobotanical uses of medicinal plants for respiratory disorders among the inhabitants of Gallies-Abbottabad, Northern Pakistan. J Ethnopharmacol. doi:https://doi.org/10.1016/j.jep.2014.08.005
- Khaskheli AA, Talpur FN, Ashraf MA, Cebeci A, Jawaid S, Afridi HI. 2015. Monitoring the Rhizopus oryzae lipase catalyzed hydrolysis of castor oil by ATR-FTIR spectroscopy. J Mol Catal B Enzym. 113(1):56–61. doi:https://doi.org/10.1016/j.molcatb.2015.01.002
- Li Q, Fang Y, Ding X, Wu J. 2012. Force-dependent bond dissociation govern rolling of HL-60 cells through E-selectin. Exp Cell Res. 318(14):1649–1658. doi: https://doi.org/10.1016/j.yexcr.2012.05.018
- Qureshi T, Memon N, Memon SQ, Ashraf MA. 2015. Decontamination of ofloxacin: optimization of removal process onto sawdust using response surface methodology. Desalin Water Treat, Online First. https://doi.org/10.1080/19443994.2015.1006825
- Sarangapani KK, Yago T, Klopocki AG, Lawrence MB, Fieger CB, Rosen SD, McEver RP, Zhu C. 2004. Low force decelerates L-selectin dissociation from P-selectin glycoprotein ligand-1 and endoglycan. J Biol Chem. 279(3):2291–2298. doi:https://doi.org/10.1074/jbc.M310396200
- Surhio MA, Talpur FN, Nizamani SM, Amin FA, Bong CW, Lee CW, Ashraf MA, Shahd MR. 2014. Complete degradation of dimethyl phthalate by biochemical cooperation of the Bacillus thuringiensis strain isolated from cotton field soil. RSC Adv. (4):55960. doi: https://doi.org/10.1039/C4RA09465D
- Tabassum N, Rafique U, Balkhair KS, Ashraf MA. 2014. Chemodynamics of methyl parathion and ethyl parathion: adsorption models for sustainable agriculture. Biomed Res Int. Article ID 831989. doi:https://doi.org/http://dx.doi.org/10.1155/2014/831989