Abstract
The number of chromosomes and karyotype of Hibiscus mutabilis f. mutabilis were analyzed with the root tip squash method. The results showed that H. mutabilis f. mutabilis is diploid, the chromosome number of the somatic cell is2n = 92 and the index of relative length is 16L + 12M2 + 56M1 + 8S. The karyotype formula is K(2n) = 2x = 92 =86m + 6sm and the asymmetrical index of the karyotype is 54.54%. The karyotype is 2C.
Introduction
Hibiscus mutabilis f. mutabilis is also called the Fu rong flower, Ju shuang flower, Zui jiu ru rong and Hua mu. It belongs to the Malvaceae Hibiscus genus of deciduous shrubs or small trees, native to China. Hibiscus mutabilis f. mutabilis is a well-known traditional Chinese flower and an important late autumn flowering plant because of its large, bright flowers (Kayani et al. Citation2014). It has the following characteristics: flowers solitary in the axillae, eight small bracts, linear, base connate; calyx bell shaped, five lobes, ovate, pointed; white or reddish flowers becoming dark red, petals suborbicular, bearded base, flowering in August to October; capsule flat globose; seed kidney shaped, pilose on the back (Editorial Board of Chinese Academy of Sciences China Flora Citation1984). Hibiscus mutabilis f. mutabilis has a useful ecological function, playing a very significant role in slope protection. This tree provides very good garden greenery, and is often grown in parks and gardens and on roadsides. In addition, the flowers and leaves have medicinal properties, and have been used in clearing the lungs, cooling the blood and detoxification (Fu et al. Citation2004; Li et al. Citation2009).
The Hibiscus genus includes a great variety of plants with complex interspecies relationships. Besides, the chromosome number varies greatly and the polyploidy is complex. Song (Citation2001) reported the following: Hibiscus schizopetalus 2n = 42, H. mutabilis 2n = 92, H. rosa-sinensis 2n = 84, H. rosa-sinensis ‘Double Rainbow’ 2n = 105, H. rosa-sinensis ‘Flavo-plenus’ 2n = 138 and H. rosa-sinensis ‘Carminatus’ 2n = 147; H. schizopetalus is diploid, H. rosa-sinensis is tetraploid, H. rosa-sinensis ‘Double Rainbow’ is pentaploid and H. rosa-sinensis ‘Carminatus’ is heptaploid. Longley (Citation1933) reported that H. moscheutos n = 19–20. Liu et al. (Citation2009) reported that H. moscheutos 2n = 2x = 38 = 32m + 6sm and the karyotype is 2A. However, research on H. mutabilis f. mutabilis has focused mainly on aspects of pharmacological chemistry (Fu et al. Citation2004; Li et al. Citation2009; Li et al. Citation2013; Wan et al. Citation2013), tissue culture and breeding, etc. (Zheng Citation2009; Nie & Xiang Citation2012). Less research has been reported on chromosome number and karyotype analysis.
In this article, the seed of H. mutabilis f. mutabilis was taken as the study material. The chromosome number of the somatic cell was confirmed using the root tip squash technique. The karyotype was analyzed to provide a cytogenetic basis for research on aspects such as the origin, phylogeny, genetic breeding and breed improvement of H. mutabilis f. mutabilis.
Materials and methods
Experimental material
The experimental material comprised seeds collected in December 2012 from single-petal pink H. mutabilis f. mutabilis, grown at experimental stations of the College of Horticulture, Jinling Institute of Technology.
Experimental methods
Accelerating the growth of the root system
The seeds were washed, then soaked in 100 mg/l of gibberellic acid (GA3) for 24 h. They were then placed in a culture dish with moist filter paper in an incubator at 25°C to accelerate germination, and washed once a day until the root grew to about 0.5–1 cm.
Root tip processing
About 0.5 cm of the root tip was cut off between 07:30 and 08:30 h. Fifty pieces of root tip of them were placed in saturated Santochlor® insecticide at room temperature (15 ± 3°C), and 50 pieces in an ice/water mixture at 0–4°C for preprocessing. The preprocessing times are shown in .
Table 1. Root tip preprocessing schedule of Hibiscus mutabilis f. mutabilis seeds.
The preprocessed root tip was washed in distilled water. Then, 0.075 M potassium chloride aqueous solution was used to soak the root tip for 30 min at room temperature for pre-hypotension, before dissociation, followed by washing once or twice with distilled water. It was transferred to 95% alcohol and glacial acetic acid (3:1) stationary liquid for fixation for 2 h at room temperature. After washing the root tip once or twice in 70% alcohol, it was kept in 70% alcohol and put into a deep freeze at 4°C for standby application. The root tip was placed in 1 mol/l hydrochloric acid, and treated in a water bath at 60°C for about 10 min. After dissociation, it was put in redistilled water for 15 min for post-hypotension (Surhio et al. Citation2014). Then, the ordinary tableting method was used and the root tip was dyed with carbol fuchsin. It was viewed with a Nikon Eclipse 80I microscope and photographs were taken.
Karyotype analysis
Calculation of the chromosome number and karyotype analysis were carried out according to the standards of Li and Chen (Citation1985). Over 50 cells were counted, and over 85% of cells with a constant and consistent chromosome number were taken as the number of chromosomes of this variety. Five pieces of the metakinesis phase with good dispersion were selected to carry out analysis and measurement, and to gain data on chromosome parameters. Analysis of the chromosome type was conducted according to the classification system of Levan et al. (Citation1964), and that of the karyotype in accordance with the classification standard of Stebbins (Citation1971). The dissymmetrical coefficient of the karyotype was calculated according to the method of Arano. The greater the specific value, the more obvious the dissymmetry will be.
Results and discussion
Influence of material processing method on the chromosome
Different processing methods obtain different degrees of clarity of the chromosome. The chromosome obtained from the root tip after 12 h low-temperature processing is the clearest. This is because the low-temperature environment cuts off the extension of the microtubule and restrains the formation of the spindle body, making mitosis stop in the mid-phase and gain a greater number of metacinesis phases. During prophase in the experiment, concentrated hydrochloric acid and anhydrous alcohol (1:1) are used to bring about dissociation. Excessive dissociation will occur. An environment with excessive acidity gives rise to dissociation of the chromosome, so that it cannot maintain its integrity.
Chromosome number
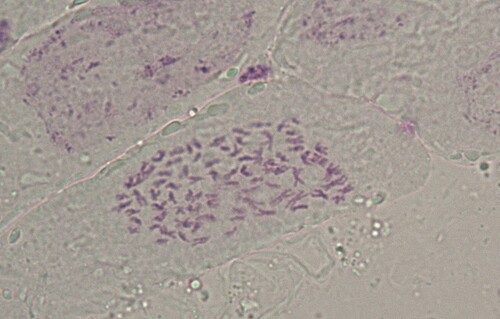
Although the chromosome number on the picture is not fixed, it lies within a certain range. By counting 50 cells, it was found that over 90% of the cells had a chromosome number of 2n = 92 (Khaskheli et al. Citation2015). Therefore, the chromosome number of the somatic cells of H. mutabilis f. mutabilis is 92 ().
Chromosome length composition and karyotype analysis
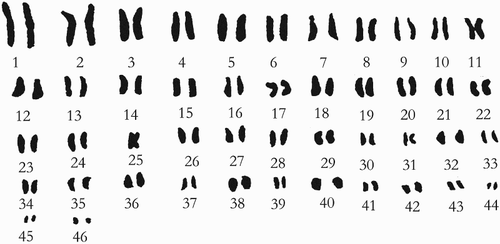
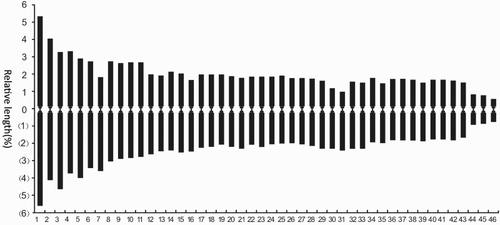
According to the form of chromosome, the relative length and arm ratio, measured by amplifying the micrograph during the microscopic examination, and the pair analysis, accurate compasses can be used to measure the length of the long and short arms of the chromosome of five cells, and obtain the average value. The arm ratio (long arm/short arm) of each chromosome can be calculated, and the position type of the centromere confirmed. In many cases, the absolute length is not a reliable value for comparison, as the preprocessing conditions and the shortening degree of the chromosome are different. Therefore, even for the same plant the absolute length measured by different authors has obvious differences. However, the relative length is a relatively stable value for comparison, and is calculated as: Relative length = (Length of each chromosome/Total length of chromosome set) × 100%. Specific parameters are shown in and Figures and . As shown in , it turns out that H. mutabilis f. mutabilis is diploid, with a chromosome length of 199.98 µm and average length of 4.35 µm, and the change range of the relative length of the chromosome is 1.24–10.89. The index of relative length is 2n = 16L + 12M2 + 56M1 + 8S. The formula of the karyotype is K(2n) = 2x = 92 = 86m + 6sm. The seventh, 30th and 31st pairs of chromosomes are mesiocentromere chromosomes. The rest are mid-centromere chromosomes. The change range of the arm ratio is 1.01–2.46. The length ratio between the longest chromosome and the shortest chromosome is 8.78. Chromosomes with an arm ratio greater than 2 account for 2.17% of the total chromosome number. The dissymmetrical coefficient of the karyotype is 54.54%. From this analysis, it can be judged that the karyotype classification of H. mutabilis f. mutabilis is 2C.
Table 2. Chromosome relative length, arm ratio and type of Hibiscus mutabilis f. mutabilis.
Conclusion
When calculating a plant's chromosome number, morphology is one of the most stable cytological features. The size, form and structure of the chromosome are diverse. Different classification groups have different karyotypes, which is reflected not only between plant families, subfamilies, genera and species, but also in different populations of the same type (Shi et al. Citation2009). Karyotype analysis can be used to study and provide a basis for biological systems development and relative relations (Tabassum et al. Citation2014). Compared with simple shape classification, chromosome data can address the problem of conventional shape classification being difficult to solve (Hu et al. Citation2002; Yan et al. Citation2008; Yang et al. Citation2008; Zhu & Wang Citation2008). The results of the present research indicate that the chromosome number of H. mutabilis f. mutabilis is 2n = 92, consistent with Song's (Citation2001) results. However, a few somatic chromosome numbers do not conform with 92, maybe because natural variation in H. mutabilis f. mutabilis has occurred during the process of biological evolution. The index of relative length is 16L + 12M2 + 56M1 + 8S and the karyotype formula is K(2n) = 2x = 92 = 86m + 6sm. Chromosomes with an arm ratio greater than 2 account for 2.17% of the total chromosome number. The dissymmetrical coefficient of the karyotype is 54.54% and the karyotype is classified as type 2C. It is generally acknowledged that plants with symmetrical chromosomes belong to the primitive type, and those with dissymmetrical chromosomes to a more evolved type. Therefore, H. mutabilis f. mutabilis belongs to a relatively advanced type with respect to evolution (Qureshi et al. Citation2015).
Funding
This work is supported by Qing Lan Project of Jiangsu Province (2014), the National Science Foundation of China [no. 51208467] and the Welfare Technological Application Research project of the Science and Technology Agency of Zhejiang Province [no. 2012C33004].
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Arano, H. 1963. Cytological studies in subfamily Carduoideae (Compositae) of Japan. IX. The karyotype analysis and phylogenic considerations on Pertya and Ainsliaea. Botanical Magazine (Tokyo). 76:32–39. doi: https://doi.org/10.15281/jplantres1887.76.32
- Editorial Board of Chinese Academy of Sciences China Flora. 1984. Flora of China. 49(2):61–83. Science and Technology Press, Beijing.
- Fu S, Luo S, Zhou L, Zhang F. 2004. Effect of leaves of Hibiscus mutabilis L. on renal ischemia reperfusion injury of rat model. Guangxi Sci. 11(2):131–133.
- Hu J, Su Z, Yue B, et al. 2002. Progress of karyotype analysis method in plants research. Journal of Sichuan Teachers College (Natural Science). 23(3):239–244.
- Kayani S, Ahmad M, Zafar M, Sultana S, Khan MPZ, Ashraf MA, Hussain J, Yaseen G. 2014. Ethnobotanical uses of medicinal plants for respiratory disorders among the inhabitants of Gallies-Abbottabad, Northern Pakistan. J Ethnopharmacol. doi:https://doi.org/10.1016/j.jep.2014.08.005
- Khaskheli AA, Talpur FN, Ashraf MA, Cebeci A, Jawaid S, Afridi HI. 2015. Monitoring the Rhizopus oryzae lipase catalyzed hydrolysis of castor oil by ATR-FTIR spectroscopy. J Mol Catal B: Enzym. 113(1):56–61. doi:https://doi.org/10.1016/j.molcatb.2015.01.002
- Levan A, Fredga K, Sandberg A. 1964. Nomenclacture for centromeric position on chromosomes. Hereditas. 52:201–220. doi: https://doi.org/10.1111/j.1601-5223.1964.tb01953.x
- Li M, Chen R. 1985. A suggestion on the standardization of karyotype analysis in plants. J Wuhan Botanical Res. 3(4):297–302.
- Li C, Liu S, Wu D, et al. 2009. Study on bacteriostasis of extracts of Hibiscus mutabili leaf. Sci Technol Food Ind. 3(11):97–99.
- Li C, Zgeng F, Tian B, et al. 2013. Antibacterial effect of extraction from Hibiscus mutabilis leaf on E. coli O1 and Staphylococcus aureus 91053. Sci Technol Food Ind. 34(1):57–59.
- Liu K, Wang X, Chi J, et al. 2009. Mitotic karyotyping and meiotic observation in Hibiscus moscheutos L. J Plant Genetic Resour. 10(2):286–289.
- Longley AE. 1933. Chromosome in Gossypium and related genera. J Agric Res. 46:217–227.
- Nie G, Xiang Q. 2012. Study on characteristics and landscape applications of Hibiscus mutabilis L. Horticulture & Seed. 6:81–83.
- Qureshi T, Memon N, Memon SQ, Ashraf MA. 2015. Decontamination of ofloxacin: optimization of removal process onto sawdust using response surface methodology. Desalin Water Treat. doi:https://doi.org/10.1080/19443994.2015.1006825.
- Shi L, Li H, Gao R, et al. 2009. Chromosome number and karyotype analysis of Althaea rosea. Jiangsu Agricultural Sciences. 5:173–174.
- Song J. 2001. Studies on the chromosome number, ploidy relationship and relative relationship of several plants in hibisucus. Shantou University, Shantou, China.
- Stebbins GL. 1971. Chromosomal evolution in higher plants. London: Edward Arnold.
- Surhio MA, Talpur FN, Nizamani SM, Amin FA, Bong CW, Lee CW, Ashraf MA, Shahd MR. 2014. Complete degradation of dimethyl phthalate by biochemical cooperation of the Bacillus thuringiensis strain isolated from cotton field soil. RSC Advances. 4:55960. doi: https://doi.org/10.1039/C4RA09465D
- Tabassum N, Rafique U, Balkhair KS, Ashraf MA. 2014. Chemodynamics of methyl parathion and ethyl parathion: adsorption models for sustainable agriculture. Biomedical Research International. doi:https://doi.org/10.1155/2014/831989.
- Wan D, Mei Q, Pan S. 2013. Determination of total flavonoids content in Hibisci mutabilis folium from different harvest periods. J South-Central Univ Nat (Nat. Sci. Edition). 32(4):46–49.
- Yan S, An Y, Sun R, et al. 2008. Research progress of karyotype analysis and chromosome micro-dissection technology. Biotechnology Bulletin. 4:70–74.
- Yang D, Zhu W, Lin J, et al. 2008. Mapping of QTLs for phenol reaction using chromosome segment substitution lines in rice (Oryza sativa L.). Jiangsu J. of Agr.Sci. 24(6):751–755.
- Zheng Z. 2009. Study on the formation of flower color from Hibiscus mutabilis. Agricultural University of Hunan.
- Zhu W, Wang C. 2008. A review on chromosomal segment substitution lines in crops. Jiangsu J of Agr.Sci. 24(6):963–968.